Abstract
Recent studies have demonstrated that in peripheral lymphoid tissues of normal mice and healthy humans, 1% to 5% of αβ T-cell receptor–positive (TCR+) T cells are CD4−CD8− (double-negative [DN]) T cells, capable of down-regulating immune responses. However, the origin and developmental pathway of DN T cells is still not clear. In this study, by monitoring CD4 expression during T-cell proliferation and differentiation, we identified a new differentiation pathway for the conversion of CD4+ T cells to DN regulatory T cells. We showed that the converted DN T cells retained a stable phenotype after restimulation and that furthermore, the disappearance of cell-surface CD4 molecules on converted DN T cells was a result of CD4 gene silencing. The converted DN T cells were resistant to activation-induced cell death (AICD) and expressed a unique set of cell-surface markers and gene profiles. These cells were highly potent in suppressing alloimmune responses both in vitro and in vivo in an antigen-specific manner. Perforin was highly expressed by the converted DN regulatory T cells and played a role in DN T-cell–mediated suppression. Our findings thus identify a new differentiation pathway for DN regulatory T cells and uncover a new intrinsic homeostatic mechanism that regulates the magnitude of immune responses. This pathway provides a novel, cell-based, therapeutic approach for preventing allograft rejection.
Introduction
In the peripheral lymphoid tissues of normal mice and humans, 1% to 5% of αβ T-cell receptor–positive (TCR+) T cells are CD4−CD8− (double-negative [DN]) T cells.1,2 MRL/Mpj-lpr/lpr mice have a mutant Fas gene and a massive lymphadenopathy consisting of an age-related accumulation of DN αβTCR+ T cells.3,4 The DN T cells have been shown to possess the capacity to regulate auto- and alloimmune responses and induce immune tolerance.5–8 However, the origin of peripheral DN T cells is still unclear. The heterogeneity of DN T- ells in the expression of surface markers suggests that several maturation/differentiation pathways may exist. In murine models, several studies have demonstrated that DN αβ TCR+ T cells can be derived directly from CD8+ T cells.9–12 Other studies suggest that DN αβ TCR+ natural killer T cells (NKT cells) arise extrathymically from bone marrow (BM).13 More recently, Ford et al reported that DN T regulatory cells can develop outside the thymus, but not from mature CD8+ T-cell precursors.14 However, a differentiation pathway of peripheral DN T cells from CD4+ T cells was not identified
In this report, we monitored CD4 expression during CD4+ T-cell proliferation and differentiation and identified a new pathway for the generation of a DN regulatory T-cell subset. This pathway uncovered a new intrinsic homeostatic mechanism that regulates the magnitude of immune responses to alloantigen both in vitro and in vivo. Our observations will permit the development of novel, cell-based, therapeutic approaches for the prevention of allograft rejection and for the treatment of autoimmune diseases.
Materials and methods
Mice
Male C57BL/6 (H-2b), C57BL/6 congenic for CD45.1, C57BL/6 TEa TCR-transgenic, C57BL/6 perforin gene knock-out (KO), C57BL/6 RAG−/−, DBA/2 (H-2d), C3H (H-2k), and B6D2F1 (H-2b/d) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Foxp3gfp knock-in C57BL/6 mice were provided by Dr Wenda Gao (Boston, MA).15 All mice were maintained in the animal facilities of Harvard Institutes of Medicine.
Reagents and antibodies
Recombinant mouse interleukin-2 (IL-2), IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were obtained from Biosource (Camarillo, CA). CD4+ T-cell enrichment column, T-cell enrichment column, and recombinant mouse IL-15 were obtained from R&D Systems (Minneapolis, MN). Fluorochrome-conjugated antibodies to mouse CD3, CD4, CD8, CD25, CD28, CD40, CD44, CD45.1, CD69, CD86, Ter119, B220, CD11b, CD11c, Gr1, NK1.1, TCRβ, TCRγδ, and isotype controls were obtained from eBioscience (San Diego, CA). Annexin V–PE was purchased from BD Pharmingen (San Diego, CA). CD4+CD25+ regulatory T cell (Treg) isolation kits, anti-PE microbeads, and magnetic bead separation columns were obtained from Miltenyi Biotec (Auburn, CA). Mitomycin C was obtained from Sigma (St Louis, MO).
Purification of CD4+, CD4+CD25+, CD4+CD25−, and CD4−CD8− DN T cells
Single-cell suspensions were prepared from the spleens and lymph nodes, and red blood cells (RBCs) were removed using RBC lysis buffer (Qiagen, Valencia, CA). CD4+ T cells were isolated by CD4+ T-cell enrichment columns (negative selection), followed by CD4+ positive selection. CD4+CD25+ Treg's were isolated using CD4+CD25+ Treg isolation kits. CD4+CD25− T cells were isolated with T-cell enrichment columns and subsequent deletion of CD25+, Ter119+, B220+, CD8+, CD11b+, TCRγδ+, and NK1.1+ cells. CD3+CD4−CD8− fractions were sorted following mixed lymphocyte reaction (MLR) using a Dakocytomation MoFlo cytometer (Dako, Carpinteria, CA) at the Beth Israel Deaconess Medical Center Flow Cytometry Core Facility.
Purification and maturation of DCs
BM cells were harvested from femurs and tibias. RBCs were removed using RBC lysis buffer; then, GR1+, Ter119+, and B220+ cells were depleted using magnetically conjugated antibodies. Purified BM cells were cultured with 20 ng/mL rGM-CSF and treated with LPS on day 5. Mature dendritic cells (mDCs) were separated by positive selection of CD86 and CD40 on day 6.
MLRs
CFSE (Molecular Probes, Eugene, OR)–labeled CD4+CD25+ Treg's, CD4+CD25− T cells, or CD4+ T cells were incubated for up to 5 to 6 days in 96-well round-bottom plates with mDCs at a ratio of 100 000 T cells to 25 000 DCs, or with mitomycin C–treated antigen-presenting cells (APCs) at a ratio of 100 000 T-cells to 100 000 APCs in culture. Recombinant rIL-2 or rIL-15 was added to the culture at a concentration of 5 ng/mL or 500 ng/mL, respectively.
In vivo analysis of proliferating CD4+ T cells
CFSE-labeled CD4+ T cells (20 × 106) from CD45.1 congenic C57BL/6 mice were transferred into B6D2F1 or C57BL/6 RAG−/− mice by tail vein injection. Mice were killed 3 or 7 days later; single-cell suspensions were prepared from harvested spleens and lymph nodes and stained with mAbs against CD45.1, CD3, and CD4.
