Opportunistic infections contribute to morbidity and mortality after peripheral blood progenitor cell (PBPC) transplantation and are related to a deficient T-cell compartment. Accelerated T-cell reconstitution may therefore be clinically beneficent. Keratinocyte growth factor (KGF) has been shown to protect thymic epithelial cells in mice. Here, we evaluated immune reconstitution after autologous CD34+ PBPC transplantation in rhesus macaques conditioned with myeloablative total body irradiation in the absence or presence of single pretotal body irradiation or repeated peritransplant KGF administration. All KGF-treated animals exhibited a well-preserved thymic architecture 12 months after graft. In contrast, thymic atrophy was observed in the majority of animals in the control group. The KGF-treated animals showed higher frequencies of naive T cells in lymph nodes after transplantation compared with the control animals. The animals given repeated doses of KGF showed the highest levels of T-cell receptor excision circles (TRECs) and the lowest frequencies of Ki67+ T cells, which suggest increased thymic-dependent reconstitution in these animals. Of note, the humoral response to a T-cell–dependent neo-antigen was significantly higher in the KGF-treated animals compared with the control animals. Thus, our findings suggest that KGF may be a useful adjuvant therapy to augment T-cell reconstitution after human PBPC transplantation.
Introduction
Opportunistic infections caused by protracted immunodeficiency contribute to morbidity and mortality after peripheral blood progenitor cell (PBPC) transplantation. Thus, the recovery of the immune system after transplantation impacts significantly on the outcome of transplantation. To a great extent the integrity of a new immune system relies on the capacity of the thymus to generate naive T cells; this is especially true for the CD4+ T-cell compartment.1,–3 Only the naive T-cell pool has the broad repertoire of T-cell receptor (TCR) specificities necessary to generate new immune responses.4,–6 In contrast to thymic repopulation, the normalization of T-cell numbers by peripheral expansion of the pre-existing T-cell pool results in a narrow TCR repertoire, which may impair the ability to induce new immune responses. Injury to the thymic microenvironment is considered one of the major factors responsible for slow T-cell immune reconstitution after transplantation and any interventions preserving thymic function could improve transplant outcomes.4,,,–8
Recombinant human keratinocyte growth factor (KGF; Palifermin, Kepivance; Amgen, Thousand Oaks, CA) is currently licensed for the reduction of the incidence and duration of oral mucositis after transplantation for hematologic malignancies.9 Exogenous KGF administration has also been shown to protect thymic epithelial cells (TECs) from injury induced by chemotherapy, radiation, or oxidative stress in murine models.10,,–13 Prevention of thymic injury leads to improved thymopoiesis, as TECs nurture hematopoietic precursors, and thereby ensure thymocyte development and maturation of newly generated T cells.11,13,–15 KGF is a known stimulator of epithelial cell growth.16,17 The KGF receptor is expressed on TECs that produce IL-7, and as KGF increases IL-7 transcripts in the thymus, induction of IL-7 production is a possible effector mechanism.13 Another might be increased resistance against apoptosis or enhancement of epithelial cell recovery as observed in other epithelial tissues after KGF treatment.18,,–21 KGF's effect as a protective and trophic factor for TECs supports the rationale for its ability to ensure thymocyte proliferation and maturation. A greater mass of true epithelial space should translate into greater de novo production of naive T cells in the time period early after transplantation as the TEC–thymocyte interaction is essential for T-cell development. This hypothesis is supported by the observation that effective development of T cells needs seeding of precursor cells to a regularly structured thymic microenvironment14 and the finding that exogenous KGF given to lethally irradiated, bone marrow reconstituted recipients resulted in supranormal thymocyte numbers.13 However, the effects of KGF in rodents may be different compared with humans and thus careful evaluation of this agent in a nonhuman primate model of immune reconstitution after PBPC transplantation is critical for preclinical development. In addition, the use of nonhuman primates allows serial sampling of blood and lymph nodes (LNs) as well as postmortem analysis of the thymus, which is impossible in clinical trials. Furthermore, these autologous primate studies can differentiate the effects of KGF on immune function from factors complicating analysis in clinical trials, including the impact of the underlying disease necessitating transplantation, previous therapies, and alloreactivity.
Thus, in this study we evaluated the effect of KGF treatment on thymic architecture and T-cell immune reconstitution after myeloablative total body irradiation (TBI) and autologous PBPC transplantation in healthy rhesus macaques. Our findings suggest that KGF protects thymic architecture and thus improves de novo T-cell immune reconstitution, even leading to an improved humoral response to a T-cell-dependent neo-antigen. Therefore, KGF may be a useful adjuvant therapy to reduce morbidity and mortality after PBPC transplantation via effects on the thymus and enhanced immune reconstitution.
Materials and methods
Animals
All animals were colony-bred rhesus macaques (Macaca mulatta) of Indian origin, maintained and used in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services [DHHS] publication NIH85-23). The protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute (NHBLI), National Institutes of Health (NIH), Bethesda, MD. Healthy animals of either sex at age 3 or 5 years were selected (Table S1, available on the Blood website; see the Supplemental Tables and Figures link at the top of the online article). These animals were free of known infectious or immunologic diseases.
