Abstract
We analyzed outcomes after hematopoietic cell transplantation (HCT) in 257 patients, 3 to 72.7 years old (median, 43 y), with secondary myelodysplastic syndrome (MDS) including those with transformation to acute myeloid leukemia (tAML). Conditioning regimens included high-dose total-body irradiation (TBI)/chemotherapy (n = 83); busulfan (BU)/cyclophosphamide (CY) (BUCY, n = 122; with BU targeting [tBUCY], n = 93); fludarabine (Flu) with tBU (FLUtBU; n = 12); Flu plus 200 cGy TBI (n = 26); and miscellaneous regimens (n = 14). Donors were HLA-identical or partially mismatched family members in 135 and unrelated individuals in 122 patients. Five-year relapse-free survival was highest (43%) and nonrelapse mortality lowest (28%) among tBUCY-conditioned patients. Outcomes were compared with results in 339 patients who received transplants for de novo MDS/tAML, and a multivariate analysis failed to show significant differences in outcome between the 2 cohorts. Relapse probability and relapse-free survival correlated significantly with disease stage (P < .001) and karyotype (P < .001). Relapse incidence was lower (P = .003) and relapse-free survival superior (P = .02) with unrelated donor transplants. The data suggest that overall inferior outcome in patients with secondary MDS/tAML was related to the frequency of high-risk cytogenetics. For both cohorts, transplantation outcomes improved over the time interval studied.
Introduction
As treatment of cancer and other diseases with irradiation and cytotoxic chemotherapy has proven curative, secondary myelodysplastic syndromes (MDSs) have been diagnosed with increasing frequency. The prognosis of treatment-related MDS is generally poor. While as many as half of the patients with secondary MDS respond to induction chemotherapy, remissions are of short duration and only a small proportion will be cured.1–3 Hematopoietic cell transplantation (HCT) from a suitably HLA-matched donor offers an alternative with the potential for cure.4,5
An initial report from our Center on patients who developed MDS (including those who transformed from MDS to acute myeloid leukemia [tAML]) following prior cytotoxic therapy6 or an antecedent hematologic disorder (we will refer to these patients as having secondary MDS/tAML) indicated that HCT at an early stage of their disease (with low marrow myeloblast counts) was associated with a higher probability of long-term survival in remission than patients who received transplants for more advanced disease.7 This analysis also suggested that the type of transplant conditioning regimen had a major impact on long-term survival. Conditioning regimens have been modified over the years with the aim of reducing toxicity while maintaining or improving efficacy. These efforts have included the development of reduced-intensity or nonmyeloablative regimens with the intent of offering HCT to older patients or patients with comorbid conditions that would have precluded them from receiving more conventional high-dose conditioning regimens. We undertook the present analysis in a large cohort of patients with secondary MDS/tAML in an attempt to identify additional risk factors and to determine whether more recently developed conditioning regimens had an impact on transplant-related complications and long-term relapse-free survival. Finally, we compared outcome in this patient cohort to recently reported results in patients with de novo MDS, again including those who had transformed from MDS to AML (we will refer to these patients as having de novo MDS/tAML).8
Patients, materials, and methods
Secondary MDS/tAML
Patients.
Secondary MDS/tAML was defined as disease arising in patients previously treated with irradiation, chemotherapy, or both for lymphohematopoietic or other disorders or patients with antecedent hematopoietic disorders, even if they had not received cytotoxic therapy. Thus, this cohort comprises patients with treatment-related MDS/tAML and patients in whom MDS/tAML evolved from the pathophysiology of their primary disease. Data are summarized in Tables 1 and 2. Between April 1980 and June 2006, 251 consecutive patients with secondary MDS/tAML received transplants at the Fred Hutchinson Cancer Research Center (FHCRC). Ninety of these patients had been included in a previous report.7 There were 134 males and 123 females, 3 to 72.7 years old (median, 43 y; 3.0-71.6 y [median, 42.4 y] for patients conditioned with “conventional” transplant regimens, and 37.4-72.7 y [median 56.9 y] for patients conditioned with a nonmyeloablative regimen of fludarabine [Flu] + 200 cGy total-body irradiation [TBI]). Disease stages were categorized according to the French-American-British (FAB) classification9 for patients with less than 20% myeloblasts. However, all patients with 20% myeloblasts or more were considered as having tAML, based on the World Health Organization (WHO) classification.6 Cytogenetic risk was assigned for MDS and tAML patients based on the categories of the International Prognostic Scoring System (IPSS).11 Among 103 patients whose MDS had transformed to tAML, 29 underwent HCT without further therapy; 74 patients received induction chemotherapy, and 23 of these had declines in myeloblast counts to less than 20% (< 5% in 9, and 5%-19% in 11), whereas 51 proceeded to HCT without having shown responses.
