Abstract
JAK2 617V>F mutation occurs in a homozygous state in 25% to 30% of patients with polycythemia vera (PV) and 2% to 4% with essential thrombocythemia (ET). Whether homozygosity associates with distinct clinical phenotypes is still under debate. This retrospective multicenter study considered 118 JAK2 617V>F homozygous patients (104 PV, 14 ET) whose clinical characteristics were compared with those of 587 heterozygous and 257 wild-type patients. Irrespective of their clinical diagnosis, homozygous patients were older, displayed a higher leukocyte count and hematocrit value at diagnosis, and presented larger spleen volume. Aquagenic pruritus was significantly more common among homozygous PV patients. JAK2 617V>F homozygosity associated with more frequent evolution into secondary myelofibrosis in both PV and ET. After adjustment for sex, age, leukocyte count, and previous thrombosis in a multivariate analysis, homozygous ET patients displayed a significantly higher risk of cardiovascular events (hazard ratio [HR] 3.97, 95% confidence interval [CI] 1.34–11.7; P = .013) than wild-type (HR = 1.0) or heterozygous patients (HR = 1.49). No significant association of JAK2 617V>F homozygosity with thrombosis risk was observed in PV. Finally, JAK2 617V>F homozygous patients were more likely to receive chemotherapy for control of disease. We conclude that JAK2 617V>F homozygosity identifies PV or ET patients with a more symptomatic myeloproliferative disorder and is associated with a higher risk of major cardiovascular events in patients with ET.
Introduction
Patients with Philadelphia-negative chronic myeloproliferative disorders (CMPDs)1 harbor a recurrent mutation in the Janus tyrosine kinase 2 (JAK2) gene, consisting of a valine-to-phenylalanine change at position 617 (JAK2 617V>F) in the JH2 pseudo-kinase domain.2-5 The JAK2 617V>F mutation is present in the vast majority of patients with polycythemia vera (PV)2-5 and in 40% to 60% of those with essential thrombocythemia (ET)6-8 or myelofibrosis with myeloid metaplasia (MMM).9,10 JAK2 617V>F can also occasionally be detected in “atypical” MPD, myelodysplastic syndromes, chronic myelomonocytic leukemia, systemic mastocytosis, chronic neutrophilic leukemia, and eosinophilic disorders.11-13 Mutant JAK2 is a constitutively active tyrosine kinase that confers growth factor independence in transfected hematopoietic cell lines2-5 and is at the basis of the phenomenon of erythropoietin-independent erythroid colony (EEC) growth that is a hallmark of most PV patients and is also less frequently reported in patients with ET.14 A key role for the “gain-of-function”15 of mutated JAK2 in the pathogenesis of CMPD is supported by in vitro studies, which showed enhanced JAK-STAT signaling in cells bearing the mutant allele, and by in vivo animal models in which erythrocytosis3,16 and eventually myelofibrosis16,17 developed following transplantation of hematopoietic cells transfected with mutant JAK2 into mice
About 25% to 30% of PV and MMM patients, and 2% to 4% of ET patients, harbor the mutation in the homozygous state, a condition where at least 51% of JAK2 alleles in granulocytes are mutated as the result of a mitotic recombination process.2-5 While a consistent number of reports have investigated the impact of the presence of 617V>F mutation per se on the clinical and hematologic profile of CMPD patients, a few studies have specifically addressed the consequences of JAK2 617V>F mutation when harbored in the homozygous state.18 The complete loss of normal JAK2 allele is expected to have profound effects on cell phenotype. As a matter of fact, in cotransfection experiments, the wild-type (WT) allele restored Epo dependence in cells that had become Epo-independent following JAK2 617V>F transfection.3 Consistently, by using quantitative allele determination techniques, the load of mutant allele in the granulocytes of PV patients meaningfully correlated with the degree of abnormal expression of 2 JAK2-regulated genes, PRV-1 and NF-E2.19,20 Furthermore, both increased number of circulating CD34+ cells and higher membrane expression of leukocyte alkaline phosphatase (LAP)21 were found in association with the highest JAK2 617V>F load in PV patients. However, whether homozygosity for JAK2 mutation would also influence clinical phenotype is still unclear, although some observations suggested that defined clinical aspects were actually dependent on mutant allele burden.4,18 In fact, when compared with their heterozygous counterparts, homozygous patients with PV displayed significantly higher hemoglobin levels, more frequent pruritus, greater tendency to fibrotic transformation, and higher PRV-1 levels; however, the number of homozygous patients considered in that study was low (n = 13).18 Finally, in familial MPD, the presence of JAK2 617V>F homozygosity was associated with hematologic complications including myelofibrosis and acute myeloid leukemia (AML) transformation in both PV and ET patients.22
In this study, we collected data from a large multicenter series of patients with PV and ET and we used a retrospective design with the aim of addressing the issue of whether the clinical and hematologic phenotype of V617F homozygous patients differed from that of the WT or heterozygous counterpart and, conceivably, whether the characterization of mutational state in PV or ET patients conveys relevant clinical information for their management.