Restimulation assays
C57BL/6 CD4+ and DN T cells sorted from MLR cultures were recultured with DBA/2 or C3H mDCs with or without rIL-2 (10 ng/mL), rIL-4 (10 ng/mL), and rIL-15 (500 ng/mL).
Suppression assays
CFSE-labeled CD45.1 naive CD4+CD25− T cells from C57BL/6 mice (1 × 105/well) were cocultured with DBA/2 or C3H mDCs (0.25 × 105/well) in 96-well plates for 5 days. Various numbers of DN T cells were added to MLRs as regulatory cells.
Adoptive transfer model of skin transplantation
C57BL/6 (H-2b) DN T cells (1 × 105) obtained from in vitro culture, in combination with 1 × 105 C57BL/6 naive CD4+CD25− T cells, were transferred to C57BL/6 RAG−/− mice by tail vein injection. On same day, full-thickness 1 cm2 tail skin allografts from DBA/2 (H-2d) or C3H (H-2k) mice were transplanted onto the recipient mice. Graft survival was analyzed by daily visual inspection with rejection defined as complete necrosis and loss of viable skin tissue.
Islet allograft transplantation
C57BL/6 (H-2b) mice were rendered diabetic using a single intraperitoneal injection of streptozotocin (250 mg/kg) 5 to 7 days prior to transplantation. DBA/2 (H-2d) or C3H (H-2k) islet transplantation was performed as described.16 C57BL/6 DN T cells (13 × 106), obtained from in vitro culture of naive C57BL/6 CD4+CD25− T cells with mature DBA/2 DCs plus rIL-15 for 6 days, were transferred to islet allograft recipients on the same day of transplantation. Graft survival was assessed by monitoring blood glucose levels with rejection defined as blood glucose levels greater than 16.65 mM (300 mg/dL) for 2 consecutive measurements.
Flow cytometric analysis
Cells from culture or host mice were harvested at various time points and analyzed for proliferation and expression of various cell-surface markers using an LSRII flow cytometer (BD Biosciences, Palo Alto, CA). Data were analyzed using FlowJo software (Treestar, Ashland, OR).
Real-time PCR
Messenger RNA was extracted from cells using an RNeasy mini-kit (Qiagen). Reverse transcription to cDNA was performed using TaqMan reverse transcription reagents obtained from Applied Biosystems (Foster City, CA). Specific message levels were quantified by real-time polymerase chain reaction (PCR) using the ABI 7700 Sequence Detection System (Applied Biosystems). All primers and probes for target genes were purchased from Applied Biosystems.
Statistical analysis
The effects of DN T cells on skin and islet allograft survival were statistically analyzed using a log-rank test.
Results
Peripheral CD4+ T cells convert to CD4− cells in vitro and in vivo
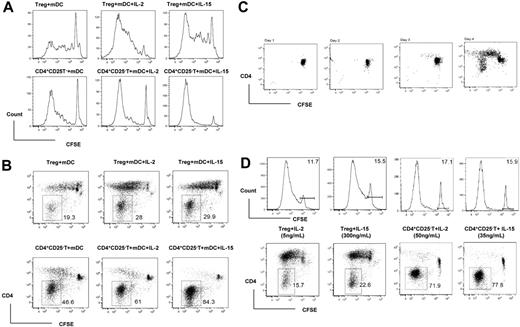
Recent studies showed that mature BM DCs, but not splenic DCs or other types of APCs, have the ability to reverse the anergic state of CD4+CD25+ Treg's in syngeneic and allogeneic systems in vitro.17–19 In an attempt to study the effects of T-cell growth factors (TCGFs) on the activation and proliferation of CD4+CD25+ Treg's and CD4+CD25− T cells in vitro, we used LPS-matured allogeneic BM DCs18 with or without TCGFs in an MLR as described in detail in “Materials and methods.” CFSE-labeled C57BL/6 CD4+CD25+ and CD4+CD25− T cells cocultured with mature allogeneic DCs proliferated vigorously during a 6-day MLR, and the addition of rIL-2 or rIL-15 further enhanced the proliferation (Figure 1A). Interestingly, we found that a significant proportion of the proliferated cells were CD4−. The percentage of CD4− cells ranged from 19.3%, when CD4+CD25+ T cells were cultured with mDCs, to 84.3%, when CD4+CD25− T cells were cultured with mDCs plus rIL-15 (Figure 1B).
rIL-2 and rIL-15 enhance the conversion of peripheral CD4+ T cells to CD4− cells via allogeneic mature BM DC stimulation. (A) Flow cytometry of CFSE-labeled T-cell proliferation induced by allogeneic mDCs and cytokines. CD4+CD25− (CD4+CD25−) and CD4+CD25+ (Treg) T cells from C57BL/6 mice were stimulated with mature DBA/2 DCs plus rIL-2 or rIL-15 for 5 days. CD3+ T cells were gated and used for CFSE analysis. (B) Flow cytometry of CD4− cells converted from C57BL/6 CD4+ T cells via mature DBA/2 DC stimulation. Numbers beside outlined areas indicate the percentage of cells in the designated gate. (C) CD4− cells appeared only after several cycles of cell proliferation induced by allogeneic mDCs. (D) At equivalent concentration to support alloantigen-triggered CD4+ T-cell proliferation, rIL-2 and rIL-15 exert similar potency to enhance the conversion of CD4+ into CD4− T cells. The horizontal bars gate the nondividing cells, and the numbers refer to the percentage of these cells.
rIL-2 and rIL-15 enhance the conversion of peripheral CD4+ T cells to CD4− cells via allogeneic mature BM DC stimulation. (A) Flow cytometry of CFSE-labeled T-cell proliferation induced by allogeneic mDCs and cytokines. CD4+CD25− (CD4+CD25−) and CD4+CD25+ (Treg) T cells from C57BL/6 mice were stimulated with mature DBA/2 DCs plus rIL-2 or rIL-15 for 5 days. CD3+ T cells were gated and used for CFSE analysis. (B) Flow cytometry of CD4− cells converted from C57BL/6 CD4+ T cells via mature DBA/2 DC stimulation. Numbers beside outlined areas indicate the percentage of cells in the designated gate. (C) CD4− cells appeared only after several cycles of cell proliferation induced by allogeneic mDCs. (D) At equivalent concentration to support alloantigen-triggered CD4+ T-cell proliferation, rIL-2 and rIL-15 exert similar potency to enhance the conversion of CD4+ into CD4− T cells. The horizontal bars gate the nondividing cells, and the numbers refer to the percentage of these cells.