Study design
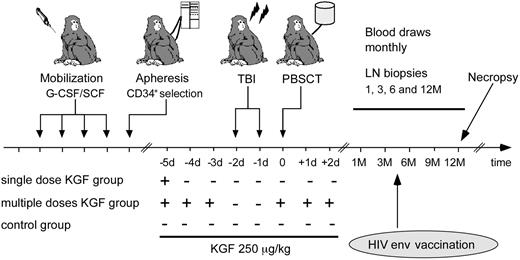
One group of animals (n = 4) received 250 μg/kg body weight KGF palifermin, Kepivance, Amgen) by intravenous bolus on day −5 (day 0 = day of transplantation); a second group of animals (n = 2) received KGF days −5, −4, and −3 before TBI, as well as on day 0, +1, and +2 based on the dosage scheme in murine studies and clinical trials.9,11 Besides mild erythema of the palms after KGF treatment, no side effects were observed. In addition, at 12 months after treatment no epithelial tumor development in any of the animals was observed. These animals were compared with an age-matched control group that was transplanted but not treated with KGF (n = 5; Figure 1). All animals were vaccinated 3.5 months after transplantation with 1012 particles of a replication-incompetent Adenovirus serotype 5 vector encoding for HIV-1 envelope protein gp140 formulated in sterile saline and delivered into the quadriceps muscle by use of a needle and syringe.22
Study design. Animals were mobilized with granulocyte colony–stimulating factor/SCF for 4 days and PBMCs collected by leukapheresis, followed by CD34+ selection. They underwent transplantation after TBI. We compared 3 groups: one group received KGF once before, the second 3 times before, and the third 3 times after conditioning. These animals were compared with a control group that underwent transplantation without KGF treatment. All were vaccinated with an HIV env vaccine 3.5 months after transplantation, and followed for 12 months after transplantation. They were euthanized and underwent necropsy to collect all lymphoid tissue at the end of study.
Study design. Animals were mobilized with granulocyte colony–stimulating factor/SCF for 4 days and PBMCs collected by leukapheresis, followed by CD34+ selection. They underwent transplantation after TBI. We compared 3 groups: one group received KGF once before, the second 3 times before, and the third 3 times after conditioning. These animals were compared with a control group that underwent transplantation without KGF treatment. All were vaccinated with an HIV env vaccine 3.5 months after transplantation, and followed for 12 months after transplantation. They were euthanized and underwent necropsy to collect all lymphoid tissue at the end of study.
Mobilization and collection of CD34+ hematopoietic progenitor cells
PBPCs were mobilized with recombinant human granulocyte colony-stimulating factor (10 μg/kg; Amgen) in combination with stem cell factor (SCF) (200 μg/kg; Amgen) administered by subcutaneous injection daily for 4 days. Mobilized PBPCs were collected by leukapheresis as described23 and isolated using density gradient centrifugation. CD34+ cell enrichment was performed using the 12.8 IgM anti-CD34 biotinylated antibody and MACS streptavidin microbeads (Miltenyi Biotec, Auburn, CA). The CD34+ cell concentration in peripheral blood and the purity of the MACS-sorted CD34+ cells were analyzed by flow cytometry. The cells were frozen in 90% fetal bovine serum mixed with 10% dimethyl sulfoxide. The viability of the cells after thawing was less than 95% in all animals measured by trypan blue staining (Table S1). All animals received CD34+ selected cells.
Transplantation conditions
The animals received 500 cGy TBI daily for 2 days (total 1000 cGy), delivered at a rate of 8.8 cGy/min via an 860-Co teletherapy irradiator (Eldorado). The next day, cryopreserved autologous CD34+ selected PBPCs (purity 85%-96%) were re-infused. The CD34+ cell numbers infused into each animal were matched among the 3 groups (Table S1). The graft was thawed and re-infused via a central venous catheter. A day later, the animals were started on granulocyte colony-stimulating factor 5 μg/kg/d intravenously until the total white blood cell count reached 6 × 109/L.
Collection of PB and LN samples
Blood was drawn and a lymph node was removed for baseline analysis before mobilization and transplantation. All animals were followed for 1 year after transplantation (Figure 1). Blood was drawn every month up to 9 months and thereafter at 12 months after transplantation. Inguinal or axillary LNs were removed at 1, 3, 6, and 12 months after transplantation. Twelve to 14 months after transplantation the animals were euthanized and all lymphoid tissue was collected.
Cell preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from citrated venous blood by density gradient sedimentation using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Mononuclear cells were isolated from lymph node samples by gentle mechanical disruption of tissues in RPMI (HyClone Laboratories, Logan, UT) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin, and streptomycin. The cells were passed through a 100-μm mesh filter to remove any remaining tissue fragments.