Patients, materials, and methods
Study population
A retrospective study was performed within the GIMEMA (Italian Group for Malignant Hematologic Disorders of the Adult) MPD Working Party, which has been established among tertiary hematology centers in Italy; 12 centers participated in this retrospective study (listed in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and reported 1035 patients. The only criterion adopted for recruitment into the study was that patients had been typed for the presence of JAK2 617V>F mutation in granulocytes, collected at diagnosis or at any time point thereafter, according to the allele-specific oligonucleotide (ASO) polymerase chain reaction (PCR) and BsaXI digestion methodology (see “JAK2 617V>F genotyping”).2 The Consorzio Mario Negri Sud (Santa Maria Imbaro, Italy) acted as the study coordinating center. Data collection was centralized through an electronic, web-based database that allowed consistency and validity checks of remote data entry. Periodically, in-depth checks of the database were performed by running SAS batch programs to find out either any potential inconsistency or invalid data (SAS 9.1 software; SAS Institute Inc., Cary, NC). Data queries were addressed to the investigators and solved with them by the coordinating center. The study was conducted in accordance with the Declaration of Helsinki on medical research in humans. Patients gave informed consent for the molecular analysis for JAK2 genotyping and for being included in observational studies. Approval was obtained from the participating institutions' ethics committees.
The diagnosis of different clinical CMPD entities was accomplished according to either the Polycythemia Vera Study Group (PVSG)23 or World Health Organization (WHO) criteria.24 Postpolycythemic or postthrombocythemic evolution into myelofibrosis with myeloid metaplasia (PPV-MMM or PET-MMM) was diagnosed in patients with PV or ET, according to clinical and pathologic findings outlined in “Clinical outcomes.”
JAK2 617V>F genotyping
The mutational state for JAK2 617V>F was determined using the ASO-PCR assay, exactly as described by Baxter et al,2 on DNA obtained from granulocytes isolated from peripheral-blood samples using conventional gradient centrifugation technique. To determine whether the mutation was carried in the homozygous state, digestion of PCR products with BsaXI restriction enzyme was performed.2
Study objectives
The aim of the study was to evaluate whether patients harboring the homozygous JAK2 617V>F mutation displayed a unique disease phenotype according to common hematologic parameters and clinical characteristics. Controls were represented by wild-type and JAK2 617V>F heterozygous patients. The following variables were included in the analysis: age, sex, blood-cell count at diagnosis and at the time when blood was sampled for genotype determination, duration of disease, presence and extent of splenomegaly, presence of constitutional symptoms or of aquagenic pruritus, treatment instituted during the course of disease, occurrence of thrombotic vascular events and of hemorrhages, and rate of evolution into PPV/PET-MMM or into AML.