To examine the source of these CD4− cells, highly purified CD4+CD25− T cells (> 99% pure) were cultured with mature allogeneic DCs plus rIL-15. As shown in Figure 1C, the CD4− cells were not detectable at days 1, 2, and 3 of the MLR, suggesting that the CD4− cells did not arise from contamination of the culture. The CD4− cells first appeared on day 4 of the MLR accompanied by robust cell proliferation. Moreover, the CD4 phenotype was only seen in cells that had undergone 4 to 5 rounds of alloantigen-triggered CD4 T-cell proliferation (determined by CFSE intensity), indicating that the CD4 cells were converted from proliferated CD4 T cells (Figure 1C). The same kinetic change was found in the conversion of CD4+CD25+ T cells to CD4− cells (data not shown).
To quantitatively evaluate the impact of rIL-2 and rIL-15 on the conversion of CD4+ T cells to CD4− cells, we titrated the doses of rIL-2 and rIL-15 in MLRs. We noted that markedly different doses of the 2 cytokines were required to achieve equivalent degrees of enhancement of the alloantigen-triggered proliferation in CD4+CD25+ versus CD4+CD25− T cells (Figure 1D). For example, to produce approximately 85% divided cells from CD4+CD25− T cells required 50 ng/mL rIL-2 or 35 ng/mL rIL-15, while CD4+CD25+ T cells required 5 ng/mL rIL-2 or 300 ng/mL rIL-15 (Figure 1D). However, we found that when we compared dosages that supported equivalent degrees of alloantigen-triggered proliferation, the 2 cytokines induced a similar degree of conversion to CD4− T cells (approximately 70% for CD4+CD25− T cells, and approximately 20% for CD4+CD25+ T cells; Figure 1D).
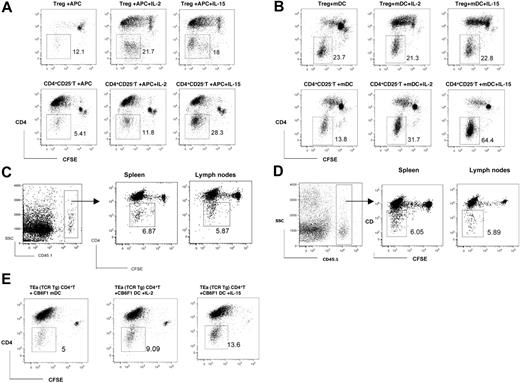
We further examined the conversion of CD4+ T cells to CD4− T cells in the MLR by using mitomycin C–treated allogeneic APCs or syngeneic mDCs. We found that both CFSE-labeled CD4+CD25− and CD4+CD25+ T cells from the spleen and lymph nodes of naive congenic CD45.1 C57BL/6 mice can be converted to CD4− cells after a 6-day stimulation with mitomycin C–treated DBA/2 allogeneic APCs (Figure 2A) or syngeneic mDCs (Figure 2B) in vitro. The addition of rIL-2 or rIL-15 in the culture significantly enhanced the conversion (Figure 2A).
In vivo or in vitro conversion of peripheral CD4+ T cells to CD4− T cells via auto- or alloantigen-triggered or homeostatic proliferation. (A) Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells via mitomycin C–treated DBA/2 APC stimulation in vitro. (B) Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells via syngeneic mDC stimulation in vitro. (C) CFSE-labeled C57BL/6 (CD45.1) CD4+ T cells were transferred to B6D2F1 mice by intravenous injection. Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells harvested from spleens and lymph nodes of B6D2F1 mice on day 3. (D) CFSE-labeled C57BL/6 (CD45.1) CD4+ T cells were transferred to syngeneic Rag KO mice by intravenous injection. Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells harvested from spleens and lymph nodes of syngeneic Rag KO mice on day 7. (E) Flow cytometry of CD4− cells converted from C57BL/6 TEa transgenic CD4+ T cells via CB6F1 mDC stimulation in vitro. Numbers refer to the percentages of gated CD4− cells.
In vivo or in vitro conversion of peripheral CD4+ T cells to CD4− T cells via auto- or alloantigen-triggered or homeostatic proliferation. (A) Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells via mitomycin C–treated DBA/2 APC stimulation in vitro. (B) Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells via syngeneic mDC stimulation in vitro. (C) CFSE-labeled C57BL/6 (CD45.1) CD4+ T cells were transferred to B6D2F1 mice by intravenous injection. Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells harvested from spleens and lymph nodes of B6D2F1 mice on day 3. (D) CFSE-labeled C57BL/6 (CD45.1) CD4+ T cells were transferred to syngeneic Rag KO mice by intravenous injection. Flow cytometry of CD4− cells converted from C57BL/6 (CD45.1) CD4+ T cells harvested from spleens and lymph nodes of syngeneic Rag KO mice on day 7. (E) Flow cytometry of CD4− cells converted from C57BL/6 TEa transgenic CD4+ T cells via CB6F1 mDC stimulation in vitro. Numbers refer to the percentages of gated CD4− cells.
We also tracked the CD4 expression during alloantigen-triggered and homeostatic proliferation in vivo. We adoptively transferred CFSE-labeled highly purified CD4+ T cells (> 99% pure) from congenic CD45.1 naive C57BL/6 mice into semiallogeneic B6D2F1 or syngeneic immune-deficient Rag KO mice. As Figure 2C shows, the congeneic CD45.1+ CD4+ T cells underwent a robust proliferation in allogeneic B6D2F1 hosts 3 days after adoptive transfer. After 4 to 5 rounds of proliferation, 5.87% and 6.87% of CD45.1+ CD4+ T cells harvested from the lymph nodes and spleen of B6D2F1 hosts converted to CD4− T cells, respectively (Figure 2C). A similar pattern of in vivo homeostatic proliferation and conversion of the congeneic CD45.1+ CD4+ T cells harvested from syngeneic Rag KO hosts, 7 days after adoptive transfer, were demonstrated in Figure 2D.
