Differentiation of hematopoietic stem cells (HSCs) after birth is largely restricted to the bone marrow cavity, where HSCs are associated closely with osteoblasts (OBs). How OBs localize HSCs to the endosteal niche remains unclear. To explore adhesive interactions between HSCs and OBs, a cell blot analysis was used that revealed 2 major bands that corresponded to monomers and multimers of annexin II (Anxa2). Immunohistochemistry revealed that OBs and marrow endothelial cells express Anxa2 at high levels. Function-blocking studies confirmed that Anxa2 mediates HSC adhesion mainly via the N-terminal portion of the Anxa2 peptide. Adhesion of HSCs to OBs derived from Anxa2-deficient animals (Anxa2−/−) was significantly impaired compared with OBs obtained from wild-type animals (Anxa2+/+). Moreover, fewer HSCs were found in the marrow of Anxa2−/− versus Anxa2+/+ animals. Short-term lodging, engraftment, and survival of irradiated mice with whole marrow cells were substantially inhibited by N-terminal peptide fragments of Anxa2 or anti-Anxa2 antibodies. Similar findings were noted in long-term competitive repopulation studies. Collectively, these findings reveal that Anxa2 regulates HSC homing and binding to the bone marrow microenvironment and suggest that Anxa2 is crucial for determining the bone marrow niche of HSCs.
Introduction
A complex exchange of regulatory signals orchestrates differentiation and self-renewal of hematopoietic stem cells (HSCs) in the marrow. These signals are produced mainly by the stromal compartment of the bone marrow, and recent studies have revealed that osteoblasts (OBs) comprise a crucial component of the niche.1,,,,,–7 One function of OBs is to produce many of the molecules that regulate the growth of HSCs. Another is to localize HSCs to bone marrow. The molecules used by OBs to localize HSCs to the endosteal surfaces remain unclear.
Our previous work demonstrated that the adhesion of HSCs to OBs is central for the survival of HSCs on OBs.8,9 Specifically it was found that adhesion between HSCs and OBs is mediated by cell-to-cell rather than cell-to-matrix interactions, and trypsin or calcium chelators disrupted the binding interactions.8,9 Moreover, there appear to be 2 separate types of interactions: those that occur immediately upon binding, which are largely unknown, and those that established secondarily to support HSC survival.9 OBs express many types of molecules that may facilitate adhesive interactions between OBs and HSCs. These include VCAM-1 and ICAM-1,10,,,,–15 CD44, CD164, and osteopontin.7,16,–18 In addition, N-cadherin, Wnt signaling pathways, Notch-1/Jagged-1 interactions, and Ang-1/Tie2–mediated events are believed to be crucial to establishing HSCs within a particular niche.3,–5,19
To determine which molecules regulate the establishment of the marrow niche for HSCs, we performed an open search of the HSC microenvironment in bone marrow using a cell-blotting technique.20,21 We found that annexin II (Anxa2) expressed by OBs regulates the initial adhesion of HSCs. Immunohistochemistry confirmed that Anxa2 is expressed by OBs at endosteal surfaces and within endothelial marrow sites. Antibodies or peptides that block Anxa2 function, overexpression of Anxa2 protein, and use of interfering siRNAs to knock down Anxa2 expression demonstrated that Anxa2 regulates adhesive interactions between OBs and HSCs in vitro. Transplantation of bone marrow and purified HSCs into mice in the presence of Anxa2 inhibitors resulted in less homing and engraftment of the transplanted cells into the marrow. Moreover, fewer HSCs were identified in the marrow of Anxa2-deficient (Anxa2−/−) animals, and HSCs adhered less vigorously to Anxa2−/− OBs versus Anxa2+/+ OBs. These findings further define the specific adhesive mechanisms that underlie the localization of HSCs to the endosteal niche. Furthermore, because endothelial cells and OBs regulate the trafficking of HSCs, the expression of Anxa2 by OBs and by marrow endothelia suggests that Anxa2 plays a crucial role in regulating HSC homing and intramarrow trafficking.
Materials and methods
Approval was obtained from the University of Michigan's institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Cells and culture
All culture media included supplemental penicillin/streptomycin (1% vol/vol), l-glutamine (1% vol/vol), and FBS (10% vol/vol) (Invitrogen, Carlsbad, CA). The human osteosarcoma cell lines MG-63 and SaOS-2 (ATCC, Rockville, MD) were grown in DMEM. Human bone marrow endothelial cells (HBMEs) were grown in RPMI.22 The MC3T3-E1 clone 4 cells were obtained from Dr R. Franceschi (University of Michigan, Ann Arbor, MI) and were maintained in αMEM.23 The human acute myelogenous leukemia cell line KG1a (ATCC) was cultured in IMDM.
HSC isolation
Human Lin−CD34+ marrow cells were obtained from healthy adult volunteers under a protocol approved by the University of Michigan's IRB. The mononuclear cells were isolated by density separation on Ficoll-Hypaque (specific gravity, 1.077), and lineage depletion was performed using a cocktail of biotin-conjugated antibodies against lineage antigens (CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123, and glycophorin A). Positive immunoselection was performed using a CD34 (QBEND/10) MicroBead kit (Miltenyi Biotec, Auburn, CA). Normal human OBs were obtained by explant outgrowth as described previously.1
Bone marrow cells were flushed from femurs and tibias with Hanks buffered salt solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum HBSS (Gibco, Grand Island, NY). Cells were triturated and filtered through nylon screen (40 μm; BD Falcon, Bedford, MA) to obtain single-cell suspensions. Cells were incubated first with antibody cocktail of anti-CD150PE (Clone TC15–12F12.2; BioLegend, San Diego, CA), CD48FITC (Clone BCM-1) and CD41FITC (Clone MWReg30), C-kitBIO (Clone 2B8), and Sca-1APC (Clone E13–161.7) for 20 minutes on ice, then rinsed and stained with antibiotin microbeads (Miltenyi Biotec) and streptavidin-APC-Cy7–conjugated secondary antibody for another 20 minutes. Cells were enriched for c-kit+ using an AutoMACS machine (Miltenyi Biotec). The enriched cells were resuspended in 2 mg/mL 7-AAD (eBioscience, San Diego, CA) to discriminate live from dead cells. Only live (7-AAD) cells were included in analyses and sorts. Hematopoietic stem cells were sorted on a FACS Vantage dual laser flow-cytometer (Becton Dickinson, San Jose, CA) by gating on cells that are CD150+CD48−CD41−Sca-1+C-kit+. In some cases, murine Lin−Sca1+ cells were obtained by first depleting the marrow cells with biotinylated antibodies against CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), and Ter-119 and using antibiotin microbeads (Miltenyi Biotec). Magnetic labeling using an Anti-Sca-1 MicroBead Kit was used for positive immunoselection (Miltenyi Biotec).
Cell blot adhesion and proteomic analysis
Osteosarcoma membrane lysates were run on native gels of lithium dodecyl sulfate polyacrylamide (LDS-PAGE), and blotted to PVDF.20,21 After blocking with 1% BSA, biotin-labeled KGla cells were used to probe the membranes for 2 hours at 37°C. The membranes were then fixed and avidin-HRP and VIP substrate kit used for detection following the manufacturer's directions (Vector Laboratories, Burlingame, CA).
Replicate blots were used to identify the cell binding bands on LDS-PAGE gels and were purified. The bands were sequenced by mass spectrometry (MS). Spectra were acquired on an Applied Biosystems 4700 Proteomics Analyzer (Foster City, CA) using 4700 Explorer Software (version 1.0, Applied Biosystems). The 4 most intense peaks from each MS spectrum (S/N < 7) were selected for subsequent MS/MS acquisition. Automated database searches were performed using GPS Explorer from Applied Biosystems using Mascot (version 1.8; Matrix Science, London, United Kingdom).
