Abstract
Taxane derivatives such as paclitaxel elicit their antitumor effects at least in part by induction of apoptosis, but the underlying mechanisms are incompletely understood. Here, we used different cellular models with deficiencies in key regulators of apoptosis to elucidate the mechanism of paclitaxel-induced cell death. Apoptosis by paclitaxel was reported to depend on the activation of the initiator caspase-10; however, we clearly demonstrate that paclitaxel kills murine embryonic fibroblasts (MEFs) devoid of caspase-10 as well as human tumor cell lines deficient in caspase-10, caspase-8, or Fas-associating protein with death domain. In contrast, the lack of Apaf-1 or caspase-9, key regulators of the mitochondrial pathway, not only entirely protected against paclitaxel-induced apoptosis but could even confer clonogenic survival, depending on the cell type and drug concentration. Thus, paclitaxel triggers apoptosis not through caspase-10, but via caspase-9 activation at the apoptosome. This conclusion is supported by the fact that Bcl-2–overexpressing cells and Bax/Bak doubly-deficient MEFs were entirely resistant to paclitaxel-induced apoptosis. Interestingly, also the single knockout of Bim or Bax, but not that of Bak or Bid, conferred partial resistance, suggesting a particular role of these mediators in the cell-death pathway activated by paclitaxel.
Introduction
Paclitaxel (Taxol; Calbiochem, Darmstadt, Germany) is one of the most effective antitumor drugs and is approved for chemotherapy of a variety of human malignancies, including breast, ovarian and non-small cell lung cancer.1 Like other taxane derivatives, paclitaxel binds to the β-subunit of tubulin, thus preventing microtubule depolymerization that is required for proper mitosis and cell division. During mitosis, the spindle checkpoint ensures proper chromosome segregation by blocking the anaphase onset until all chromosomes are attached to microtubules and tension across the kinetochores is generated. Paclitaxel inhibits the dynamic instability of the mitotic spindle, leading to impaired chromosome alignment. Consequently, the cells become arrested by the spindle checkpoint at the G2/M phase and then die eventually by an apoptotic pathway, called mitotic catastrophe.2 Cells that escape this pathway can be also arrested by a second, postmitotic checkpoint, which is activated by aberrant division of cells with multipolar spindles, and leads to cell death in polyploid cells following paclitaxel treatment.3 Although both p53-dependent and -independent mechanisms have been implicated in cell death during aberrant mitosis, the exact mechanisms of paclitaxel-induced apoptosis are currently unknown.
Apoptosis is largely controlled by a family of aspartate-specific cysteine proteases, called caspases, that function as initiators and executioners of the apoptotic process.4,5 Caspases are activated by two major signaling routes, the extrinsic death receptor and the intrinsic mitochondrial pathway, that both depend on the formation of large multiprotein complexes.4,6 Initiator caspase-8 and caspase-10, a close homolog of caspase-8 in humans, are the key mediators of the extrinsic pathway. In a simplified model, binding of death ligands such as TNF (tumor necrosis factor), CD95L, or TRAIL (TNF-related apoptosis-inducing ligand) to their respective death receptors leads to receptor oligomerization. This event then results in the recruitment of the adaptor protein Fas-associating protein with death domain (FADD) and caspase-8/10 into a death-inducing signaling complex (DISC). In the DISC, the initiator caspases are activated by dimerization and autoproteolytic cleavage. Subsequently, caspase-8 and caspase-10 cleave and activate caspase-3, resulting in the further cleavage of several cellular targets that are responsible for the morphologic manifestations of cell death. The intrinsic pathway, in contrast, is regulated at the mitochondria, which release cytochrome c and other proapoptotic factors during different forms of cellular stress.4,6 In the cytosol, cytochrome c binds to Apaf-1 and, in concert with the initiator caspase-9, forms the apoptosome. Caspase-9 is then activated at this complex and triggers the caspase cascade and subsequent apoptosis.
The release of cytochrome c is controlled by members of the Bcl-2 family that are characterized by 1 to 4 Bcl-2 homology (BH) domains.7 Bcl-2 proteins are classified into 2 major groups: the prosurvival members (eg, Bcl-2, Bcl-xL) and the proapoptotic members, which are further subdivided into the multidomain proteins (eg, Bax, Bak) and the BH3-only proteins (eg, Bad, Bim, Bid). Both Bax and Bak undergo a conformational change in response to apoptotic stimuli, leading to their assembly into multimers with channel-forming properties in the mitochondrial membrane. The conformational change of Bak or Bax is inducible by BH3-only proteins, which act as allosteric regulators of Bcl-2 proteins and as sensors of apoptotic signaling.8 For example, during death receptor–mediated apoptosis, Bid is cleaved into the death-promoting p15 fragment, tBid, which is targeted to mitochondria and binds to either Bax or Bak, resulting in their activation. Other BH-3–only proteins are activated by posttranscriptional or posttranslational mechanisms. Bim, for example, exists in several splice variants with different proapoptotic activities, such as BimS, BimL, and BimEL. In normal cells, BimL and BimEL are kept inactive by their sequestration to the microtubule network.9 Certain apoptotic stimuli, including paclitaxel, lead to the phosphorylation-induced activation of Bim, which results in its displacement from microtubuli and translocation to the mitochondria.
Previous studies have indicated an essential role of initiator caspase-10 in apoptosis induction by paclitaxel, which was proposed to be activated independently of death receptors and downstream of cytochrome c release.10,11 A similar postmitochondrial activation loop has been described for caspase-8 and is suggested to enhance the cytotoxic effect of irradiation and chemotherapeutic drugs.12-14 The conclusion that caspase-10 is required for paclitaxel-induced cell death was mainly based on protective effects of a so-called “caspase-10–specific” peptide inhibitor AEVD-fmk. However, tetrapeptide-based inhibitors are relatively unspecific and presumably unable to distinguish the contribution of an individual caspase.15 Therefore, we thought that the impact of caspase-10 on paclitaxel-induced apoptosis required further clarification.
In the present study, we used several models with deficiencies in key regulators of apoptosis in order to elucidate the mechanisms of paclitaxel-induced cell death. We demonstrate that not only death receptors but also caspase-10 are dispensable for paclitaxel-induced cell death. In contrast, paclitaxel-induced apoptosis requires caspase-9 and a functional apoptosome. Further upstream, we show that Bcl-2–overexpressing cells and Bax/Bak doubly deficient cells were entirely protected against paclitaxel-induced apoptosis. Interestingly, a single knock-out of Bim or Bax also conferred partial resistance, suggesting a particular role of these mediators in the mechanism of paclitaxel action.
Materials and methods
Cell culture and treatments
SHEP and SH-SY5Y neuroblastoma cells and MCF-7 breast carcinoma cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (PAA Laboratories, Linz, Austria). MCF-7 cells reconstituted with caspase-3 and clones stably expressing caspase-10 in the absence or presence of caspase-316 were cultured in the same medium supplemented with either 400 μg/mL neomycin and/or 300 μg/mL zeocin (Invitrogen, Karlsruhe, Germany). Jurkat T cells deficient in caspase-8 or FADD17,18 were kindly provided by J. Blenis (Harvard Medical School, Boston, MA). Jurkat cells overexpressing Bcl-2 as well as Jurkat cells deficient in caspase-9 and caspase-9–reconstituted clones have been published.12,19 All Jurkat cell lines were cultured in RPMI-1640 medium. Wild-type and knock-out mouse embryonic fibroblasts (MEFs) were kindly provided by A. Strasser (The Walter and Eliza Hall Institute, Melbourne, Australia), S. J. Korsmeyer (Harvard Medical School, Boston, MA), F. Cecconi (University Tor Vergata, Rome, Italy) and C. Borner (University of Freiburg, Germany), and maintained in Dulbecco modified Eagle medium (DMEM). Cells were seeded at a density of 1 × 105 cells per well in 6-well plates and treated for 24 to 96 hours with different concentrations of paclitaxel (Calbiochem) in the presence or absence of the pan-caspase inhibitor QVD-oPh (MP Biomedicals, Eschwege, Germany).
Immunoblotting
Cells were lysed in buffer containing 1% NP-40, 50 mM Tris [pH 7.5], 150 mM NaCl, 0.5 mM EDTA, 2 mM DTT, and complete protease inhibitor cocktail. The lysates were then separated on 10% to 15% linear polyacrylamide gels and transferred onto Hybond PVDF membranes (Amersham Biosciences, Uppsala, Sweden). Proteins were detected by enhanced chemiluminescence. The following primary antibodies were used: caspase-10 (clone 4C1; MBL International, Nagoya, Japan), human caspase-8 (clone 12F5; BioCheck, Münster, Germany), mouse caspase-8 (clone 1G12; Alexis, Lausen, Switzerland), β-actin (clone AC-74; Sigma-Aldrich, Steinheim, Germany), mouse Apaf-1 (18H2; Alexis), caspase-3 (goat polyclonal antibody; R&D Systems, Wiesbaden, Germany), FADD (clone 1; BD Biosciences Pharmingen, Heidelberg, Germany), human caspase-9 (rabbit polyclonal antibody; Cell Signaling Technologies, Danvers, MA), mouse caspase-9 (rabbit polyclonal antibody; Cell Signaling Technologies) and Bcl-2 (clone C-2; Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase–coupled secondary antibodies were purchased from Promega (Mannheim, Germany), Molecular Probes (Leiden, the Netherlands), and Santa Cruz Biotechnology, respectively.
