Abstract
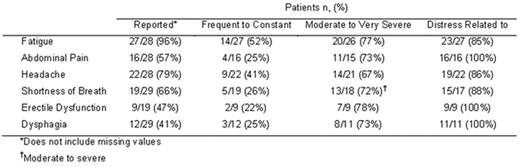
In paroxysmal nocturnal hemoglobinuria (PNH), lack of the GPI-anchored terminal complement inhibitor CD59 from erythrocytes renders them susceptible to chronic hemolysis, which is central to the signs and symptoms of PNH. Patients are at elevated risk for thrombosis, experience anemia that may require transfusion support, and suffer from fatigue that can be severe. Patients often have a poor quality of life resulting from PNH related symptoms including pain, dyspnea, dysphagia and erectile dysfunction, which negatively impact quality of life. The prevalence and severity of symptoms were explored in the context of a multi-national content validation study, of patients not receiving eculizumab therapy, employing the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and the European Organization for Research and Treatment of Cancer Quality of Life Core 30 (EORTC QLQ-C30) instruments. Symptom questions were asked of 29 PNH patients (19 men, 10 women, mean age 41.2±13.2 years) from the United Kingdom, United States, France and Spain. More than half (52%) had PNH for over 5 years. Most (76%) reported never having had a blood clot, 31% reported not receiving any medication for their PNH, and 59% reported either that they had never been transfused or had not received transfusion within the last year for PNH. Patients viewed overall quality of life, global health, functioning, fatigue, pain, and shortness of breath as important PNH-related signs/symptoms. Both the FACIT-Fatigue and EORTC instruments were relevant and adequate in assessing the level of fatigue and other quality of life measures in PNH. The burden of disease in this multicultural and diverse cohort of patients was significant: 76% were forced to modify their daily activities to manage their PNH and 17% were unemployed due to PNH. Nearly all (96%) complained of fatigue and more than half reported abdominal pain, headache and shortness of breath (Table). Patients also commonly reported dysphagia (41%) and erectile dysfunction (47% in males). Most patients reported these PNH-related symptoms as moderate to very severe, and a substantial majority reported distress associated with the symptoms. Significant disease burden was identified in a diverse population of PNH patients, most of which had minimal or no transfusion requirements and a low incidence of thrombosis. Therapy that controls hemolysis and thereby improves fatigue, pain, shortness of breath, dysphagia and erectile dysfunction may prove beneficial for PNH patients with these disease characteristics.
Author notes
Disclosure:Employment: Dr Kroon and Ms. Severino are employees of Alexion Pharmaceuticals, Inc. Consultancy: Drs. Meyers, Weitz, and Hill have served as consultants to Alexion Pharmaceuticals, Inc. Ownership Interests:; Dr Kroon and Ms. Severino own stock in Alexion Pharmaceuticals, Inc. Research Funding: Dr. Weitz has received research funds from Alexion Pharmaceuticals, Inc. Honoraria Information: Drs. Meyers and Hill have received lecture fees from Alexion Pharmaceuticals, Inc. Membership Information: Drs. Meyers, Weitz, and Hill have served on advisory boards for Alexion Pharmaceuticals, Inc.