Binding assays
Our lab has previously performed extensive studies with HSCs and OBs using the more traditional adhesion assays.8 We found that HSCs rapidly alter their expression of adhesion molecules upon contact with OBs within the time frame and conditions of most adhesion assays.9 This makes blocking these interactions difficult if not impossible using the conventional assays. Therefore, to find new adhesive molecules, we developed models that limit protein turnover and reduce the interactions to those that occur rapidly before secondary adhesive events are recruited. Cell-cell binding assays were performed cells labeled with 2.5 μg/mL of the lipophilic dye carboxyfluorescein diacetate (CFDA; Molecular Probes, Eugene, OR) in IMDM for 30 minutes at 37°C, and washed in PBS essentially as described in Crean et al.8 Thereafter, the cells were rested for 30 minutes to reduce nonspecific background, and subsequently resuspended in PBS to deliver 105 cells/well in the adhesion assay if available. Adhesion assays were performed in PBS containing Ca+2/Mg+2 where the hematopoietic cells were added to a final reaction volume of 100 μL, the plates were spun at 57g for 5 minutes at 4°C and allowed to incubate for an additional 10 minutes at 4°C. The nonadherent cells were removed in 3 subsequent washes and the remaining fluorescence was quantified in a 96-well fluorescent plate reader. In some cases, the cells were incubated in 10 μg/mL anti-Anxa2 (Clone 5; BD Pharmingen, San Diego, CA), anti–N-cadherin (clone 32; BD BioSystems, San Diego, CA), anti-CD164 (clone N6B6; BD Biosciences, San Jose, CA), anti–Jagged-1 (clone 18 832; R&D Systems, Minneapolis, MN), or MOPC-21 (IgG control; Sigma, St Louis, MO) antibodies for 15 minutes on ice prior to seeding onto the monolayers.
Anxa2 binding assays were performed with purified bovine lung Anxa2 (Biodesign International, Saco, ME), 0 to 50 μg of 1 of 7 Anxa2 synthetic peptides or BSA plated into 96-well plates for 24 hours at 4°C, washed, and then blocked with 0.1% BSA for 2 hours. The Anxa2 peptides corresponded to residues 1 to 12, 13 to 24, 25 to 36, 42 to 54, 114 to 125, 199 to 210, and 274 to 285. A random peptide was also synthesized for the first 12 amino acid residues (TVLLHEICKSSL).
Anxa2 silencing and overexpression
A 60-bp oligonucleotide, containing 19-nucleotides to a portion of the human Anxa2 (141CTAACTTTGATG CTGAGCG159) and its reverse complement sequences separated by a 9-nucleotide spacer sequence were subcloned into the BglII and HindIII restriction sites of the 5.4-kb plasmid pSUPER containing the H1-RNA promoter (OligoEngine, Seattle, WA). MG-63 and SaOS-2 cells were transfected with a scrambled control and siRNA Anxa2 vectors using Superfect or Effectene (Qiagen, Valencia, CA), and single-cell clones were selected using G418 (500 μg/mL; Invitrogen). Transient transfection studies were performed on day 3.
Anxa2 overexpression or siRNA knockdown was monitored by immunoblot using primary Anxa2 antibodies or α-Tubulin (B&D Biosciences Pharmingen or Sigma). A horseradish peroxidase–conjugated secondary antibody was used for detection (Santa Cruz Biotechnologies, Santa Cruz, CA).
Immunohistochemistry
Murine bones were harvested and fixed in 10% buffered formalin and decalcified in EDTA, and 2 to 3 μM paraffin-embedded slides were prepared and stained with antibody to Anxa2 (Clone 5, 1 mg/mL; B&D Biosciences) or an IgG control (Sigma) in conjunction with a HRP-AEC staining system kit following the manufactures protocols (R&D Systems).
In vivo animal models
Experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals (UCUCA). The impact of Anxa2 on the short-term homing capabilities of mouse bone marrow mononuclear cells was evaluated after 24 hours by transplanting 1 × 107 CFDA-labeled congenic cells into C57BL6 mice. The percentage engraftment was determined by evaluating percentage fluorescent cells per 106 bone marrow mononuclear cells in a FACS Vantage dual-laser flow cytometer (Becton Dickinson).
For short-term survival assays, C57BL6 mice were irradiated twice with 570 cGy and injected with either none (negative control) or 2 × 105 bone mononuclear marrow donor cells into the left retro-orbital sinus. Anti-Anxa2 antibody, antibody to p11 (Anxa2 binding partner), an IgG antibody control, or an N-terminal peptide corresponding to the 1 to 12 amino acids or a scrambled peptide control each at 0.2 μg/20 g animal was administered into the right retro-orbital sinus. At 2 and 24 hours after transplantation, the animals were dosed again with the experimental treatments delivered intraperitoneally. Moribund animals were humanely killed as required. Survival observations were maintained for 20 days.
Competitive long-term bone marrow transplantations (CLT-BMTs) were used to determine the effect of Anxa2 blockade on HSC homing. HSCs isolated on the basis of the SLAM family of receptors (CD150 and CD48).24,–26 HSCs derived from Ly-5.1 (CD45.1) mice using a maximum of HSCs were transplanted into Ly-5.2 (CD45.2) congenic C57BL6 mice to follow long-term repopulation capabilities.27 Recipient mice were exposed twice to 570 cGy given 3 hours apart in a gamma cell 40 cesium source. One hour later the mice were injected intravenously with a mixture of a radioprotective dose (RPD) of Ly-5.2 cells (2 × 105 cells) and 36 Ly-5.1 HSCs in 100 μL in the right retro-orbital sinus. Antibody to Anxa2 or a synthetic peptide corresponding to the 12 N-terminus amino acids of Anxa2 both at 10 μg/kg (0.2 μg/animal) was injected into the left sinus (or mixed controls (control IgG and scrambled peptide)). Sixteen weeks after transplantation, the percentages of blood cells bearing the Ly5.2 (clone 104) and Ly-5.1 (clone A20.1) phenotypes were determined through peripheral blood obtained from the tail veins of individual recipient mice stained together with B220 (6B2), Mac-1 (M1/70), CD3 (KT31.1), and Gr-1 (8C5) lineage markers. Samples were analyzed by FACS Vantage dual laser flow-cytometer (Becton Dickinson).
Anxa2−/− animals
Ling et al generated the Anxa2-deficient (Anxa2−/−) animals used in our study.28 Dr K. A. Hajjar (Weill Medical College of Cornell University, New York, NY) graciously provided our laboratory with a pair of the homozygous Anxa2−/− mice for breeding.
Statistical analyses
Analysis of variance was used to determine significance to a level of P < .05. Survival was analyzed by the Kaplan-Meier method and a log-rank test was used for univariate analysis of the data. The end points for this analysis were survival at last follow-up.
Results
In previous investigations, we observed that there are 2 types of adhesive interactions that regulate HSCs binding to OBs: those dependent upon constitutively expressed molecules, and those that are established after contact. By limiting the adhesion assay to a relatively short incubation period (15 minutes) under conditions that do not favor transcription (4°C), we were able to study events mediated by molecules that are expressed constitutively. In the present study, we extended these observations using a cell-blotting technique20,21 to identify molecules that regulated adhesion between HSCs and OBs, initially examining the adhesion of KG1a cells (a CD34+ hematopoietic cell line) to monolayers of MG-63 or SaOS-2 cells (human osteosarcoma cell lines),8 and then verifying the results using primary cells.