In order to determine that the CD4+ T cell–converted CD4− cells and the CD4+ T cells from which they are derived are class II–restricted, we used the TEa mouse, a MHC II–restricted CD4 TCR transgenic system. TCR stimulation is delivered by culturing Vβ6+CD4+ T cells from C57BL/6 TEa TCR-transgenic mice with C57BL/6 × BALB/c F1 (CB6F1) DCs. TEa TCR-transgenic CD4+ T cells recognize I-Ed peptides presented by I-Ab MHC II molecules. Transgenic T cells are therefore capable of responding to I-Ed peptides present on the CB6F1 DCs. As Figure 2E shows, Vβ6+ CD4+ T cells from C57BL/6 TEa TCR-transgenic mice proliferated vigorously during a 6-day MLR with CB6F1 DCs. After 4 to 5 rounds of proliferation, 5% of Vβ6+CD4+ T cells converted to CD4− T cells, and the addition of rIL-2 or rIL-15 in the culture significantly enhanced the conversion.
A unique phenotype and gene profile
To characterize the converted CD4− cells, we examined the expression of cell-surface markers on CD4− cells obtained after MLR. The converted CD4− cells express a unique set of cell-surface markers, as shown in Figure 3. The CD4− T cells are CD4−, CD8−, CD3+, TCRβ+, NK1.1−, CD44+, CD25+, CD69+, and CD28+ (Figure 3A). Since the converted CD4− cells arise from CD4+ T cells and are CD8− and CD3+, we have named them CD4+ T-cell–converted DN T cells.
CD4− cells converted from CD4+ T cells show distinctive cell-surface marker and cytokine expression profiles. (A) The induced CD4− T cells were stained with antibodies to indicate cell-surface markers. (B) The relative expression of CD4 and CD8 genes was determined by real-time RT-PCR on different cell populations. Results shown here represent 3 independent experiments. (C) Most of the DN T cells are annexin V−, although most of the activated CD4+ T cells are annexin V+. The horizontal bars gate annexin V staining positive cells, and the numbers refer to the percentages of these cells. (D) CD4+Foxp3-GFP− and CD4+Foxp3−GFP+ T cells from Foxp3-GFP knock-in C57BL/6 mice were stimulated with mature DBA/2 DC alone or plus rIL-15 for 6 days. The DN T cells are GFP− (Foxp3−). (E) The relative expression of indicated genes was determined by real-time RT-PCR on different cell populations. Results shown here represent 4 independent experiments. *CD4+CD25− T DN: DN T cells converted from CD4+CD25− T cells. **Treg DN: DN T cells converted from CD4+CD25+ Treg's.
CD4− cells converted from CD4+ T cells show distinctive cell-surface marker and cytokine expression profiles. (A) The induced CD4− T cells were stained with antibodies to indicate cell-surface markers. (B) The relative expression of CD4 and CD8 genes was determined by real-time RT-PCR on different cell populations. Results shown here represent 3 independent experiments. (C) Most of the DN T cells are annexin V−, although most of the activated CD4+ T cells are annexin V+. The horizontal bars gate annexin V staining positive cells, and the numbers refer to the percentages of these cells. (D) CD4+Foxp3-GFP− and CD4+Foxp3−GFP+ T cells from Foxp3-GFP knock-in C57BL/6 mice were stimulated with mature DBA/2 DC alone or plus rIL-15 for 6 days. The DN T cells are GFP− (Foxp3−). (E) The relative expression of indicated genes was determined by real-time RT-PCR on different cell populations. Results shown here represent 4 independent experiments. *CD4+CD25− T DN: DN T cells converted from CD4+CD25− T cells. **Treg DN: DN T cells converted from CD4+CD25+ Treg's.
To determine the mechanism of the disappearance of CD4 expression on the cell surface, we analyzed CD4 gene expression of converted CD4− T cells by using real-time reverse transcription (RT)–PCR. As shown in Figure 3B, the CD4 gene was highly expressed in CD4+ T cells. In contrast, there was no detectable CD4 gene expression in converted CD4− T cells. In addition, the CD8 gene was highly expressed in CD8+ T cells, but not in CD4+ and converted DN T cells. Thus, the CD4 gene was silent in converted DN T cells.
Previous studies have reported that activation-induced cell death (AICD) is a routine consequence of T-cell activation, and that apoptotic events occur after a discrete number of T-cell divisions.20 We compared the frequency of apoptotic cells between the proliferated CD4+ and DN T-cell subsets. Among the proliferated CD4+ T cells, 54% were staining positive for annexin V+ after a 5-day MLR (Figure 3C). Surprisingly, there were only 6.12% annexin V+–staining cells among converted DN T cells, even though they have gone through 4 to 8 rounds of cell division (Figure 3C), suggesting that the converted DN T cells were resistant to AICD.
The forkhead family transcription factor Foxp3 acts as the natural Treg cell lineage specification factor and thus identifies Treg's independently of CD25 expression.21 Using a gene-targeting approach, Foxp3gfp knock-in mice were generated, in which a bicistronic EGFP reporter was introduced into the endogenous Foxp3 locus,15 allowing us to track Foxp3 expression. Interestingly, we found that CD4+CD25+Foxp3gfp+ T cells from Foxp3gfp knock-in mice lost their Foxp3gfp expression when they switched to DN T cells, while CD4+CD25−Foxp3gfp− T cells remained Foxp3gfp− when they switched to DN T cells (Figure 3D). Thus, the converted DN T cells from both CD4+CD25− and CD4+CD25+ origin were Foxp3−.
Next we used the quantitative real-time PCR technique to further analyze the gene expression profile of converted DN T cells. As shown in Figure 3D, neither DN T cells converted from CD4+CD25− nor from CD4+CD25+ T cells express Foxp3, and only expressed the Treg-related genes CTLA4 and TGFβ at low levels (Figure 3E). The IL-2, IL-4, and IFNγ genes were highly expressed by activated CD4+ T cells, but were expressed at low levels by DN T cells. Interestingly, DN T cells expressed high levels of the cytotoxic lymphocyte–related genes perforin and granzyme B. Thus, DN T cells converted from both CD4+CD25− and CD4+CD25+ T cells shared a similar gene expression profile that was distinctive from naive CD4+CD25−, naive CD4+CD25+ Treg's, and activated CD4+ T cells.
Converted DN T cells are Treg's
To analyze the functional properties of CD4+-converted DN T cells, we isolated CD4+ and converted DN T cells from MLR by cell-sorting. Upon restimulation in a secondary MLR with the same strain of mature DCs (DBA/2) as used in the primary MLR, C57BL/6 CD4+ T cells proliferated vigorously, and the addition of rIL-2, rIL-4, or rIL-15 further enhanced proliferation (Figure 4A). In contrast, DN T cells were hyporesponsive when restimulated by mDCs. Interestingly, rIL-2 and rIL-15, but not rIL-4, completely restored the responsiveness of DN T cells (Figure 4A). Moreover, DN T cells retained a stable CD4− phenotype after restimulation with mDCs, even after robust proliferation by restimulation with mDCs plus IL-2 or IL-15 (Figure 4B).