Clinical outcomes
The presence of splenomegaly was objectively determined on the basis of an ultrascan demonstrating an enlarged organ with a longitudinal diameter of at least 12 cm; according to this objective measurement, patients were further divided in 2 groups, those with an organ diameter between 12 and 15 cm and those with diameter greater than 15 cm. Major thrombotic events included ischemic stroke; transient ischemic attack; myocardial infarction; angina pectoris; deep venous thrombosis including cerebral thrombosis, abdominal vein thrombosis, or Budd-Chiari syndrome; and pulmonary embolism.25 Thrombotic events were recorded only if they occurred after diagnosis or in the 2 preceding years26 and if they were objectively documented or there were unequivocal medical records available in case of angina or transient ischemia.27 Only major bleeding events were considered and included fatal hemorrhages, nonfatal intracranial bleeding, or any other hemorrhages requiring surgery or causing hemoglobin level reduction of 20 g/L (2 g/dL) or greater and/or needing red blood cell transfusion of at least 2 packed units. Transition from PV or ET into PPV/PET-MMM was defined as the presence of a recently developed blood leukoerythroblastic picture, associated with spleen enlargement, and substantiated by the finding of diffuse bone marrow fibrosis in ad-hoc bone marrow biopsy.27 Diagnosis of evolution into acute leukemia was established according to the criteria of the French-American-British (FAB) cooperative group.28 As to treatments, for the purposes of this study we considered only whether or not patients had been treated with cytoreductive drugs, based on physician's decision at the top of current treatment strategies; indications for chemotherapy were the control of hematocrit (target, 0.45 [≤ 45%] in male, 0.42 [≤ 42%] in female), thrombocytosis (target, ≤ 600 × 109/L) or leukocytosis, or the control of progressive and/or symptomatic spleen enlargement; also whether patients were being phlebotomized, alone or in association with chemotherapy, was recorded. Patients considered at high risk (age ≥ 60 y, and/or previous thrombotic events) were treated with chemotherapy according to current clinical practice.25,29
Statistical analysis
Univariate analysis was performed to evaluate differences in proportions by the chi-square and Fisher exact tests. Differences in continuous variables were tested using t test, 1-way analysis of variance (anova), and the Tukey test for multiple comparisons, as appropriate. Continuous variables have been categorized using median and quartile values at baseline. Two different multivariable approaches have been adopted for data analysis. First, Cox proportional hazards models using values measured at diagnosis were used to assess whether the level of exposition to a potential risk predictor captured at diagnosis could be found to be a statistically significant marker of increased probability of recurrence of thrombosis and hemorrhage. The multivariable models to assess this hypothesis have been fitted after adjusting for age, sex, leukocytosis, hematocrit, platelet count, splenomegaly, and JAK2 mutation. Second, the database was explored using unconditional logistic regression models to assess the factors that were associated with an increased probability of receiving chemotherapy as pharmacologic cytoreductive strategy. This analysis included the same variables listed previously in this paragraph. Continuous variables have been analyzed using median levels at baseline as cutoff values. Cox proportional hazards event-free survival curves, adjusted for covariates, were plotted using the corrected group prognosis method. Analyses were performed with SAS 9.1 software. All probability values are 2 tailed (< .05).