Phenotyping of cells by immunofluorescent staining and flow cytometric analysis
As described, 0.25 to 1.0 × 106 cells from PBMC and lymph node samples were resuspended in wash buffer (phosphate-buffered saline/0.5% bovine serum albumin) and incubated with directly conjugated monoclonal antibodies (mAbs) (Table S2) as described.24 For intracellular analysis of Ki67 expression, cells were first stained for the cell surface markers CD4, CD8β, and CD95 before incubation in 2 × fixation/permeabilization solution (BD Biosciences, San Jose, CA) and then stained with Ki67 Ab. Four-parameter flow cytometric analysis was performed on a FACS Calibur (BD Biosciences). List mode files were analyzed using FlowJo software (Treestar Inc., San Carlos, CA). CD4+ naive T cells were identified as a uniform CD95lowCD28high and β7 integrinint population as previously described25 (Figure 2B,C). Effector memory CD4+ T cells were identified as CD95high β7 integrinint and CD28− and the remaining of CD4+ T cells as central memory T cells. Naive CD8+ T cells were identified as CD95lowCD28intCD11alow, and the remaining CD8+ T cells were defined as memory phenotype.
Reconstitution of T cells in lymph nodes after transplantation. (A) Frequencies of CD3+ T cells in lymph nodes before and after transplantation; animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. (B) and (C) Flow cytometry plots show T cell phenotyping in a representative animal before and 1 month after transplantation. (D) and (E) Frequencies of naive CD4+ and CD8+ T cells in lymph nodes obtained from the animals over time. Cells were gated firstly on small lymphocytes using scatter parameters, then on CD3+ T cells, and then subsets were analyzed within CD4+ or CD8+ populations using FlowJo software (TreeStar). Data are displayed are as median and interquartile range (IQR). For the 2 animals receiving multiple doses, only median is plotted as an IQR would be statistically unreliable. Significant P indicated for comparisons between all KGF-treated animals and controls.
Reconstitution of T cells in lymph nodes after transplantation. (A) Frequencies of CD3+ T cells in lymph nodes before and after transplantation; animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. (B) and (C) Flow cytometry plots show T cell phenotyping in a representative animal before and 1 month after transplantation. (D) and (E) Frequencies of naive CD4+ and CD8+ T cells in lymph nodes obtained from the animals over time. Cells were gated firstly on small lymphocytes using scatter parameters, then on CD3+ T cells, and then subsets were analyzed within CD4+ or CD8+ populations using FlowJo software (TreeStar). Data are displayed are as median and interquartile range (IQR). For the 2 animals receiving multiple doses, only median is plotted as an IQR would be statistically unreliable. Significant P indicated for comparisons between all KGF-treated animals and controls.
Flow cytometric cell sorting for T cells and TREC analysis
CD4+ and CD8+ T cells were stained as described and sorted on a modified FACSVantage SE/DiVa (Becton Dickinson) by gating on either CD4+ or CD8+ cells within the CD3+ population. This resulted in less than 98% pure populations of T-cell subsets. Cell pellets were frozen and TREC levels were measured by quantitative polymerase chain reaction directly on cell lysates as previously described.5 Polymerase chain reaction was performed on ABI Prism 7700 sequence detector (Applied Biosystems).
Histology and immunohistochemistry
At necropsy, tissues from the following sites were harvested: tonsils, thymus, lymph nodes, spleen, Peyer patches, and bone marrow. A portion of each tissue was snap-frozen in OCT and kept at −80°C; the remaining tissue was fixed in 10% formalin and paraffin-embedded. Immunohistochemical staining with anti-CD3 (LabVision), anti-CD20, anti-high-molecular-weight keratin, and anti-Ki67 (all from DakoCytomation) were performed using Envision Plus detection system (DakoCytomation) with DAB as chromogen on a DakoAutomated immunostainer.
CDR3 spectratyping
CDR3 spectratyping was performed on PBMCs as previously described with modifications.26 cDNA was amplified with Vbeta-specific-5′ and FAM-labeled C-3′ primers previously described27 and analyzed on a 3100 Genetic Analyzer using capillary electrophoresis in the presence of standards labeled with ROX dye. Acquired data were analyzed using Genescan and Genotyper software.
Quantification of spectratyping results
To quantify the deviation of the peak spectrum of a given Vβ from a Gaussian distribution and not simply rely on qualitatively visual judgment, we developed a new method to calculate Skewness and Kurtosis of the Vβ's distribution of the peak areas. We subtracted the Kurtosis of a Gaussian distribution (which is 3) from the Kurtosis calculated for the Vβ peak area distributions to compare them to Gaussians.
Skewness is defined as μ3/σ, where μ3 is the third moment about the sample mean and σ is the standard deviation.3 For a sample size of n the skewness would be
Skewness measures how strongly the data deviate from a situation where there are equivalent weights of high and low peaks. A Gaussian (and any symmetric distribution) would have a skewness of 0. Negative skewness means a heavy left tail of the distribution, positive skewness a heavier right tail. To obtain a skewness score for a given monkey we averaged over the absolute values of the skewness of all of its Vβ samples.
Kurtosis is defined as μ4/σ where μ4 is the fourth moment about the sample mean and σ is the standard deviation.4 For a sample size of n the kurtosis would be
Kurtosis measures whether a distribution is flat or has isolated peaks. Data sets with a distinct peak near the mean and heavy tails will have a high kurtosis. Flat data sets will have a low kurtosis.
