Abstract
Interleukin 6 (IL6) trans-signaling has emerged as a prominent regulator of immune responses during both innate and acquired immunity. Regulation of IL6 trans-signaling is reliant upon the release of soluble IL6 receptor (sIL6R), which binds IL6 to create an agonistic IL6/sIL6R complex capable of activating cell types that would not normally respond to IL6 itself. Here we show that intrinsic and extrinsic apoptotic stimulation by DNA damage, cytokine deprivation, and Fas stimulation promotes shedding of sIL6R. Apoptosis-induced shedding of the IL6R was caspase dependent but PKC independent, with inhibition of ADAM17 preventing IL6R shedding. Such insight is relevant to the control of acute inflammation, where transition from the initial neutrophil infiltration to a more sustained population of mononuclear cells is essential for the resolution of the inflammatory process. This transitional event is governed by IL6 trans-signaling. This study demonstrates that IL6R is shed from apoptotic human neutrophils. In vivo studies in a murine inflammation model showed that neutrophil depletion resulted in reduced local sIL6R levels and a concomitant decrease in mononuclear cells, suggesting that apoptosis-induced IL6R shedding from neutrophils promotes IL6 trans-signaling and regulates the attraction of monocytic cells involved in the clearance of apoptotic neutrophils.
Introduction
IL6 exhibits proinflammatory and anti-inflammatory properties and plays a central role in host defense against infection and tissue injury. Activities of IL6 are pleiotropic, stimulating a wide range of biologic processes including B-cell maturation, hepatocyte regeneration, and neuronal growth.1
IL6 first binds to a specific IL6 membrane receptor (IL6R) and this complex associates with 2 molecules of the ubiquitously expressed signaling receptor gp130.2 The formation of this complex leads to intracellular activation of the transcription factors STAT1 and STAT3 and of the Raf/Ras/MAPK pathway.3 Interestingly, only a subset of cells in the human body including many leukocytes and hepatocytes expresses the IL6R. Cells expressing only gp130 but lacking membrane-bound IL6R are not responsive to IL6. However, these cells can be stimulated by IL6 bound to a soluble form of the IL6R (sIL6R) in a process called trans-signaling.4 In acute bacterial peritonitis, the soluble IL6R orchestrates the switch from neutrophil to mononuclear cell infiltration by modulating inflammatory chemokine expression in peritoneal mesothelial cells.5 Moreover, it has been demonstrated that many pathophysiological effects of IL6 depend on trans-signaling.6 The soluble IL6R can be generated by shedding or alternative splicing. Several inducers of IL6R shedding such as the phorbol ester PMA,7 bacterial pore-forming toxins,8 bacterial metalloproteinases,8 C-reactive protein,9 and cholesterol depletion10 have been identified. PMA-induced shedding of the IL6R implicated activation of protein kinase C (PKC),7 whereas IL6R shedding induced by bacterial toxins and cholesterol depletion was independent of PKC activation.10,11 IL6R shedding induced by PMA and cholesterol depletion is dependent on the metalloproteinase ADAM17 (adisintegrin and metalloproteinase), and to a lesser extent on the related ADAM10.10 Gene targeting of ADAM10 and ADAM17 resulted in embryonic lethality, indicating the importance of shedding processes during growth and development.12,13
Apoptosis is a suicidal process that may occur during all stages of life (eg, development and aging).14 Apoptosis is characterized by cell shrinkage, nuclear condensation, and fragmentation as well as phosphatidylserine exposure within the outer leaflet of the cell membrane. Furthermore, a family of proteases named caspases is activated by cleavage from inactive precursors. Apoptosis is a tightly controlled process that can be induced by Fas stimulation, cytokine withdrawal, glucocorticoid treatment, heat shock, and UV irradiation.15 During apoptosis, shedding of membrane proteins including E-cadherin and L-selectin,16,17 tumor necrosis factor receptor 1,18 and the complement membrane cofactor protein CD4619 has been observed and ADAM proteases have been implicated in the release of surface molecules during apoptosis.17,19
Here, we show that the induction of apoptosis by stimuli as diverse as DNA damage, cytokine withdrawal, Fas stimulation, and UV light leads to caspase-dependent activation of the ADAM17 (but not ADAM10) protease, which is independent of PKC activation and PARP cleavage. This, in turn, promotes shedding of the membrane-bound IL6R, thereby generating sIL6R. Using neutrophils as a cellular model, studies presented herein propose that apoptosis-induced shedding of the IL6R facilitates IL6 trans-signaling. In this context, we propose that IL6 trans-signaling in stromal tissue cells promotes recruitment of mononuclear cells involved in the nonphlogistic phagocytosis of the neutrophil infiltrate. Such a mechanism may provide an explanation for the controlled resolution of inflammatory response.
Materials and methods
Cell culture
HepG2 cells were obtained from the American Type Culture Collection (Rockville, MD/Manassas, VA). Ba/F3 cells stably transfected with human GP130 (Ba/F3-gp130 cells) or GP130 and IL6R cDNAs (Ba/F3-gp130/IL6R cells) have been previously described.20,21 Hyper IL6, a chimeric protein of IL6 covalently linked to the soluble IL6R, was produced as described previously.20
Antibodies and chemicals
For the Western blot detection of PKCδ, sc-937 mAB was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-PARP and anti–caspase 3 antibodies were from Cell signaling (Beverly, MA). For detection of IL6R, the anti-IL6R 14-18 antibody (H.L., unpublished results, January 2007) was used. For the detection of ADAM17, a carboxyl terminal ADAM17 antibody was used (Chemicon, Hampshire, United Kingdom). The agonistic Fas antibody CH-11 was from Upstate (Charlottesvile, VA). The anti–β-actin antibody was from abCAM (Cambridge, United Kingdom). The antimouse IL6R D7715A7 mAB was from BIOZOL (Eching, Germany). The hIL6R enzyme-linked immunosorbent assay (ELISA) kit was purchased from R&D systems (Wiesbaden, Germany). Allophycocyanin-conjugated goat antimouse mAB was from Dianova (Hamburg, Germany). Sheep anti–mouse IgG horseradish peroxidase and goat anti–rabbit IgG horseradish peroxidase were from Amersham Biosciences (Buckinghamshire, United Kingdom). The annexin V/propidium iodide staining kit was from BD Bioscience (Heidelberg, Germany); the metalloprotease inhibitors GI254023X (ADAM10), GW280264X (ADAM10 and ADAM17), and marimastat were a generous gift from Glaxo Smith Kline (Stevenage, United Kingdom).22 TAPI-2, z-LEHD-FMK, and PMA were from Calbiochem (Schwalbach am Taunus, Germany). z-VAD-FMK, z-DEDV-FMK, and z-IETD-FMK were from Bachem (Weil am Rhein, Germany). Doxorubicin, cycloheximide, GF109203X, and Gö6976 were from Sigma-Aldrich (Deisenhofen, Germany). The DN-ADAM17 variant was cloned as previously described.23
Transfection, transduction, and selection of cells
HepG2 cells were transfected using DEAE dextran as previously described.24 The transfection efficiency as visualized after 24 hours by GFP expression (Axiovert 200 Microscope; Zeiss, Jena, Germany) was approximately 80%.
Induction of cellular apoptosis and immunoprecipitation of IL6R
Ba/F3 cells were washed 3 times with PBS and seeded out at a density of 1 × 106 cells/mL.20 Apoptosis was induced by addition of 500 ng/mL doxorubicin or by cytokine withdrawal. HepG2 cells transfected with the p409-hIL6R plasmid were seeded out 24 hours after transfection at a density of 3 × 105 cells/mL. For induction of apoptosis, HepG2 cells were treated with 100 μM cycloheximide (CHX) for 120 minutes prior to stimulation with the anti-CD95 antibody CH-11. All other inhibitors were added 30 minutes prior to stimulation. For induction of apoptosis, freshly isolated neutrophils were seeded out at a density of 5 × 106 cells/mL and treated with UV (200,000 μJ/cm2) or anti-Fas antibody (500 ng/mL) and were incubated for 4 hours or 6 hours at 37°C. Cell lysis and immunoprecipitation of the IL6R in supernatants and cell lysates followed by Western blotting were performed as described before.10
Enzyme-linked immunosorbent assay (ELISA) for mIL6R
Microtitre plates (Greiner Microlon, Solingen, Germany) were coated with antimurine IL6R pAB AF1830 (0.8 μg/mL; R&D Systems) in PBS. After blocking, 100-μL aliquots of cell lysates or culture supernatants were added. IL6R bound to the plate was detected by biotinylated goat antimouse IL6R pAB BAF1830 (working concentration, 0.2 μg/mL; R&D Systems) followed by streptavidin-horseradish peroxidase (R&D Systems). The enzymatic reaction was performed with soluble peroxidase substrate (BM blue POD from Roche, Mannheim, Germany) at 37°C and the absorbance was read at 450 nm on a SLT Rainbow plate reader (Tecan, Maennedorf, Switzerland).