DN T cells are anergic upon restimulation by mDCs and are potent in suppressing same-alloantigen–triggered naive CD4+CD25− T-cell proliferation. (A) DN T cells and CD4+ T cells isolated from primary MLRs stimulated by DBA/2 mDCs plus IL-15 were restimulated by mature DBA/2 DCs plus the indicated cytokines for 4 days. Proliferation was determined by [3H] TdR incorporation and shown as means of 3 independent experiments. Error bars represent standard deviation. (B) DN T cells retain the DN phenotype 4 days after restimulation with mDCs with or without IL-2 or IL-15. (C) C57BL/6 DN T cells induced by mature DBA/2 DCs potently suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by the same alloantigens (mature DBA/2 DCs). The horizontal bars gate the nondividing cells, and the numbers refer to the percentages of these cells. (D) C57BL/6 DN T cells induced by mature DBA/2 DCs suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by third party alloantigens (mature C3H DCs) at lower efficacy.
DN T cells are anergic upon restimulation by mDCs and are potent in suppressing same-alloantigen–triggered naive CD4+CD25− T-cell proliferation. (A) DN T cells and CD4+ T cells isolated from primary MLRs stimulated by DBA/2 mDCs plus IL-15 were restimulated by mature DBA/2 DCs plus the indicated cytokines for 4 days. Proliferation was determined by [3H] TdR incorporation and shown as means of 3 independent experiments. Error bars represent standard deviation. (B) DN T cells retain the DN phenotype 4 days after restimulation with mDCs with or without IL-2 or IL-15. (C) C57BL/6 DN T cells induced by mature DBA/2 DCs potently suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by the same alloantigens (mature DBA/2 DCs). The horizontal bars gate the nondividing cells, and the numbers refer to the percentages of these cells. (D) C57BL/6 DN T cells induced by mature DBA/2 DCs suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by third party alloantigens (mature C3H DCs) at lower efficacy.
We further analyzed the function of DN T cells by determining if converted DN T cells suppress alloantigen-triggered proliferation of naive CD4+CD25− T cells. As shown in Figure 4C, CFSE-labeled naive congenic CD45.1 C57BL/6 CD4+CD25− T cells underwent vigorous proliferation during a 5-day MLR with either allogeneic DBA/2- or C3H-derived mDCs (Figure 4C-D). We found that DN T cells converted from both naive C57BL/6 CD4+CD25− and CD4+CD25+ T cells in a primary MLR with DBA/2 mature DCs exerted powerful inhibition on naive CD4+CD25− T cells stimulated with the same alloantigen. The addition of rIL-2 and rIL-15 in a primary culture, which significantly enhanced the conversion of CD4+ to DN T cells, did not have adverse effects on the potency of DN T cells to suppress alloantigen-triggered naive CD4+CD25− T-cell proliferation in a secondary MLR (Figure 4C). Moreover, the potency of suppression of DN T cells tended to be alloantigen specific. As shown in Figure 4D, DN T cells converted during DBA/2 alloantigen stimulation suppressed C3H alloantigen–triggered naive CD4+CD25− T-cell proliferation in a secondary MLR with lower efficacy. The differences of efficacy were more profound when naive CD4+CD25− T cells were cocultured with DN T cells at a 4:1 ratio (100 000 CD4+CD25− T cells to 25 000 DN T cells; Figure 4D).
AICD is an important intrinsic mechanism that controls the magnitude of immune responses.22,23 To determine how converted DN T cells may exert their regulatory effects, we analyzed the impact of DN T cells on AICD of proliferated CD4+ T cells by measuring the apoptotic events among proliferated CD4+ T cells cocultured with mDCs with or without DN T cells. As shown in Figure 5A, there were 24.8% annexin V+ cells among proliferated CD4+ T cells when CD4+CD25− T cells were cocultured with mature allogeneic DCs alone. In contrast, there were 75.7% annexin V+ cells among proliferated CD4+ T cells when DN T cells were added to the coculture at a 1:1 ratio (T effector cell to DN T cell). Thus, DN T cells exaggerated cell death of proliferated CD4+ T cells in MLR.
DN T cells enhance AICD of proliferated CD4+ T cells, and perforin plays a role in DN T-cell–mediated cell death. (A) Annexin V staining of C57BL/6 CD4+ T cells stimulated by mature DBA/2 DCs. C57BL/6 DN T cells greatly increased the annexin V+ frequency in proliferating C57BL/6 CD4+ T cells. In the upper panels, the horizontal bars gate the dividing cells, and the numbers refer to the percentage of these cells; in the lower panels, the horizontal bars gate annexin V staining positive cells, and the numbers refer to the percentages of these cells. (B) CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T cells were stimulated by mature DBA/2 DCs. The suppressor function of C57BL/6 DN T cells converted from wild-type and perforin KKO mice was compared. Results shown here represent 3 independent experiments. The horizontal bars gate the nondividing cells, and the numbers refer to the percentages of these cells. (C) DN T-cell–mediated suppression of CD4+CD25− T cell proliferation was attenuated in the absence of perforin. Results shown here are means of 3 independent experiments. (D) Annexin V staining of C57BL/6 CD4+CD25− T cells stimulated by mature DBA/2 DCs. DN T cells converted from wild-type, but not perforin KO C57BL/6 mice greatly increased the annexin V+ population in proliferating C57BL/6 CD4+ T cells.
DN T cells enhance AICD of proliferated CD4+ T cells, and perforin plays a role in DN T-cell–mediated cell death. (A) Annexin V staining of C57BL/6 CD4+ T cells stimulated by mature DBA/2 DCs. C57BL/6 DN T cells greatly increased the annexin V+ frequency in proliferating C57BL/6 CD4+ T cells. In the upper panels, the horizontal bars gate the dividing cells, and the numbers refer to the percentage of these cells; in the lower panels, the horizontal bars gate annexin V staining positive cells, and the numbers refer to the percentages of these cells. (B) CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T cells were stimulated by mature DBA/2 DCs. The suppressor function of C57BL/6 DN T cells converted from wild-type and perforin KKO mice was compared. Results shown here represent 3 independent experiments. The horizontal bars gate the nondividing cells, and the numbers refer to the percentages of these cells. (C) DN T-cell–mediated suppression of CD4+CD25− T cell proliferation was attenuated in the absence of perforin. Results shown here are means of 3 independent experiments. (D) Annexin V staining of C57BL/6 CD4+CD25− T cells stimulated by mature DBA/2 DCs. DN T cells converted from wild-type, but not perforin KO C57BL/6 mice greatly increased the annexin V+ population in proliferating C57BL/6 CD4+ T cells.