Results
Patient characteristics
The clinical characteristics at diagnosis of the 962 CMPD patients considered in the study are detailed in Table 1. There were 323 patients with PV and 639 with ET. In the original survey, 73 additional patients with a diagnosis of PV who were JAK2 617V>F wild type were reported from the Centers. However, in the light of recent demonstration that these patients may harbor different mutations in JAK2,30 they were not considered for the purposes of this analysis; therefore, in the case of PV, we only compared JAK2 617V>F homozygous versus heterozygous patients. Median time from diagnosis to blood sampling for mutational analysis was 4.2 years, and median follow-up time in the whole patient population was 5.0 years (0-27.4 years). The JAK2 617V>F mutation was found in 705 patients (73.3%), of whom 587 (61.0% of total) were heterozygous and 118 (12.3%) were homozygous. The frequency of homozygosity was 32.2% in PV and 2.2% in ET, consistent with previous reports.2-5,18 When compared with WT (in case of ET) or heterozygous patients (in case of both PV and ET; Table 1), homozygous patients displayed a significantly higher leukocyte count and hematocrit, irrespective of their unique clinical diagnosis. On the other hand, platelet count was significantly lower in homozygous than in heterozygous patients with PV. The increased number of leukocytes in PV and ET patients harboring JAK2 mutation also persisted during follow-up (not shown in detail), whereas hematocrit and platelet count normalized as the effect of phlebotomies ± cytoreduction or cytoreduction alone, respectively. The frequency of splenomegaly was significantly higher in homozygous PV or ET patients (58.8% and 72.7%, respectively) than in WT patients with ET (21.9%) or in heterozygous patients (44.8% and 27.6% for PV and ET, respectively). In particular, marked spleen enlargement (> 15 cm) was significantly more common among homozygous PV or ET patients. Finally, homozygous PV patients complained more frequently of aquagenic pruritus, whereas there was no meaningful correlation with the occurrence of systemic symptoms (Table 1).
Cardiovascular events
One hundred seventy-six patients (18.3%) had a major thrombotic event at diagnosis, with a similar frequency in PV (19.2%) and ET (17.8%) (Table 2). Arterial thrombosis predominated in both diseases, accounting for 79.0% and 59.6% of the events recorded in PV and ET, respectively. During follow-up, thrombosis occurred in 122 patients (12.7%), corresponding to 14.9% of PV and 11.6% of ET patients. A more detailed description of the type of cardiovascular event is reported in Table S1.
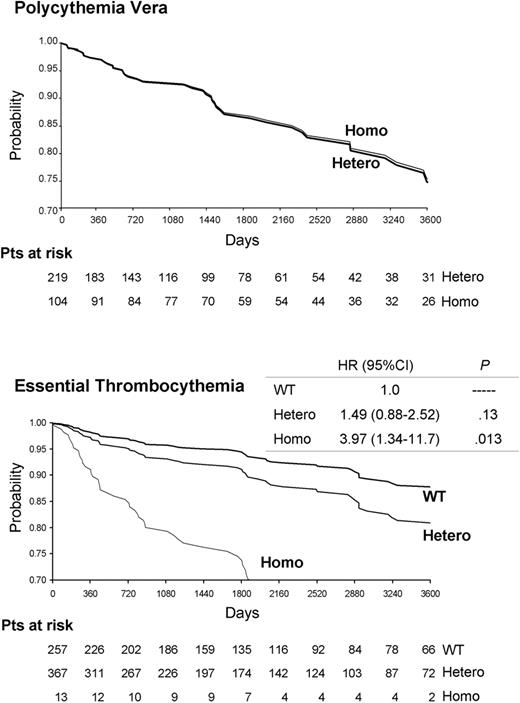
There was no significant difference in the overall rate and type of major thrombosis between heterozygous and homozygous patients with PV (Table 2; Figure 1 ). On the other hand, thrombotic events were significantly more frequent in homozygous patients with ET than in WT or heterozygous patients, both at the time of diagnosis and during follow-up (P < .05 for both). In homozygous ET patients, arterial thrombosis predominated at the time of diagnosis, whereas both arterial and venous events were equally present during follow-up (Table 2; Table S1). Of note, 3 of 14 homozygous patients (21.4%) presented at diagnosis with deep abdominal venous thromboses (Table S1). In multivariate analysis, adjusted for established risk factors for thrombosis in ET (ie, age, history of thrombosis, and leukocytosis [≥ 8.7 × 109/L])31 and for sex, JAK2 617V>F homozygosity was independently associated with occurrence of cardiovascular events (hazard risk 3.97, 95% confidence interval [CI] 1.34-11.7; P = .013 compared with WT patient).