Flow cytometry analysis
Cells (3 × 105) were washed twice with fluorescence-activated cell sorting (FACS) buffer (PBS, 0.2% BSA, 0.01% NaN3) and then incubated with 1 μg/mL anti-IL6R antibody (M91; Immunotech, Marseille, France) for 60 minutes on ice. After a single wash in FACS buffer, cells were incubated with the allophycocyanin-conjugated goat antimouse antibody for 60 minutes on ice. Cells were washed once, resuspended in 500 μL FACS buffer, and analyzed by flow cytometry (FACS-Canto; Becton Dickinson, Heidelberg, Germany).
Isolation of human peripheral blood granulocytes (PMNs) and induction of apoptosis
Peripheral blood was collected by venipuncture from healthy adult volunteers. Approval was obtained from the University Hospital Schleswig-Holstein institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. The anticoagulated blood (lithium heparin) was layered onto a histopaque gradient consisting of 2 layers: lymphocyte separation medium (PAA Laboratories, Pasching, Austria) on the top and histopaque 1119 (Sigma-Aldrich, Deisenhofen, Germany) at the bottom prior to centrifugation at 800g for 20 minutes. The granulocyte-rich layer of histopaque 1119 was collected and washed once in PBS (PAA Laboratories). The PMN pellet was resuspended in RPMI 1640 medium (Sigma-Aldrich, Deisenhofen, Germany) and further fractionated on a discontinuous percoll (Amersham Biosciences, Freiburg, Germany) gradient consisting of layers with densities of 1105 g/mL (85%), 1100 g/mL (80%), 1093 g/mL (75%), 1087 g/mL (70%), 1081 g/mL (65%) percoll. After centrifugation for 20 minutes at 800g, the interface between the 80% and 85% percoll layers was collected and washed twice in PBS. PMN purity was more than 95% as assessed by size and granularity on flow cytometry.
Air pouch
The air pouch model of local inflammation was performed with C57BL/6 mice according to Edwards et al.25 In brief, mice were anesthetized with ether and subcutaneous dorsal pouches were created by injection of 6 mL sterile air. After 3 days, the pouches were reinjected with 4 mL air. On day 6, 1 mL 1% carrageenan (Sigma, St Louis, MO) in sterile PBS was injected into the pouches. The corresponding controls received sterile PBS. Neutrophil depletion was induced with a rat antimouse mAb against the neutrophil maturation antigen, GR1 (Ly6G mAb; BD Biosciences-Pharmingen, San Diego, CA). The Ly6G mAb (100 μg) was administered (intraperitoneally) 18 hours before carrageenan injection. The neutralizing IL6R mAb (100 μg) was administered (intraperitoneally) 6 hours before carrageenan injection. At different time points after treatment, the animals were anesthetized and the pouches were washed with 3 mL PBS. The lavage fluid was immediately cooled on ice. Total neutrophils and mononuclear phagocytes were determined by FACS analysis with Ly6G mAB and F4/80 mAB (Caltag-Invitrogen, Karlsruhe, Germany), respectively. The remaining exudates were centrifuged at 3360g for 10 minutes at 4°C, and the supernatant was stored at −20°C until use.
Results
Generation of the sIL6R during intrinsic and extrinsic apoptosis
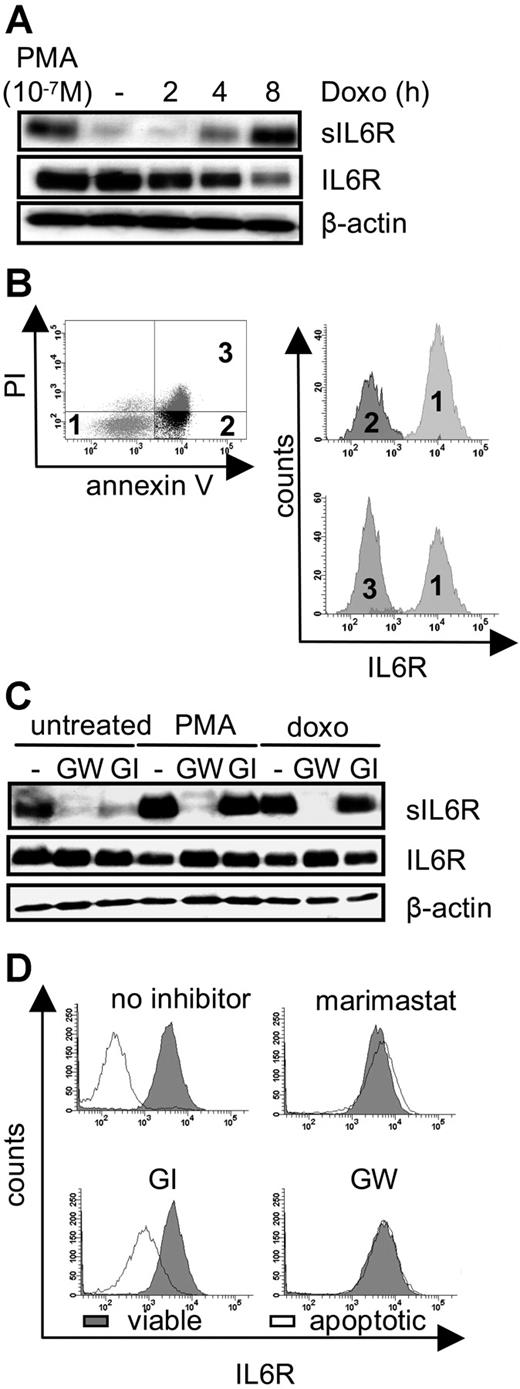
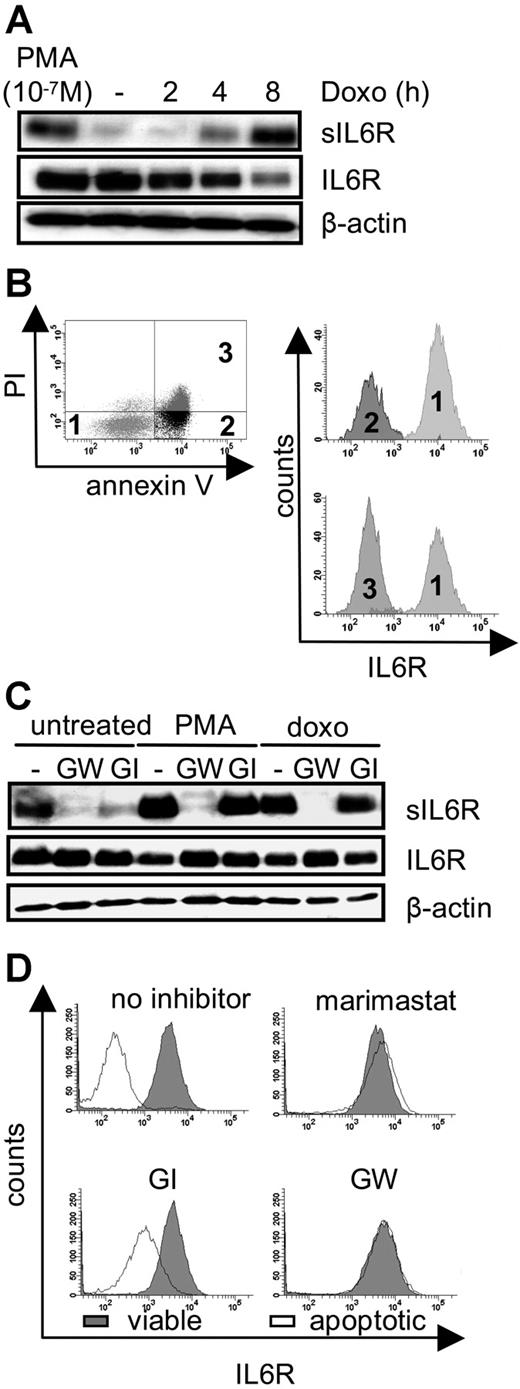
Shedding of membrane proteins can be induced by diverse stimuli such as PMA-mediated PKC activation, treatment of cells by bacterial toxins, cholesterol depletion of cellular membranes, and treatment of cells with C-reactive protein. Collectively, all these stimuli trigger membrane alterations or perturbation. We therefore reasoned that drastic membrane changes occurring during apoptosis when phosphatidylserine is exposed to the exterior of the plasma membrane might also lead to an induction of ectodomain shedding of the IL6R. We induced DNA damage in pre-B cells (Ba/F3-gp130/IL6R cells), which express the human IL6R. These cells underwent apoptosis after treatment with the chemotherapeutic drug doxorubicin. Apoptosis was assessed by flow cytometry to identify early and late apoptotic stages (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). Ninety-nine percent of the cells were apoptotic after 8 hours of doxorubicin treatment, whereas early apoptosis was detectable after 2 hours as judged by annexin V reactivity. PKCδ cleavage, which is mediated by caspase 3, was detected after 6 hours of doxorubicin treatment (Figure S1B). As indicated in Figure 1A, an increase of the soluble form of the IL6R was detectable after 4 hours of doxorubicin treatment, while the amount of membrane-bound IL6R decreased, indicating that IL6R was released from the cell surface.