Perforin, a cytotoxic lymphocyte-related cytokine, was highly expressed by DN T cells (Figure 3D), and was therefore a candidate molecule for mediating DN T-cell suppression of alloantigen-triggered CD4+CD25− T-cell proliferation. We compared the suppressive function of DN T cells converted from CD4+ T cells from wild-type C57BL/6 mice with that from perforin gene KO C57BL/6 mice. Figure 5B and C show that the ability of DN T cells derived from perforin KO mice to suppress alloantigen-triggered proliferation of naive CD4+CD25− T cells was significantly lower than that of DN T cells from wild-type mice. The inhibition rate decreased from 71.6% to 29.2% when CD4+CD25− T cells were cocultured with DN T cells at a 1:1 ratio and from 55.3% to 13.3% when CD4+CD25− T cells were cocultured with DN T cells at a 4:1 ratio (Figure 5C). Figure 5D illustrates that the addition of DN T cells derived from perforin KO mice, but not from wild-type mice, into the coculture did not increase the frequency of annexin V+ cells among activated CD4+ T cells, indicating that perforin played a role, at least in part, in DN T-cell–mediated cell death and suppression.
Potency in suppressing alloimmune responses in vivo
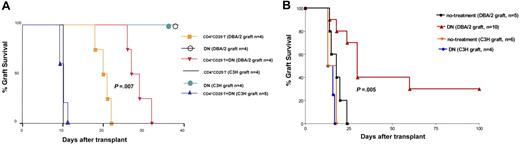
To test the functional potential of the converted DN T cells in vivo, we utilized an adoptive transfer model of skin allografts.24 C57BL/6 (H-2b) RAG−/− recipients received 100 000 naive C57BL/6 CD4+CD25− T cells with or without 100 000 DN T cells (converted from CD4+CD25− T cells of naive C57BL/6 mice by coculture with mature DBA/2 [H-2d] DCs plus rIL-15 in MLR for 6 days). An alloantigen-specific DBA/2 or control third-party strain C3H (H-2k) tail skin graft was placed on the same day. As shown in Figure 6A, adoptive transfer of 100 000 naive C57BL/6 CD4+CD25− T cells were capable of triggering acute rejection of DBA/2 or C3H skin allografts with mean graft survival times of 20 days and 10 days, respectively. In contrast, adoptive transfer of the same number of converted C57BL/6 DN T cells alone did not trigger rejection of DBA/2 or C3H skin allografts, indicating that the DN T cells were anergic upon alloantigen restimulation. However, significant prolongation of DBA/2 skin allografts occurred when equal numbers of naive C57BL/6 CD4+CD25− and DN T cells (converted from naive C57BL/6 CD4+CD25− T cells after a 6-day MLR with DBA/2 mDCs plus IL-15) were cotransferred (Figure 6A; mean graft survival time of 28.5 days; P < .001). In contrast, the cotransferred DN T cells did not protect third-party strain C3H skin allografts from acute rejection (Figure 6A; mean graft survival time of 10 days; P = .13). Thus, DN T cells were capable of suppressing naive CD4+CD25− T-cell–triggered skin allograft rejection in vivo in an alloantigen-specific manner.
DN T cells prolong alloantigen-specific MHC-mismatched skin and islet allograft survival in vivo. (A) DN T cells suppress naive CD4+CD25− T-cell–triggered skin allograft rejection in an alloantigen-specific manner. The rejection of skin grafts from DBA/2 or C3H mice transplanted into C57BL/6 RAG−/− mice was induced by adoptive transfer of naive C57BL/6 CD4+CD25− T cells. Cotransfer of C57BL/6 DN T cells suppressed the rejection more efficiently in mice that received DBA/2 grafts. Statistical analyses were performed using a log-rank test. (B) DN T cells significantly prolonged MHC-mismatched islet allograft survival in an alloantigen-specific manner in immune-competent recipients. Administration of 13 × 106 DN T cells significantly prolonged alloantigen-specific DBA/2, but not third-party C3H, islet allograft survival. Statistical analyses were performed using a log-rank test.
DN T cells prolong alloantigen-specific MHC-mismatched skin and islet allograft survival in vivo. (A) DN T cells suppress naive CD4+CD25− T-cell–triggered skin allograft rejection in an alloantigen-specific manner. The rejection of skin grafts from DBA/2 or C3H mice transplanted into C57BL/6 RAG−/− mice was induced by adoptive transfer of naive C57BL/6 CD4+CD25− T cells. Cotransfer of C57BL/6 DN T cells suppressed the rejection more efficiently in mice that received DBA/2 grafts. Statistical analyses were performed using a log-rank test. (B) DN T cells significantly prolonged MHC-mismatched islet allograft survival in an alloantigen-specific manner in immune-competent recipients. Administration of 13 × 106 DN T cells significantly prolonged alloantigen-specific DBA/2, but not third-party C3H, islet allograft survival. Statistical analyses were performed using a log-rank test.
Based on the finding of allospecific suppressive function of DN T cells in the in vivo adoptive T-cell transfer model of skin allograft, we sought to determine if the administration of a relatively small number of DN T cells as a monotherapy would have any effect on graft survival in an immunocompetent MHC–completely mismatched transplantation model. We chose a pancreatic islet transplantation model in which 13 × 106 DN T cells (converted from CD4+CD25− T cells of naive C57BL/6 mice by coculture with mature DBA/2 [H-2d] DCs plus rIL-15 in MLR for 6 days) were transferred into streptozotocin-induced diabetic C57BL/6 recipients at the time of islet cell transplantation. As shown in Figure 6B, the transfer of 13 × 106 DN T cells resulted in a statistically significant prolongation of alloantigen-specific DBA/2 strain, but not the third-party C3H strain, islet allograft survival compared with that of the untreated control group (P = .005). This demonstrates the utility of ex vivo CD4+ T-cell–converted alloantigen-specific DN Treg's as an immune modulatory therapy in preventing allograft rejection in a MHC-mismatched islet allograft model.
