Abstract
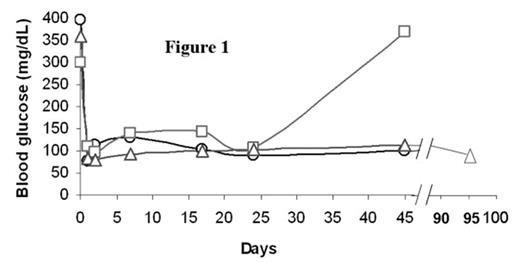
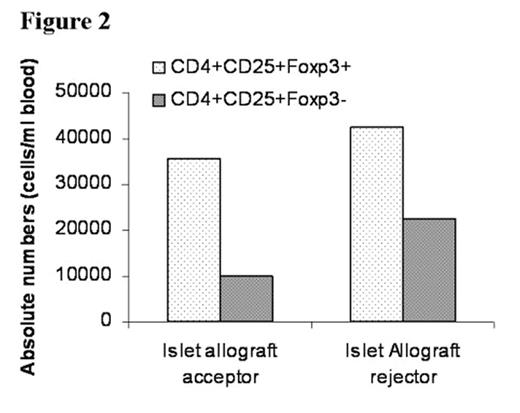
In the past several years, novel insights into the role of costimulatory molecules in generating adaptive immune responses have led to the development of new approaches for tolerance induction. We previously demonstrated that preconditioning of diabetes-prone NOD mice with anti-CD8 and combined with blockade of CD40L (anti-CD154) allowed chimerism to be established with 550 cGy TBI. Here, we investigated whether combined costimulatory blockade of CD40L, inducible co-stimulator (ICOS) and OX40L (CD134L) would enhance bone marrow chimerism and promote islet allograft survival in diabetic NOD mice. We also determined whether CD4+/CD25+/FoxP3+ regulatory T-cells (Treg) play an important role in maintenance of tolerance. Spontaneously diabetic NOD mice (blood glucose levels >350 mg/dL) were treated with anti-CD8 antibody (day -3). After 500 cGy TBI (day 0), NOD mice were transplanted with 30 × 106 B10.BR bone marrow cells (day 0) followed by transplantation of 300–400 B10.BR islets under the renal capsule and administered anti-CD154, anti-OX40L, and anti-ICOS mAbs IP (day 0, 1, 2, 3). Chimerism and peripheral blood CD4+/CD25+/FoxP3+ Treg cell expression were quantitated by flow cytometry. Urine and blood glucose levels were monitored. Nonmyeloablatively conditioned mice transplanted with islet allografts and infused with bone marrow (BM) demonstrated acceptance and continued function of islet allografts, with hyperglycemic reversal ≥ 45 (A) and 95 (B) days (Figure 1). Recipients of islet allografts and BM (∼5–12 days), islet allografts and antibody treatment (∼20 days) or islet allografts alone (∼5 days) rejected their grafts. Blood glucose returned to pretreatment hyperglycemic levels in the rejected islet allograft recipient (C) treated in the same manner as the acceptors (A and B) as well as in the control recipients. Donor chimerism at one month post-BMT was 58.5% (A), 89.7% (B) and 0% (C), respectively. Although CD4+/CD25+/FoxP3+ Treg expression was similar between the islet allograft acceptor and rejector recipients, the ratio of CD4+/CD25+/FoxP3+ Treg to CD4+/CD25+/FoxP3− T-effector cells was 2-fold greater in the islet acceptors (3.6 vs. 1.8) (Figure 2). Taken together, our data suggest that prolongation of donor-specific chimerism is associated with tolerance to islet allografts and that CD4+/CD25+/FoxP3+ Treg may be important in maintaining peripheral tolerance. Studies are underway to determine if CD4+/CD25+/FoxP3+ Treg may prevent intra-islet infiltration by autoimmune effector cells, thereby maintaining tolerance to islet allografts.
Author notes
Disclosure: No relevant conflicts of interest to declare.