Flow cytometric analyses
Flow cytometric analysis of the cell cycle and cellular DNA content was carried out essentially as described.20 Cells were lysed in hypotonic citrate buffer, stained with propidium iodide (PI; 50 μg/mL) for 10 minutes at room temperature (RT) and subsequently analyzed in the Fl-2/Fl-3 channels of a FACSCalibur flow cytometer equipped with Cell Quest II software (Becton Dickinson, Heidelberg, Germany). Phosphatidylserine (PS) exposure and PI uptake were measured using annexin V–FITC and PI (2 μg/mL) according to the manufacturer's recommendations (BD Biosciences Pharmingen). Costaining with the vital dye PI was carried out to exclude dead cells. Using standard Fl-1/Fl-2 settings, cells were measured directly on a FACSCalibur. The mitochondrial membrane potential was analyzed using the cationic, lipophilic dye 3,3′-dihexyloxacarbocyanine (DiOC6; Molecular Probes) as described.21 Live cells were stained with 40 nM DiOC6 and PI (2 μg/mL) in the dark at 37°C for 30 minutes. After washing, cells were evaluated on a FACSCalibur. Each experiment was performed independently at least 3 times and an individual experiment analyzing 10 000 cells was carried out in duplicate.
Crystal violet assay
Cell death was also measured by the crystal violet assay based on the staining of viable cells as described.16 In brief, 2 × 104 cells per well were seeded into 96-well plates and incubated with 0.1 μM paclitaxel for the indicated times at 37°C. Viable cells were subsequently stained with 20% methanol containing 0.5% crystal violet and solubilized in 33% acetic acid. The absorbance was measured at 590 nm (A590). The percentage of specific cell death is defined as 100 − (A590 of test well × 100 / A590 of untreated well). Each experiment was performed independently at least 3 times in duplicate.
Clonogenic and cell proliferation assays
Colony formation of Jurkat cell lines was evaluated using semisolid medium. Briefly, 104 cells/well were mixed with RPMI-1640 medium containing 1% methylcellulose and 30% FCS and plated on 6-well plates. After 14 days, colonies were stained with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and scored under a microscope. Colony formation was calculated for each condition in relation to values obtained for untreated cells. For measuring the colony-forming potential of adherent MEFs, 3 × 104 cells/well were seeded in 6-well plates and treated with the indicated concentrations of paclitaxel at the following day. After 48 hours, cells were washed and maintained in normal medium for another 24 hours until the untreated controls reached 90% confluency. Cells were split 1:10 and reseeded in new 6-well plates. After 6 days, cells were stained with crystal violet as described. Relative proliferation was defined as A590 of test well × 100 / A590 of untreated well.
Caspase assays
Caspase activity was measured by a fluorimetric assay using the synthetic substrate N-acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin (ac-DEVD-amc; MP Biomedicals) as described.22 Cell lysates (25 μg protein) were added to 50 μM ac-DEVD-amc in buffer (50 mM HEPES [pH 7.3], 100 mM NaCl, 10% sucrose, 0.1% CHAPS, and 10 mM DTT). Substrate cleavage was monitored for up to 180 minutes.
Fluorescence microscopy
To analyze spindle formation, cells were seeded on coverslips, treated with 1 μM paclitaxel, or left untreated. After 48 hours, cells were fixed in methanol-acetone (1:1), blocked with 4% bovine serum albumin (BSA) in phosphate-buffered saline (BSA/PBS) for 1 hour, and stained with anti–α-tubulin (clone DM 1A; Sigma-Aldrich) at 4°C overnight. After washing with PBS, cells were stained with Alexa-488–conjugated anti–mouse IgG (Molecular Probes) for 1 hour at RT and mounted in fluorescence medium (Dako, Glostrup, Denmark) containing DAPI (4,6 diamidino-2-phenylindole; Molecular Probes). Digital pictures were captured using a Leica TCS-SP2/AOBS confocal microscope (Leica Camera AG, Solms, Germany).
(SA)–β-galactosidase staining
MEFs were seeded and treated essentially as described for the evaluation of colony formation. After the indicated treatments, cells were split 1:10 and reseeded onto coverslips in 6-well plates and maintained for another week in normal medium. Senescence-associated (SA)–β-galactosidase staining was carried out as described.23 Briefly, cells were fixed in 2% formaldehyde and 0.2% glutaraldehyde for 5 minutes at RT, washed in PBS, and incubated at 37°C in the absence of CO2 in staining solution (150 mM NaCl, 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K2Fe(CN6), 40 mM citric acid, and 12 mM sodium phosphate [pH 6.0]) containing 1 mg/mL 5-bromo-4-chloro-3-indolyl-D-galactoside. Digital pictures were captured using a ProgResC14 digital camera (Jenoptik, Jena, Germany) equipped with Openlab 3.5.1 software (Improvision, Tübingen, Germany) and using a Zeiss Axiovert 135 microscope (100×/1.3 oil objective; Zeiss AG, Jena, Germany).
Results
AEVD-based substrates are not specific for caspase-10
Paclitaxel-induced apoptosis has been reported to require the activation of initiator caspase-10.10,11 This conclusion was mainly derived from experiments using “caspase-10–specific” tetrapeptide inhibitors and substrates, matching the preferred caspase-10 cleavage sequence AEVD. Caspases recognize tetrapeptide sequences (P4–1) and cleave their substrates after the P1 aspartate residue. There is, however, evidence from experiments with peptide libraries that, due to a considerable overlap between the individual consensus sequences, tetrapeptide-based small molecules might be unspecific and therefore unable to distinguish a role of a certain caspase.24 To investigate this assumption, we used recombinant caspase-3 and caspase-10 proteins and compared their ability to cleave the fluorogenic substrates AEVD and IETD that are supposedly the consensus cleavage sequences of caspase-10 and caspase-8, respectively (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both caspases cleaved the IETD-based substrate to a roughly similar extent. However, compared with caspase-3, caspase-10 was clearly less effective in cleaving the alleged caspase-10–specific AEVD peptide, suggesting that analysis of AEVD cleavage is not an appropriate tool to delineate the involvement of caspase-10 in certain apoptotic pathways.
MEFs devoid of caspase-10 are sensitive to paclitaxel-induced apoptosis
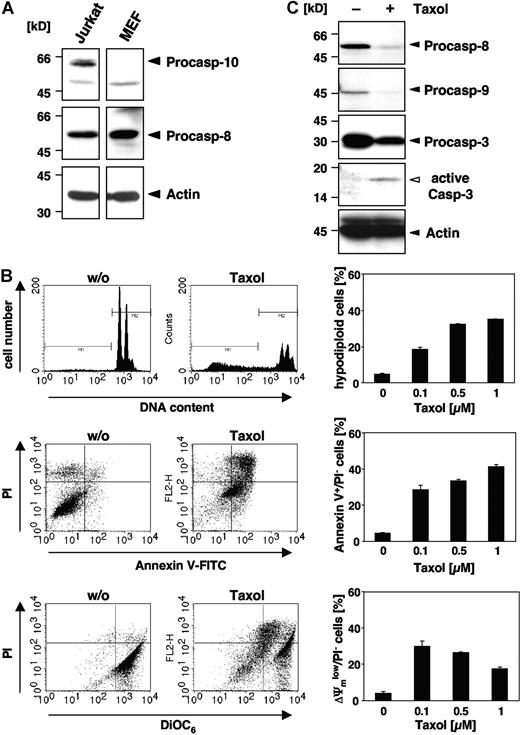
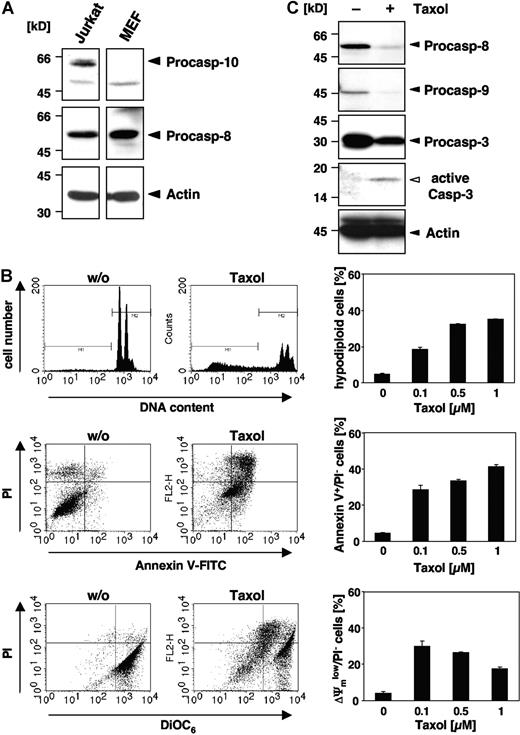
Although some reports have implicated caspase-10 in apoptosis of murine cells,25-27 there is clear evidence that the caspase-10 gene is absent in the mouse genome.28,29 Therefore, we wondered whether paclitaxel could induce apoptosis in murine cells or, alternatively, trigger other death mechanisms. Western blot analysis confirmed that caspase-8 but not caspase-10 was expressed in MEFs, whereas both initiator caspases were detected in human Jurkat T-cells (Figure 1A). When MEFs were treated for 48 hours with increasing doses of paclitaxel, apoptotic DNA fragmentation, as assessed by flow cytometric detection of hypodiploid cells, was clearly induced (Figure 1B). DNA fragmentation was already observed at a concentration of 0.1 μM paclitaxel and was increased dose dependently. Furthermore, phosphatidylserine that is exposed on the outer plasma membrane during apoptosis was detectable by flow cytometric measurement of annexin V–FITC binding (Figure 1B). Another typical feature of apoptotic cells, the breakdown of the mitochondrial transmembrane potential, was also associated with the death of MEFs (Figure 1B). Moreover, Western blot analysis revealed cleavage and activation of effector caspase-3 in MEFs upon stimulation with paclitaxel, which was associated with a loss of the caspase-8 and caspase-9 proforms (Figure 1C). These results clearly demonstrate that paclitaxel can induce apoptosis in murine cells despite of their lack of caspase-10.
