Abstract
The hypoxia-inducible factor (HIF) pathway is crucial in mitigating the deleterious effects of oxygen deprivation. HIF-α is an essential component of the oxygen-sensing mechanisms and under normoxic conditions is targeted for degradation via hydroxylation by HIF–prolyl hydroxylases. Several HIF–prolyl hydroxylase inhibitors (PHIs) induced erythropoietin (epo) expression in vitro and in mice, with peak epo expression ranging from 5.6- to 207-fold above control animals. Furthermore, several PHIs induced fetal hemoglobin (HbF) expression in primary human erythroid cells in vitro, as determined by flow cytometry. One PHI, FG-2216, was further tested in a nonhuman primate model without and with chronic phlebotomy. FG-2216 was orally bioavailable and induced significant and reversible Epo induction in vivo (82- to 309-fold at 60 mg/kg). Chronic oral dosing in male rhesus macaques was well tolerated, significantly increased erythropoiesis, and prevented anemia induced by weekly phlebotomy. Furthermore, modest increases in HbF-containing red cells and reticulocytes were demonstrated by flow cytometry, though significant increases in HbF were not demonstrated by high-pressure liquid chromatography (HPLC). HIF PHIs represent a novel class of molecules with broad potential clinical application for congenital and acquired anemias.
Introduction
The hypoxia-inducible factor (HIF) pathway plays a protective role in regulating genes that mitigate the effects of low oxygen tension. Under normoxic conditions, oxygen-sensitive HIF-α isoforms are rendered inactive via proline hydroxylation by HIF-specific prolyl hydroxylases (HIF-PHs), which lead to binding of von Hippel–Lindau protein and targeted degradation through the ubiquitin-proteasome pathway. Under hypoxic conditions, where less oxygen substrate is available for proline hydroxylation by HIF-PHs, HIF-α isoforms are stabilized, heterodimerize with HIF-β, and translocate to the nucleus where they bind to hypoxia-responsive element (HRE) motifs.1–3 In cooperation with other transcriptional coactivators, HIF induces transcription of genes that ameliorate the effects of hypoxia, including EPO and its receptor, transferrin and its receptor, glucose transporters and glycolytic enzymes, and vascular endothelial growth factor (VEGF).4
A relationship between fetal hemoglobin (HbF) levels and hypoxia has been reported for nearly half a century: increased HbF levels are associated with intrauterine hypoxia,5 maternal smoking,6 postnatal hypoxemia from congenital heart disease,7,8 and anemia of prematurity.9 Additionally, infants born at high altitude demonstrate enhanced erythropoiesis and elevated HbF levels compared with infants born at sea level.10 Evidence for postnatal induction of HbF through a hypoxia pathway also exists in several species. Camelids adapt to hypoxia through increased fetal hemoglobin levels, with adult alpacas demonstrating HbF levels of 55% at high altitude.11 In young baboons, significant increases in HbF levels occurred after phenylhydrazine induced hemolysis or hypobaric hypoxia.12 While the magnitude of the HbF response may be genetically determined,13 HbF levels could be maintained long-term by continued erythropoietic stress.14 Indeed, a relationship between the HIF pathway and HbF expression has been proposed recently, and putative HIF-binding sites have been described in the locus control region of the globin gene cluster.15 Thus modulating HIF-α, the critical and labile subunit(s) in the HIF pathway orchestrating the response to hypoxia, represents a new direction to investigate for HbF induction.
Stabilization of HIF-α through inhibition of HIF-PHs may have therapeutic potential in the treatment of the β-hemoglobinopathies. For example, the hypoxic environment during fetal development is protective for individuals with sickle cell anemia; however, following the transition to normoxia at birth, fetal hemoglobin levels fall with a gradual replacement of the γ-globin chain by the abnormal β-globin chain, rendering the pathologic hemoglobin (Hb) tetramer prone to polymerization upon deoxygenation. The polymerized Hb leads to impaired deformability and sickling of red blood cells, which lodge in end arterioles, producing the classic and most prominent feature of the disorder, repeated vasoocclusive crises. Individuals who coinherit mutations resulting in hereditary persistence of fetal hemoglobin (HPFH) are partially or completely protected from the consequences the β-globin disorders, including sickle cell anemia.16,17 The protection afforded by HPFH in the β-thalassemias and hemoglobinopathies has maintained HbF induction as a treatment goal.