Intrinsic induction of apoptosis induces ADAM17-mediated IL6R shedding. (A) Ba/F3-gp130/IL6R cells were treated for 2 hours, 4 hours, and 8 hours with doxorubicin (500 ng/mL) or 2 hours with the phorbol ester PMA (100 nM). Soluble IL6R was immunoprecipitated from conditioned media using the polyclonal anti–serum 6.226 and subsequently visualized by Western blot analysis using the anti-IL6R mAb 14-18. Reciprocal Western blot analysis of cell lysates was performed using mAb 14-18 and an anti–β-actin mAb, which was used as a loading control. (B) Ba/F3-gp130/IL6R cells were treated for 6 hours with doxorubicin, and multiparameter FACS staining was performed with propidium iodide and antibodies against annexin V, and the anti-IL6R (M91 mAb). Histograph assignments are as follows: (1) viable cells, (2) early apoptotic cells, and (3) late apoptotic cells. (C) Ba/F3-gp130/IL6R cells were treated for 8 hours with 500 ng/mL doxorubicin or for 2 hours with 100 nM PMA in the presence or absence of the metalloprotease inhibitors GW280264X (3 μM) and GI254023X (3 μM). Inhibitors were added 30 minutes prior to stimulation. Western blot analysis was used as outlined for panel A to monitor levels of sIL6R, IL6R, and β-actin. (D) Ba/F3-gp130/IL6R cells were treated as described in panel C and multiparameter FACS analysis was performed as before. The intensity of IL6R staining was monitored in both viable (gray histograms) and apoptotic (white histograms) cells in the presence or absence of the indicated inhibitors.
Intrinsic induction of apoptosis induces ADAM17-mediated IL6R shedding. (A) Ba/F3-gp130/IL6R cells were treated for 2 hours, 4 hours, and 8 hours with doxorubicin (500 ng/mL) or 2 hours with the phorbol ester PMA (100 nM). Soluble IL6R was immunoprecipitated from conditioned media using the polyclonal anti–serum 6.226 and subsequently visualized by Western blot analysis using the anti-IL6R mAb 14-18. Reciprocal Western blot analysis of cell lysates was performed using mAb 14-18 and an anti–β-actin mAb, which was used as a loading control. (B) Ba/F3-gp130/IL6R cells were treated for 6 hours with doxorubicin, and multiparameter FACS staining was performed with propidium iodide and antibodies against annexin V, and the anti-IL6R (M91 mAb). Histograph assignments are as follows: (1) viable cells, (2) early apoptotic cells, and (3) late apoptotic cells. (C) Ba/F3-gp130/IL6R cells were treated for 8 hours with 500 ng/mL doxorubicin or for 2 hours with 100 nM PMA in the presence or absence of the metalloprotease inhibitors GW280264X (3 μM) and GI254023X (3 μM). Inhibitors were added 30 minutes prior to stimulation. Western blot analysis was used as outlined for panel A to monitor levels of sIL6R, IL6R, and β-actin. (D) Ba/F3-gp130/IL6R cells were treated as described in panel C and multiparameter FACS analysis was performed as before. The intensity of IL6R staining was monitored in both viable (gray histograms) and apoptotic (white histograms) cells in the presence or absence of the indicated inhibitors.
As a positive control, Ba/F3-gp130/IL6R cells were treated for 2 hours with PMA, a well-known inducer of IL6R shedding.27 Doxorubicin-treated Ba/F3-gp130/IL6R cells stained with annexin V, propidium iodide, and with an anti-IL6R antibody were analyzed by flow cytometry. As illustrated in Figure 1B, annexin V–positive cells and annexin V/propidium iodide double-positive cells showed a similar loss of membrane-bound IL6R, indicating that shedding of the IL6R is an early apoptotic event.
Shedding of the IL6R is mediated by the metalloprotease ADAM17
It was previously described that shedding of the IL6R after PMA treatment or cholesterol depletion was mediated by the metalloprotease ADAM17 and to a lesser extent by ADAM10.10 Cotreatment of the doxorubicin-treated Ba/F3-gp130/IL6R cells with the broad spectrum metalloprotease inhibitor marimastat completely blocked generation of the sIL6R (Figure S1C). In an earlier study we had described 2 inhibitors, which differentially blocked the activity of ADAM17 and ADAM10. GW280264X (GW) blocked both ADAM10 and ADAM17, whereas GI254023X (GI) inhibited ADAM10 100 times better than ADAM17.22 Western blot and FACS analysis revealed that IL6R shedding from apoptotic Ba/F3 cells treated with doxorubicin was completely blocked by the ADAM10/ADAM17-specific inhibitor GW280264X but not by the ADAM10-specific inhibitor GI254023X (Figure 1C,D), indicating that ADAM17 was responsible for apoptosis-induced shedding of the IL6R. Neither metalloproteinase inhibitor affected apoptosis, indicating that shedding is not a prerequisite for apoptosis (data not shown).
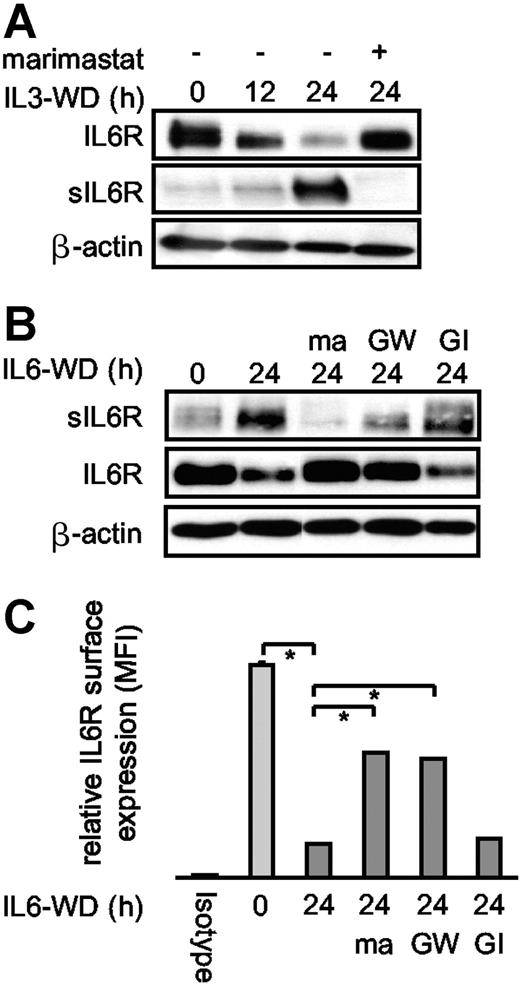
Proliferation of the factor-dependent murine pre-B-cell line Ba/F3-gp130/IL6R can be stimulated by IL6 or IL3. Cytokine withdrawal, like doxorubicin treatment, induces apoptosis via the intrinsic apoptotic pathway. We found that intrinsic apoptosis following cytokine withdrawal induces shedding of the IL6R. In line with data from doxorubicin-treated Ba/F3-gp130/IL6R cells, ectodomain shedding after cytokine withdrawal was completely inhibited by marimastat and almost completely by the ADAM10/ADAM17-specific inhibitor GW280264X but not by the ADAM10-specific inhibitor GI254023X (Figure 2A-C). The residual IL6R shedding after blocking of ADAM10 and ADAM17 might be caused by (a) yet-unidentified protease(s), which also mediates constitutive shedding, or (b) incomplete inhibition of ADAM17 and ADAM10 by the GW280264X inhibitor concentrations used in this experiment, since this could not be observed after PMA- and doxorubicin-induced shedding of the IL6R in Ba/F-gp130-IL6R-cells (for comparison, see Figure 1C).