To obtain a kurtosis score for a given monkey, we averaged over the absolute values of the (kurtosis-3) of all of its Vβ samples. A large kurtosis score thus indicates that the Vβ spectra of the monkey had an overall strong deviation from Gaussian distributions (Table 1).
Analysis of humoral responses against HIV-1 envelope
HIV envelope protein supernatant was coated overnight on Immunol-2 HB microtiter plates (Thermo Labsystems, Milford, MA). Plates were blocked (20% fetal bovine serum/1% bovine serum albumin-buffered solution) for 1 hour at 37°C. Duplicate wells of serial dilutions of the monkey sera (1:200, 800, 3200, 12 800) were incubated 2 hours at 37°C, followed by Biotin labeled antimonkey IgG/IgA/IgM (Rockland Immunochemicals, Gilbertsville. PA; 1 hour, 37°C), Streptavidin-horseradish peroxidase (HRPO) (30 minutes, room temperature; KPL, Gaithersburg, MD), and tetramethyl benzidine substrate (30 minutes, room temperature; KPL). Mean optical densities (Ods) for each dilution were corrected for the mean OD of the same dilution of the animal matched pre-immunization sample. End point titers for each animal were established as the last dilution with a pre-immunization corrected OD less than 0.2.
Statistical analysis
Statistical analysis was conducted with the program S-plus (StatSci Division, MathSoft Inc.). Unless otherwise specified, simple 2-group comparisons were conducted using rank-based nonparametric tests, such as the Wilcoxon test. To examine differences in responses of interest over time, separate linear models were fit to each animal using all measurements between 30 and 180 days after the procedure, and the slopes of these models were compared between groups. These models were used to test for group differences in T-cell populations, and the percentage of T cells that were Ki67+.
Results
Transplantation and engraftment
Healthy adolescent rhesus macaques underwent mobilization of PBPC with granulocyte colony-stimulating factor and SCF (Figure 1) and PBMCs were collected by leukapheresis. The animals were thereafter conditioned with myeloablative TBI and transplanted with purified autologous CD34+ PBPC (Figure 1 and Table S1). Four animals received KGF at a single dose of 250 μg/kg body weight before transplantation and 2 animals received repetitive treatment of KGF 3 times before and 3 times after conditioning, simulating the regimen used for autologous PBPC transplants in humans.9,21 The KGF-treated animals were compared with an age and CD34+ cell dose-matched control group of 5 monkeys concurrently transplanted without KGF treatment. All animals engrafted (leucocytes < 109/L, neutrophils < 0.5 × 109/L) 8 to 13 days after transplantation.
KGF treatment increases naive T-cell reconstitution
One month after undergoing TBI and PBPC transplantation, all animals experienced a dramatic depletion of PB and LN T cells (Figures S1A and S2A, respectively). In all animals there was a rapid recovery of CD8+ T cells, whereas the recovery of CD4+ T cells showed slower kinetics. This led to an inversion of the CD4/CD8 ratio during the first 3 months after transplantation (data not shown), as previously reported in humans28,29 and in rhesus macaques.24 There was no difference between treatment groups in reconstitution of total T-cell numbers in peripheral blood; therefore, the phenotype and frequency of defined T-cell subsets was analyzed in further detail.24,25 As all animals studied were adolescents at time of study entry, naive T cells were the most prevalent T-cell phenotype both in the CD4+ and CD8+ T cell populations before transplantation (Figure 2B,C). After transplantation there was a marked inversion of the ratio of naive to memory CD4+ and CD8+ T cells in all animals. The number of naive T cells gradually increased over time in all groups (Figure 2D,E for LN; Figures S1B,C for PB). Notably, CD4+ and CD8+ naive T-cell frequencies in LN were significantly higher in the KGF-treated compared with untreated animals at the 3-month time point (P = .013 for CD4+ T cells and P = .01 for CD8+ T cells). The frequency of naive CD4+ T cells in PB was significantly higher in the KGF-treated compared with untreated animals at the 3-month time point (P = .009). In peripheral blood, however, absolute numbers of naive CD4+ or CD8+ T cells in the KGF-treated animals were not significantly higher by 6 to 12 months (P = .178 for CD4+ T cells, P = .247 for CD8+ T cells; Figure S1).