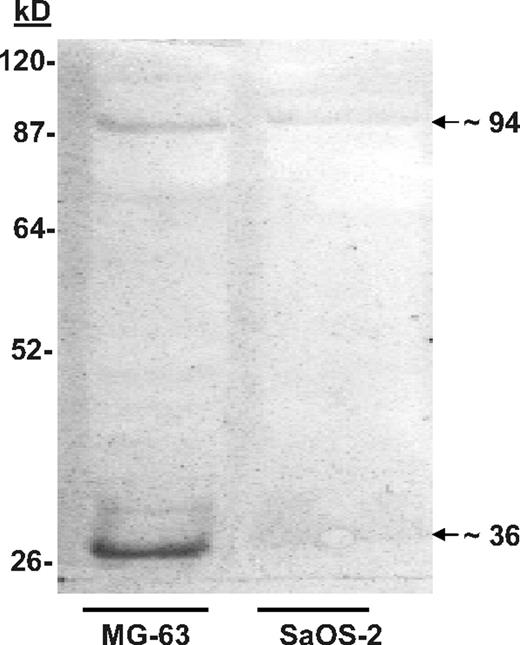
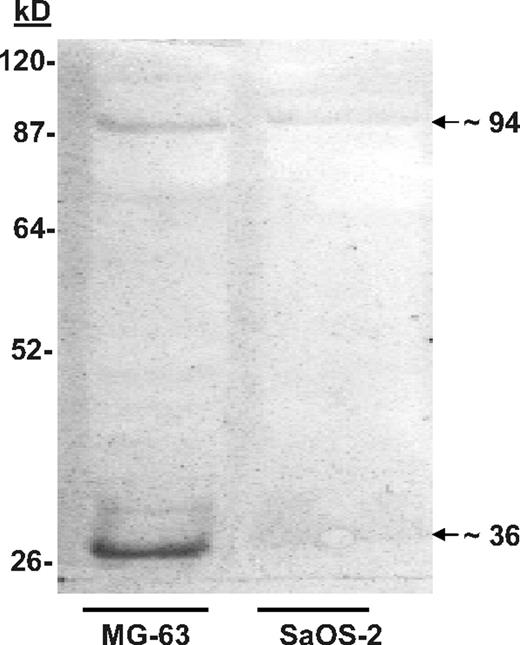
Nondenaturing discontinuous gels were used to separate proteins derived from MG-63 and SaOS-2 cells. The membrane blots were then probed with biotin-labeled KG1a cells. Binding was visualized using streptavidin-conjugated horseradish peroxidase (HRP). MG-63 proteins that bound KG1a cells were identified at approximately 36, 70 to 75, and approximately 94 kDa (Figure 1). A similar pattern of binding, albeit not as robust, was obtained using proteins extracted from SaOS-2 cells (Figure 1), which was reminiscent of the of in vitro adhesion assays in which SaOS-2 cells bound significantly fewer KG1a cells than MG-63.8 When the KG1a cells were treated with the glycosylation inhibitor tunicamycin (which blocks N-linked transfer of sugars to proteins), the intensity of the adhesion-associated bands was diminished (not shown), which also resembled the results of adhesion assays reported previously.8
Cell blot–identified annexin II (Anxa2) expressed on osteoblasts (OBs) mediates adhesion of KG1a cells. SaOS-2 or MG-63 membrane lysates were run on native lithium dodecyl sulfate–polyacrylamide gels before being blotted onto polyvinylidene fluoride membranes.20,21 After blocking the membranes with bovine serum albumin (BSA), the membranes were probed with biotin-labeled KGla cells for 2 hours at 37°C. The membranes were then fixed and the location of the cells identified with streptavidin-conjugated horseradish peroxidase. The figure is presented as an enhanced image for visualization. (The original figure is provided in Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article.) Adhesion of the biotin-labeled KG1a cells to proteins in the MG-63 membrane lysates revealed a band at approximately 36 and approximately 94 kDa.
Cell blot–identified annexin II (Anxa2) expressed on osteoblasts (OBs) mediates adhesion of KG1a cells. SaOS-2 or MG-63 membrane lysates were run on native lithium dodecyl sulfate–polyacrylamide gels before being blotted onto polyvinylidene fluoride membranes.20,21 After blocking the membranes with bovine serum albumin (BSA), the membranes were probed with biotin-labeled KGla cells for 2 hours at 37°C. The membranes were then fixed and the location of the cells identified with streptavidin-conjugated horseradish peroxidase. The figure is presented as an enhanced image for visualization. (The original figure is provided in Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article.) Adhesion of the biotin-labeled KG1a cells to proteins in the MG-63 membrane lysates revealed a band at approximately 36 and approximately 94 kDa.
Mass spectrometry (MS) spectra, tandem MS acquisition, and automated database searches were performed on the protein bands derived from replicate blots. Tryptic fragments of the protein purified from the approximately 36-kDa band yielded the sequences WISIMTER, SYSPYDMlLESIR, and GKSLYYFIQQDTK; these sequences were identical to amino acids 213 to 220, 234 to 245, and 312 to 324 of the Anxa2 monomer. Similar analyses were performed on the protein purified from the approximately 94-kDa band. These results suggested that the proteins in the approximately 94-kDa bands represented multimers of Anxa2 complexed with p11 (∼ 11-kDa). Reliable sequence could not however be obtained from the 70- to 75-kDa bands.
Anxa2 is localized to OB surfaces in bone marrow
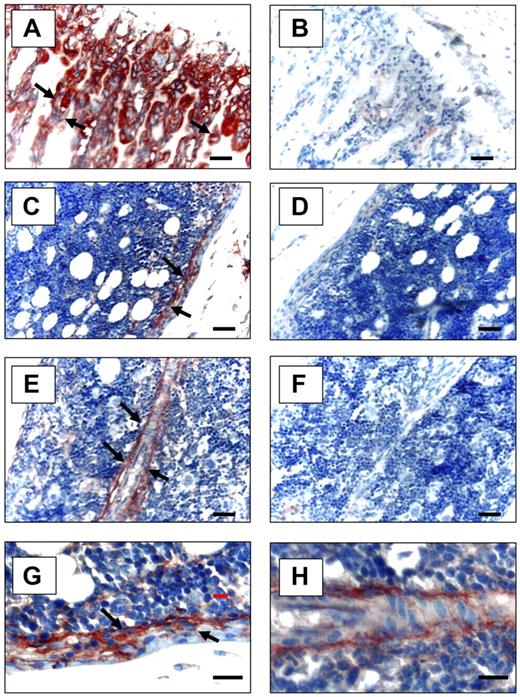
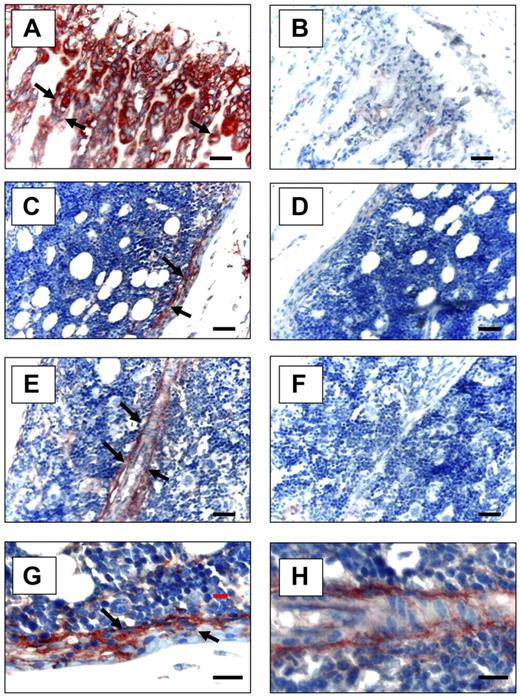
To determine whether Anxa2 is expressed at sites relevant to the localization of HSCs to the bone marrow niche, immunohistochemistry for Anxa2 was used. Anxa2 immunoreactivity was intense at the endosteal osteoblastic surfaces of murine marrow closest the growth plate (Figure 2A,C,E), whereas no signal was observed in the absence of the specific anti-Anxa2 antibody (Figure 2B,D,F). This pattern directly paralleled the immunolocalization of SDF-1 to the endosteal surfaces and specifically marked OBs.29,30 Bonemarrow endothelial cells were also immunoreactive for Anxa2 (Figure 2E).