Cardiovascular event-free survival (days), according to the JAK2 617V>F mutational state and adjusted for sex, age, leukocytosis, and history of thrombosis in patients with polycythemia vera or essential thrombocythemia. The hazard ratio (HR), the 95% confidence interval (95% CI), and the corresponding P value are shown on the right for ET patients. WT, Hetero, and Homo indicate patients who were wild-type, heterozygous, or homozygous for the JAK2 617V>F mutation, respectively.
Cardiovascular event-free survival (days), according to the JAK2 617V>F mutational state and adjusted for sex, age, leukocytosis, and history of thrombosis in patients with polycythemia vera or essential thrombocythemia. The hazard ratio (HR), the 95% confidence interval (95% CI), and the corresponding P value are shown on the right for ET patients. WT, Hetero, and Homo indicate patients who were wild-type, heterozygous, or homozygous for the JAK2 617V>F mutation, respectively.
Hemorrhages at diagnosis were manifested by 55 patients (5.7%), similarly distributed in PV (5.3%) and ET (6.0%); we found no association with JAK2 mutational state. On the other hand, 45 patients (4.7%) presented major hemorrhages during follow-up; the frequency was higher in homozygous patients (21.4%) than in wild-type or heterozygous ET patients (3.1% and 3.8%, respectively; P < .01; data not shown in detail).
Treatment
A total of 214 patients (22.3%) were treated with phlebotomies during the course of their disease, accounting for 58.4% of those with PV and 4.1% of those with ET, whereas chemotherapy was administered (alone or in association with phlebotomies) to 497 patients (51.8%), including 59.3% of PV and 48.0% of ET patients (Table 3). The proportion of PV patients requiring chemotherapy was significantly higher among homozygous than heterozygous patients (67.3% versus 55.5%; P = .044). In multivariate analysis, JAK2 mutational state was the most significant factor associated with chemotherapy treatment (odds ratio [OR] 2.00, CI 95% 1.10-3.65, P = .023). In the case of ET, 71.4% of homozygous patients needed chemotherapy compared with less than 50% of either wild-type or heterozygous patients; however, the difference only approached the significance level (P = .055). In multivariate analysis, age (OR 4.38, CI 95% 2.95-6.50, P < .001) and platelet count higher than 850 × 109/L (OR 2.38, CI 95% 1.53-3.70, P < .001) were significant risk factors for chemotherapy in ET.
Hematologic transformation
Disease in 35 patients, 15 with PV and 20 with ET, transformed into MMM. MMM transformation in PV occurred more frequently among homozygous (11.5%) than heterozygous patients (1.4%, P < .001). Additionally, 14.3% of homozygous ET patients developed myelofibrosis compared with 1.6% of WT patients (P = .001) and 4.7% of the heterozygous counterpart (P = .011). Acute myelogenous leukemia developed in 1 heterozygous patient with ET.
Discussion
This study aimed at determining whether patients with PV or ET harboring JAK2 617V>F mutation in the homozygous state displayed unique characteristics in comparison to their heterozygous or WT counterparts (in case of ET). This information might support the hypothesis that assessment of JAK2 mutational status actually provides relevant information for clinical practice. Previous studies reported that homozygosity is displayed by approximately 2% of ET patients and 25% to 30% of PV patients, a figure comparable with that found in this study (2.2% and 32.2%, respectively). As a matter of fact, although the issue of potential clinical impact of JAK2 617V>F homozygosity has been addressed by others,4,5,18 the low number of subjects evaluated in each of the previous studies and the fact that in most of them patients with PV or ET were grouped together, rather than being analyzed independently, may account for conflicting results reported (a summary of these studies is presented in Table S2). Therefore, we designed a cooperative study among Italian hematology centers referred to as the GIMEMA-MPD WP, which resulted in the collection of a relevant number of homozygous subjects (n = 118) and a large control population represented by JAK2 617V>F WT patients with ET (n = 257) or patients heterozygous for the mutation (n = 587) with either PV or ET.