KGF increases TREC levels and decreases peripheral T-cell proliferation
To estimate thymic output, we measured TREC within sorted CD4+ and CD8+ T cells from PB obtained before and after transplantation. In all animals 1 month after transplantation TREC levels were very low compared with pretransplantation levels (Figure 3A,B), but began to increase by 3 months after transplantation. The animals given multiple doses of KGF had significantly higher TREC levels compared with untreated animals both in CD4+ T cells and CD8+ T cells 5 months and later after transplantation (P < .05), suggesting that these animals had overall greater thymic output.5,30 As a group, however, KGF-treated animals did not have significantly higher TREC levels than untreated animals. Thymic-independent peripheral expansion of T cells presents an alternative pathway for immune reconstitution that would be characterized by an increased frequency of circulating proliferating T cells. We therefore examined the frequency of dividing PB T cells by staining for the nuclear cell cycle-associated antigen Ki67. Before transplantation, the frequency of circulating Ki67+ CD4+ and Ki67+ CD8+ T cells was low (< 7%) in all groups (Figure 3C,D). At 1 month after transplantation the frequency of Ki67+ T cells had dramatically increased. The frequencies of both Ki67+ CD4+ and Ki67+ CD8+ T cells in peripheral blood were not significantly higher in the 2 KGF-treated groups compared with the control group at the end of the first month after transplantation (P = .329 for Ki67+ CD4+ T cells and P = .126 for Ki67+ T cells CD8+). Notably, the lowest frequencies of Ki67+ T cells were observed in the animals treated with multiple doses of KGF. After the peak of Ki67+ T cells observed at 1 month in all study groups, the numbers of Ki67+ T cells gradually declined and reached pretransplant levels by 5 to 7 months after transplantation as T-cell counts increased. Rebound of Ki67+ CD4+ T cells in LNs between months 1 and 6 was significantly lower in the animals treated with KGF compared with control animals (P = .05), and was similar for CD8+ T cells (P = .05). The differences were most notable and significant at the first month post transplantation (P = .004 for CD4+ T cells, P = .004 for CD8+ T cells). Taken together these data suggest that the KGF-treated animals had higher thymic output of de novo generated T cells than control animals and thus experienced less peripheral T-cell expansion to achieve the same degree of reconstitution of absolute T-cell numbers.
Thymic output and T-cell proliferation. (A) and (B) Graphs show changes in the frequency of CD4+ and CD8+ T cells containing TREC before and after transplantation. (C) and (D) Graphs show the frequency of Ki67+ CD4+ and Ki67+ CD8+ T cells in blood before and after transplantation. (E) and (F) Graphs show the frequency of Ki67+ CD4+ and T CD8+ T cells in lymph node before and after transplantation. Animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. Data are displayed are as median and IQR. For the 2 animals receiving multiple doses, only median is plotted as an IQR would be statistically unreliable. Significant P indicated for comparisons between multiple-dose-KGF–treated or all KGF-treated animals and controls.
Thymic output and T-cell proliferation. (A) and (B) Graphs show changes in the frequency of CD4+ and CD8+ T cells containing TREC before and after transplantation. (C) and (D) Graphs show the frequency of Ki67+ CD4+ and Ki67+ CD8+ T cells in blood before and after transplantation. (E) and (F) Graphs show the frequency of Ki67+ CD4+ and T CD8+ T cells in lymph node before and after transplantation. Animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. Data are displayed are as median and IQR. For the 2 animals receiving multiple doses, only median is plotted as an IQR would be statistically unreliable. Significant P indicated for comparisons between multiple-dose-KGF–treated or all KGF-treated animals and controls.
Broader T-cell receptor repertoire diversity with KGF treatment
To further determine the source and quality of phenotypically naive CD4+ and CD8+ T cells after transplantation, we evaluated the diversity of TCRs at 1 year after transplantation using TCRB CDR3 spectratyping.31 A limited TCR repertoire suggests that most T cells have expanded from a limited pool of parent T cells, whereas a broader repertoire suggests a greater contribution from de novo generated naive T cells. The breadth of the TCR repertoire was derived from the spectratyping data by ranking the animals according to the product of the skewness and kurtosis of their repertoires, 2 measures of diversity, as described in the Methods. The majority of KGF-treated animals showed a broader TCR repertoire compared with the untreated animals; indeed, the top 4 ranked repertoires were all from KGF-treated animals (Table 1). However, the difference in TCR repertoire between KGF-treated and control animals did not reach statistical significance. One KGF-treated animal (RQ3602) had a highly skewed repertoire, even with well-preserved thymic architecture, and this precluded deriving any statistically significant correlation between KGF treatment and TCR diversity from this small sample size.