Conditioning consisted of 9.2 to 15.75 Gy TBI and cyclophosphamide (CY; 120 mg/kg)12 or busulfan (BU; 14-16 mg/kg) and CY (120 mg/kg; BUCY)13 in 122 patients. In 93 of these patients, BU doses were adjusted to reach target plasma steady-state levels of 800 to 900 ng/mL, as described.14 Twelve patients received Flu plus BU,15 and 26 patients received a nonmyeloablative regimen consisting of Flu (3 × 30 mg/m2) and 200 cGy TBI.16,17 Fourteen patients received miscellaneous chemotherapy regimens (Table 3).
Donors and sources of hematopoietic stem cells.
Donors were HLA-identical or partially identical family members in 135 patients and HLA-identical or partially mismatched unrelated donors in 122 patients. The source of stem cells was marrow in 150 patients and peripheral blood progenitor cells (PBPCs) obtained after G-CSF mobilization in 102 patients; 5 patients received transplants with unrelated umbilical cord blood cells (Table 3).
GVHD and supportive care.
Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine (CSP) plus methotrexate (MTX)18 in most patients and various regimens of CSP,19 MTX,18 FK506 (tacrolimus), and mycophenolate mofetil (MMF)20 in the remaining patients.21 Treatment of acute GVHD consisted of corticosteroids, CSP, antithymocyte globulin, or other agents dependent upon protocols active at the time. Infection prophylaxis and therapy, transfusion support, and other supportive care were provided according to standard procedures.10,22
Causes of death.
In patients who died with progressive or recurrent disease after HCT, relapse was considered the cause of death. In all other cases, the cause of death provided by the attending physician was listed.
Comparison with de novo MDS/tAML
In addition to the analysis of risk factors for the outcome in patients with secondary MDS/tAML, we compared these results with those in patients with de novo MDS/tAML (as defined in “Introduction”) who received transplants at our Center (Table 1).8 This cohort consisted of 339 patients, 1 to 69 years old (median, 47.3 y), who received transplants between February 1990 and November 2003. Donors were HLA-identical siblings in 145 patients, partially mismatched family members in 20, HLA-identical unrelated individuals in 98, and HLA-nonidentical unrelated individuals in 72 patients (Table 3). For this comparative analysis to arrive at comparable patient cohorts that received transplants during the same time interval, we excluded patients who received cord blood (n = 5), patients conditioned with miscellaneous regimens (n = 24), patients with unknown cytogenetics (n = 34), and patients with secondary MDS/tAML who received transplants before 1990 (n = 20). Thus, results in 217 patients with secondary and 312 patients with de novo MDS/tAML were compared. Among 121 patients in the de novo cohort whose disease had transformed to tAML, 34 went to HCT without further therapy; 87 patients received induction chemotherapy, and 37 responded (< 5% myeloblasts in 10, and 5% to 19% myeloblasts in 27), while 50 proceeded to HCT without having responded to chemotherapy.
All patients gave informed consent according to the procedures required by the Institutional Review Board of the FHCRC and in accordance with the Declaration of Helsinki.
Statistical methods
The overall probabilities of survival and relapse-free survival were calculated using the method of Kaplan and Meier.23 The incidences of relapse and nonrelapse mortality were estimated from the cumulative incidence curves.24 Cox regression analysis was used to estimate hazard ratios relative to a given reference group.25 Nonrelapse mortality and relapse were treated as competing risks in such analyses. All P values are derived from hazard ratio analyses and are 2 sided. Results were analyzed as of June 30, 2006.
Results
Patients with secondary MDS/tAML
Engraftment.