Expression of Anxa2 protein in murine long bones. Mouse tibias were fixed in 10% formalin at 4°C before being decalcified in 10% EDTA (pH 7.4) and embedded in paraffin wax. Immunolocalization of Anxa2 protein (arrows) was visualized using a monoclonal antibody that was raised against Anxa2 or an IgG control antibody and a 9 ethyl-carbazole chromogen kit. Anxa2-immunostained tibias (20 × magnification) (A,C,E) and control immunoreactivity (B,D,F). Scale bars represent 50 μm. (A-B) Anxa2 expression at the epiphyseal growth plates. (C-D) Anxa2 expression in the diaphysis regions. (E-F) Anxa2 expression in bone marrow endothelial cells. (G-H) Higher magnification of panels C and E. Images at 20× (A-F) and 40× (G, H) via a 0.75 NA oil objective. A Nikon E800 (Diagnostic Instruments, Sterling Heights, MI) camera and Image-Pro Plus software (MediaCybernetics, Bethesda, MD) were used.
Expression of Anxa2 protein in murine long bones. Mouse tibias were fixed in 10% formalin at 4°C before being decalcified in 10% EDTA (pH 7.4) and embedded in paraffin wax. Immunolocalization of Anxa2 protein (arrows) was visualized using a monoclonal antibody that was raised against Anxa2 or an IgG control antibody and a 9 ethyl-carbazole chromogen kit. Anxa2-immunostained tibias (20 × magnification) (A,C,E) and control immunoreactivity (B,D,F). Scale bars represent 50 μm. (A-B) Anxa2 expression at the epiphyseal growth plates. (C-D) Anxa2 expression in the diaphysis regions. (E-F) Anxa2 expression in bone marrow endothelial cells. (G-H) Higher magnification of panels C and E. Images at 20× (A-F) and 40× (G, H) via a 0.75 NA oil objective. A Nikon E800 (Diagnostic Instruments, Sterling Heights, MI) camera and Image-Pro Plus software (MediaCybernetics, Bethesda, MD) were used.
Anxa2 regulates binding
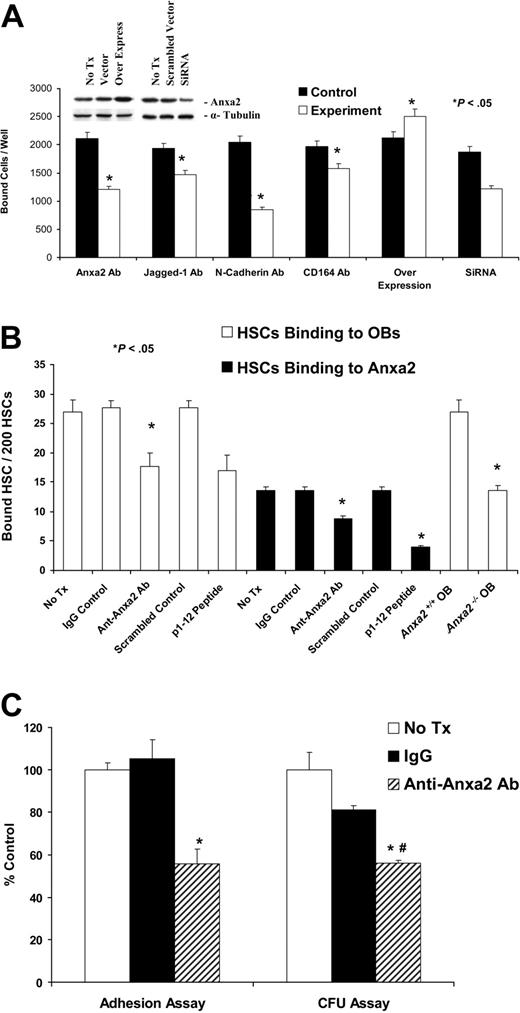
In vitro cell adhesion assays were performed to directly establish the relevance of Anxa2 to adhesion. Initially, KG1a cells were allowed to adhere to SaOS-2 monolayers. Because previous studies demonstrated that Jagged-1, N-cadherin, and CD164 are crucial for regulating the adhesion of HSCs to bone marrow cells and to OBs in particular,3,4,31 neutralizing antibodies that targeted these molecules were included as controls. Adhesion of KG1a cells to SaOS-2 monolayers was lower in the presence of the neutralizing antibodies compared with IgG-matched controls (Figure 3A). When the binding assay was repeated in the presence an anti-Anxa2 antibody, adhesion was also inhibited significantly (reduction, ∼ 48%) (Figure 3A). Overexpression of Anxa2 by cDNA transfection produced an increase in expression by 2- to 3-fold (Figure 3A insert) that resulted in an increase in 28% more KG1a cells adhering to the SaOS-2 cells. The silencing of Anxa2 expression by siRNA significantly the reduced adhesion of KG1a cells to SaOS-2 cells compared with the scrambled siRNA controls (Figure 3A). Similar observations were made when MG-63 or human bone marrow endothelial cells and murine OBs were used as the adherent target cell (data not shown). Further studies were subsequently performed with the KG1a/osteosarcoma system, which demonstrated that Anxa2 itself acts as an adhesion ligand, and peptide mapping identified the N-terminal 1 to 12 amino acids of Anxa2 as the active binding site (Figure S2).
Anxa2 regulates the adhesion of early blood cells to human and murine OBs. (A) KG1a cells (1 × 105) were deposited directly onto SaOS-2 monolayers in the presence or absence of antibodies that blocked the function of Jagged-1, N-cadherin, CD164, or Anxa2 or isotype-matched controls or a synthetic peptide corresponding to the 12 N-terminal amino acids (p1-12 peptide) of Anxa2 or a scrambled control for 15 minutes at 4°C. Where indicated, overexpression vectors or short interfering RNA (siRNA) knock down of Anxa2 was used to alter Anxa2 expression. After removing the nonadherent cells, the number of adherent cells was quantified using a fluorescence plate reader. Data are presented as the mean ± SD percentage of adherent cells. Inserts show the results of the Western blot analysis of overexpression or siRNA knockdown of Anxa2 in MG-63 and SaOS-2 cells. (B) Two hundred murine HSCs were layered on murine wild-type or Anxa2−/− OBs in the presence or absence of an anti-Anxa2 antibody (1 μg/mL). Adhesion was quantified after washing away the nonadherent cells by direct fluorescent cell enumeration. (C) Lin−CD34+ bone marrow progenitor cells (1 × 103) were deposited directly onto primary human OBs in the presence or absence of an antibody to Anxa2 or isotype-matched controls. After removing nonadherent cells, the number of adherent cells was quantified. Approximately one third (32% ± 8% or 320 ± 80 of 1000) of the input cells established functional adhesive interactions after 15 minutes at 4°C that were not disrupted by washing. Progenitor cell assays in methylcellulose were performed on CD34+ bone marrow progenitor cells recovered following trypsinization. As the individual frequencies of colony-forming unit–granulocyte-macrophage (CFU-GM), colony-forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM), and burst-forming unit–erythroid (BFU-E) did not differ significantly among the groups, data were normalized to the value of the untreated controls and are presented as the mean ± SD total colony-forming units per well as a percentage of the value of the untreated controls (n = 5). *P < .05 versus the no-treatment control, and #P < .05 versus the IgG control. Data are presented as the percentage of adherent cells relative to the percentage of cells that adhered under the control conditions (IgG control).