Discussion
Accumulating evidence supports the existence of several diverse populations of Treg's that are engaged in peripheral tolerance.23 These different regulatory populations function in different ways; some are naturally produced, while others are locally induced as a result of specific types of immune responses.23 Further characterization of the function and development of these Treg's will contribute to our understanding of the intrinsic and extrinsic mechanisms during an acquired process that maintain peripheral tolerance.
By monitoring the CD4 expression during CD4+ T-cell proliferation and differentiation, we identified a differentiation pathway of a DN Treg subset. We provide evidence that after 4 to 5 rounds of antigen-triggered or homeostatic CD4+ T-cell proliferation, CD4+ T cells can convert to DN T cells, and IL-2 and IL-15 enhanced this conversion. Moreover, DN T cells retained a stable phenotype after restimulation with mDCs plus IL-2 or IL-15 (Figure 4B). The disappearance of cell-surface CD4 molecules on converted DN T cells, identified by fluorescence-activated cell-sorter (FACS) analysis (Figure 3A), was shown to be a result of CD4 gene silencing in converted DN T cells as determined by real-time PCR (Figure 3B). Interestingly, the DN T cells converted from either CD4+CD25− T cells or CD4+CD25+ Treg's share common phenotypes and gene profiles that were distinctive from CD4+CD25+ Treg's, naive CD4+CD25− T cells, and activated CD4+ T cells (Figure 3A-E). Moreover, the CD4+-converted DN T cells were αβ TCR+CD28+CD44+NK1.1−, which differ from thymus derived γδ TCR+ DN T cells and from BM-derived DN natural suppressor T cells that express NK1.125–27 (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). The CD4+-converted DN T cells also differ from DN T-cell clones described by Zhu-Xu Zhang that did not express CD28 and CD44 at any time point after activation,2 and DN T cells converted from CD8+ T cells that were activated in the presence of IL-4.28 Although several studies have reported that in the peripheral lymphoid tissues of normal mice and healthy humans, 1% to 5% of total lymphocytes are αβ-TCR+ DN T cells.1,2 Nevertheless, the origin of peripheral DN T cells is still unclear, and the differentiation of peripheral DN T cells from activated CD4+ T cells has not been previously described.
In this study, we provide evidence that after 4 to 5 rounds of antigen-triggered proliferation or homeostatic proliferation, peripheral mature CD4+ T cells could convert to DN T cells, which express a unique set of surface markers and gene profiles. This study further identifies the CD4+-converted DN T cells as functional Treg's. DN T cells are anergic upon restimulation with mature allogeneic DCs, and the addition of rIL-2 and rIL-15, but not rIL-4, completely reinstate their responsiveness. Although the DN T cells converted from both CD4+CD25+Foxp3+ Treg's and CD4+CD25− Foxp3− T cells are Foxp3−, they were highly potent in suppressing alloimmune responses in vitro and in vivo in an alloantigen-specific manner. It was notable that on a per-cell basis comparison, the converted DN T cells were more potent in suppressing alloantigen-trigged proliferation of naive CD4+CD25− T cells than naive and proliferated CD4+CD25+ Treg's (data not shown). Moreover, this study provides evidence that 1 mechanism DN T cells use to suppress alloantigen-triggered naive CD4+CD25− T-cell proliferation is through exaggeration of AICD in activated CD4+ T cells. Perforin, a cytotoxic lymphocyte-related cytokine, was highly expressed by DN T cells and also played a role in DN T-cell–mediated suppression.
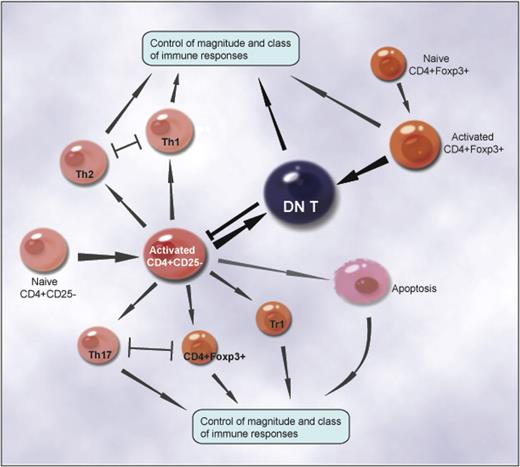
The immune system is a homeostatic organization that must regulate itself to avert insufficient immunity and suppress excessive responses.23 The expression of the CD4 glycoprotein on mature T cells defines a major functional subset of T cells. Upon activation, naive CD4+CD25− T cells are capable of differentiating into T helper 1 (TH1), TH2, and TH17 effectors, or CD4+CD25+Foxp3+, Tr1 regulatory cells, depending on the nature and strength of TCR and costimulation signaling and the cytokine milieu of the microenvironment.15,29–33 The intrinsic homeostatic mechanisms that occur during the initial antigen-induced activation of CD4+ T cells control the magnitude and class of immune responses, including the emergence of TH1, TH2, and TH17 effectors and CD4+CD25+Foxp3+, Tr1 regulatory cells.23 The dichotomy of Th1 and Th2 T-cell subsets,34–37 the reciprocal differentiation of Treg's, TH17 effectors,15 and AICD22,23 illustrate the complexity of intrinsic homeostatic mechanisms that control the magnitude and class of immune responses to infectious organisms and tissue inflammation (Figure 7).
The intrinsic homeostatic mechanisms that occur during the initial antigen-induced activation of CD4+ T cells control the magnitude and class of immune responses, including the emergence of TH1, TH2, and TH17 effectors and CD4+CD25+Foxp3+-, Tr1-, and CD4+-converted DN regulatory cells. The dichotomy of TH1 and TH2 T-cell subsets, the reciprocal differentiation of Treg's and TH17 effectors, and AICD elucidate how the intrinsic homeostatic mechanisms control the magnitude and class of immune responses to infectious organisms and tissue inflammation. A new pathway of differentiating previously unidentified DN Treg's represents a negative feedback mechanism that regulates the magnitude of immune responses.
The intrinsic homeostatic mechanisms that occur during the initial antigen-induced activation of CD4+ T cells control the magnitude and class of immune responses, including the emergence of TH1, TH2, and TH17 effectors and CD4+CD25+Foxp3+-, Tr1-, and CD4+-converted DN regulatory cells. The dichotomy of TH1 and TH2 T-cell subsets, the reciprocal differentiation of Treg's and TH17 effectors, and AICD elucidate how the intrinsic homeostatic mechanisms control the magnitude and class of immune responses to infectious organisms and tissue inflammation. A new pathway of differentiating previously unidentified DN Treg's represents a negative feedback mechanism that regulates the magnitude of immune responses.