Among patients with secondary MDS/tAML, 23 died before day 28 and could not be evaluated for engraftment. Among the remaining patients, the median time to achieve granulocyte counts of more than 0.5 × 109/L for at least 3 days was 18 days (range, 8-35 d) and platelet counts of more than 20 × 109/L for at least 7 days without transfusion was 21 days (range, 7-78 d).
GVHD.
Of 249 evaluable patients with secondary MDS/tAML, 165 (67%) developed grades II-IV acute GVHD and 147 (57%) developed chronic GVHD.
Relapse.
The 5-year cumulative incidence of relapse, by disease stage, was 33% for tAML, 36% for RAEB [refractory anemia with excess blasts], and 12% for RA/RARS [refractory anemia/refractory anemia with ringed sideroblasts] patients (P < .001; Table 4). Among patients with tAML, the incidence of relapse was not reduced in patients who had received and responded to induction chemotherapy before HCT. In fact, the hazard ratio (HR) of relapse was lower in patients who had not received therapy (HR 0.46; P = .03) or had been treated but had not responded (HR 0.49; P = .02). One might question the inclusion of patients with a primary diagnosis of AML (Table 4), although these patients' MDS was clearly different from the original AML. In any event, results of an analysis that excluded these 6 patients were not different from results in the entire cohort of 257 patients (not shown). Older patients (≥ 50 years) were more likely to relapse than younger patients (HR 1.80; P = .02).
By IPSS cytogenetic risk category (at time of transplantation), the 5-year cumulative incidence of relapse was 16% for patients with low-risk, 30% for patients with intermediate-risk, and 35% for patients with high-risk disease (P < .001). The 5-year cumulative relapse incidence was 9% for patients previously treated with immunosuppressive therapy, 22% for patients who had previously undergone HCT, 30% for those who had received chemotherapy, and 40% for chemoradiotherapy-treated patients (P < .001). An additional risk factor was the primary disease itself (P < .001). The impact of the different HCT conditioning regimens on relapse incidence did not reach statistical significance (P = .08).
Survival.
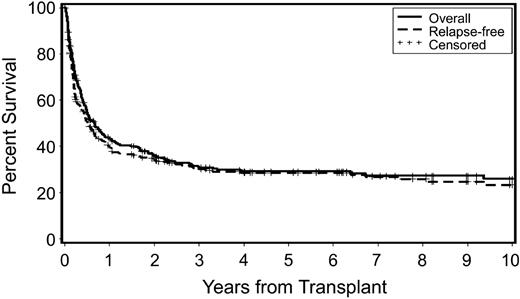
Overall and relapse-free survivals are shown in Figure 1. As patients who relapsed generally died with progressive disease, these survival curves are comparable. At the time of latest contact, 92 patients with secondary MDS/tAML were alive 6 months to 23.6 years (median, 3.8 y) after HCT.
Probability of overall survival and relapse-free survival in patients who received transplants for secondary MDS/tAML. Censored indicates censored observations at the date of last contact.
Probability of overall survival and relapse-free survival in patients who received transplants for secondary MDS/tAML. Censored indicates censored observations at the date of last contact.
The 5-year relapse-free survival for the entire group was 29%; by disease stage, survival was 19% for tAML, 25% for RAEB, and 41% for RA/RARS (P < .001; Figure 2). By transplant regimen, relapse-free survival was 23% for patients conditioned with FluTBI (200 cGy), 24% for Flu/BU, 18% for high-dose TBI/CY, 28% for BUCY, and 43% for tBUCY-conditioned patients (P = .001). The best 5-year overall and relapse-free survival was achieved in patients with RA/RARS (58% and 53%, respectively). Older patients were less likely to survive (HR 1.65; P = .001) than younger patients.
Probability of relapse-free survival by disease stage at the time of transplantation in patients with secondary MDS/tAML. Censored indicates censored observations at the date of last contact; RA/RARS, refractory anemia/refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; tAML, transformation to AML; and tAML Rx, patients with tAML who received chemotherapy before HCT and were categorized as RA or RAEB at the time of HCT.
Probability of relapse-free survival by disease stage at the time of transplantation in patients with secondary MDS/tAML. Censored indicates censored observations at the date of last contact; RA/RARS, refractory anemia/refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; tAML, transformation to AML; and tAML Rx, patients with tAML who received chemotherapy before HCT and were categorized as RA or RAEB at the time of HCT.