Anxa2 regulates the adhesion of early blood cells to human and murine OBs. (A) KG1a cells (1 × 105) were deposited directly onto SaOS-2 monolayers in the presence or absence of antibodies that blocked the function of Jagged-1, N-cadherin, CD164, or Anxa2 or isotype-matched controls or a synthetic peptide corresponding to the 12 N-terminal amino acids (p1-12 peptide) of Anxa2 or a scrambled control for 15 minutes at 4°C. Where indicated, overexpression vectors or short interfering RNA (siRNA) knock down of Anxa2 was used to alter Anxa2 expression. After removing the nonadherent cells, the number of adherent cells was quantified using a fluorescence plate reader. Data are presented as the mean ± SD percentage of adherent cells. Inserts show the results of the Western blot analysis of overexpression or siRNA knockdown of Anxa2 in MG-63 and SaOS-2 cells. (B) Two hundred murine HSCs were layered on murine wild-type or Anxa2−/− OBs in the presence or absence of an anti-Anxa2 antibody (1 μg/mL). Adhesion was quantified after washing away the nonadherent cells by direct fluorescent cell enumeration. (C) Lin−CD34+ bone marrow progenitor cells (1 × 103) were deposited directly onto primary human OBs in the presence or absence of an antibody to Anxa2 or isotype-matched controls. After removing nonadherent cells, the number of adherent cells was quantified. Approximately one third (32% ± 8% or 320 ± 80 of 1000) of the input cells established functional adhesive interactions after 15 minutes at 4°C that were not disrupted by washing. Progenitor cell assays in methylcellulose were performed on CD34+ bone marrow progenitor cells recovered following trypsinization. As the individual frequencies of colony-forming unit–granulocyte-macrophage (CFU-GM), colony-forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM), and burst-forming unit–erythroid (BFU-E) did not differ significantly among the groups, data were normalized to the value of the untreated controls and are presented as the mean ± SD total colony-forming units per well as a percentage of the value of the untreated controls (n = 5). *P < .05 versus the no-treatment control, and #P < .05 versus the IgG control. Data are presented as the percentage of adherent cells relative to the percentage of cells that adhered under the control conditions (IgG control).
To verify whether Anxa2 mediates the adhesion of normal cells, HSCs were isolated on the basis of the SLAM family of receptors (CD150 and CD48) and Sca-1.24,–26 The binding of fluorescently labeled HSCs to primary murine OBs and Anxa2 itself was assessed in the presence or absence of 1 μg/mL anti-Anxa2 antibody or 1 μg/mL of the Anxa2 N-terminal peptide (or controls). After washing away the nonadherent cells, quantification was performed manually in a blinded fashion using a fluorescent microscope. The data demonstrate that the binding of HSCs to OBs, or Anxa2 itself, was significantly inhibited using either antibody or the N-terminal Anxa2 peptide (Figure 3B). When the binding of HSCs to OBs derived from wild-type (Anxa2+/+) or Anxa2-deficient (Anxa2−/−) animals was evaluated, it was noted that significantly more HSCs bound to the Anxa2+/+ OBs than to the Anxa2−/− OBs (Figure 3B).
To determine if Anxa2 plays a similar role in regulating binding of cells, normal human Lin−CD34+ bone marrow progenitors, the cells were permitted to bind to primary human OBs. Nearly 32% ± 8% or 320 ± 80 of 1000 input cells established functional adhesive interactions after 15 minutes at 4°C that were not disrupted by washing. Progenitor assays were preformed on the bound cells, because there is considerable heterogeneity within the Lin−CD34+ population. Colony-forming units were quantified after 2 weeks. Treatment of cells with IgG alone reduced adhesion to 80% of that of the untreated controls, whereas inclusion of the anti-Anxa2 antibody caused a 56% reduction in adhesion (Figure 3C). There were no alterations in the resulting colony morphotypes relative to the control (unpublished data, YJ3/06). These results suggest that Anxa2 is crucial for the establishment of adhesive interactions between hematopoietic progenitors and OBs.
Anxa2 regulates the homing of bone marrow cells
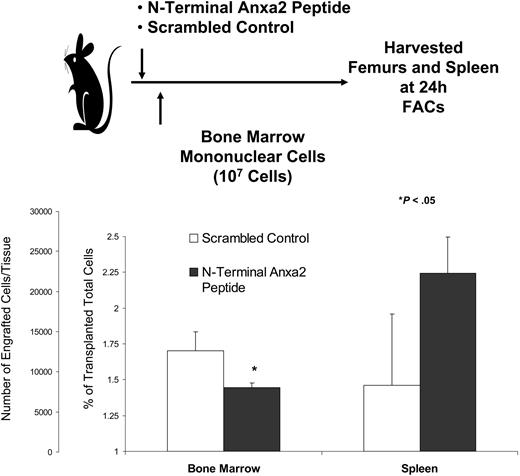
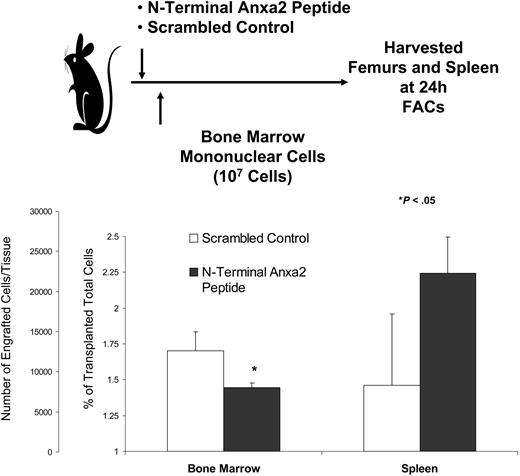
To evaluate if Anxa2 blockade would influence marrow trafficking, short-term homing of murine marrow cells was evaluated. Immediately prior to transplantation, recipient mice were inoculated with a synthetic peptide that corresponded to the N-terminal 1 to 12 amino acids of Anxa2 or a scrambled control peptide. The level of engraftment was assessed in the long bones at 24 hours by fluorescence-activated cell sorting (FACs) analysis. As shown in Figure 4, treatment of the mice with the N-terminal Anxa2 peptide significantly reduced the level of engraftment in the marrow.
Anxa2 regulates stem cell homing to bone marrow. Fluorescently labeled bone marrow mononuclear cells were transplanted via intravenous injection into nonablated mice that had been inoculated with either a N-terminal Anxa2 peptide or a scrambled control at a concentration of 10 μg/kg (0.2 μg/animal). The percentage engraftment of the transplanted cells was quantified at 24 hours by FACs analysis by gating on 106 cells. *P < .05 versus the control. The data demonstrate that blocking Anxa2 results in fewer labeled cells in the marrow.
Anxa2 regulates stem cell homing to bone marrow. Fluorescently labeled bone marrow mononuclear cells were transplanted via intravenous injection into nonablated mice that had been inoculated with either a N-terminal Anxa2 peptide or a scrambled control at a concentration of 10 μg/kg (0.2 μg/animal). The percentage engraftment of the transplanted cells was quantified at 24 hours by FACs analysis by gating on 106 cells. *P < .05 versus the control. The data demonstrate that blocking Anxa2 results in fewer labeled cells in the marrow.