For the purposes of this study, we hold the notion of homozygosity as it is currently used in the clinical diagnostic setting, that is, to indicate that the percentage of mutant allele in a granulocytic cell population is greater than 50%. In addition, all patients included in this study were genotyped using allele-specific oligonucleotide (ASO) PCR and BsaXI restriction analysis, as described by Baxter et al,2 to ensure consistency in patients' genotyping. However, it must also be stressed that this definition of homozygosity does not actually distinguish between true homozygosity in a normal background from a predominance of heterozygous cells; indeed, using clonal assays, homozygous progenitors were also reported to consistently occur in “heterozygous” PV patients.32
The retrospective design of this study might be questioned concerning the time lapse occurring between the clinical diagnosis and the time when mutational analysis was performed, because of the possibility of a time-dependent change in mutational state. However, a recent study in ET patients found no evidence for significant modifications of JAK2 mutant clone burden over a median follow-up time of 47 months,33 whereas none of 50 JAK2 617V>F–negative ET patients, enrolled in the PT-1 trial and re-evaluated after a median of 77 months, became mutation positive.34 A substantial stability was also reported in 88% of 44 patients with idiopathic or secondary myelofibrosis studied, although in 3 of 44 patients progression from WT to heterozygosity and from heterozygosity to homozygosity was observed in the absence of overt signs of disease evolution.35 Overall, while evidence for a time-dependent increase in clonal dominance has been provided in few cases,21,36 at present there is no longitudinal study with a statistically consistent number of subjects and/or for a reasonable length of time to unequivocally support the thesis that JAK2 mutational status varies significantly with time.
Irrespective of their unique clinical diagnosis, patients with JAK2 617V>F homozygosity displayed significantly enhanced proliferation of the erythroid and myeloid lineages, generally with impairment of the megakaryocytic one. This observation is in keeping with previous reports (Table S2) and notably with the results of murine models in which erythrocytosis and leukocytosis, but not thrombocytosis, developed after transplantation with JAK2 617V>F–transfected cells.16,17,37,38 Also, the significantly higher rate of evolution into secondary MMM observed among homozygous patients with PV or ET is consistent with the 2-phase evolution of the murine models, in which a PV-like disease manifested shortly after transplantation of transfected cells was followed by changes suggestive of evolution toward myelofibrosis. Of note, these animal models closely mimicked a “true” homozygous condition, since they were obtained by introducing donor stem cells containing only the mutant transgene into recipient mice. Conceivably, the finding of a significantly higher frequency and degree of spleen volume enlargement in homozygous patients could also be interpreted as reflecting the extent of myeloproliferation.
JAK2 617V>F mutant patients with ET were older than their WT counterpart (P < .05), whereas there was no significant difference between homozygous and heterozygous patients with PV or ET; the preferential occurrence of JAK2 mutation in patients of older age has been observed in large studies in ET,7,8 whereas data in PV are scanty18 and the impact of homozygosity is poorly defined. On the other hand, no statistically significant difference in disease duration was observed depending on mutational status, although it tended to be longer in homozygous patients with either PV or ET.
The presence of JAK2 617V>F homozygous mutation pointed to patients more likely to display a symptomatic disease, since they were referred more frequently with aquagenic pruritus (in case of PV), presented more often with the highest degree of spleen enlargement, and had a higher rate of evolution into secondary MMM. An increased frequency of pruritus in homozygous PV patients had already been noted in a smaller series18 and might be considered a very characteristic finding associated with the homozygous PV state; the pathogenetic mechanisms underlying this intriguing association are unknown and merit further investigation. Finally, it was found that homozygous PV patients were more likely to require a cytostatic approach for controlling their disease than the heterozygous counterpart; a similar trend, almost at the level of statistical significance, was also documented in patients with ET.