Paclitaxel induces apoptotic cell death in murine cells lacking caspase-10. (A) Caspase-10 is not detected in cells of murine origin. Lysates of MEFs and human Jurkat T cells were immunoblotted with caspase-10–, caspase-8–, and β-actin–specific antibodies. Arrowheads indicate the positions of procaspase-10, procaspase-8, and β-actin. (B) MEFs die by apoptosis in response to paclitaxel. MEFs were incubated for 48 hours with paclitaxel and then analyzed for DNA fragmentation using PI staining of hypodiploid nuclei (top panels), phosphatidylserine exposure using double staining with annexin-V–FITC and PI (middle panels), and loss of mitochondrial membrane potential (ΔΨm) using the potentiometric dye DiOC6 (bottom panels). Representative fluorescence-activated cell sorter (FACS) analyses of untreated MEFs or cells treated with 1 μM paclitaxel are shown (left panels). Hypodiploid nuclei are contained in the marker region M1; normal nuclei with a higher DNA content are shown in M2. The bar diagrams (right panels) summarize the dose response of MEFs treated with the indicated concentrations of paclitaxel and show the mean values (± SD) of hypodiploid nuclei, vital PI-excluding cells with phosphatidylserine exposure, or loss of ΔΨm. (C) Paclitaxel treatment induces caspase activation. Immunoblot analysis of cell lysates from MEFs that were either left untreated or incubated for 48 hours with 1 μM paclitaxel. The positions of the procaspases and β-actin are indicated by closed arrowheads. Cleaved active caspase-3 is marked by an open arrowhead.
Paclitaxel induces apoptotic cell death in murine cells lacking caspase-10. (A) Caspase-10 is not detected in cells of murine origin. Lysates of MEFs and human Jurkat T cells were immunoblotted with caspase-10–, caspase-8–, and β-actin–specific antibodies. Arrowheads indicate the positions of procaspase-10, procaspase-8, and β-actin. (B) MEFs die by apoptosis in response to paclitaxel. MEFs were incubated for 48 hours with paclitaxel and then analyzed for DNA fragmentation using PI staining of hypodiploid nuclei (top panels), phosphatidylserine exposure using double staining with annexin-V–FITC and PI (middle panels), and loss of mitochondrial membrane potential (ΔΨm) using the potentiometric dye DiOC6 (bottom panels). Representative fluorescence-activated cell sorter (FACS) analyses of untreated MEFs or cells treated with 1 μM paclitaxel are shown (left panels). Hypodiploid nuclei are contained in the marker region M1; normal nuclei with a higher DNA content are shown in M2. The bar diagrams (right panels) summarize the dose response of MEFs treated with the indicated concentrations of paclitaxel and show the mean values (± SD) of hypodiploid nuclei, vital PI-excluding cells with phosphatidylserine exposure, or loss of ΔΨm. (C) Paclitaxel treatment induces caspase activation. Immunoblot analysis of cell lysates from MEFs that were either left untreated or incubated for 48 hours with 1 μM paclitaxel. The positions of the procaspases and β-actin are indicated by closed arrowheads. Cleaved active caspase-3 is marked by an open arrowhead.
Human tumor cell lines deficient in caspase-8/10 expression undergo paclitaxel-induced apoptotic cell death
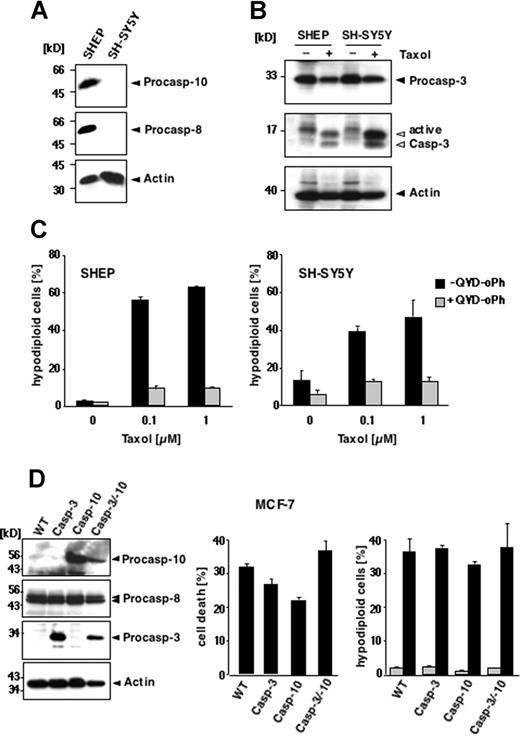
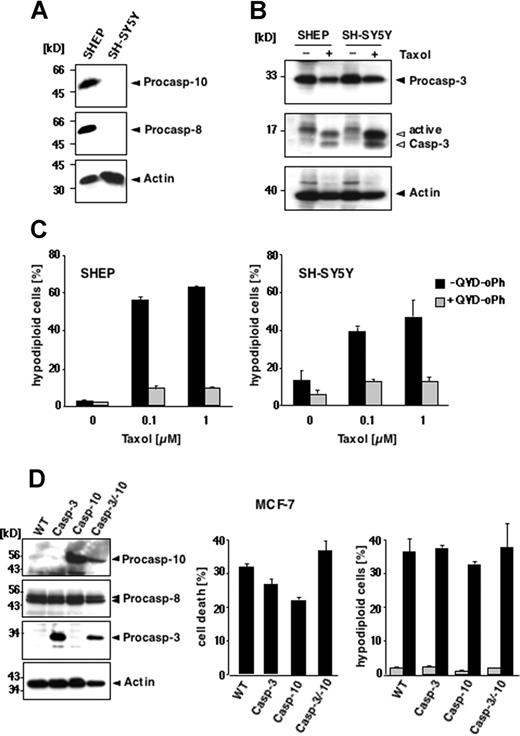
These results demonstrate that paclitaxel can induce apoptosis in murine cells lacking caspase-10. Nevertheless, human cells might differ from murine cells in their paclitaxel responsiveness. To test whether human cells require caspase-10 for paclitaxel-mediated apoptotic signaling, we used neuroblastoma cell lines that are characterized by a frequent loss of death receptor–associated initiator caspase expression.30 We compared SH-SY5Y cells that are deficient in caspase-8 and caspase-10 with SHEP cells that express both initiator caspases (Figure 2A). Regardless of the presence or absence of caspase-10, cells were susceptible to paclitaxel. Caspase-3 was activated in both the caspase-8/10–expressing and -deficient cells (Figure 2B), which also displayed the typical morphologic signs of apoptosis (data not shown). After 48 hours, DNA fragmentation occurred in both cell lines at 0.1 μM paclitaxel, reaching a plateau at 1 μM with 47% apoptotic caspase-8/10–deficient SH-SY5Y cells and 63% caspase-8/10–proficient SHEP cells (Figure 2C). Similar results were obtained with the caspase-8/10–deficient small-cell lung carcinoma cell line H2171 (data not shown). Treatment with 0.1 μM paclitaxel for up to 72 hours also revealed a time-dependent increase of apoptotic cells in all cell lines tested (data not shown). Moreover, all cell lines were completely rescued from apoptosis by the pancaspase inhibitors QVD-oPh (Figure 2C) and zVAD-fmk (data not shown), suggesting that paclitaxel-induced apoptosis is caspase dependent. Thus, these results demonstrate that expression of caspases-8 or caspase-10 is not necessary for the susceptibility of cells to paclitaxel.
Loss of caspase-8/10 expression does not impede paclitaxel-induced apoptosis in human tumor cell lines. (A-C) Apoptosis in SHEP and SH-SY5Y cells. (A) Caspase-8 and caspase-10 are not expressed in SH-SY5Y neuroblastoma cells, as demonstrated by immunoblot analysis using antibodies against caspase-10, caspase-8, and β-actin. (B) Paclitaxel induces caspase-3 activation regardless of caspase-8 and caspase-10 expression. Lysates of SHEP and SH-SY5Y cells were subjected to immunoblot analysis using caspase-3 and β-actin antibodies. The positions of the uncleaved proteins are indicated by closed arrowheads; cleaved fragments of caspase-3 are indicated by open arrowheads. (C) SHEP (left panel) and SH-SY5Y cells (right panel) were treated with increasing concentrations of paclitaxel for 48 hours in the presence or absence of 10 μM pancaspase inhibitor QVD-oPh. Apoptosis was assessed by FACS analysis of hypodiploid nuclei and is given as means (± SD). (D) Reconstitution of caspase-10 does not enhance the sensitivity of MCF-7 cells to paclitaxel. Left panel shows status of caspase expression in the different MCF-7 cells. Lysates of parental cells (WT) and cell clones stably expressing caspase-3, caspase-10, or both were immunoblotted for the indicated proteins. Middle panel shows that reconstitution of caspase-10 or caspase-3 does not sensitize cells to paclitaxel-mediated toxicity. Parental cells and the caspase-3– and/or caspase-10–reconstituted clones were treated for 48 hours with 0.1 μM paclitaxel. The percentage of cell death was assessed by crystal violet staining and compared with the untreated controls. Right panel shows parental and the reconstituted derivatives, stably expressing caspase-3, caspase-10, or both, treated with 0.1 μM paclitaxel for 72 hours. Apoptotic, hypodiploid cells were measured by flow cytometry. The percentage of apoptotic cells is given as mean (± SD).