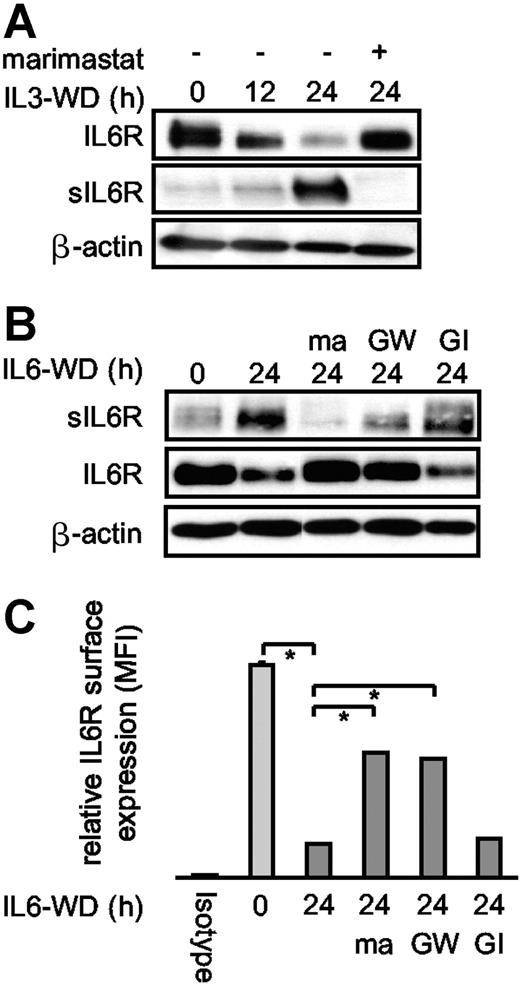
Cytokine deprivation of Ba/F3 cells induced apoptosis and IL6R shedding. (A) IL3-dependent Ba/F3-gp130/IL6R cells were washed 3 times and cultured for 12 hours and 24 hours in cytokine-free medium to induce apoptosis. Control cell cultures were maintained over the 24-hour period in the presence of IL3 (IL3WD, 0 hour; WD, withdrawal). Marimastat (10 μM) was added as indicated. Lysates and immunoprecipitated supernatants were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot with anti-IL6R antibody 14-18. (B) IL6-dependent Ba/F3-gp130/IL6R cells were treated and analyzed as described in panel A. The metalloprotease inhibitors marimastat (10 μM), GI254023X (3 μM), and GW280264X (3 μM) were added as indicated. (C) IL6-dependent Ba/F3-gp130/IL6R cells were treated as described in panel A. Cells were washed and stained with the anti-IL6R antibody M91 and analyzed by flow cytometry. The black bar represents isotype control staining, the light gray bar represents IL6R surface expression of viable cells, and the dark gray bars represent IL6-deprived cells in the presence or absence of the metalloprotease inhibitors described in panel B. Values represent the mean (± SD) from 3 independent experiments (P ≤ .01).
Cytokine deprivation of Ba/F3 cells induced apoptosis and IL6R shedding. (A) IL3-dependent Ba/F3-gp130/IL6R cells were washed 3 times and cultured for 12 hours and 24 hours in cytokine-free medium to induce apoptosis. Control cell cultures were maintained over the 24-hour period in the presence of IL3 (IL3WD, 0 hour; WD, withdrawal). Marimastat (10 μM) was added as indicated. Lysates and immunoprecipitated supernatants were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot with anti-IL6R antibody 14-18. (B) IL6-dependent Ba/F3-gp130/IL6R cells were treated and analyzed as described in panel A. The metalloprotease inhibitors marimastat (10 μM), GI254023X (3 μM), and GW280264X (3 μM) were added as indicated. (C) IL6-dependent Ba/F3-gp130/IL6R cells were treated as described in panel A. Cells were washed and stained with the anti-IL6R antibody M91 and analyzed by flow cytometry. The black bar represents isotype control staining, the light gray bar represents IL6R surface expression of viable cells, and the dark gray bars represent IL6-deprived cells in the presence or absence of the metalloprotease inhibitors described in panel B. Values represent the mean (± SD) from 3 independent experiments (P ≤ .01).
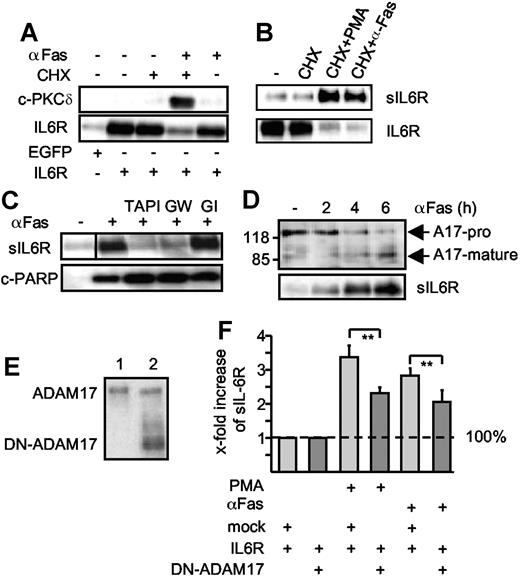
To place these studies in a more physiological context, we next analyzed apoptosis induced by the death receptor Fas. HepG2 cells were treated with cycloheximide in combination with an agonistic anti-Fas antibody to induce apoptosis.28 Apoptosis induction was visualized by the appearance of cleaved caspase 3, PKCδ, and PARP (Figure 3A,C). HepG2 cells were transiently transfected with an expression plasmid coding for the human IL6R. Western blot analysis of apoptotic HepG2 cells in comparison with viable HepG2 cells revealed that the level of membrane-bound IL6R was reduced after Fas stimulation (Figure 3A,B). Again, shedding of the IL6R was completely inhibited by a broad spectrum metalloproteinase inhibitor (TAPI) and by the ADAM10/ADAM17-specific inhibitor GW280264X but not by the ADAM10-specific inhibitor GI254023X (Figure 3C). Since PARP cleavage was not inhibited, blockade of IL6R shedding did not interfere with the progression of apoptosis (Figure 3C). From the fact that cycloheximide treatment of cells did not affect IL6R shedding, we concluded that activation of ADAM17 was not due to enhanced ADAM17 protein synthesis, but to activation of pre-existing ADAM17 molecules. This was further underlined by Western blot analyses showing ADAM17 processing upon apoptosis induction (Figure 3D). Before Fas stimulation, ADAM17 was detected mainly in its unprocessed proform, whereas at the time of onset of IL6R shedding the active mature ADAM17 protein appeared.