T- and B-cell immune responses after neo-antigen challenge
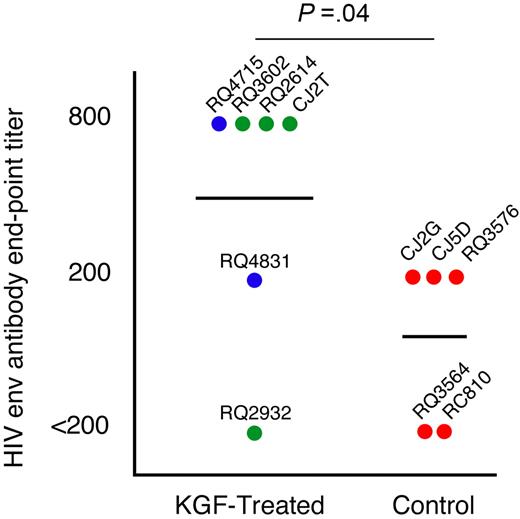
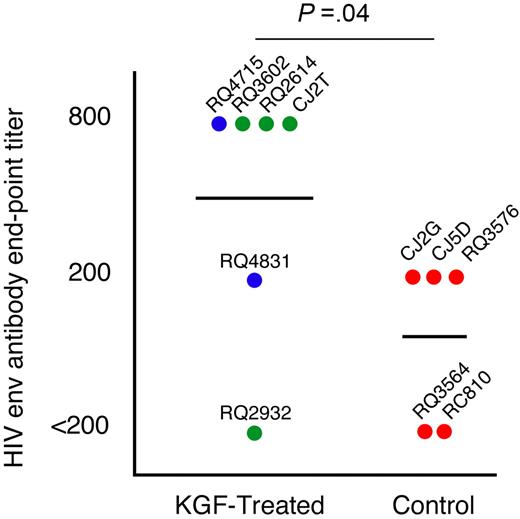
We challenged the animals with a T-cell-dependent neo-antigen at 3.5 months after transplantation by immunization with a replication-incompetent adenovirus vector encoding the HIV-1 envelope glycoprotein gp140. Most animals, irrespective of treatment, showed very low or undetectable T-cell cytokine responses against HIV-1 envelope, perhaps due to the lack of a boost as well as the very early time point of vaccination after transplantation (data not shown). To address the functional capability of inducing humoral immunity, we monitored the levels of neutralizing antibodies elicited against HIV-1 envelope. In most animals peak levels of antibodies were observed at 6 weeks after immunization.32 Five of the 6 KGF-treated and 3 of 5 control animals had detectable levels of antibodies against HIV-1 envelope (Figure 4). Notably, the KGF-treated animals had significantly higher median antibody titers than the control animals (P = .04 by a 1-sided Mann-Whitney test), suggesting that they were capable of mounting a superior humoral response to a T-cell-dependent neo-antigen.
Humoral immune responses. Antibody responses elicited by a single immunization with a recombinant Adenovirus vector construct encoding for HIV-1 envelope protein 3.5 months after transplantation were measured by ELISA for antibody levels to HIV-1 envelope. All serum samples were diluted to find end-point titers and were corrected based on the last dilution of a sample pre-vaccination for each animal. The highest end-point titer for each monkey is depicted in the graph. Animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. The identifications of individual animals are shown.
Humoral immune responses. Antibody responses elicited by a single immunization with a recombinant Adenovirus vector construct encoding for HIV-1 envelope protein 3.5 months after transplantation were measured by ELISA for antibody levels to HIV-1 envelope. All serum samples were diluted to find end-point titers and were corrected based on the last dilution of a sample pre-vaccination for each animal. The highest end-point titer for each monkey is depicted in the graph. Animals given multiple doses of KGF are indicated with blue symbols, those given a single dose of KGF with green symbols, and control animals with red symbols. The identifications of individual animals are shown.
Preserved thymic architecture with a greater frequency of double-positive thymocytes in KGF-treated animals
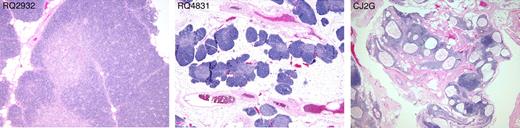
Because KGF primarily enhances epithelial cell growth and thereby may protect TECs, we performed histologic and phenotypic analysis of the thymus 12 to 14 months after transplantation to determine whether preserved thymic architecture might underlie the beneficial reconstitutive effects of KGF treatment described. Slides were analyzed while blinded to the treatment groups and thymic architecture was scored according to 3 grades (Figure 5 and Figure S2). Grade 1 thymi had completely intact architecture with preserved lobular structures and well-defined cortical and medullary areas with high cellularity. Six animals were scored at this grade. One animal (RQ4715) had received multiple doses of KGF and the others (RQ3602, RQ2932, CJ2T, RQ2614) had received a single dose. In addition, one control animal (RQ3576) showed thymic structure of this grade. Grade 2 thymi had areas of well-preserved architecture and high cellularity but with some degree of thymic atrophy in other areas. One animal that received multiple doses of KGF (RQ4831) and one untreated animal (CJ5D) exhibited thymi of this type. Grade 3 thymi showed severe thymic atrophy, defined by fat replacement, decreased cortical thickness, cystic changes of the epithelium, and low cellularity. All 3 remaining control animals belonged to this group. In summary, all 6 KGF-treated animals had no or little thymic atrophy, whereas 3 of 5 control animals had severe thymic atrophy. The weight of the thymi at autopsy did not correlate with the quality of the thymic architecture (Table 1). In addition, a Fisher exact test on the association between KGF exposure (yes/no) and thymus grade (1, 2, or 3) gave a P of .04, suggesting that the preservation of thymic architecture correlated with KGF treatment.
Thymic histology. Hemotoxylin and eosin stains of the thymus of 3 representative animals at necropsy 12 to 14 months after transplantation. Thymus (left panel) scored as grade 1: well-preserved architecture with well-defined cortex and medulla, no atrophic changes. Grade 2 thymus (middle panel): some degree of atrophic changes consisting of fat replacement of lobules and preservation of cortex and medulla and islets of cell-dense tissue containing many thymocytes. Grade 3 thymus (right panel): marked atrophic changes and cystic degeneration. All photos were taken with an Olympus BX41 microscope, objective 4 × /0.13 UPlanFl.