Loss of caspase-8/10 expression does not impede paclitaxel-induced apoptosis in human tumor cell lines. (A-C) Apoptosis in SHEP and SH-SY5Y cells. (A) Caspase-8 and caspase-10 are not expressed in SH-SY5Y neuroblastoma cells, as demonstrated by immunoblot analysis using antibodies against caspase-10, caspase-8, and β-actin. (B) Paclitaxel induces caspase-3 activation regardless of caspase-8 and caspase-10 expression. Lysates of SHEP and SH-SY5Y cells were subjected to immunoblot analysis using caspase-3 and β-actin antibodies. The positions of the uncleaved proteins are indicated by closed arrowheads; cleaved fragments of caspase-3 are indicated by open arrowheads. (C) SHEP (left panel) and SH-SY5Y cells (right panel) were treated with increasing concentrations of paclitaxel for 48 hours in the presence or absence of 10 μM pancaspase inhibitor QVD-oPh. Apoptosis was assessed by FACS analysis of hypodiploid nuclei and is given as means (± SD). (D) Reconstitution of caspase-10 does not enhance the sensitivity of MCF-7 cells to paclitaxel. Left panel shows status of caspase expression in the different MCF-7 cells. Lysates of parental cells (WT) and cell clones stably expressing caspase-3, caspase-10, or both were immunoblotted for the indicated proteins. Middle panel shows that reconstitution of caspase-10 or caspase-3 does not sensitize cells to paclitaxel-mediated toxicity. Parental cells and the caspase-3– and/or caspase-10–reconstituted clones were treated for 48 hours with 0.1 μM paclitaxel. The percentage of cell death was assessed by crystal violet staining and compared with the untreated controls. Right panel shows parental and the reconstituted derivatives, stably expressing caspase-3, caspase-10, or both, treated with 0.1 μM paclitaxel for 72 hours. Apoptotic, hypodiploid cells were measured by flow cytometry. The percentage of apoptotic cells is given as mean (± SD).
Reconstitution of caspase-10 does not enhance apoptosis in response to paclitaxel treatment
Although death receptor–associated initiator caspases are dispensable for paclitaxel-induced cell death, caspase-10 might be involved in a postmitochondrial amplification loop in a similar manner as it was proposed for caspase-8 in B lymphoid cells.13,14 To investigate this possibility, we chose the mammary carcinoma cell line MCF-7, which expresses functional caspase-8, but not caspase-3 or caspase-10.16,31 Because caspase-3 seems to be required for the induction of a caspase-8–mediated positive feedback loop amplifying drug-mediated cell killing,14 we reconstituted not only parental MCF-7 cells, but also a caspase-3–expressing MCF-7 cell line with caspase-10 (Figure 2D left panel). Several stable clones expressing varying caspase levels were generated and have been characterized previously.16 To address the impact of caspase-10, we assessed cell death in the parental MCF-7 cells and the caspase-reconstituted clones in response to paclitaxel using the crystal violet assay. We have previously shown that caspase-10 sensitized MCF-7 cells to TRAIL, which was more pronounced in the presence of caspase-3 (Engels et al16 and data not shown). In contrast to TRAIL, however, we found no correlation between caspase-3, caspase-8, or caspase-10 expression and the susceptibility to paclitaxel. Cell death rates ranged from 20% to 40% irrespective of reconstitution with caspase-3 or caspase-10 (Figure 2D middle panel). In the different MCF-7 clones, we also analyzed the occurrence of DNA degradation in response to paclitaxel. Due to the absence of caspase-3, MCF-7 cells lack internucleosomal DNA cleavage;31 however, they display high-molecular-weight or lysosomal DNA fragmentation that can be measured by flow cytometry. Interestingly, DNA degradation increased in response to 0.1 μM paclitaxel to a similar extent in all cells regardless of caspase-10 expression (Figure 2D right panel).
Death effector domain–containing initiator caspases and FADD are not required, nor do they enhance paclitaxel-mediated cell death
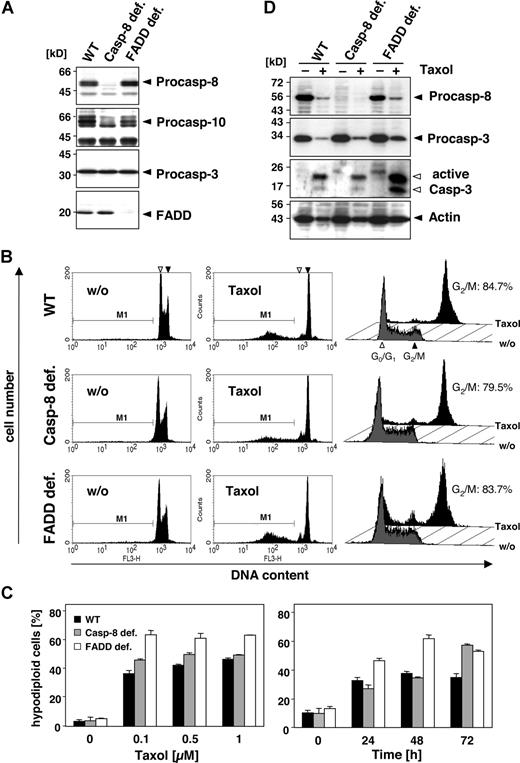
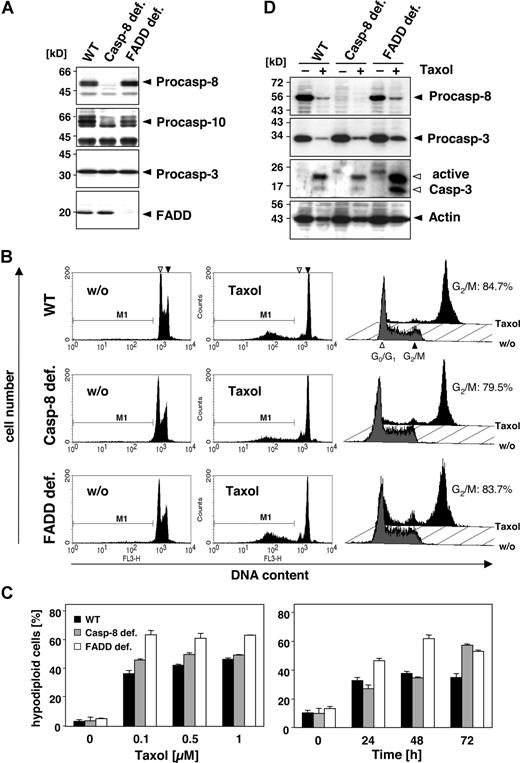
To investigate the role of caspase-10 and caspase-8 in more detail, we compared the sensitivity of wild-type, caspase-8–deficient, and FADD-deficient Jurkat T cells toward paclitaxel. Caspase-8–deficient Jurkat cells were as sensitive to the drug as the wild-type cells (Figure 3A-C). Furthermore, FADD-deficient Jurkat cells, which can neither recruit caspase-8 nor caspase-10 to the DISC, were even more sensitive toward paclitaxel than the parental or caspase-8–deficient Jurkat cells (Figure 3A-C). All cells revealed a similar time- and dose-dependent increase in paclitaxel-induced apoptosis (Figure 3C). Furthermore, regardless of FADD or caspase-8, all cell lines activated caspase-3, which for unknown reasons was even more pronounced in the FADD-deficient cells (Figure 3D). Western blot analysis also demonstrated the cleavage of caspase-8, as indicated by the loss of its proform in the parental and FADD-deficient Jurkat cells.
Death effector domain–containing initiator caspases and FADD are not required and do not enhance paclitaxel-induced cell death. (A) Lysates of Jurkat cells (WT) and their caspase-8– or FADD-deficient derivatives were subjected to immunoblot analysis. Protein bands specific for caspase-8, caspase-10, caspase-3, and FADD are indicated by arrowheads. (B) Wild-type, caspase-8–deficient, and FADD-deficient Jurkat cells were treated with 1 μM paclitaxel for 24 hours (middle panels) or left untreated (left panels). Hypodiploid nuclei indicative of paclitaxel-induced apoptosis were detected irrespectively of FADD or caspase-8 expression. The position of nuclei in G0/G1-phase (◁) and G2/M-phase (◀) is marked. Cell-cycle analysis by a linear depiction of PI staining (right panels) revealed no blockage of the paclitaxel-induced G2/M arrest due to lack of FADD expression. Gray histograms show the untreated controls, and black histograms represent paclitaxel-treated samples. Cells in the G2/M phase are given as percentage of all vital, nonapoptotic cells. (C) Treatment of the different Jurkat cell clones with increasing concentrations of paclitaxel for 72 hours (left panel) or with 0.5 μM paclitaxel for the indicated time points (right panel) revealed a dose- and time-dependent increase in hypodiploid cells, regardless of caspase-8 or FADD expression. The results show the mean values (± SD). (D) Paclitaxel-induced caspase activation is independent of FADD and caspase-8. Immunoblot analysis of lysates from wild-type and FADD- or caspase-8–deficient Jurkat cells that were treated with 1 μM paclitaxel for 48 hours or left untreated. The positions of procaspase-8, procaspase-3, and β-actin are indicated with closed arrowheads. The cleaved fragments of active caspase-3 are marked with open arrowheads.