A series of HIF PH inhibitors (PHIs) have been developed that pharmacologically inhibit HIF-PHs, thereby preventing HIF-α degradation and leading to HIF-dependent transcription.18 There are 3 HIF-PHs that regulate HIF-alpha physiologically in a nonredundant manner.19 PHIs with differential HIF-PH inhibition profiles and unique pharmacokinetic properties that led to HIF-2α isoform stabilization and selectively activate Epo expression were first identified, then screened for their HbF induction potential. This goal of both Epo and HbF induction is supported by several laboratories demonstrating that HbF induction occurs during erythroid differentiation and maturation.14,20,21 Here we present data for a panel of HIF PHIs that functionally activate HIF, lead to elevated epo expression in vitro and in mice, and promote fetal hemoglobin expression in primary human bone marrow cells. One PHI, FG-2216, induces robust erythropoietin and modest fetal hemoglobin in normal rhesus macaques when chronically administered by an oral route.
Materials and methods
The murine and rhesus experiments were approved by the animal care and use committees at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Heart, Lung, and Blood Institute (NHLBI), and Fibrogen.
Induction of epo expression and secretion in vitro
PHIs were generated as previously described.18 1G6 cells are a clonal derivative of Hep3B cells (ATCC, Manassas, VA) and were plated overnight (2.5 × 104 cells/well) in 96-well plates and washed once with Dulbecco modified Eagle medium (DMEM) plus 0.5% FBS prior to incubation with the indicated concentrations of PHIs or vehicle control (0.15% DMSO) for 24 hours. Cell-free culture supernatants were generated by centrifugation of supernatants in a conical-bottom 96-well plate for 5 minutes at 500g. The supernatant was quantitated for epo (R&D Systems, Minneapolis, MN) levels according to the manufacturer's instructions.
Determination of epo induction in vivo in normal mice
Ten-week-old male Swiss Webster mice (Simonson Labs, Gilroy, CA) were dosed intravenously with the indicated PHI at 60 mg/kg via tail vein. Blood samples were taken under general anesthesia 4 hours after dosing and heparinized plasma was collected. Samples were analyzed for epo by enzyme-linked immunosorbent assay (ELISA; R&D System) according to manufacturer's instructions. Circulating levels of plasma epo were quantitated with a human Epo ELISA kit. Epo values represent the mean plus or minus standard deviation (n = 3 mice/cohort).
Differentiation of human erythroid progenitors ex vivo
Human bone marrow–derived CD34+ cells (Cambrex, Walkersville, MD) were induced to undergo erythroid differentiation ex vivo according to a protocol modified from Fibach et al.22 Cells were cultured in 48-well plates in 350 μL of hematopoietic progenitor growth media (Cambrex) containing 50 ng/mL each of GM-CSF, IL-6, and SCF (R&D systems) for 7 days (phase 1). Nonadherent cells were then collected and replated at the same starting cell density in fresh media containing the identical cytokine cocktail plus 50 ng/mL of IL-3, in the absence or presence of 1 unit/mL recombinant human Epo for an additional 7 days (phase 2) prior to analysis of HbF-positive cells by flow cytometry. Concentrations of PHI or vehicle control, with and without hydroxyurea (HU), were added in the last 2 days of the phase 2 culture. High-pressure liquid chromatography (HPLC) was not performed for this screening set of experiments.
Fetal hemoglobin determination
Red cell HbF expression was analyzed by flow cytometry (FACS Calibur or FACScan, BD Biosciences, San Jose, CA) using Tricolor-conjugated monoclonal antibody specific for human HbF or IgG1 isotype control (CalTag, Burlingame, CA) according to manufacturer's directions. Results were expressed as percentage increase in mean fluorescence intensity (MFI) over controls. F+ cells and F+ reticulocytes were determined as previously described.23
For absolute HbF protein levels, 100 μL of EDTA-anticoagulated whole blood from each animal was pelleted, washed twice, and lysed with water, frozen at −20 for 5 minutes, and thawed at 37°C for 5 minutes. After centrifugation and filtering (Ultrafree-MC; Millipore, Bedford, MA), the supernatant was analyzed by HPLC using a PolyCAT A 35 × 4.6-mm column (PolyLC, Columbia, MD). Hemoglobins A and F were each quantified as areas under the curve and confirmed by mass spectroscopy.