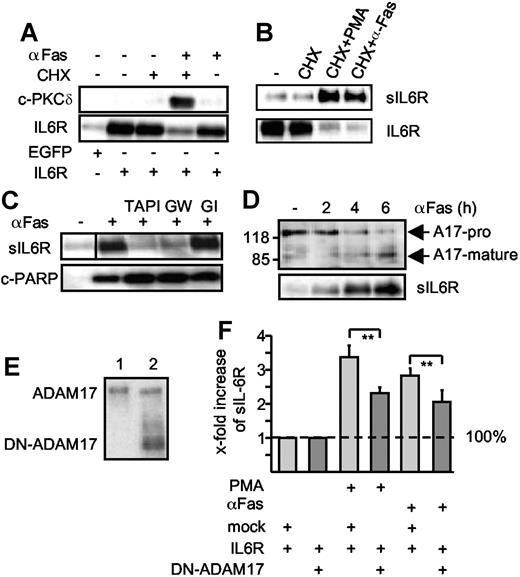
Extrinsic induction of apoptosis induces ADAM17-mediated IL6R shedding. (A) HepG2 cells were transfected with expression plasmids encoding the human IL6R or a control marker protein (EGFP). Cells were treated (6 hours) with cycloheximide (CHX, 100 μM), agonistic anti-Fas antibody CH-11 (αFas, 500 ng/mL), or a combination of cycloheximide and αFas. Cell lysates were prepared and Western blot analysis for PKCδ and IL6R was performed. (B) HepG2 cells were transfected as described in panel A and treated with CHX (100 μM) or CHX (100 μM, 6 hours) in combination with PMA (100 nM, 2 hours) or αFas (500 ng/mL, 6 hours). After stimulation, the sIL6R was immunoprecipitated from supernatants and lysates were prepared from the cell pellets. IL6R and sIL6R were subsequently monitored by Western blot analysis. (C) Apoptosis was induced in the transfected HepG2 cells with CHX alone or in combination with CH-11. The metalloprotease inhibitors TAPI (100 μM), GI254023X (3 μM), or GW280264X (3 μM) were added as indicated 30 minutes prior to stimulation. Western blot analysis of immunprecipitated sIL6R and cleaved PARP (c-PARP) was performed. A vertical line has been inserted to indicate where a gel lane was cut. This gel came from one experiment but irrelevant lanes have been cut out. (D) HepG2 cells were cotreated for 0 hour, 2 hours, 4 hours, and 6 hours with CHX and αFas. ADAM17 was immunoprecipitated from the cell lysates by addition of an anti-ADAM17 polyclonal antibody (3 μg) before detection by Western blot. Release of sIL6R was monitored as before. A17-pro indicates proform of ADAM17; A17-mature, mature form of ADAM17. (E) Western blot of membrane fractions of HepG2 cells expressing p409 empty vector (lane 1) or a dominant-negative ADAM17 mutant that lacks the pro- and catalytic domain (DN-ADAM17, lane 2). (F) HepG2 cells were transiently transfected with IL6R cDNA together with empty p409 vector or p409-DN-ADAM17 plasmid. After 48 hours, cells were treated with CHX (100 μM, 6 hours) in presence of DMSO (vehicle control, 6 hours) to monitor baseline shedding, PMA (100 nM, 2 hours) or αFas for 6 hours. The sIL6R protein in the culture media was quantified using the sIL6R-specific ELISA. The fold increase of the sIL6R is expressed as a percentage of baseline shedding (set to 100%). Baseline shedding of IL6R/mock-transfected cells was 5549.07 (± 1626.3) pg/mL and of IL6R/DN-TACE 3068.84 (± 744) pg/mL. Values represent the mean (± SD) from 3 independent experiments (*P ≤ .001 and **P ≤ .01).
Extrinsic induction of apoptosis induces ADAM17-mediated IL6R shedding. (A) HepG2 cells were transfected with expression plasmids encoding the human IL6R or a control marker protein (EGFP). Cells were treated (6 hours) with cycloheximide (CHX, 100 μM), agonistic anti-Fas antibody CH-11 (αFas, 500 ng/mL), or a combination of cycloheximide and αFas. Cell lysates were prepared and Western blot analysis for PKCδ and IL6R was performed. (B) HepG2 cells were transfected as described in panel A and treated with CHX (100 μM) or CHX (100 μM, 6 hours) in combination with PMA (100 nM, 2 hours) or αFas (500 ng/mL, 6 hours). After stimulation, the sIL6R was immunoprecipitated from supernatants and lysates were prepared from the cell pellets. IL6R and sIL6R were subsequently monitored by Western blot analysis. (C) Apoptosis was induced in the transfected HepG2 cells with CHX alone or in combination with CH-11. The metalloprotease inhibitors TAPI (100 μM), GI254023X (3 μM), or GW280264X (3 μM) were added as indicated 30 minutes prior to stimulation. Western blot analysis of immunprecipitated sIL6R and cleaved PARP (c-PARP) was performed. A vertical line has been inserted to indicate where a gel lane was cut. This gel came from one experiment but irrelevant lanes have been cut out. (D) HepG2 cells were cotreated for 0 hour, 2 hours, 4 hours, and 6 hours with CHX and αFas. ADAM17 was immunoprecipitated from the cell lysates by addition of an anti-ADAM17 polyclonal antibody (3 μg) before detection by Western blot. Release of sIL6R was monitored as before. A17-pro indicates proform of ADAM17; A17-mature, mature form of ADAM17. (E) Western blot of membrane fractions of HepG2 cells expressing p409 empty vector (lane 1) or a dominant-negative ADAM17 mutant that lacks the pro- and catalytic domain (DN-ADAM17, lane 2). (F) HepG2 cells were transiently transfected with IL6R cDNA together with empty p409 vector or p409-DN-ADAM17 plasmid. After 48 hours, cells were treated with CHX (100 μM, 6 hours) in presence of DMSO (vehicle control, 6 hours) to monitor baseline shedding, PMA (100 nM, 2 hours) or αFas for 6 hours. The sIL6R protein in the culture media was quantified using the sIL6R-specific ELISA. The fold increase of the sIL6R is expressed as a percentage of baseline shedding (set to 100%). Baseline shedding of IL6R/mock-transfected cells was 5549.07 (± 1626.3) pg/mL and of IL6R/DN-TACE 3068.84 (± 744) pg/mL. Values represent the mean (± SD) from 3 independent experiments (*P ≤ .001 and **P ≤ .01).
A dominant-negative ADAM17 variant (DN-ADAM17) lacking the metalloprotease domain has been described.23 After cotransfection of expression plasmids coding for the DN-ADAM17 variant and the IL6R, PMA-induced as well as apoptosis-induced IL6R shedding was reduced by 31% and 28%, respectively, supporting the view that ADAM17 is responsible for IL6R shedding (Figure 3E-F). Of note, IL6R shedding was not reduced to background level. This might be explained by the fact that the amount of DN-ADAM17 did not largely exceed the amount of endogenous ADAM17 in our experiments.
ADAM17 activation during apoptosis is caspase dependent but PKC independent
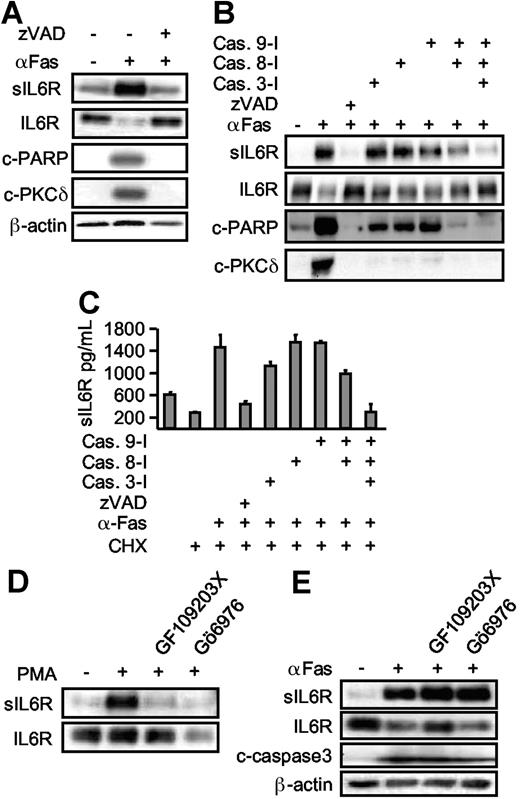
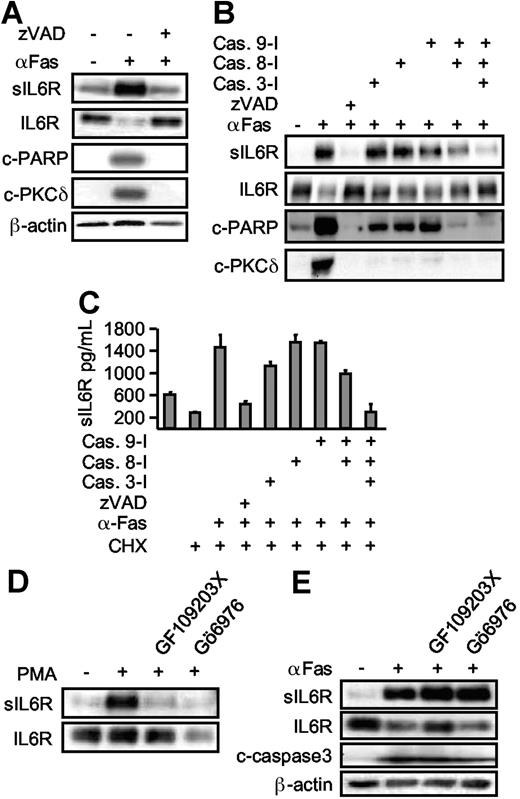
Caspases are major intracellular effectors of intrinsic and extrinsic apoptosis. To establish a link between caspase 3 activation and IL6R shedding, zVAD-fmk (a broad spectrum caspase inhibitor) was applied 30 minutes prior to cycloheximide and Fas treatment. Inclusion of zVAD-fmk prevented apoptosis and inhibited the processing of the caspase 3 substrates PARP and PKCδ (Figure 4A). At the same time, shedding of the IL6R was completely abrogated, indicating that caspase activation was required for ADAM17 activation (Figure 4A). Next, we used specific inhibitors for the initiator proteins caspase 8 and caspase 9, and for the effector protein caspase 3.29 Inhibition of none of the caspases (3, 8, or 9) alone resulted in appreciable blockade of IL6R release. Combined inhibition of caspase 8 and caspase 9 reduced IL6R shedding, however multiple inhibition of caspases 3, 8, and 9 completely abolished IL6R shedding (Figure 4B,C). These experiments confirm that upon induction of apoptosis, caspases 3, 8, and 9 are all required to mediate ADAM17 activation and IL6R shedding.