Death effector domain–containing initiator caspases and FADD are not required and do not enhance paclitaxel-induced cell death. (A) Lysates of Jurkat cells (WT) and their caspase-8– or FADD-deficient derivatives were subjected to immunoblot analysis. Protein bands specific for caspase-8, caspase-10, caspase-3, and FADD are indicated by arrowheads. (B) Wild-type, caspase-8–deficient, and FADD-deficient Jurkat cells were treated with 1 μM paclitaxel for 24 hours (middle panels) or left untreated (left panels). Hypodiploid nuclei indicative of paclitaxel-induced apoptosis were detected irrespectively of FADD or caspase-8 expression. The position of nuclei in G0/G1-phase (◁) and G2/M-phase (◀) is marked. Cell-cycle analysis by a linear depiction of PI staining (right panels) revealed no blockage of the paclitaxel-induced G2/M arrest due to lack of FADD expression. Gray histograms show the untreated controls, and black histograms represent paclitaxel-treated samples. Cells in the G2/M phase are given as percentage of all vital, nonapoptotic cells. (C) Treatment of the different Jurkat cell clones with increasing concentrations of paclitaxel for 72 hours (left panel) or with 0.5 μM paclitaxel for the indicated time points (right panel) revealed a dose- and time-dependent increase in hypodiploid cells, regardless of caspase-8 or FADD expression. The results show the mean values (± SD). (D) Paclitaxel-induced caspase activation is independent of FADD and caspase-8. Immunoblot analysis of lysates from wild-type and FADD- or caspase-8–deficient Jurkat cells that were treated with 1 μM paclitaxel for 48 hours or left untreated. The positions of procaspase-8, procaspase-3, and β-actin are indicated with closed arrowheads. The cleaved fragments of active caspase-3 are marked with open arrowheads.
In addition to its proapoptotic function, FADD has been implicated in cell-cycle control, although this role of FADD might be cell-type specific.32 Phosphorylation of FADD was suggested to mediate paclitaxel-induced G2/M phase arrest. However, Jurkat cells deficient in FADD showed no alterations in cell-cycle distribution compared with the wild-type or caspase-8–deficient counterparts (Figure 3B; right panel). After treatment with 0.1 μM paclitaxel for 24 hours, similar numbers of cells in all 3 lines (approximately 80% of the still vital cells) were arrested at G2/M, indicating that at least in Jurkat cells FADD does not affect paclitaxel-induced cell-cycle arrest.
Caspase-9 and Apaf-1 mediate paclitaxel-induced cell death
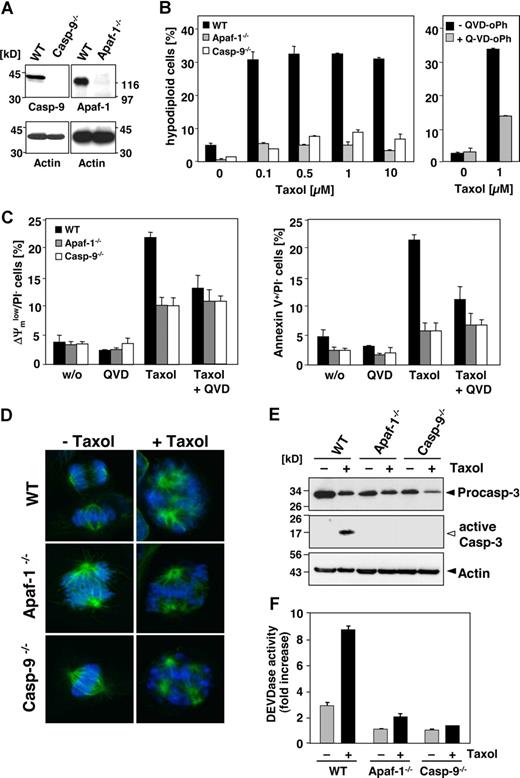
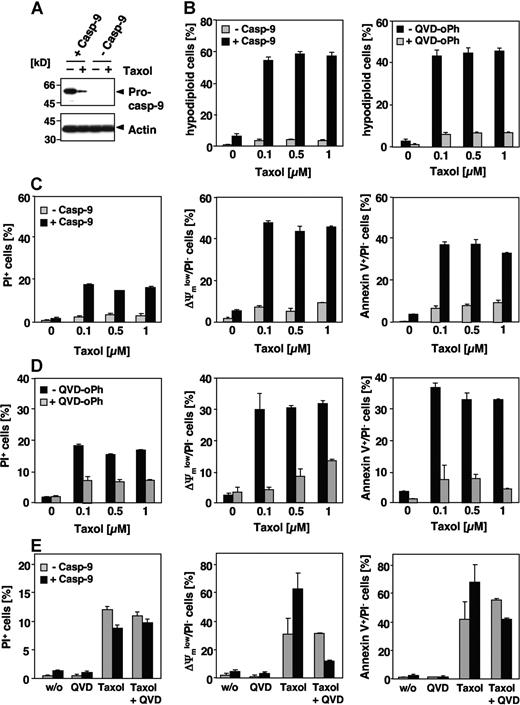
Our results suggest that paclitaxel-induced apoptosis is neither dependent on caspase-8 nor caspase-10. Chemotherapeutic drugs usually induce apoptosis by the intrinsic pathway resulting in caspase-9 activation at the apoptosome. Nevertheless, the involvement of caspase-9 in paclitaxel-induced signaling is controversial. Therefore, we analyzed paclitaxel-induced apoptosis in a Jurkat cell line deficient in caspase-9 expression and the corresponding caspase-9–reconstituted clones. Immunoblot analysis confirmed the presence or absence of caspase-9 in both cell lines (Figure 4A), whereas expression levels of other apoptosis regulators, including other caspases and Bcl-2 family and IAP proteins were unaffected (Samraj et al19 ; data not shown). Caspase-9–deficient cells were completely resistant even to high concentrations of paclitaxel, whereas Jurkat cells reconstituted with caspase-9 showed marked DNA degradation, exposure of phosphatidylserine and breakdown of the mitochondrial membrane potential after 48 hours, even at concentrations as low as 0.1 μM (Figure 4B-D). These apoptotic features were caspase dependent, as they were efficiently blocked by a pancaspase inhibitor (Figure 4B,D). We therefore asked whether caspase-9 deficiency could also provide long-term protection against paclitaxel, and performed clonogenicity assays 2 weeks after paclitaxel treatment. Compared with caspase-9–reconstituted cells, however, loss of caspase-9 did not provide long-lasting protection and could not maintain proliferative activity in the Jurkat cell system, even after paclitaxel exposure with varying concentrations and duration (data not shown). This inability to rescue Jurkat cells from death was probably a consequence of caspase-independent mitochondrial failure. Upon long-term treatment mitochondrial depolarization, PS exposure and cell death also became obvious in caspase-9–deficient cells (Figure 4E). Furthermore, in caspase-9–proficient cells, these late events were only partially blocked by the pancaspase inhibitor QVD-oPh.
Paclitaxel-induced apoptosis depends on caspase-9. (A) Effects of paclitaxel on caspase-9–deficient Jurkat cells. Lysates of caspase-9–deficient and caspase-9–reconstituted cells treated for 48 hours with 1 μM paclitaxel or left untreated were subjected to immunoblot analysis using caspase-9 and β-actin antibodies. Caspase-9 was cleaved in proficient cells upon stimulation with paclitaxel as indicated by the decrease of its proform. (B) Caspase-9–deficient and – reconstituted Jurkat cells were treated with increasing concentrations of paclitaxel for 48 hours or were left untreated. Analysis of hypodiploid nuclei (left panel) demonstrates that caspase-9–proficient but not caspase-9–deficient cells undergo apoptosis in response to paclitaxel. Treatment of caspase-9–reconstituted Jurkat cells with the pancaspase inhibitor QVD-oPh (20 μM) revealed that paclitaxel-induced DNA degradation is caspase dependent (right panel). (C,D) Caspase-9–deficient and – reconstituted Jurkat cells were treated as described in panel B. Dead cells were measured by cellular uptake of the nonvital dye PI (left panels). Mitochondrial membrane depolarization (middle panels), PS exposure (right panels), and cell death were observed only in caspase-9–proficient cells (C). Consistently, these effects can be blocked by the caspase inhibitor QVD-oPh (D). (E) Upon long-term treatment with paclitaxel, caspase-9–deficient cells also succumb to a caspase-independent cell death. Cells were treated for 4 days with 1 μM paclitaxel in the presence or absence of QVD-oPh and analyzed as described. Results in panels B-E show the mean values (± SD).
Paclitaxel-induced apoptosis depends on caspase-9. (A) Effects of paclitaxel on caspase-9–deficient Jurkat cells. Lysates of caspase-9–deficient and caspase-9–reconstituted cells treated for 48 hours with 1 μM paclitaxel or left untreated were subjected to immunoblot analysis using caspase-9 and β-actin antibodies. Caspase-9 was cleaved in proficient cells upon stimulation with paclitaxel as indicated by the decrease of its proform. (B) Caspase-9–deficient and – reconstituted Jurkat cells were treated with increasing concentrations of paclitaxel for 48 hours or were left untreated. Analysis of hypodiploid nuclei (left panel) demonstrates that caspase-9–proficient but not caspase-9–deficient cells undergo apoptosis in response to paclitaxel. Treatment of caspase-9–reconstituted Jurkat cells with the pancaspase inhibitor QVD-oPh (20 μM) revealed that paclitaxel-induced DNA degradation is caspase dependent (right panel). (C,D) Caspase-9–deficient and – reconstituted Jurkat cells were treated as described in panel B. Dead cells were measured by cellular uptake of the nonvital dye PI (left panels). Mitochondrial membrane depolarization (middle panels), PS exposure (right panels), and cell death were observed only in caspase-9–proficient cells (C). Consistently, these effects can be blocked by the caspase inhibitor QVD-oPh (D). (E) Upon long-term treatment with paclitaxel, caspase-9–deficient cells also succumb to a caspase-independent cell death. Cells were treated for 4 days with 1 μM paclitaxel in the presence or absence of QVD-oPh and analyzed as described. Results in panels B-E show the mean values (± SD).