Patients treated with immunosuppressive therapy for their primary disease, generally aplastic anemia, had a 5-year relapse-free survival of 47% compared with 35% for patients who had previously undergone HCT, 23% for those treated with chemoradiotherapy, and 20% for those given chemotherapy only (P < .001). Additional factors that significantly affected relapse-free survival were the cytogenetic risk group (P = .008), the primary disease (P = .02), and the source of stem cells (P = .04), as well as GVHD occurring after transplantation (P = .005; Table 4). There was no significant difference in survival between related and unrelated transplant recipients.
Causes of death.
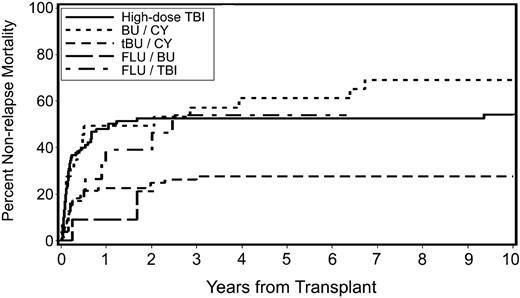
Causes of death are listed in Table 5. Overall, 176 patients with secondary MDS/tAML (67%) had died at the time of analysis. Death occurred after relapse in 65 patients, whereas 111 patients died of nonrelapse causes. The 5-year cumulative incidence of nonrelapse mortality was 54% for patients conditioned with FluTBI (200 cGy), 53% for patients conditioned with high-dose TBI/CY, 61% for patients conditioned with BUCY, and 28% for patients conditioned with tBUCY (P < .001; Figure 3). There was a suggestion of a lower incidence of death related to organ failure or infections in tBUCY-conditioned patients than in patients given other conditioning regimens (data not shown). Nonrelapse mortality was also significantly correlated with time from diagnosis to HCT (P = .02), the source of stem cells (P < .001), the year of transplantation (P < .001), and older age (P = .03; Table 4).
Cumulative incidence of nonrelapse mortality by preparative regimen in patients with secondary MDS/tAML. High-dose TBI indicates high-dose TBI-containing regimens; BU/CY, busulfan and cyclophosphamide; tBU/CY, busulfan targeted to plasma levels of 600 to 900 ng/mL and cyclophosphamide; FLU/BU, busulfan targeted to plasma levels of 600 to 900 ng/mL and fludarabine; and FLU/TBI, fludarabine and TBI (200 cGy).
Cumulative incidence of nonrelapse mortality by preparative regimen in patients with secondary MDS/tAML. High-dose TBI indicates high-dose TBI-containing regimens; BU/CY, busulfan and cyclophosphamide; tBU/CY, busulfan targeted to plasma levels of 600 to 900 ng/mL and cyclophosphamide; FLU/BU, busulfan targeted to plasma levels of 600 to 900 ng/mL and fludarabine; and FLU/TBI, fludarabine and TBI (200 cGy).
Comparison of results in patients with secondary and with de novo MDS/tAML
Results are summarized in Tables 6 and 7 and in Figures 4 and 5. Figure 4 shows relapse-free survival for patients with de novo and secondary MDS/tAML. While unadjusted data suggested increased HRs for relapse and relapse-free survival for patients with secondary MDS/tAML (Table 6), there were no significant differences between the 2 cohorts in regards to the 3 end points studied after adjustment for risk factors other than disease etiology (as listed in Table 4), and no significant differences were noted when patients with MDS and tAML were analyzed separately (data not shown). A comparison of results in patients with antecedent hematologic disorders and patients with de novo disease suggested a lower hazard of relapse, nonrelapse mortality, and relapse-free survival for patients with antecedent hematologic disorders, but following adjustments differences were not significant (Table 6).
Probability of relapse-free survival in patients with secondary and de novo MDS/tAML (unadjusted P = .03). Censored indicates censored data of patients alive and in remission at date of last contact.
Probability of relapse-free survival in patients with secondary and de novo MDS/tAML (unadjusted P = .03). Censored indicates censored data of patients alive and in remission at date of last contact.