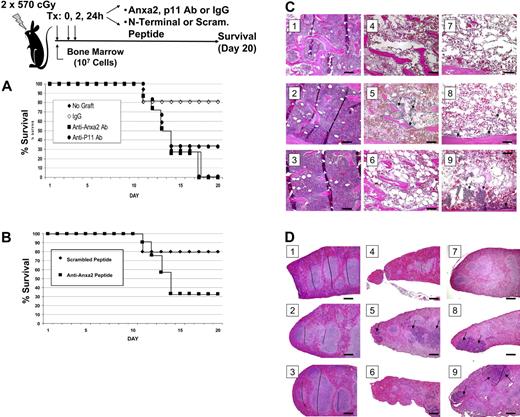
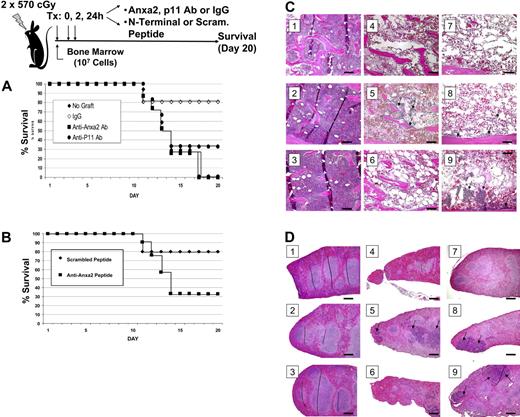
To assess the role of Anxa2 in hematopoietic reconstitution, irradiated mice received a transplant of bone marrow cells in the presence or absence of antibodies raised against Anxa2, p11 (the Anxa2-binding partner), or an isotype-matched antibody control. Additional treatment groups received a synthetic peptide that corresponded to Anxa2 N-terminal peptide or a scrambled peptide control. Survival of the mice was analyzed using the Kaplan-Meier method, and a log-rank test was used for univariate analysis of the data. Mice that did not receive a transplant either died or were killed (Figure 5A). None of the mice that received transplants and the anti-Anxa2 antibody survived to day 20, where as 80% of the mice that underwent transplantation and received the IgG control antibody survived (Figure 5A). Survival was lower in the mice that received transplants and the anti-p11 antibody compared with the controls, but this antibody did not decrease survival to the same extent as did the anti-Anxa2 antibody (Figure 5A). The survival of mice treated with the N-terminal peptide was lower that that of mice treated with the scrambled peptide (Figure 5B; and unpublished data).
Anxa2 regulates homing of bone marrow progenitor cells to bone marrow. Survival was evaluated following intravascular injection of 2 × 105 bone marrow cells into irradiated mice. Control mice received a sham injection. (A) Radiated mice received intravenous injections of anti-Anxa2 antibody, p11 (the binding partner of Anxa2), or an isotype-matched antibody control at 0, 2, and 24 hours after transplantation. (B) Recipient mice were inoculated with either N-terminal Anxa2 peptide or a scrambled peptide (control) at the time of the transplantation as well as 2 hours and 24 hours later. Survival was analyzed using the Kaplan-Meier method. Data are representative data of 3 experiments. *P < .05 versus the control. Histologic examination of the bone marrow (C) and spleens (D) of experimental animals taken at day 9. (i) Representative data of normal histology of animals receiving no treatment. (ii) Animals received an N-terminal Anxa2 peptide only. (iii) Animals were treated with an anti-Anxa2 antibody only. (iv) Animals were treated with lethal irradiation (2 doses of 570 cGy). (v) Animals were treated with irradiation and a marrow transplantation (2 × 105 cells). (vi) Animals were treated with irradiation, a marrow transplantation, and an N-terminal Anxa2 peptide. (vii) Animals were treated with irradiation, a marrow transplantation, and an anti-Anxa2 antibody. (viii) Animals were treated with irradiation, a marrow transplantation, and a scrambled peptide. (ix) Animals were treated with irradiation, a marrow transplantation, and an IgG control antibody. Regeneration of hematopoietic marrow was noted in animal marrows, and spleen was noted in animals receiving transplants and control antibody and scrambled peptide (arrows) at 20 ×. Bars indicate 100 microns. Image acquisition information is in caption to Figure 2.
Anxa2 regulates homing of bone marrow progenitor cells to bone marrow. Survival was evaluated following intravascular injection of 2 × 105 bone marrow cells into irradiated mice. Control mice received a sham injection. (A) Radiated mice received intravenous injections of anti-Anxa2 antibody, p11 (the binding partner of Anxa2), or an isotype-matched antibody control at 0, 2, and 24 hours after transplantation. (B) Recipient mice were inoculated with either N-terminal Anxa2 peptide or a scrambled peptide (control) at the time of the transplantation as well as 2 hours and 24 hours later. Survival was analyzed using the Kaplan-Meier method. Data are representative data of 3 experiments. *P < .05 versus the control. Histologic examination of the bone marrow (C) and spleens (D) of experimental animals taken at day 9. (i) Representative data of normal histology of animals receiving no treatment. (ii) Animals received an N-terminal Anxa2 peptide only. (iii) Animals were treated with an anti-Anxa2 antibody only. (iv) Animals were treated with lethal irradiation (2 doses of 570 cGy). (v) Animals were treated with irradiation and a marrow transplantation (2 × 105 cells). (vi) Animals were treated with irradiation, a marrow transplantation, and an N-terminal Anxa2 peptide. (vii) Animals were treated with irradiation, a marrow transplantation, and an anti-Anxa2 antibody. (viii) Animals were treated with irradiation, a marrow transplantation, and a scrambled peptide. (ix) Animals were treated with irradiation, a marrow transplantation, and an IgG control antibody. Regeneration of hematopoietic marrow was noted in animal marrows, and spleen was noted in animals receiving transplants and control antibody and scrambled peptide (arrows) at 20 ×. Bars indicate 100 microns. Image acquisition information is in caption to Figure 2.
Necropsies performed on day 9 revealed that the antibody alone had no effect on tissue structure or integrity of the aorta, spleen, liver, kidney, or bone marrow and ruled out a direct effect of the treatment on these organs. Bone marrow of the irradiated mice that did not receive cell transplants revealed severe swelling of the marrow sinusoids and reductions in cellularity (Figure 5C). Irradiated mice receiving a transplant and/or control antibodies exhibited partial recovery within focal areas of the marrow; recovery was nearly complete by day 20 (data not presented, YJ). Recovery of the spleen (Figure 5D) and peripheral blood cell counts paralleled the recovery exhibited by the marrow with a nadir in counts observed at day 9 and recovery by day 20 (unpublished data, YJ). Mice that received a bone marrow transplant together with the anti-Anxa2 antibody or N-terminal Anxa2 peptide exhibited a significant reduction in the size and number of recovered hematopoietic focal areas on day 9 (Figure 5D-E). These results thus suggest that OBs and/or endothelial Anxa2 plays an essential role in the homing of HSCs to their bone marrow microenvironmental niche.
To directly address the role of Anxa2 on HSC function, CLT-BMTs were used. Here HSCs were isolated from Ly-5.1 (CD45.1) mice on the basis of the SLAM family of receptors (CD150 and CD48).24,–26 Antibody to Anxa2 or the N-terminal Anxa2 peptide (both at 10 μg/kg [0.2 μg/animal]) were used to target Anxa2 activities with 36 HSCs transplanted into Ly-5.2 (CD45.2) congenic C57BL6 mice to follow long-term repopulation capabilities.27 Animals treated with antibody or N-terminal Anxa2 peptide demonstrated significantly fewer of the Ly-5.1 progeny in the peripheral blood including circulating B cells, T cells, and granulocytes at 16 weeks compared with animals treated with peptide or antibody controls (Figure 6).