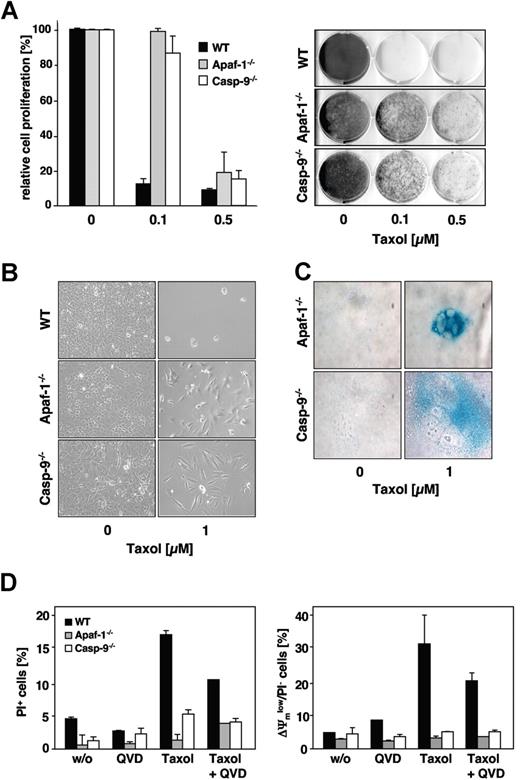
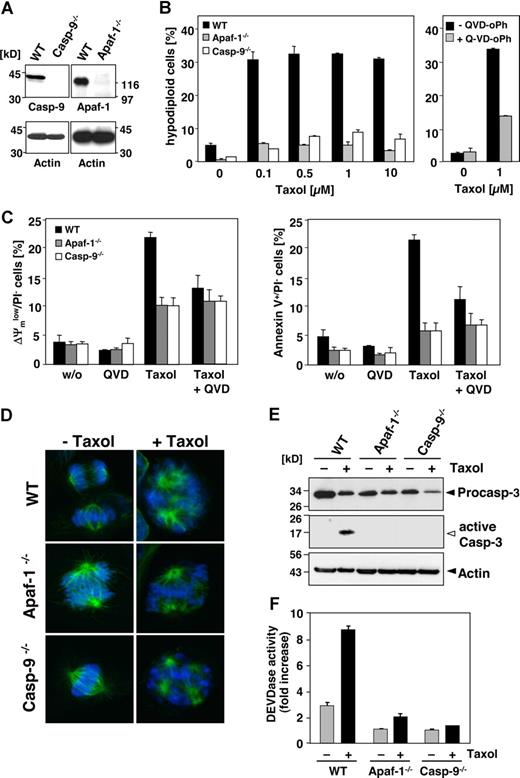
We further used MEFs deficient in either caspase-9 or Apaf-1 (Figure 5A). After 48 hours of incubation, approximately 30% of the wild-type MEFs underwent apoptosis in response to 0.1 μM paclitaxel, whereas the caspase-9–deficient counterparts were fully resistant in these short-term assays (Figure 5B). Also, MEFs from Apaf-1 knock-out animals were completely protected against even 100 times higher concentrations of paclitaxel (Figure 5B). Only in wild-type, but not in the knock-out MEFs, mitochondrial depolarization and PS exposure were triggered by paclitaxel (Figure 5C). However, exposure to paclitaxel resulted in the formation of multipolar spindles with lagging or misaligned chromosomes in all cell lines, regardless of the presence or absence of Apaf-1 or caspase-9 (Figure 5D). Nevertheless, wild-type, but not caspase-9– or Apaf-1–deficient MEFs revealed caspase-3 processing and induction of DEVDase activity in response to paclitaxel treatment (Figure 5E,F). Blockage of caspase function with the pancaspase inhibitor QVD-oPh consistently resulted in reduced DNA fragmentation, less exposure of PS, and preservation of the mitochondrial membrane potential in paclitaxel-treated wild-type cells (Figure 5B, right panel; Figure 5C). These results therefore suggest that paclitaxel induces apoptosis by the intrinsic pathway involving caspase-9 activation at the Apaf-1 apoptosome.
Paclitaxel-induced apoptosis depends on caspase-9 and Apaf-1. (A) Left panel shows status of caspase-9 or Apaf-1 expression in wild-type and the caspase-9−/− and Apaf-1−/− MEFs as assessed by immunoblot analysis. (B) Caspase-9 and Apaf-1 knock-out MEFs are resistant to paclitaxel-induced apoptosis. Wild-type, caspase-9−/−, or Apaf-1−/− MEFs were treated with increasing concentrations of paclitaxel for 48 hours or left untreated (left panel). Apoptosis was assessed by measurement of hypodiploid nuclei and is given as the mean (± SD). Right panel shows that apoptosis in wild-type MEFs treated with paclitaxel is blocked by the caspase inhibitor QVD-oPh (20 μM). (C) Paclitaxel treatment disrupts the mitochondrial membrane potential (left panel) and triggers PS exposure (right panel) in wild-type, but not in caspase-9– or Apaf-1–deficient cells. These effects are largely caspase dependent. Cells were treated with 1 μM paclitaxel for 48 hours in the presence or absence of QVD-oPh. Results show the mean values (± SD). (D) Paclitaxel induces the formation of multipolar spindles with lagging and misaligned chromosomes independently of caspase-9 or Apaf-1 expression. Microtubuli (green) were detected by immunofluorescence staining with anti–α-tubulin and chromosomes (blue) with the DNA dye DAPI and analyzed by confocal microscopy. (E) Active caspase-3 is only detected in paclitaxel-treated wild-type, but not in caspase-9– or Apaf-1–deficient MEFs. Exposure of the caspase-3 blot was increased from 30 seconds to 2 minutes in order to detect active caspase-3 fragments (◁). Noncleaved caspase-3 and actin are marked (◀). (F) DEVDase activity is not induced in paclitaxel-treated MEFs deficient in caspase-9 or Apaf-1. Results are shown as mean values (± SD).
Paclitaxel-induced apoptosis depends on caspase-9 and Apaf-1. (A) Left panel shows status of caspase-9 or Apaf-1 expression in wild-type and the caspase-9−/− and Apaf-1−/− MEFs as assessed by immunoblot analysis. (B) Caspase-9 and Apaf-1 knock-out MEFs are resistant to paclitaxel-induced apoptosis. Wild-type, caspase-9−/−, or Apaf-1−/− MEFs were treated with increasing concentrations of paclitaxel for 48 hours or left untreated (left panel). Apoptosis was assessed by measurement of hypodiploid nuclei and is given as the mean (± SD). Right panel shows that apoptosis in wild-type MEFs treated with paclitaxel is blocked by the caspase inhibitor QVD-oPh (20 μM). (C) Paclitaxel treatment disrupts the mitochondrial membrane potential (left panel) and triggers PS exposure (right panel) in wild-type, but not in caspase-9– or Apaf-1–deficient cells. These effects are largely caspase dependent. Cells were treated with 1 μM paclitaxel for 48 hours in the presence or absence of QVD-oPh. Results show the mean values (± SD). (D) Paclitaxel induces the formation of multipolar spindles with lagging and misaligned chromosomes independently of caspase-9 or Apaf-1 expression. Microtubuli (green) were detected by immunofluorescence staining with anti–α-tubulin and chromosomes (blue) with the DNA dye DAPI and analyzed by confocal microscopy. (E) Active caspase-3 is only detected in paclitaxel-treated wild-type, but not in caspase-9– or Apaf-1–deficient MEFs. Exposure of the caspase-3 blot was increased from 30 seconds to 2 minutes in order to detect active caspase-3 fragments (◁). Noncleaved caspase-3 and actin are marked (◀). (F) DEVDase activity is not induced in paclitaxel-treated MEFs deficient in caspase-9 or Apaf-1. Results are shown as mean values (± SD).
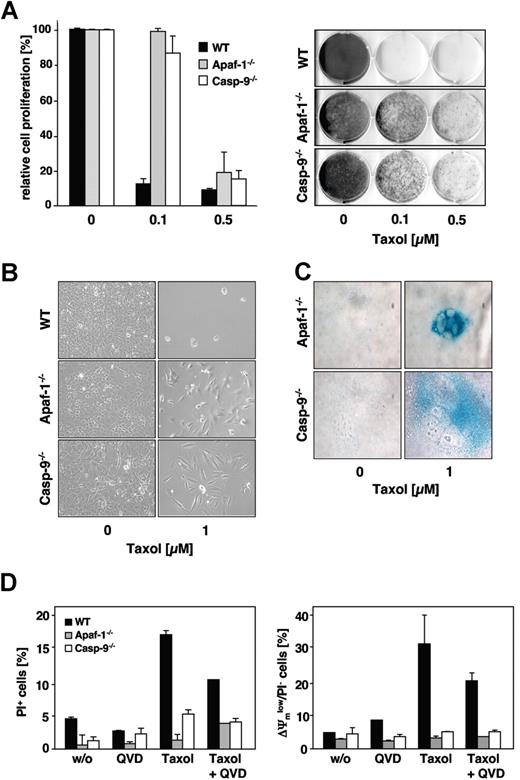
Importantly, and in contrast to Jurkat cells, the lack of caspase-9 or Apaf-1 expression resulted also in a marked long-term survival advantage of paclitaxel-treated MEFs 1 week after removal of the drug (Figure 6A). Presumably due to defects in proper mitotic spindle formation, the paclitaxel-treated knock-out cells grew more slowly compared with untreated controls. As detected by the increased β-galactosidase activity and typical morphologic changes, some of the apoptosis-incompetent cells, however, underwent senescence, while other cells regained their proliferative capacity (Figure 6B,C). Consistent with this and in marked contrast to Jurkat cells, after long-term paclitaxel treatment, caspase-9– and Apaf-1–deficient MEFs revealed a preserved mitochondrial membrane potential and no increase in overall cell death compared with untreated cells (Figure 6D). Thus, in MEFs, the absence of caspase-9 or Apaf-1 obviously conferred a survival advantage.