Thymic histology. Hemotoxylin and eosin stains of the thymus of 3 representative animals at necropsy 12 to 14 months after transplantation. Thymus (left panel) scored as grade 1: well-preserved architecture with well-defined cortex and medulla, no atrophic changes. Grade 2 thymus (middle panel): some degree of atrophic changes consisting of fat replacement of lobules and preservation of cortex and medulla and islets of cell-dense tissue containing many thymocytes. Grade 3 thymus (right panel): marked atrophic changes and cystic degeneration. All photos were taken with an Olympus BX41 microscope, objective 4 × /0.13 UPlanFl.
Flow cytometric analysis of the thymi at autopsy (12-14 monthsafter transplant) showed that the predominant population of thymocytes in all KGF-treated animals consisted of CD4+ CD8+ double-positive thymocytes (Figure 6). In contrast, in all 3 untreated animals where tissue was saved for analysis, double-negative CD4− CD8− cells comprised the major population of thymocytes (Figure 6). Hence, there was a significant difference in the distribution of double-negative and double-positive thymocytes between the KGF-treated and untreated animals (P = .047 by a rank-based permutation test on double-positive and double-negative proportions simultaneously modeled after O'Brien.33 The data suggest that KGF promoted the development of thymocytes from double-negative to CD4+ CD8+ double-positive thymocytes. Thus the preservation of thymic architecture correlates with KGF treatment and also correlates with improved naive CD4+ and CD8+ T-cell reconstitution.
Thymic subsets. Flow cytometric analysis of the thymocyte subsets at time of necropsy in the 3 different groups. SP indicates single-positive (CD4+ or CD8+); DP, double-positive (CD4+ CD8+); DN, double-negative (CD4−CD8−). The thymus of animals CJ5D and RQ3564 were not available for this analysis.
Thymic subsets. Flow cytometric analysis of the thymocyte subsets at time of necropsy in the 3 different groups. SP indicates single-positive (CD4+ or CD8+); DP, double-positive (CD4+ CD8+); DN, double-negative (CD4−CD8−). The thymus of animals CJ5D and RQ3564 were not available for this analysis.
In addition, when the animals were ranked according to their thymic histology, those with preserved thymic structure (grades 1 and 2) showed significantly better reconstitution of absolute counts of peripheral blood naive CD4+ T cells at months 3 (P = .017), 4 (P = .017), 6 (P = .017), and 8 (P = .033), as well as the mean over 6 to 12 months (P = .009) than the animals with poor thymic architecture (grade 3; Figure S3A). The recovery of naive CD8+ T cells also tended to be greater in the animals with preserved thymic architecture and was significantly so at 6 months (P = .033; Figure S3B). Furthermore, poor thymic architecture, as determined histologically, was also associated with a poor ranking in terms of TCR repertoire diversity (Figure S4). While these observations do not bear on our conclusions regarding the effect of KGF, they serve to confirm the known association between thymic function and histology, naive T-cell numbers, and repertoire diversity.
Finally, there were no histologically discernible differences in the architecture or cellularity of LNs, tonsils, spleen, bone marrow, or Peyer patches between the KGF-treated and control animals. LNs showed follicular and paracortical hyperplasia in all animals, and the white pulp of the spleen was hyperplastic with prominent follicles in all animals except 2 control animals (RQ3564, RC810) that showed regressive changes in the white pulp such as small germinal centers with hyaline changes (unpublished data).
Discussion
Patients after PBPC transplantation experience a prolonged period with increased risk of infections. Poor host immunity and presence of resistant organisms compromise the treatment of infections leading to increased morbidity and mortality after transplantation. The slow recovery of newly developed, naive T cells is currently considered to be one of the most important determinants of immune competence after PBPC transplantation.34 Thus, approaches to improve T-cell immune reconstitution, particularly at the earliest time points after transplantation, are warranted.
Our major findings were as follows: (1) treatment with KGF resulted in a well-preserved thymic architecture at 1 year after transplantation compared with atrophic changes in most of the untreated animals; (2) a normal pattern of thymocyte development was observed in KGF-treated but not control animals by 12 to 14 months after transplantation; (3) preserved thymic architecture was associated with significantly higher PB naive CD4+ and CD8+ T-cell counts; (4) KGF treatment led to significantly higher naive CD4+ and CD8+ T-cell frequencies in LNs at the early 3-month time point; (5) multiple-dose KGF treatment led to significantly higher CD4+ and CD8+ T-cell TREC frequencies at time points after 5 months; (6) KGF treatment led to a significantly lower rebound of Ki67+ CD4+ T cells in LNs between months 1 and 6, and these differences were most notable and significant at 1 month after transplantation; (7) the majority of KGF-treated animals showed a broader TCR repertoire compared with the control animals 1 year after transplantation; and (8) KGF-treated animals showed a significantly increased antibody response to neo-antigen immunization compared with the control group.