Impact of cytogenetic risk category on outcome in patients with secondary and with de novo MDS/tAML. (A) Probability of relapse. Low secondary indicates low risk in secondary group; low de novo, low risk in de novo group; int secondary, intermediate risk in secondary group; int de novo, intermediate risk in de novo group; high secondary, high risk in secondary group; and high de novo, high risk in de novo group. (B) Probability of relapse-free survival by cytogenetic risk for patients with secondary and de novo MDS/tAML. Cytogenetic risk groups according to IPSS criteria.
Impact of cytogenetic risk category on outcome in patients with secondary and with de novo MDS/tAML. (A) Probability of relapse. Low secondary indicates low risk in secondary group; low de novo, low risk in de novo group; int secondary, intermediate risk in secondary group; int de novo, intermediate risk in de novo group; high secondary, high risk in secondary group; and high de novo, high risk in de novo group. (B) Probability of relapse-free survival by cytogenetic risk for patients with secondary and de novo MDS/tAML. Cytogenetic risk groups according to IPSS criteria.
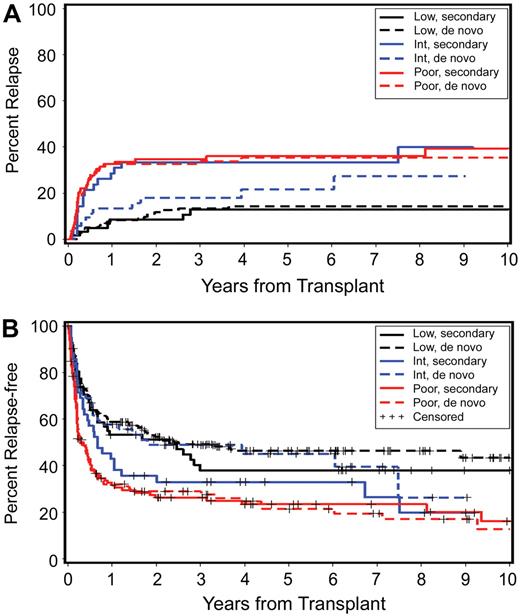
Detailed results of a multivariate analysis are summarized in Table 7. Disease stage (P < .001) and cytogenetic risk group (P < .001) were the 2 most significant risk factors for relapse (Figure 5A) and relapse-free survival (Figure 5B). Relapse was less frequent (HR 0.59; P = .01) with HC transplants from unrelated donors, but this was not reflected in a significant improvement in relapse-free survival (HR 0.83; P = .13). Transplant conditioning regimens had an impact on relapse (P = .06), with a HR of 2.54 for patients conditioned with FlutBU (with high-dose TBI serving as reference), and nonrelapse mortality (P = .03), with the lowest HR of 0.54 observed for patients conditioned with tBUCY. The inclusion of ATG into BU-containing regimens (n = 25) had no significant effect on outcome (data not shown). Overall there was no significant difference in relapse-free survival between different conditioning regimens (P = .10). Higher nonrelapse mortality correlated with earlier years of HCT (P = .05). Nonrelapse mortality was also higher among older patients (HR 1.65; P = .001) and this was reflected in a lower probability of relapse-free survival (HR 1.49; P = .001).
Relapse rates among patients with good-risk and poor-risk cytogenetics were similar for de novo and secondary MDS/tAML (Figure 5A), indicating that it was the patients' karyotype that was the dominant factor in determining outcome rather than de novo or secondary etiology. Of note, however, for patients with “intermediate-risk” cytogenetics (by IPSS criteria), the relapse rate was higher among those with secondary MDS/tAML (n = 42) than those with de novo MDS/tAML (n = 52; Figure 5A; HR 1.99; P = .06). Review of the individual karyotypes indicated that the clonal abnormalities that placed patients into the intermediate-risk group differed between patients with de novo and with secondary MDS/tAML. For example, trisomy 8 was more frequent in the de novo cohort (n = 17; 33%) than among patients with secondary MDS/tAML (n = 6; 13%). Conversely, 11q23 was more frequent among patients with secondary MDS/tAML (n = 9; 19%) than among patients with de novo disease (n = 3; 6%). These differences in clonal cytogenetic abnormalities within the intermediate-risk group are likely to have contributed to differences in outcome.