Clonogenic growth after paclitaxel withdrawal is enhanced in MEFs lacking caspase-9 or Apaf-1 expression. (A) Clonogenicity assay of wild-type, caspase-9−/− and Apaf-1−/− MEFs. Cells were treated with the indicated concentrations of paclitaxel. After 48 hours, cells were washed, split 1:10, and kept in normal medium. After 7 days of further incubation, cells were stained with crystal violet and subsequently scanned using an Epson Perfection 3200 scanner and processed using Adobe Photoshop 7.0 software (Adobe, San Jose, CA) (right panel). The relative proliferation compared with untreated controls was calculated as the mean (± SD) by measurement of the dye absorbance (left panel). (B) Caspase-9– and Apaf-1–deficient cells, but not wild-type cells, retain their proliferative capacity. Cells were treated as described in panel A and analyzed by light microscopy using a Zeiss Axiovert 135 microscope (40×/0.6 Korr-objective), a ProgResC14 digital camera, and Openlab 3.5.1 software. (C) After prolonged cultivation of paclitaxel-treated caspase-9– or Apaf-1–deficient cells, a proportion of the cells undergo senescence as assessed by staining for senescence-associated β-galactosidase (blue). Staining of treated (1 μM paclitaxel for 48 hours) and untreated cells was analyzed after further cultivation in normal medium for 2 weeks. (D) Even long-term paclitaxel treatment triggers mitochondrial depolarization (right panel) and cell death (left panel) only in wild-type cells, but not in caspase-9– or Apaf-1–deficient cells. In wild-type cells, these effects are partially caspase dependent. MEFs were incubated with 1 μM paclitaxel for 4 days and subsequently analyzed by flow cytometry. Results show the mean values (± SD).
Clonogenic growth after paclitaxel withdrawal is enhanced in MEFs lacking caspase-9 or Apaf-1 expression. (A) Clonogenicity assay of wild-type, caspase-9−/− and Apaf-1−/− MEFs. Cells were treated with the indicated concentrations of paclitaxel. After 48 hours, cells were washed, split 1:10, and kept in normal medium. After 7 days of further incubation, cells were stained with crystal violet and subsequently scanned using an Epson Perfection 3200 scanner and processed using Adobe Photoshop 7.0 software (Adobe, San Jose, CA) (right panel). The relative proliferation compared with untreated controls was calculated as the mean (± SD) by measurement of the dye absorbance (left panel). (B) Caspase-9– and Apaf-1–deficient cells, but not wild-type cells, retain their proliferative capacity. Cells were treated as described in panel A and analyzed by light microscopy using a Zeiss Axiovert 135 microscope (40×/0.6 Korr-objective), a ProgResC14 digital camera, and Openlab 3.5.1 software. (C) After prolonged cultivation of paclitaxel-treated caspase-9– or Apaf-1–deficient cells, a proportion of the cells undergo senescence as assessed by staining for senescence-associated β-galactosidase (blue). Staining of treated (1 μM paclitaxel for 48 hours) and untreated cells was analyzed after further cultivation in normal medium for 2 weeks. (D) Even long-term paclitaxel treatment triggers mitochondrial depolarization (right panel) and cell death (left panel) only in wild-type cells, but not in caspase-9– or Apaf-1–deficient cells. In wild-type cells, these effects are partially caspase dependent. MEFs were incubated with 1 μM paclitaxel for 4 days and subsequently analyzed by flow cytometry. Results show the mean values (± SD).
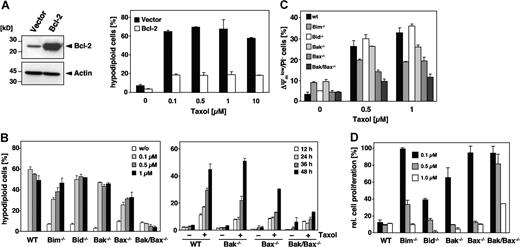
Paclitaxel-induced apoptosis is inhibited by Bcl-2 and facilitated by Bim and Bax
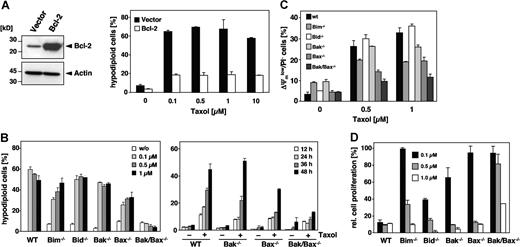
Pro- and antiapoptotic members of the Bcl-2 family are upstream regulators of the mitochondrial signaling pathway. Consistent with the notion that paclitaxel uses this pathway was our finding that overexpression of Bcl-2 (Figure 7A) or Bcl-xL (data not shown) rescued Jurkat cells from apoptosis, even after a 48-hour treatment with 10 μM paclitaxel. Bcl-2 counteracts the proapoptotic multidomain proteins Bak and Bax, whose activation results in the release of cytochrome c. To further analyze the involvement of specific Bcl-2 proteins, we treated MEFs derived from Bak and Bax single knock-out mice as well as from Bak/Bax double knock-out mice with increasing concentrations of paclitaxel for 48 hours. Unlike wild-type MEFs, Bak/Bax doubly deficient cells were completely protected (Figure 7B; left panel). Also, a time course experiment revealed a clear-cut protection of Bak/Bax doubly deficient cells (Figure 7B; right panel). The protection against paclitaxel was also confirmed by the lack of caspase-3 activation (data not shown). Bak single knock-out MEFs were equally sensitive as the wild-type cells and underwent apoptosis with a similar dose and time dependency. Interestingly, although Bax and Bak are assumed to have redundant functions, the single knock-out of Bax but not of Bak provided partial protection against paclitaxel-induced DNA fragmentation, which was particularly evident at low concentrations (Figure 7B). This was further substantiated by the analysis of mitochondrial membrane depolarization in response to the drug that was less pronounced in Bax-deficient cells (Figure 7C). Clonogenicity assays furthermore demonstrated a survival advantage of Bax single and Bak/Bax double knock-out cells compared with Bak-deficient or wild-type MEFs (Figure 7D). These results therefore indicate that paclitaxel-induced apoptosis might be preferentially mediated by Bax.
Involvement of Bcl-2 family proteins in paclitaxel-induced cell death. (A) Bcl-2 protects cells against the apoptosis-inducing effects of paclitaxel. Left panel shows immunoblot analysis of the status of Bcl-2 expression in Jurkat cells that were either stably transfected with Bcl-2 or the corresponding vector control. Right panel shows that Bcl-2–overexpressing cells and the corresponding vector control cells were subjected to increasing concentrations of paclitaxel for 48 hours as indicated. Flow cytometric analysis of hypodiploid cell nuclei revealed a strong protective effect of Bcl-2 against up to 10 μM paclitaxel. Results show the mean values (± SD). (B) Single knock-out of Bax or Bim confers partial resistance, while the double knock-out of Bax and Bak completely inhibits paclitaxel-induced apoptosis. MEFs derived from Bim, Bid, Bak, and Bax single knock-out as well as from Bak/Bax double knock-out mice were incubated with increasing concentrations of paclitaxel for 48 hours. Results from subsequent flow cytometric analysis of hypodiploid cell nuclei are depicted as a bar diagram of mean values (± SD; left panel). The right panel shows a time course analysis of apoptosis in Bax and Bak single and double knock-out MEFs that were treated for the indicated times with 1 μM paclitaxel. (C) Mitochondrial depolarization in response to paclitaxel is blocked in Bak/Bax double knock-out cells, and decreased in Bax or Bim single knock-out cells. MEFs were treated for 24 hours with the indicated concentrations of paclitaxel, stained with DiOC6/PI, and analyzed by flow cytometry. Results are presented as mean values (± SD). (D) Clonogenicity assays of wild-type and knock-out MEFs were performed as described in Figure 6A. After 7 days in normal medium, proliferation of paclitaxel-treated cells was measured by crystal violet staining and calculated as the mean (± SD) relative to the untreated controls.