Rhesus macaques, protocol for oral administration of FG-2216, and large-volume phlebotomy
Male rhesus macaques (Macaca mulatta), 3 to 6 years of age weighing 4 to 7 kg, were housed and handled according to the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources. Animals were sedated using ketamine for oral gavage with FG-2216 and large-volume phlebotomy.
Dosing of FG-2216, in either 0.5% carboxymethylcellulose (CMC) suspension or capsules, was initiated at 40 mg/kg (3 animals) administered orally twice a week for a minimum of 6 to 8 weeks. The dose was then increased to 60 mg/kg (4 animals) twice a week for another 6 to 8 weeks. Large-volume phlebotomy (15%-20% blood volume/wk) with iron supplementation was then initiated with continued dosing of FG-2216 (4 animals) for an additional 6 to 8 weeks. Three control animals received no drug but were similarly phlebotomized.
Induction of Epo after oral administration of FG-2216 in nonhuman primates
Serial plasma samples were obtained after the first dose of drug at 40 and 60 mg/kg, with time points including 0 (predose), 2, 4, 8, 10, 12, 24, 48, and 96 hours after dosing. Circulating levels of plasma Epo were determined with a human Epo ELISA kit.
Weekly laboratory testing
Blood for complete blood counts (CBCs) and full chemistry panels was obtained prior to the first FG-2216 dosing of each week. CBCs were performed using a Celldyne 3500 (Abbott Laboratories, Abbott Park, IL). Electrolyte, renal, and hepatic panels were determined from serum using Johnson & Johnson Vitros 250 (Biostad, Sainte Julie, QC). Reticulocyte counts were determined by flow cytometry as previously described (BD Biosciences).
Statistics
Statistical analyses were performed using the Student t test, with a P value less than .05 deemed significant.
Results
Induction of HIF-dependent epo expression by PHIs in vitro
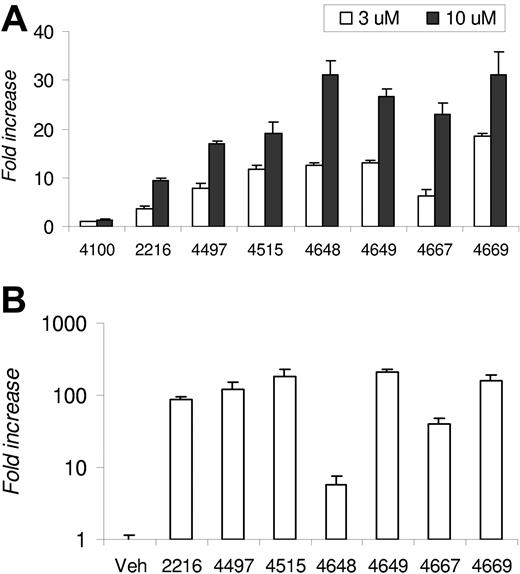
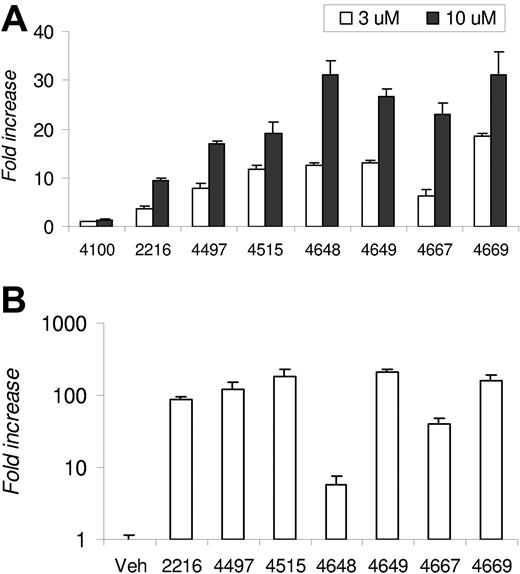
A proprietary library of small-molecule HIF PHIs, which reversibly and differentially inhibit the enzymatic activity of HIF-specific prolyl and asparaginyl hydroxylases, is being developed. The capacity of a select panel of PHIs to stabilize HIF-α and activate HIF transcriptional activity under normoxic conditions was tested in 1G6 cells (data not shown). Since many PHIs induced HIF stabilization, we screened an identical panel of PHIs for their capacity to functionally activate HIF-dependent transcription by examining induction of endogenous epo expression in 1G6 cells, since the parental line (Hep3B) was previously shown to exhibit elevated epo expression in response to hypoxia.24 The epo levels ranged from 3 to 12 mIU/mL in DMSO-treated cell cultures and from 30 to 400 mIU/mL in PHI-treated cultures. Since the experiments were performed on different days, the data were pooled and presented as fold induction above DMSO, which was set at 1. All PHIs except FG-4100 led to dose-dependent epo secretion, with increases ranging from 3.6- to 18.7-fold at the 3-μM dose and 9.3- to 31.0-fold at the 10-μM dose (Figure 1A).