A depleted T-cell compartment may be reconstituted through either a thymic-dependent or thymic-independent pathway.35,36 The thymic-dependent pathway results in de novo generation of naive T cells with a more diverse TCR repertoire, whereas the thymic-independent pathway relies on peripheral expansion of mature T cells with a consequently more limited TCR repertoire.37 The latter tends to dominate the early time period after transplantation but is self-limiting as these T cells undergo apoptosis.38,39 Our data support the hypothesis that KGF promotes the thymic-dependent pathway through several lines of evidence that are outlined. The most dramatic effect of KGF appears to be the preservation of thymic architecture and promotion of normal thymocyte developmental subsets. If such maintenance of the microenvironment leads to increased thymic output one would expect a higher frequency of naive T cells in the periphery with less reliance on the thymic-independent pathway of peripheral expansion to achieve immune reconstitution. Indeed, in support of this, we observed a lower frequency of Ki67+ T cells in the LN of KGF-treated animals as well as higher frequencies of naive T cells in LNs. The observation that in PB naive CD4+ T cells were significantly higher in KGF-treated compared with control animals only at the 3-month time point may be because of the fact that only a tiny proportion of total body T cells is located in the circulation. In dynamic circumstances such as during immune reconstitution after transplantation, it may be that the blood does not provide an accurate reflection of the situation in LNs and other organized lymphoid tissues where the bulk of naive T cells reside. Certainly, this has been shown to be the case in HIV infection, both in the acute phase and during immune reconstitution with antiretroviral therapy.40,41 Along these lines, TREC levels in circulating T cells in KGF-treated animals were not significantly higher than in controls. It is notable, however, that the 2 animals that received multiple doses of KGF had significantly greater circulating T-cell TREC levels than controls at 5 to 12 months after transplantation.
Clearly, the aim of augmenting early thymic output to reconstitute the naive T-cell pool is to reduce morbidity and mortality from infections and posttransplantation tumor relapse. The response to a T-cell-dependent neo-antigen is a useful surrogate marker for the improvement of de novo immune reconstitution. The current recommendation for vaccination in the clinical setting is to wait 6 to 12 months after transplantation to increase the probability of an immune response.42 Thus, it is promising that the KGF-treated animals in our study had significantly increased humoral responses to neo-antigen presented early after transplantation compared with the control group; this improved immunity might also apply to endogenous pathogens such as herpes viruses.43 This finding, together with the observations discussed, are particularly encouraging when taking into account that even in this large animal model with small groups, the majority of parameters of immune reconstitution measured were significant and suggested a clearly beneficial effect of KGF. That some measures of immune reconstitution were improved at later time points may be because of KGF having a primarily qualitative effect, rather than simply speeding up the reconstitution process as such. Importantly, short-course KGF treatment was well-tolerated, free of side effects, and effective. Other available approaches, including IL-7, may require prolonged administration, would not be expected to stimulate TECs directly, and may have side effects.18,19,44 Whether KGF would have the same beneficial effects in the allogeneic transplantation setting needs to be confirmed either in large animal models or in clinical trials. In mice, KGF has been shown to improve T-cell immune reconstitution, and decrease incidence and severity of graft-versus-host disease in allogeneic transplantation,11,45 even in older subjects.12
Taken together, our data are in line with findings in mouse models and suggest that preservation of thymic architecture is a major determinant of naive T-cell reconstitution after PBPC transplantation and that treatment with KGF enhances this. Of note is the preservation of thymic architecture 1 year after transplantation even after a single dose of KGF. Older individuals with a natural involution of the thymus5,30 undergoing transplantation as well as patients undergoing haploidentical transplantation have a high risk for graft-versus-host disease and prolonged immune deficiency.46 Thus, it is especially in these settings that KGF would be relevant not only as a therapeutic agent against mucositis and potentially for the prevention of graft-versus-host disease10,47 but also to promote de novo T-cell generation from the thymus, particularly in the early stages after transplantation. In conclusion, our results provide a rational framework for the further evaluation of the effects of KGF in augmenting immune reconstitution in humans, not only after transplantation but also in other situations of profound T-cell deficiency such as in HIV infection.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work could not have been done without the dedication of the animal care staff of 5 Research Court, particularly Allen Krouse and Mark E. Metzger. The authors also thank David Ambrozak, Evan McGruder, Robert T. Bailer, Fernando Garcia, and Dennis Johnson for their assistance. The authors thank Amgen USA for supplying G-CSF, SCF, and KGF. The authors thank Thomas Fountaine III for help with immune histologic stainings. The authors are grateful to Dr Gary Nabel for providing the HIV-1 vector and Dr John Mascola for providing serum samples from animals with detectable titers after such vaccination for this study.
This work was supported in part by the intramural program of the National Institutes of Health and in part by R01 HL073794 (B.R.B.). R.S. was supported by a postdoctoral grant of Deutsche Krebshilfe/Mildreed-Scheel-Stiftung.
National Institutes of Health (NIH)
Authorship
Contribution: R.S. and K.L. contributed equally to this manuscript. R.S., K.L., F.J.G., S.P., J.M., R.D., C.C., and K.C. performed research. M.N. and M.M.S. performed statistical and mathematical analysis of data. R.K., B.B., C.D., and D.D. designed research. All authors were involved in analysis of the data and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Douek, Human Immunology Section, Vaccine Research Center, NIAID, NIH/DHHS, 40 Convent Dr, Rm 3509, Bethesda, MD, 20892; e-mail: ddouek@nih.gov.