Apoptosis-induced shedding of the IL6R is caspase dependent, but PKC independent. (A) HepG2 cells expressing hIL6R were treated (6 hours) with CHX alone or in combination with αFas. The pan-caspase inhibitor zVAD-fmk (100 μM) was added as indicated. Western blot analysis of immunoprecipitated sIL6R was compared with an analysis of PARP (c-PARP, cleaved PARP), PKCδ (c-PKCδ, cleaved PKCδ), and β-actin. (B) HepG2 cells were treated as described in panel A. The inhibitor (each at 100 μM) for caspase 3 (zDEDV-fmk), caspase 8 (zIETD-fmk), and caspase 9 (zLEHD-fmk) was added 30 minutes prior to αFas stimulation. Western blot analysis was performed as before. (C) ELISA analysis of sIL6R production in the presence of caspase inhibitors. Values represent the mean (± SD) from 3 independent experiments. (D) HepG2 cells expressing hIL6R were incubated with PMA for 2 hours or preincubated with the pan-PKC inhibitor GF109203X (5 μM) or the inhibitor of Ca2+-dependent PKC Gö6976 (1 μM). Levels of immunoprecipitated sIL6R and cellular IL6R were determined by Western blot. (E) Comparative analysis of αFas-treated HepG2 cells in the presence of GF109203X (5 μM) and Gö6976 (1 μM).
Apoptosis-induced shedding of the IL6R is caspase dependent, but PKC independent. (A) HepG2 cells expressing hIL6R were treated (6 hours) with CHX alone or in combination with αFas. The pan-caspase inhibitor zVAD-fmk (100 μM) was added as indicated. Western blot analysis of immunoprecipitated sIL6R was compared with an analysis of PARP (c-PARP, cleaved PARP), PKCδ (c-PKCδ, cleaved PKCδ), and β-actin. (B) HepG2 cells were treated as described in panel A. The inhibitor (each at 100 μM) for caspase 3 (zDEDV-fmk), caspase 8 (zIETD-fmk), and caspase 9 (zLEHD-fmk) was added 30 minutes prior to αFas stimulation. Western blot analysis was performed as before. (C) ELISA analysis of sIL6R production in the presence of caspase inhibitors. Values represent the mean (± SD) from 3 independent experiments. (D) HepG2 cells expressing hIL6R were incubated with PMA for 2 hours or preincubated with the pan-PKC inhibitor GF109203X (5 μM) or the inhibitor of Ca2+-dependent PKC Gö6976 (1 μM). Levels of immunoprecipitated sIL6R and cellular IL6R were determined by Western blot. (E) Comparative analysis of αFas-treated HepG2 cells in the presence of GF109203X (5 μM) and Gö6976 (1 μM).
We next asked whether PKCs are involved in IL6R shedding during apoptosis, because PKCs are known to activate ADAM17 after PMA treatment and PKCs are known downstream targets of caspases. Here, we used a nonselective PKC inhibitor (GF109203X [5 μM]) and a selective PKCα/β inhibitor (Gö6976 [1 μM]). Both inhibitors effectively blocked PMA-induced, but not Fas-induced, shedding (Figure 4D-E), suggesting that upon apoptosis induction, ADAM17 was activated in a PKC-independent manner. As expected, inhibition of PKCs did not inhibit caspase 3 activation, since PKCs are believed to act downstream of caspase 3.
IL6R shedding is not required to terminate IL6-induced STAT3 phosphorylation during apoptosis but can induce IL6 trans-signaling in neighboring nonapoptotic cells
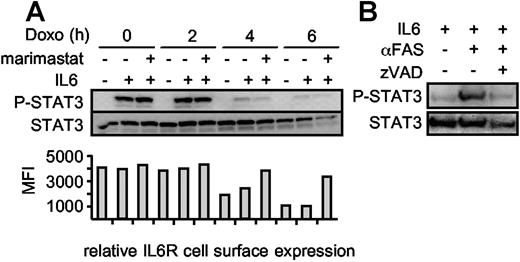
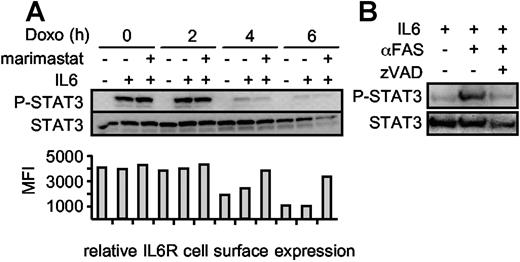
We treated Ba/F3-gp130/IL6R cells with doxorubicin in the presence or absence of marimastat for 0 hour, 2 hours, 4 hours, and 6 hours and subsequently stimulated the cells for 10 minutes with IL6 to induce STAT3 phosphorylation. Upon induction of apoptosis, the cells became less susceptible to IL6 stimulation as indicated by decreasing STAT3 phosphorylation after 4 hours, whereas at that time point the overall level of STAT3 did not change (Figure 5A). After 6 hours, the STAT3 protein content seemed to be slightly reduced, which was very likely caused by progression of apoptosis. Blocking IL6R shedding during apoptosis did not increase the amount of phosphorylated STAT3, indicating that the role of IL6R shedding is not termination of STAT3 activation. Therefore, we hypothesized that the role of IL6R shedding during apoptosis might rather be to stimulate surrounding nonapoptotic cells via IL6 trans-signaling. To test this, we used conditioned supernatant from viable and apoptotic HepG2 cells to stimulate Ba/F3-gp130 cells. These cells can induce STAT3 phosphorylation only following stimulation with IL6 in the presence of the sIL6R.20 As shown in Figure 5B, only conditioned supernatant from apoptotic HepG2 cells induced STAT3 phosphorylation in Ba/F3-gp130 cells, indicating that the sIL6R generated during apoptosis is biologically active and can promote IL6 trans-signaling.
STAT3 phosphorylation is abrogated during apoptosis. (A) Ba/F3-gp130/IL6R cells were washed 3 times and incubated for 0 hour, 2 hours, 4 hours, and 6 hours with 500 ng/mL doxorubicin in serum-free medium in the absence of IL6. The metalloprotease inhibitor marimastat was added as indicated to prevent IL6R shedding at a concentration of 10 μM. After the incubation period, the cells were centrifuged and resuspended in medium containing IL6 (200 ng/mL) or without IL6 as control for 10 minutes. The cells were immediately lysed and the proteins were immunoblotted with an anti–p-STAT3 antibody. Reprobing with an anti-STAT3–specific antibody ensured comparable protein loading. The relative IL6R cell surface expression was analyzed by flow cytometry (B) Supernatants of viable, αFas (CH-11)–treated and αFas + zVAD-fmk–treated HepG2 cells expressing the hIL6R were supplemented with 200 ng/mL IL6. After an incubation period of 30 minutes, the supernatants were used for stimulation of Ba/F3-gp130 cells for 10 minutes. Detection of p-STAT3/STAT3 was performed as described in panel A.