The collaborative design of this study allowed collection of a discrete number of the very infrequent homozygous ET patients, a category not considered in other studies. These patients had higher leukocyte but comparable platelet counts when compared with both WT and heterozygous patients and presented a more conspicuous spleen enlargement. We paid special care in reviewing the clinical records of these subjects to ensure that they indeed fulfilled WHO diagnostic criteria for ET and were not subtle PV (Table S3). Their hematocrits, ferritin levels, and serum Epo levels were within normal range; red cell mass was found to be normal in the 5 patients in whom it was performed as part of routine diagnostic assessment, and none of the 6 evaluable patients had endogenous erythroid colonies. Original diagnosis of ET had been made according to WHO criteria in 12 of them and to PVSG criteria in the remaining 2; independent analysis of bone marrow biopsy, available in 13 of 14, confirmed that WHO criteria for ET were satisfied, excluding both PV and “prefibrotic myelofibrosis.” Finally, after a mean follow-up of 8.6 (± 4.5) years, no patient had signs of evolution to PV. Therefore, to the best of our efforts in assessing diagnosis, these 14 patients should be considered to have “true” ET according to current criteria. We report here that this specific group of patients displayed an exceedingly high rate of thrombosis, both arterial and venous. It should be noted that the frequency of cardiovascular events in this group was statistically higher than in homozygous PV patients (P < .01), since thrombosis occurred 12-fold and 2.5-fold more frequently, respectively, at diagnosis and during follow-up. An association between JAK2 617V>F mutation and risk of venous thromboses has been described in a large study that compared 362 mutant with 414 WT patients with ET,7 although no information was provided about homozygous patients. In addition, a recent study of 179 ET patients found that the frequency of thrombosis in JAK2 617V>F mutant was significantly increased and comparable to that of PV patients.39 Also in our study, the frequency of thrombosis at diagnosis was double in heterozygous ET patients than in WT patients (21.7% vs 10.5%), although the difference did not attain the significance level; however, the difference reached the significance level if all mutant (heterozygous plus homozygous) ET patients were compared with a WT counterpart. Older age and higher leukocyte count31 displayed by homozygous ET patients might have represented confounding factors in the maintained association of homozygosity with a higher rate of thrombosis, both at diagnosis and during follow-up (Table 2); however, adjusted multivariate analysis confirmed that homozygosity for JAK2 617V>F represented a significant independent risk factor for thrombosis, with a hazard risk of about 4 when compared with both WT and heterozygous patients. Should these findings be further corroborated in a prospective trial involving a consistent number of homozygous patients, we suggest that homozygosity in ET might represent a novel risk factor for thrombosis, in addition to age, previous thrombosis, and leukocytosis.31
In summary, the findings of this study indicate that, beyond its role in the diagnostic approach to CMPD, identification of JAK2 617V>F homozygous patients might provide the clinician with meaningful prognostic information, especially when homozygosity occurs in the setting of a diagnosis of ET. Furthermore, they suggest the opportunity of careful patient stratification on the basis of this molecular variable in the design and interpretation of future clinical trials.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Associazione Italiana per la Ricerca sul Cancro, Milano; Ente Cassa di Risparmio di Firenze; and Ministero Italiano della Università e Ricerca (COFIN, Progetti Cofinanziati (nos. 2006067001-003)).
Authorship
Contribution: A.M.V. and T.B. designed the research, analyzed data, and wrote the manuscript with the contribution of E.A., P.G., A.R., G.B., R.M., R.M.M., and A.B. G.F., V.G., F.F., M.L.R., V.D.S., S.C., A.T., M.R., G.S., V.L., E.R., E.P., and L.G. contributed patients, discussed data, and gave final approval to the manuscript.
A complete list of the GIMEMA-MPD WP investigators is provided in Document S1 as a data supplement to the online version of this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandro M. Vannucchi, Department of Hematology, University of Florence, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.