In this study, we uncovered a new intrinsic homeostatic mechanism that regulates the magnitude of immune responses. Our results show that a subset of proliferated CD4+ T cells convert to DN regulatory cells after 4 to 5 rounds of either antigen-triggered or homeostatic proliferation in vitro and in vivo (Figure 7). The converted DN regulatory cells were resistant to AICD. These cells also potently suppressed antigen-specific alloimmune responses both in vitro and in vivo. The strength of TCR signaling (mDCs vs micomycin C–treated APCs), the duration of the stimulation (1-3 days vs 4-7 days), and the cytokine milieu (IL-2 and IL-15 vs IL-4) all play important roles in this differentiation pathway of DN regulatory T cells. This previously unknown differentiation pathway of DN Treg's represents a negative feedback mechanism that regulates the magnitude of immune responses (Figure 7). As DN T cells can be converted from both naive CD4+CD25− T cells and CD4+CD25+ Treg's, this pathway favors regulation. In addition, the antigen specificity of converted DN T cells may render them more potent in the regulation of secondary immune responses. Tracking the differentiation pathway of CD4+-converted DN T cells in pathologic and physiologic conditions that trigger CD4+ T-cell proliferation, such as persistent infection, tissue injury, auto- or alloantigen stimulation, and lymphopenia, may lead to a greater understanding of the potentially significant role of CD4+-converted DN T cells and this new intrinsic homeostatic mechanism in infection, tumor immunology, autoimmunity, and transplantation. Indeed, we were able to isolate native αβ TCR+CD28+CD44+NK1.1− DN T cells from the spleen and lymph nodes of naive C57BL/6 mice, and these DN T cells suppress alloantigen-triggered naive CD4+CD25− T cell proliferation (Figure S2). However, to fully understand the physiologic and pathologic significance of DN T cells in vivo, further studies are warranted. The MRL/Mpj-lpr/lpr mice, which have a mutant Fas gene and an age-related lymphoproliferation and accumulation of DN αβ-TCR+ T cells,3,4 may provide an autoimmune model to study the differentiation pathway of CD4+-converted DN T cells in vivo.
As Treg's play important roles in the maintenance of peripheral tolerance, the therapeutic potential for the transfer of Treg's has been explored in autoimmune and transplantation models.38–41 However, the precise definition and an antigen specificity of regulatory cells, as well as the feasibility of obtaining a sufficient number of cells, are notable obstacles for clinical application.
In this study, we identified a new pathway to generate antigen-specific DN T cells from peripheral CD4+ T cells. The isolation of tens of millions of highly purified CD4+ T cells from the peripheral blood of a patient is within reach of current clinical technology. By stimulating highly purified CD4+ T cells with syngeneic or allogeneic CD3− mature BM DCs or APCs plus rIL-2 and/or rIL-15, we should be able to convert tens of millions of antigen-specific DN T cells within a few days. The converted DN Treg's could be easily purified by a simple CD3+ and CD4− selection technique and used as cell-based therapeutic approach for treating autoimmune diseases and preventing allograft rejection. Our results using CD4+-converted DN T cells in skin and islet transplantation models prove the concept and its feasibility (Figure 6).
In summary, our findings identify a new differentiation pathway of a DN Treg subset, uncover a new intrinsic homeostatic mechanism that regulates the magnitude of immune responses, and further provide a potentially novel, cell-based, therapeutic approach for the prevention of allograft rejection and the treatment of autoimmune diseases.
Authorship
Author contributions: D.Z. designed and conducted research, collected and analyzed data; W.Y. performed research, and collected and analyzed data; N.D., Y.T., and A.M. performed research; and X.X.Z. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Xin Xiao Zheng, Transplant Research Center, Beth Israel Deaconess Medical Center, Harvard Medical School, HIM 1028, 77 Ave Louis Pasteur, Boston, MA 02115; e-mail: xzheng@bidmc.harvard.edu.
The online version of this article contains a data supplement.
Supported by grants provided by the National Institutes of Health (X.X.Z.) and the Juvenile Diabetes Research Foundation (X.X.Z., D.Z.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr Yang Shi (Harvard Medical School, Boston) and Dr Vijay Kuchroo (Harvard Medical School, Brigham and Women's Hospital, Boston) for reviewing the manuscript, Dr Wenda Gao for providing Foxp3gfp knock-in C57BL/6 mice, Dr James Kenny for advice about DC preparation, and Dr Xu Huang for illustration design.


![Figure 4. DN T cells are anergic upon restimulation by mDCs and are potent in suppressing same-alloantigen–triggered naive CD4+CD25− T-cell proliferation. (A) DN T cells and CD4+ T cells isolated from primary MLRs stimulated by DBA/2 mDCs plus IL-15 were restimulated by mature DBA/2 DCs plus the indicated cytokines for 4 days. Proliferation was determined by [3H] TdR incorporation and shown as means of 3 independent experiments. Error bars represent standard deviation. (B) DN T cells retain the DN phenotype 4 days after restimulation with mDCs with or without IL-2 or IL-15. (C) C57BL/6 DN T cells induced by mature DBA/2 DCs potently suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by the same alloantigens (mature DBA/2 DCs). The horizontal bars gate the nondividing cells, and the numbers refer to the percentages of these cells. (D) C57BL/6 DN T cells induced by mature DBA/2 DCs suppress CFSE-labeled C57BL/6 (CD45.1) CD4+CD25− T-cell proliferation triggered by third party alloantigens (mature C3H DCs) at lower efficacy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-10-050625/4/m_zh80090700190004.jpeg?Expires=1768151850&Signature=Lu70Z3EgzHKyZJ0~6aOGDwZqAX934IL9FnYPmjGpfvac3wCJmBqNuJVVUGbcLqiZMGDxZTLrcv17yAO1xbgTe-espHUY0XuhHhB5yCs6aWOXDmxH5VGWDeWeWWAXlOeRsaZY8S3QZ6ixWEFHXyMTvv3buAotBkNnT7QwA52BeAVQBOauvrxhaZ~rkt8ZqCdipuxDip7oehwIQtGIZthUWJJykshAKnW-7lXEiP~X15zidipEJ7nn6J~M3ZtR8g0QQeMUTD0N9d1b1yJ4Sg5geWoMZ662kJj4oi9lmkTJpV1MwhwYUjCIScjhlZxwPYrS0Qh4jhLeRCNZW1jQG-ZXcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)