STAT3 phosphorylation is abrogated during apoptosis. (A) Ba/F3-gp130/IL6R cells were washed 3 times and incubated for 0 hour, 2 hours, 4 hours, and 6 hours with 500 ng/mL doxorubicin in serum-free medium in the absence of IL6. The metalloprotease inhibitor marimastat was added as indicated to prevent IL6R shedding at a concentration of 10 μM. After the incubation period, the cells were centrifuged and resuspended in medium containing IL6 (200 ng/mL) or without IL6 as control for 10 minutes. The cells were immediately lysed and the proteins were immunoblotted with an anti–p-STAT3 antibody. Reprobing with an anti-STAT3–specific antibody ensured comparable protein loading. The relative IL6R cell surface expression was analyzed by flow cytometry (B) Supernatants of viable, αFas (CH-11)–treated and αFas + zVAD-fmk–treated HepG2 cells expressing the hIL6R were supplemented with 200 ng/mL IL6. After an incubation period of 30 minutes, the supernatants were used for stimulation of Ba/F3-gp130 cells for 10 minutes. Detection of p-STAT3/STAT3 was performed as described in panel A.
sIL6R is released from apoptotic primary human neutrophils and has the capacity to direct transition from neutrophil to mononuclear cell infiltration during acute inflammation
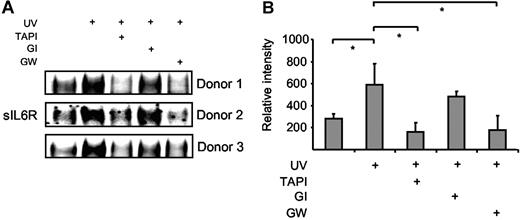
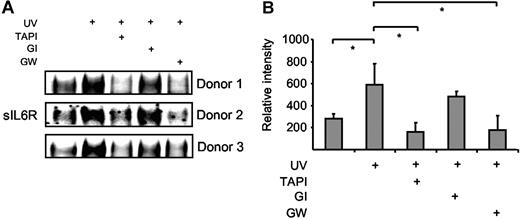
Neutrophils undergo apoptosis during resolution of inflammation, which is regulated by proapoptotic mediators such as TNFα and Fas ligand.30 Human neutrophils were isolated from blood of healthy donors and apoptosis was subsequently induced by Fas stimulation, UV irradiation, or doxorubicin treatment. Fas stimulation and UV treatment induced apoptosis of neutrophils with 45% and 90% of the cells being annexin V positive after 6 hours (Figure S2A).31 Cell culture supernatants were prepared 4 hours after Fas stimulation or UV irradiation, and the levels of sIL6R were assessed by Western blotting. As indicated in Figure 6A-B, sIL6R was shed from apoptotic neutrophils after UV irradiation to a larger extent than from untreated cells. Fas stimulation of neutrophils yielded comparable results (data not shown). The generation of sIL6R during apoptosis could be inhibited by TAPI (10 μM) and by the ADAM10/ADAM17-specific inhibitor GW280264X (3 μM) but not by the ADAM10-specific inhibitor GI254023X (3 μM) (Figure 6A-B). These data indicate that also in primary human neutrophils ADAM17, and not ADAM10, is responsible for the shedding of the IL6R during apoptosis.
Human neutrophils release IL6R upon induction of apoptosis. Peripheral blood neutrophils from 3 different donors were adjusted to 106 cells/mL and were UV treated (200 000 μJ/cm2) or left untreated for 6 hours. The metalloprotease inhibitors TAPI (10 μM), GI254023X (3 μM), or GW280264X (3 μM) were added 30 minutes prior to UV stimulation. (A) Immunoprecipitated sIL6R was analyzed by Western blot as before. (B) Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD), and relative values represent the mean (± SD; *P ≤ .05) from the 3 independent donor experiments shown in panel A.
Human neutrophils release IL6R upon induction of apoptosis. Peripheral blood neutrophils from 3 different donors were adjusted to 106 cells/mL and were UV treated (200 000 μJ/cm2) or left untreated for 6 hours. The metalloprotease inhibitors TAPI (10 μM), GI254023X (3 μM), or GW280264X (3 μM) were added 30 minutes prior to UV stimulation. (A) Immunoprecipitated sIL6R was analyzed by Western blot as before. (B) Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD), and relative values represent the mean (± SD; *P ≤ .05) from the 3 independent donor experiments shown in panel A.
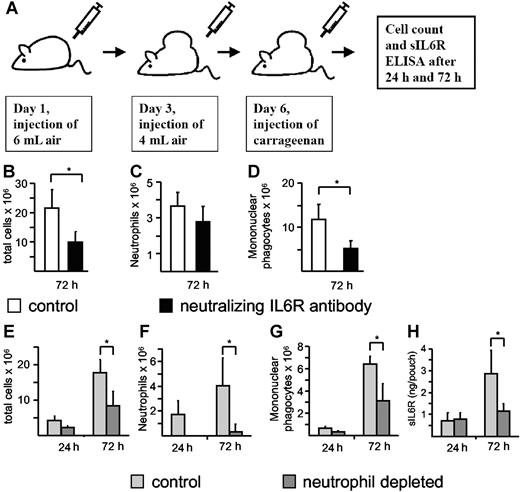
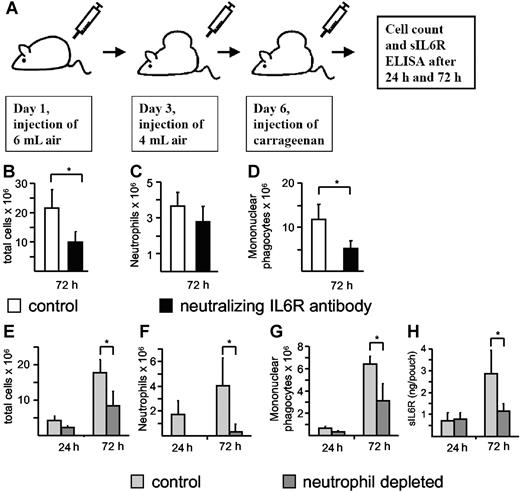
During acute inflammation, resolution of the neutrophil infiltrate is associated with a subsequent recruitment of mononuclear cells.5 It has been described previously that neutralization of the IL6R reduced the infiltration of immune cells in the murine carrageenan air pouch model of acute inflammation, but it was not demonstrated whether this reduction affected neutrophilic or mononuclear phagocytic cells.32,33 To demonstrate the importance of IL6R signaling during acute inflammation and to discriminate between the neutrophil and mononuclear cell population, we also used the air pouch model (Figure 7A). Therefore, we tested whether neutralization of the IL6R inhibited the infiltration of neutrophils or mononuclear phagocytic cells or both. A neutralizing antimurine IL6R antibody (100 μg) was administered 6 hours before carrageenan injection. The mice were killed 72 hours after induction of the acute inflammation, and total cell numbers and cell numbers of neutrophils and mononuclear phagocytes were determined. As depicted in Figure 7B-D, neutralization of IL6R resulted in a marked decrease of total cell infiltration, especially of mononuclear cells and to a lesser extent of neutrophils. These results indicate that the IL6R plays a major role in the recruitment of mononuclear cells during an acute inflammation. It should be noted that we cannot exclude at this moment that the nonsignificant reduction of neutrophil infiltration in the anti-IL6R antibody–treated animals is due to a dispensable role of IL6 signaling for initial neutrophil infiltration or due to a suboptimal dose of the applied neutralizing antibody. However, it has been shown in a peritonitis model of acute inflammation that the transitional event, which is characterized by the switch from the neutrophil-dominated phase to the mononuclear cell–dominated phase, relies on the sIL6R and thereby on IL6 trans-signaling.5 Consequently, we reasoned that liberation of sIL6R from apoptotic neutrophils would direct this process. Using this approach, we observed high numbers of infiltrating neutrophils at 24 hours and 72 hours after injection of carrageenan, with increased mononuclear phagocytic cells at 72 hours (Figure 7E-G). These changes were associated with increased detection of sIL6R in air pouch lavage fluid (Figure 7H). Significantly, antibody-mediated depletion of circulating neutrophils not only reduced neutrophil infiltration, but also resulted in a concomitant decrease in both local sIL6R levels and mononuclear phagocytic cell numbers (Figure 7E-H). These data suggest that infiltrating neutrophils are the primary source of sIL6R, and furthermore support the notion that the initial neutrophil infiltration is required to promote mononuclear cell recruitment.