Reduced chimerism occurs in BMT when Anxa2 is blocked during transplantation. Competitive long-term bone marrow transplantations (CLT-BMTs) were used to determine the effect of Anxa2 blockade on HSC engraftment. HSCs isolated on the basis of the SLAM family of receptors (CD150 and CD48) derived from Ly-5.1 (CD45.1) mice using a maximum of 36 HSCs were transplanted into Ly-5.2 (CD45.2) congenic C57BL6 mice along with a mixture of a radioprotective dose of Ly-5.2 cells (2 × 105 cells). Antibody to Anxa2, or a synthetic peptide corresponding to the N-terminal Anxa2 peptide at 10 μg/kg (0.2 μg/animal), or IgG and scrambled peptide controls were used to block Anxa2 activity. Sixteen weeks after transplantation the percentages of marrow cells bearing the Ly-5.2 and Ly-5.1 phenotypes were determined in peripheral blood analyzed by flow cytometry. The data demonstrate that targeting Anxa2 prevents HSC engraftment. Data are representative of 2 independent investigations where *P < .05 versus the control.
Reduced chimerism occurs in BMT when Anxa2 is blocked during transplantation. Competitive long-term bone marrow transplantations (CLT-BMTs) were used to determine the effect of Anxa2 blockade on HSC engraftment. HSCs isolated on the basis of the SLAM family of receptors (CD150 and CD48) derived from Ly-5.1 (CD45.1) mice using a maximum of 36 HSCs were transplanted into Ly-5.2 (CD45.2) congenic C57BL6 mice along with a mixture of a radioprotective dose of Ly-5.2 cells (2 × 105 cells). Antibody to Anxa2, or a synthetic peptide corresponding to the N-terminal Anxa2 peptide at 10 μg/kg (0.2 μg/animal), or IgG and scrambled peptide controls were used to block Anxa2 activity. Sixteen weeks after transplantation the percentages of marrow cells bearing the Ly-5.2 and Ly-5.1 phenotypes were determined in peripheral blood analyzed by flow cytometry. The data demonstrate that targeting Anxa2 prevents HSC engraftment. Data are representative of 2 independent investigations where *P < .05 versus the control.
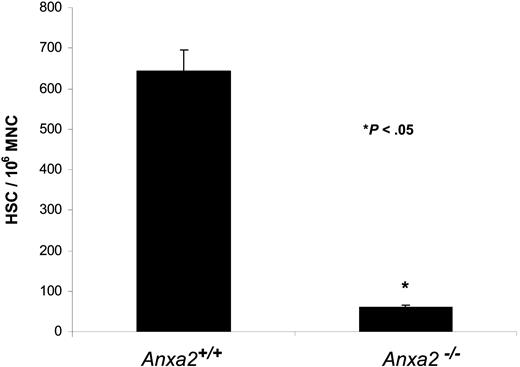
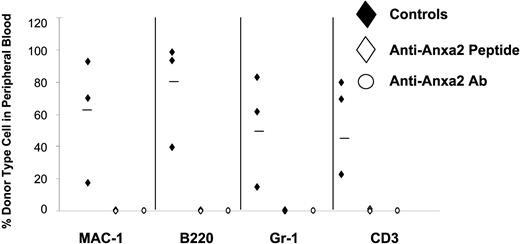
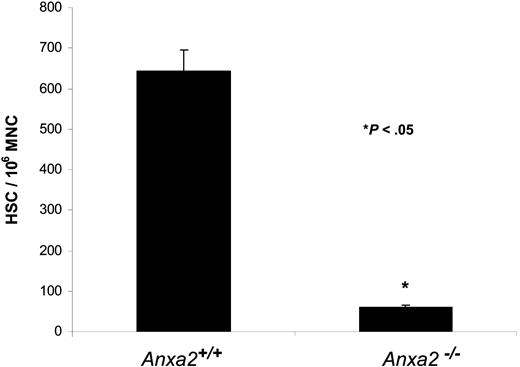
Finally, HSC frequencies were determined in Anxa2-deficient (Anxa2−/−) and age-matched wild-type animals using limiting dilution in competitively repopulated hosts. The variation in the proportion of positive animals with each dose of test cells (2 × 104, 2 × 103) was analyzed by Poisson statistics to measure HSC frequency. The Anxa2−/− animals had nearly a log-fold fewer HSCs per 106 mononuclear marrow cells in their marrow compared with age-matched wild-type animals (Figure 7).
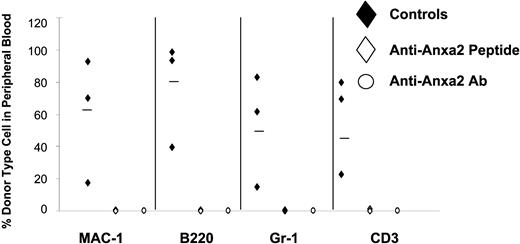
Reduced number of HSCs are present in the bone marrow of Anxa2-deficient (Anxa2−/−) mice. Competitive long-term bone marrow transplantations (CLT-BMT) were used to determine HSC frequency in murine wild-type or Anxa2−/− mice. Lethally irradiated Ly-5.1 mice (570 cGy twice in 3 hours) were injected intravenously with 2 doses bone marrow mononuclear cells derived from the Anxa2 knock-out or wild-type animals together with a radioprotective dose of 2 × 105 bone marrow cells (Ly-5.1). The proportions of Ly-5.2 cells in peripheral blood of the different lineages (MAC-1, B220, Gr-1, or CD3 cells) were analyzed by 4-color flow cytometry at 16 weeks. The variation in the proportion of animals not displaying Ly5.2-positive cells (0.1% cut off) in the peripheral blood was analyzed by Poisson statistics to measure HSC frequency. The Anxa2−/− animals had nearly a log-fold fewer HSCs per 106 mononuclear marrow cells compared with age-matched wild-type animals.
Reduced number of HSCs are present in the bone marrow of Anxa2-deficient (Anxa2−/−) mice. Competitive long-term bone marrow transplantations (CLT-BMT) were used to determine HSC frequency in murine wild-type or Anxa2−/− mice. Lethally irradiated Ly-5.1 mice (570 cGy twice in 3 hours) were injected intravenously with 2 doses bone marrow mononuclear cells derived from the Anxa2 knock-out or wild-type animals together with a radioprotective dose of 2 × 105 bone marrow cells (Ly-5.1). The proportions of Ly-5.2 cells in peripheral blood of the different lineages (MAC-1, B220, Gr-1, or CD3 cells) were analyzed by 4-color flow cytometry at 16 weeks. The variation in the proportion of animals not displaying Ly5.2-positive cells (0.1% cut off) in the peripheral blood was analyzed by Poisson statistics to measure HSC frequency. The Anxa2−/− animals had nearly a log-fold fewer HSCs per 106 mononuclear marrow cells compared with age-matched wild-type animals.
Discussion
In the present study, we demonstrate that Anxa2 plays a crucial role in establishing the HSC niche by regulating the adhesion of HSCs to OBs and endothelial cells. Initially, Anxa2 was identified using an open, functional screen in which OB membranes were isolated, separated on nondenaturing gels, and probed with biotin-labeled KG1a. Immunohistochemistry demonstrated that Anxa2 is expressed preferentially at the endosteal and endothelial surfaces. Subsequently, we demonstrated that the adhesion of HSCs to OBs and bone marrow endothelial cells were inhibited by antibodies against Anxa2 and by siRNA knockdown of Anxa2, whereas overexpression of Anxa2 enhanced the adhesion. Adhesion of HSCs to OBs derived from Anxa2-deficient animals (Anxa2−/−) was significantly impaired compared with adhesion to OBs obtained from wild-type animals (Anxa2+/+). Moreover, fewer HSCs were found in the marrow of Anxa2−/− versus Anxa2+/+ animals, and blocking Anxa2 prevented the adhesion of human Lin−CD34+ bone marrow cells to primary human OBs. In in vivo studies, short-term lodging, engraftment, and survival of irradiated mice with mononuclear cells (MNCs) was substantially inhibited by peptides targeting the N-terminus of Anxa2 or anti-Anxa2 antibodies. Similar findings were noted in long-term competitive repopulation studies using HSCs. Furthermore, we recently found that Lin−CD34+ bone marrow cells and KG1a cells both express mRNA for a punitive Anxa2 receptor (Anxa2r)32 and antibody to the Anxa2r inhibited the adhesion of KG1a cells to the MG-63 and SaOS-2 osteosarcoma cell lines (unpublished data, YJ). Together these data strongly suggest that Anxa2 plays an important role in the localization of HSCs to the marrow.