Discussion
HCT is a therapeutic modality with curative potential for patients with treatment-related or secondary MDS/tAML.26,27 However, published data show poor long-term survival even with HCT.28,29 We undertook the present analysis in an attempt to better define risk factors for transplantation outcome in patients with secondary MDS/tAML and to delineate differences in comparison to patients with de novo disease. As anticipated, relapse-free survival for the entire cohort of patients with secondary disease was lower than among patients with de novo MDS/tAML. However, there were no significant differences in regards to relapse, nonrelapse mortality, and relapse-free survival when the data were adjusted for risk factors other than disease etiology.
The pretransplantation characteristics that most significantly affected relapse-free survival among patients with secondary MDS/tAML were disease stage, cytogenetic risk group, type of therapy given for the original disease, transplant conditioning regimen, and patient age, as also reported for patients with de novo MDS.30 Unexpectedly, in patients whose MDS had transformed to tAML and who were treated and responded to pretransplantation induction chemotherapy, the HR for relapse was higher than among untreated patients or patients who had been treated but failed to achieve responses. However, as nonrelapse mortality tended to be lower in patients who had responded to pre-HCT chemotherapy, survival was comparable for the 3 subgroups. This was true for patients with both secondary and de novo disease. The observations on relapse were counterintuitive. Quite likely there was a bias in selecting patients for pre-HCT chemotherapy, and referring physicians may have decided to send patients for HCT on the basis of their performance level rather than remission status. Also, among patients who are not in remission, only those with a more smoldering course tend to proceed to HCT, whereas patients with rapid disease progression may be excluded. These questions cannot be addressed here satisfactorily and only emphasize the need for prospective controlled trials. It should be noted, nevertheless, that the transplantation outcome observed here was at variance with that reported by Yakoub-Agha et al,31 who found improved relapse-free survival in patients with secondary MDS who had responded to pre-HCT induction chemotherapy, although responses in that study were defined as complete remissions rather than a reduction in myeloblast counts to the level of “MDS” (< 20%; see “Patients and methods”).
The next most significant risk factor for transplantation outcome was the disease karyotype. As reported by Nevill et al32 and from our Center previously,33 patients with high-risk cytogenetics as defined by IPSS criteria had a significantly higher risk of relapse and lower probability of survival than patients with good-risk cytogenetics. The best strategy to reduce the posttransplantation incidence of relapse has yet to be defined. One factor that can be manipulated is the type of conditioning regimen used for HCT. In the present analysis the lowest relapse frequency was seen in patients conditioned with BUCY, whereas nonrelapse mortality was lowest in patients who received targeted BU (combined with either Flu or CY). Patients conditioned with tBUCY had the highest probability of relapse-free survival. The lowest probability of relapse-free survival was seen in patients conditioned with high-dose TBI–containing regimens. However, the use of those regimens correlated with earlier transplantation years and, as discussed already, this was the time interval with the highest rate of nonrelapse mortality. Additional changes in supportive care that occurred over the course of these studies may have contributed to improvement of transplantation results in recent years.
A nonmyeloablative regimen combining low-dose TBI (200 cGy) with Flu was used in 26 older patients or patients with comorbid conditions who were considered ineligible for “conventional” transplant regimens. Considering the high-risk characteristics of these patients, the incidence of nonrelapse mortality was low. It should be noted, however, that nonrelapse mortality for all patients declined over the time interval studied as conventional regimens were also being modified. Therefore, while acknowledging that patients who received nonmyeloablative regimens had high-risk pretransplantation characteristics, the rate of nonrelapse mortality did not differ substantially, for example, between FluTBI- (200) and tBUCY-conditioned patients.
There was a trend for a reduced incidence of organ failure and infection-related mortality in the patients who had received transplants more recently, following conditioning without the use of high-dose TBI. Of course, the development of new conditioning regimens has been accompanied by other changes in supportive care, for example the use of novel antifungal agents and ganciclovir for prophylaxis and therapy of cytomegalovirus infections, which have been shown to have a positive impact on outcome. Another parallel development was the increasing use of PBPCs, which was associated with significantly reduced nonrelapse mortality (HR 0.49; P < .001) and improved survival (HR 0.73; P = .04) in patients with secondary MDS/tAML (Table 4 univariate analysis), although the difference was no longer significant in multivariate analysis (Table 6).