Involvement of Bcl-2 family proteins in paclitaxel-induced cell death. (A) Bcl-2 protects cells against the apoptosis-inducing effects of paclitaxel. Left panel shows immunoblot analysis of the status of Bcl-2 expression in Jurkat cells that were either stably transfected with Bcl-2 or the corresponding vector control. Right panel shows that Bcl-2–overexpressing cells and the corresponding vector control cells were subjected to increasing concentrations of paclitaxel for 48 hours as indicated. Flow cytometric analysis of hypodiploid cell nuclei revealed a strong protective effect of Bcl-2 against up to 10 μM paclitaxel. Results show the mean values (± SD). (B) Single knock-out of Bax or Bim confers partial resistance, while the double knock-out of Bax and Bak completely inhibits paclitaxel-induced apoptosis. MEFs derived from Bim, Bid, Bak, and Bax single knock-out as well as from Bak/Bax double knock-out mice were incubated with increasing concentrations of paclitaxel for 48 hours. Results from subsequent flow cytometric analysis of hypodiploid cell nuclei are depicted as a bar diagram of mean values (± SD; left panel). The right panel shows a time course analysis of apoptosis in Bax and Bak single and double knock-out MEFs that were treated for the indicated times with 1 μM paclitaxel. (C) Mitochondrial depolarization in response to paclitaxel is blocked in Bak/Bax double knock-out cells, and decreased in Bax or Bim single knock-out cells. MEFs were treated for 24 hours with the indicated concentrations of paclitaxel, stained with DiOC6/PI, and analyzed by flow cytometry. Results are presented as mean values (± SD). (D) Clonogenicity assays of wild-type and knock-out MEFs were performed as described in Figure 6A. After 7 days in normal medium, proliferation of paclitaxel-treated cells was measured by crystal violet staining and calculated as the mean (± SD) relative to the untreated controls.
Because BH3-only proteins are essential triggers of apoptosis upstream of Bax and Bak, we also investigated MEFs deficient for Bim and Bid. Bim interacts with the microtubule-associated dynein complex and has been implicated in paclitaxel-mediated apoptosis of epithelial tumor cells.33 However, a comparison of the role of different Bcl-2 proteins has not yet been performed. Indeed, Bim deficiency provided a partial resistance against paclitaxel-induced DNA fragmentation that was more pronounced at low drug concentrations (Figure 7B). Bim loss also partly protected against mitochondrial depolarization (Figure 7C), and provided a survival advantage if low doses of paclitaxel were applied (Figure 7D). In contrast, Bid knock-out MEFs displayed a similar sensitivity as wild-type cells. As Bid links death receptor–associated initiator caspases with the mitochondrial pathway, this result again argues against a role of the extrinsic pathway in paclitaxel-mediated cytotoxicity. In conclusion, our findings suggest a particular role of Bim and Bax in the cytotoxic action of paclitaxel compared with other Bcl-2 proteins. Moreover, at low drug concentrations, the loss either of Bax, Apaf-1, or caspase-9 sustains a long-lasting, cell-type–dependent protection and survival advantage.
Discussion
Although paclitaxel induces stabilization of microtubules, blockade of the cell cycle, and eventually cell death, its exact mechanism of cytotoxic action is unknown. In the present study, we examined the requirement of crucial components of the death machinery for paclitaxel-induced apoptosis by using various genetic models. Previous studies pointed to the requirement of caspase-10 activation downstream of mitochondria in paclitaxel-induced apoptosis, a conclusion that was largely based on results obtained with tetrapeptide caspase inhibitors.10,11 These inhibitors bind to the catalytic cleft of caspases but, because of a large overlap between the consensus sequences of caspases, they are useless to discriminate certain caspases in living cells. For instance, the alleged caspase-10–specific AEVD tetrapeptide inhibits caspase-8 efficiently (Ki = 1.6 nM), and inhibits to a lesser extent caspase-3 (Ki = 42 nM), but only inhibits caspase-10 (Ki = 320 nM) weakly.24 To clarify the role of caspase-10 and FADD in paclitaxel-induced apoptosis, we therefore used (1) murine cells lacking the caspase-10 gene; (2) neuroblastoma cells missing caspase-10 expression due to gene silencing; and (3) caspase-10–deficient MCF-7 cells. In all models, we clearly demonstrate that caspase-10 is dispensable for paclitaxel-induced apoptosis.
The role of FADD in paclitaxel-mediated cell death is also controversially discussed. Overexpression of a FADD mutant that is unable to recruit caspase-8/10 has been reported to protect against paclitaxel-induced apoptosis,10,34 whereas other studies did not find any involvement of FADD.13,14 Moreover, it was suggested that, besides its established role in death signaling, FADD is involved in cell-cycle regulation.32,35-37 Phosphorylation of FADD was shown to sensitize cells to paclitaxel-induced cell-cycle arrest at the G2/M boundary. In our experiments, however, paclitaxel-treated cells deficient in FADD were still able to arrest at G2/M and showed no cell-cycle alterations compared with wild-type cells. More important, we also show that FADD is not required for paclitaxel-induced apoptosis, as cytotoxicity was not compromised in FADD-deficient cells.
While these data clearly argue against a role of death receptors and its components FADD, caspase-8 and caspase-10, we provide convincing genetic evidence that paclitaxel-mediated apoptosis solely relies on the mitochondrial pathway. Several studies have found caspase-9 activation during paclitaxel-induced apoptosis,14,38,39 whereas others could not detect cleavage of caspase-9.10,40 Our data clearly suggest that caspase-9 is indispensable for paclitaxel-induced apoptosis. Caspase-9– and Apaf-1–deficient MEFs were protected even after incubation with high doses of paclitaxel, while wild-type cells were completely eradicated by a 100-fold lower dose. Furthermore, Bcl-2 overexpression efficiently rescued Jurkat cells from death, even in the presence of up to 10 μM paclitaxel for at least 48 hours, while 65% of control cells died at a 100-fold lower concentration. Consistent with this, Bak−/−/Bax−/− cells were completely protected from paclitaxel-induced apoptosis. Bak and Bax are generally assumed to substitute for each other, since deficiency for both genes is required to render cells resistant to a number of proapoptotic agents.41 Interestingly, the single knock-out of Bax but not Bak also conferred partial protection against paclitaxel. This suggests that Bax is preferentially activated during paclitaxel-induced apoptosis, even though Bak might in part compensate for Bax deficiency. So far, only a few studies proposed that Bax and Bak exert nonredundant roles and might serve different functions depending on the stimulus. For instance, Bak has been suggested to play a pivotal role in apoptosis induced by gliotoxin or microbial infection,42,43 whereas Bax but not Bak has been implicated in cell death induced by the BH3-only protein Nbk.44
Previous studies suggested that paclitaxel induces a G2/M arrest, resulting in phosphorylation and inactivation of Bcl-2 by JNK.45,46 Phosphorylation-induced inhibition of Bcl-2 has been proposed to be critical for paclitaxel-induced cell death and subsequent Bax activation.47 However, more recent studies raise doubts that such a scenario is the cause of paclitaxel-induced cell death.48 Bak/Bax activation is mediated by BH3-only proteins that activate proapoptotic Bcl-2 proteins either directly or indirectly by the sequestration of antiapoptotic Bcl-2 proteins. Among the BH3-only proteins, Bim, Bid, and Puma are direct activators of Bax/Bak.49,50 Bid is a substrate of caspase-8 and caspase-10 and mediates the coupling of extrinsic and intrinsic pathways. However, we found no difference in paclitaxel sensitivity between Bid-deficient and -proficient cells, arguing again against a contribution of death receptors. Interestingly, we noticed that Bim knock-out MEFs were partially protected, at least at low concentrations of paclitaxel, suggesting that Bim, which is normally sequestered at microtubules, might be preferentially targeted by paclitaxel treatment.
The inhibition of apoptosis is generally believed to be a major determinant of resistance to chemotherapy. Recent findings, however, show that caspase inhibitors may not protect cancer cells from cytotoxic agents, but rather switch drug-induced apoptosis to an alternative stress response and caspase-independent cell death.51,52 Our experiments in Jurkat cells show that the absence of caspase-9 completely inhibited apoptosis, but paclitaxel treatment nevertheless induced disruption of the mitochondrial membrane potential and subsequent cell death, even in the absence of caspase activation. Moreover, the deficiency of caspase-9 was unable to rescue Jurkat cells in long-term survival assays. Consistent with this, the loss of mitochondrial membrane potential was only partially caspase dependent, and not fully prevented in cells with blocked caspase activation. Thus, the caspase-independent cell death observed in Jurkat cells obviously occurs as a consequence of mitochondrial failure and an inability to maintain cellular ATP levels.
Unlike in Jurkat cells, we observed that in MEFs deficient in Apaf-1 or caspase-9 even high concentrations of paclitaxel did not result in apoptosis, but instead triggered premature senescence. Most interesting was the finding that in MEFs, the absence of caspase-9 or Apaf-1 could indeed confer a survival advantage, as a proportion of the cells retained their proliferative activity associated with a sustained mitochondrial transmembrane potential. This situation might be reminiscent to sympathetic neurons or GAPDH-overexpressing HeLa cells that release cytochrome c but can restore mitochondrial functions, provided that caspase activation is blocked.53-55 Why this recovery following cytochrome c release does not occur in all cell types, such as Jurkat T cells, is an intriguing question. A likely explanation could be a different dependence of cells on glycolytic metabolism. Certainly, the survival advantage of cells with blocked caspase activation has important implications for tumor therapy, as either a deficiency of proapoptotic mediators or an elevated expression of caspase inhibitors has been observed in cancer. In fact, a recent study showed that expression of a dominant caspase-9 mutant could enhance spreading of tumor cells in an animal model.56
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank Mathis T. Niemann, Ingo Schmitz, Ajoy K. Samraj, Christoph Borner, Simone Fulda, Henning Walczak, Andreas Villunger, and Andreas Strasser for valuable materials and helpful discussion.
This work was supported by the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, and an intramural grant from the Medical Faculty of the University Düsseldorf.
Authorship
Contribution: K.J. and S.P. performed research and analyzed data. R.U.J. contributed to the design of experiments. K.S.O. contributed new reagents and wrote the paper. U.F designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ute Fischer, Institute of Molecular Medicine, University of Düsseldorf, Building 23.12, Universitätsstr. 1, D-40225 Düsseldorf, Germany; e-mail:ute.fischer@uni-duesseldorf.de.