Stem cell homing is a rapid event that depends largely on endothelial cells.33,,,–37 Stem cell lodging within extravascular spaces is dominated by events that occur at the OB surface.36,–38 For example, engrafting stem cells appear to migrate preferentially to the endosteal surfaces, whereas mature, terminally differentiated, and lineage-committed cells redistribute selectively away from the endosteal surfaces into the central marrow.7,35,39 Thus, the homing, retention, and egress of HSCs into and out of the marrow likely represents a dynamic balance between cell-cell adhesions and diverse coupling systems in an environment within which physical adhesion and developmental signals serve to support survival, growth, and differentiation.40 The expression of Anxa2 by both OBs and bone marrow endothelial cells suggests that Anxa2 may play a crucial role in regulating both the homing and lodging of HSCs. However, Anxa2 is believed to be an endothelial cell coreceptor for plasminogen and tissue plasminogen activator. Treatment of rat carotid arteries with wild-type Anxa2 prevented thrombosis in response to oxidative injury with ferric chloride, whereas treatment with a mutant form of the protein had no such effect.28 In humans with acute promyelocytic leukemia, overexpression of Anxa2 by leukemia cells may lead to deregulation of plasmin generation and a hyperfibrinolytic hemorrhagic state,41 which suggests that Anxa2 is involved in maintaining hemostatic balance.
Recently, the gene that encodes Anxa2 in mice was targeted to analyze the physiological role of Anxa2.28 Homozygous Anxa2−/− mice were viable, exhibited fibrin deposition in the microvasculature, and failed to clear injury-induced arterial thrombi.28 Postnatal angiogenesis was markedly impaired in the Anxa2−/− mice, and this was attributed to a failure to localize plasmin activity to the endothelial cell surface and a failure to activate selected matrix metalloproteinases.28 Other studies have revealed that the number of circulating Flk-1+, Sca-1+ endothelial and hematopoietic precursor cells was approximately 20-fold lower in Anxa2−/− mice than in wild-type controls. We have not yet been able to achieve under the conditions used in the present study reproducible and durable engraftment of HSCs into Anxa2−/− mice. This suggests that Anxa2 is a key molecule in transplantation, whose real role is revealed by the stress of bone marrow transplantation. Further investigations are clearly required, however.
In the bone marrow, HSCs are know to associate with at least 2 separate niches, the endosteal niche (which is regulated by OB3,,,–7 ) and the vascular niche (which is regulated by endothelial cells42,43 ). Given the distribution of Anxa2 in both niches, Anxa2 likely plays several roles in regulating hematopoiesis. Specifically, while Anxa2 expressed on endothelial cells regulates the plasmin/plasminogen system and may play a role in anchoring key components of the fibrinolytic cascade,44,–46 endothelial-expressed Anxa2 may also regulate the initial attachment of HSCs to the endothelium. The endosteum, on the other hand, is a site of constant bone remodeling characterized by the generation of high concentrations of calcium ions (Ca2+). Anxa2 expressed on OBs is known to regulate osteoclastic activations and mineralization.47,48 Recently Adams et al demonstrated that the calcium receptor (CaR) is critical for HSC localization or function in the endosteal niche.49 Lin−, Sca-1+, cKit+ cells express the calcium receptor (CaR), and the bone marrow of CaR-deficient mice has markedly fewer LSK cells than do wild-type littermates. Moreover, the marrow of CaR-deficient mice contains fewer cells capable of initiating long-term cultures, while the number of LSK cells in the spleen and the blood of CaR-deficient mice is increased. Thus, Anxa2 regulates mineralization and calcium turnover by virtue of its activities on osteoclasts and OBs, and makes it an ideal candidate for regulating HSC functions as well. Anxa2 is therefore likely a key regulator of both niches and probably participates in initial niche determination, while subsequent cues are generated by additional cell adhesion regulators and site-specific expression of regulatory molecules such as cytokines and growth factors.
Previously we reported that sialated N-linked glycoproteins mediate the initial tethering of HSCs to OBs.8 However, after these adhesions are established, secondary adhesive events provide firm cell-cell contact to OBs that assure the survival of HSCs.9 Based upon antibody and recombinant ligand studies, engagement of LFA-1 and VLA-4 receptors on progenitor cells appears a likely candidate for transducing survival signals in the presence of soluble factors from OBs. Here it was demonstrated that recombinant ligands for LFA-1 (ICAM-1) or VLA-4 (VCAM-1, FN) together with osteoblast-conditioned medium are critical for the maintenance of early blood cells. Other findings suggest that CD164 and jagged-1 and perhaps even Anxa2 stabilize the adherent cells and/or provide maintenance cues to adherent cells (which involves VLA-4 [α4β1] and VLA-5 [α5β1] receptors).9 This is consistent with several recent reports that suggested that N-cadherin, Ang-1, and Jagged-1 are important players in the aforementioned processes.3,–5 In addition, the relationship between survival and niche determination is highly complex and is based on the engagement of Tie-2, N-cadherin, and osteopontin.6,7 All of these interactions are likely necessary for HSC functions, and therefore Anxa2 represents a prime candidate as a pleiotropic regulator of both the endosteal and vascular niches.
In summary, using an unbiased functional screen, we have found that Anxa2 provides a major substrate for HSC adhesion within the bone marrow microenvironment. Inhibition of this interaction in the context of HSC reconstitution leads immediately to defective HSC homing, and to subsequent hematopoietic failure and death. Our findings that Anxa2 is expressed on both bone marrow endothelial cells and OBs indicates that Anxa2 likely plays an important role in multiple bone marrow microenvironmental niches, including vascular42,50 and an endosteal3,,,–7 niches that regulate the survival of HSCs in bone marrow. Therefore, Anxa2 may play a central role in bone marrow engraftment and in the multistep process of stem cell trafficking and homing in general.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by DAAD190310168 and DE13701.
Proteomics was provided by the Michigan Proteome Consortium (http://www.proteomeconsortium.org), which is supported in part by funds from the Michigan Life Sciences Corridor. The human Anxa2 cDNA and Anxa2r were kindly provided by Drs G. W. Lu and G. D. Roodman (University of Pittsburgh, PA). We are grateful to Dr K. A. Hajjar (Weill Medical College of Cornell University, New York, NY) for providing the Anxa2−/− mice. Dr B. Seshi (UCLA) provided assistance with the cell blotting. Drs S. Morrison, M. Kiel, J. Ferrara, C. Y. Wang, and L. K. McCauley provided helpful comments and critiques (University of Michigan). This work is dedicated to the memory of Kirk Koepsel and Ellesmere Jane.
Authorship
Contribution: Y.J., A.H., Y.-X.S., and Y.S. performed cell cultures, adhesion assays, and animal experiments; J.W. performed cell blotting and identification of Anxa2; J.S., Z.W., and Y.S. performed transplant studies; J.W. performed vector construction; S.G.E., P.H.K., and R.S.T. performed scientific oversight and data interpretation. Y.J. and J.W. contributed equally to the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Russell S. Taichman, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, 1011 North University Ave, Ann Arbor, MI 48109-1078; e-mail: rtaich@umich.edu.