As suggested by earlier reports,7 treatment of the patient's primary disease was a significant factor for transplantation outcome. The type of prior therapy was closely linked to the primary diagnosis (ie, patients with antecedent hematologic disorders were generally not treated with cytotoxic therapy and had a significantly better outcome [lower relapse rate and better relapse-free survival] than patients with hematologic malignancies or solid tumors as primary disease). However, as for the entire cohort of patients with secondary MDS, transplantation outcome did not differ significantly from that in patients with de novo MDS. Of note was the observation that patients who had undergone hematopoietic cell transplantation (in the majority autologous) as therapy for their primary disease fared better than patients treated previously with nontransplantation chemotherapy. The reason for this is not clear, although it is conceivable that patients who received transplants experienced more complete lymphohematopoietic reconstitution and were at lower risk of infectious complications.
In addition to pretransplantation factors, the development of GVHD had a significant impact on survival. While recent data suggest that the use of thymoglobulin in the preparative regimen may reduce the incidence of GVHD in patients who received transplants for MDS,34,35 the small group of patients in the present study who had received thymoglobulin failed to derive a significant advantage.
Taken together, our data indicate that results of HCT for patients with secondary MDS/tAML have improved over time and were only marginally inferior to those in patients with de novo disease.8 Multivariate analyses showed that the impact of risk factors on transplantation outcome was similar for the 2 cohorts (Tables 6, 7). The major risk factors identified for secondary MDS/tAML, such as disease stage, cytogenetic risk groups, conditioning regimen, and year of transplantation, were also significant for the success of HCT in patients with de novo disease. Thus, the etiology of MDS by itself (de novo versus secondary) did not determine outcome but rather this was determined by the underlying disease characteristics. This assessment is further substantiated by the results in patients with intermediate-risk cytogenetics, the spectrum of which differed considerably between patients with secondary MDS/tAML (higher relapse rate) and those with de novo disease (lower relapse rate).36 While the numbers for each individual clonal abnormality were too small to allow for a meaningful statistical comparison, the pattern suggested that it was the given karyotype that determined the probabilities of relapse and relapse-free survival (Figure 5A,B). We had previously observed that patients with de novo MDS/tAML who were categorized as intermediate risk by the criteria of the IPSS could be subcategorized in regards to the probability of relapse and relapse-free survival by the severity of flow-cytometric aberrancies of marrow cells.37 It is not unlikely that aberrant phenotypes of MDS marrow cells correlate with gene expression and karyotype. Others38 have shown a stronger correlation of gene-expression profiles with karyotype than with the proportion of myeloblasts in the marrow of patients with MDS. Further studies are warranted to confirm these correlations and their impact on prognosis.
Thus, the present study confirmed that HCT provides curative therapy for a proportion of patients with secondary MDS/tAML. Furthermore, with appropriate adjustments for risk factors, transplantation outcome in patient with secondary MDS/tAML was comparable to that in patients with de novo disease. These results suggested that the disease biology rather than its etiology determined the prognosis. Conceivably, close monitoring of patients who are at risk of developing secondary MDS would lead to early recognition of the disease and transplantation before disease evolution. The best outcome in the present study was observed with a “conventional” tBUCY conditioning regimen. The use of reduced-intensity conditioning regimens has been successful in improving transplantation results in various disorders. Whether similar success can be achieved in patients with MDS/tAML remains to be determined and is being addressed in prospective trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Joanne Greene and Elizabeth Soll for maintaining the patient database, and Bonnie Larson and Helen Crawford for manuscript preparation.
This work was supported by National Institutes of Health grants HL36444, CA15704, CA78902, and CA18029 (Bethesda, MD). C.K.C. was supported by a grant from Shanghai Sixth People's Hospital.
National Institutes of Health
Authorship
Contribution: C.K.C. compiled the data on secondary MDS/tAML and drafted the manuscript. B.E.S. carried out all statistical analyses. B.L.S. compiled and verified the data on de novo MDS/tAML and revised the manuscript. E.M.B. carried out all cytogenetic studies. H.M.S. was responsible for marrow interpretation. M.E.F. was responsible for long-term follow-up. B.M.S., R.P.W., R.A.N. J.E.S., A.B., J.A.H., B.E.C., R.S., and F.R.A. provided patient care and critically reviewed the analysis and the manuscript. H.J.D. designed the study, provided input to the statistical analysis, and provided guidance for manuscript revisions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mail Stop D1–100, PO Box 19024, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.