Neutrophil depletion during acute inflammation leads to a reduction of sIL6R. (A) Scheme of the air pouch model of local inflammation in C57/BL6 mice as outlined in “Materials and methods.” IL6R neutralization was induced with the anti-mIL6R D7715A7 mAB (100 μg/mouse), which was administered (intraperitoneally) 6 hours before carrageenan injection. Seventy-two hours after carrageenan injection, total cell numbers (B) and cell numbers of neutrophils (C) and mononuclear phagocytes (D) were determined. Values represent the mean (± SD) from 5 different mice (*P ≤ .05). Neutrophil depletion was induced with the Ly6G mAB, which was administered (intraperitoneally) 18 hours before carrageenan injection. Twenty-four hours and 72 hours after carrageenan injection, total cell numbers (E) and cell numbers of neutrophils (F) and mononuclear phagocytes (G) were determined. sIL6R (H) was quantified using ELISA. Values represent the mean (± SD) from 4 different mice (*P ≤ .05).
Neutrophil depletion during acute inflammation leads to a reduction of sIL6R. (A) Scheme of the air pouch model of local inflammation in C57/BL6 mice as outlined in “Materials and methods.” IL6R neutralization was induced with the anti-mIL6R D7715A7 mAB (100 μg/mouse), which was administered (intraperitoneally) 6 hours before carrageenan injection. Seventy-two hours after carrageenan injection, total cell numbers (B) and cell numbers of neutrophils (C) and mononuclear phagocytes (D) were determined. Values represent the mean (± SD) from 5 different mice (*P ≤ .05). Neutrophil depletion was induced with the Ly6G mAB, which was administered (intraperitoneally) 18 hours before carrageenan injection. Twenty-four hours and 72 hours after carrageenan injection, total cell numbers (E) and cell numbers of neutrophils (F) and mononuclear phagocytes (G) were determined. sIL6R (H) was quantified using ELISA. Values represent the mean (± SD) from 4 different mice (*P ≤ .05).
Discussion
Programmed cell death or apoptosis is essential in multicellular organisms for development, tissue turnover, and host defense. Here we show that the induction of intrinsic and extrinsic apoptotic pathways leads to an activation of the metalloproteinase ADAM17, which subsequently leads to IL6R shedding. Although shedding of the IL6R is an early apoptotic process, blockade of ADAM17 did not inhibit apoptosis, indicating that ADAM17 activity is not required for apoptosis.
We demonstrate that, most likely cleavage of the prodomain of ADAM17 is involved in ADAM17 activation, since the amount of processed ADAM17 protein increased during apoptosis. ADAM17 has been found to be transcriptionally up-regulated and to be translocated to the cell membrane during apoptosis.17,34 We detected increased IL6R shedding in apoptotic HepG2 cells treated with the protein synthesis inhibitor cycloheximide. In HepG2 cells, we could therefore exclude that ADAM17 protein synthesis was needed for apoptosis-induced shedding of the IL6R. Studies with transfected COS-7 cells revealed that only a minor fraction of ADAM17 protein was present at the cell surface, with the majority being localized to a perinuclear region,35 and being translocated to the cell surface upon activation.36
Induction of apoptosis requires a complex interplay of signaling molecules, receptors, and enzymes. Activation of the caspase cascade comprising initiator caspases, such as caspase 8 and caspase 9, and executor caspases, such as caspase 3, finally led to apoptosis characterized by phosphatidylserine exposure, DNA fragmentation, and apoptotic body formation.14 We show here that ADAM17 activation is dependent on the combined activation of caspases 8, 9, and 3 but independent of PKC, a known mediator of PMA-induced shedding. Even though blocking of individual caspases did not significantly reduce IL6R shedding, combined inhibition blocked shedding of the IL6R. Although we cannot rule out nonspecific effects of the individual caspase inhibitors, our results point to a so-far-unidentified common downstream target of caspases 3, 8, and 9 involved in governing ADAM17 activity.
Neutrophils are characterized by a short half-life that is extended in the inflamed tissue by proinflammatory cytokines, such as IL6, granulocyte-macrophage colony-stimulating factor, interleukin 8 (IL8), and Gro-α, and their contact with lipopolysaccharides.30,37 Interestingly, the amount of apoptotic neutrophils after Fas stimulation was not altered in the presence of IL6 or IL6/sIL6R (Figure S2A). Our finding indicates that during apoptosis sIL6R-shedding does not necessarily render the cells unresponsive to IL6 but it rather seems to induce IL6 trans-signaling in neighboring nonapoptotic cells. This was further highlighted by the finding that during apoptosis, the mRNA of the negative signaling protein SOCS3 was up-regulated in neutrophils and gp130 was down-regulated in hepaocytes, indicating the induction of a negative feedback loop for gp130 signaling.34,38 Therefore, ADAM17-mediated IL6R shedding might be an additional mechanism by which ADAM17 can potentially promote the resolution of inflammation.
There is increasing evidence that apoptosis plays an important role during acute inflammation that is stimulated by agonists such as TNFα and Fas ligand.30,39 Whereas macrophages are important for the resolution of inflammation, neutrophils are essential for initiation and execution of the acute inflammatory response and are the predominant immune cells in the early stage of infection. Although the inflammatory response is highly beneficial to the host, coordinated termination is critical to limit tissue damage.40 Neutrophil apoptosis and uptake by macrophages likely facilitate resolution of inflammation. Initial studies in IL6-deficient mice suggest that IL6 is involved in this process,41 however the mechanism underlining this involvement remains unknown.
It has been reported that in apoptotic neutrophils ADAM17 expression was up-regulated17 and that airway neutrophils are activated by bacteria leading to the release of sIL6R possibly via ADAM17 activation.42 Our data now make us speculate that induction of apoptosis, irrespective of the activation stimuli, will lead to activation of the metalloproteinase ADAM17, resulting in enhanced IL6R shedding from the neutrophil surface.
Appropriate control of the inflammatory infiltrate is reliant upon the coordinated regulation of inflammatory chemokines together with a balanced apoptotic clearance of immune cells. Previously, we and others have shown that IL6 trans-signaling in stromal cells including endothelial cells, mesothelial cells, smooth muscle cells, and fibroblasts differentially regulates the expression of inflammatory chemokines including CXCL8/IL8 and CCL2/MCP1.5,43–46 Through clinical evaluation of local sIL6R levels in various inflammatory conditions, it is evident that a close association exists between the number of infiltrating neutrophils and sIL6R levels.5 In this context, IL6R shedding from neutrophils has been associated with activation by C-reactive protein, chemokines, and other chemotactic agents,5,9,47,48 whereas gp130 is not subject to proteolysis by ADAM proteases.49 In this present report, we offer an alternative mechanism whereby apoptosis-induced shedding of IL6R from the neutrophil surface facilitates formation of an IL6/sIL6R complex and directs IL6 trans-signaling presumably on endothelial cells to promote recruitment of mononuclear phagocytic cells involved in the nonphlogistic removal of apoptotic neutrophils. Thus, shedding processes in apoptotic neutrophils may have profound effects on the outcome of the inflammatory response. Notably, such a cascade of neutrophils and mononuclear cells has been shown to be nonfunctional in IL6−/− mice.5
Since ADAM17 is responsible not only for shedding of the IL6R but has also been implicated in the cleavage of, for example, TNFα, TGFα, and various adhesion molecules as well as of some cytokine and growth factor receptors,50 it will be interesting to analyze whether all these substrates are also shed upon activation of this protease after induction of apoptosis. Therefore, further experiments will be needed to fully understand the importance of this metalloprotease activation step for the progress and outcome of immune responses. Such consequences might include altered adhesion, rolling, and attraction of neutrophils as well as changes in the activation pattern of other immune cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany to J.S. and S.R.-J. (SFB415, Project B5).
We thank Stefanie Schnell for excellent technical assistance. We thank Ulrich Klostermeyer, Inken Beck, and Hilmar Lemke (Department of Biochemistry, Christian-Albrechts-University, Kiel, Germany) for stimulating discussions and technical support. Andreas Ludwig (Institut für Kardiovaskuläre Molekularbiologie, Technische Hochschule Aachen, Germany) and David Becherer (Glaxo Smith Kline) are acknowledged for the generous gift of the metalloprotease inhibitors GI254023X, GW280264X, and marimastat. We also thank Christian Idel (Institute of Medical Microbiology and Hygiene, University of Lübeck, Germany) for skillful technical help and discussions.
Authorship
Contribution: A.C. and B.R. performed the experiments; K.P., H.L., T.L., C.A.F., and S.A.J. contributed techniques and materials and analyzed data; S.R.-J. and J.S. designed the study and wrote the paper; all authors checked the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Rose-John, Department of Biochemistry, Christian-Albrechts-University, Kiel, Olshausenstrasse 40, D-24098 Kiel, Germany; e-mail: rosejohn@biochem.uni-kiel.de.