Epo induction by PHIs. (A) Fold increase of epo in 1G6 cells following 24-hour incubation with the indicated PHI at 3 or 10 μM. The data are presented as fold induction above vehicle control (DMSO), which was set at 1. The epo levels observed in DMSO-treated cell cultures ranged from 3 to 12 mIU/mL and from 30 to 400 mIU/mL in PHI-treated cultures. (B) The mean fold increase in logarithmic scale of plasma epo levels in Swiss Webster mice 4 hours after intravenous administration of PHI (n = 3 mice per group). Results are expressed as fold increase over levels determined in vehicle control–treated animals. Error bars are SE.
Epo induction by PHIs. (A) Fold increase of epo in 1G6 cells following 24-hour incubation with the indicated PHI at 3 or 10 μM. The data are presented as fold induction above vehicle control (DMSO), which was set at 1. The epo levels observed in DMSO-treated cell cultures ranged from 3 to 12 mIU/mL and from 30 to 400 mIU/mL in PHI-treated cultures. (B) The mean fold increase in logarithmic scale of plasma epo levels in Swiss Webster mice 4 hours after intravenous administration of PHI (n = 3 mice per group). Results are expressed as fold increase over levels determined in vehicle control–treated animals. Error bars are SE.
Elevation of endogenous epo after PHI administration in normal mice
The PHIs with the capacity to elevate epo expression in vitro were tested for their capacity to elevate circulating plasma epo levels in vivo. Normal mice were administered a single dose of the indicated PHI intravenously at 60 mg/kg via tail vein, and plasma epo levels were determined by human ELISA after 4 hours, which we previously determined was the approximate peak for circulating epo after intravenous dosing. As compared with mice administered vehicle control, PHI dosing led to induction of epo levels that ranged from 5.6- to 207-fold above that observed from cohorts treated with vehicle control (Figure 1B). The absolute epo levels induced in vivo notwithstanding, many pharmacodynamically active PHIs with the capacity to induce epo in vitro and in vivo were identified.
Induction of HbF expression in primary human erythroid progenitors
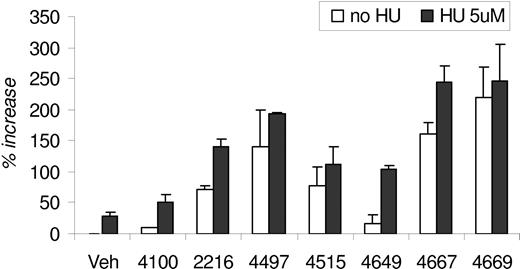
The modified Fibach 2-phase liquid culture system22 was originally developed to recapitulate erythroid differentiation and maturation of primary human erythroid cells. Generally, PHIs had little to modest negative effects on progenitor proliferation. The greatest inhibitory effect was less than 25% during assay optimization using FG-4497 (data not shown). We also determined that PHI addition to phase 1 (Epo-independent phase) showed weak to minimal effects on HbF induction, whereas PHI addition to phase 2 (Epo-dependent phase) was optimal for induction of HbF, as determined by flow cytometry, with minimal effects on cell growth. Due to its antiproliferative effects, HU was routinely added only during the last 48 hours of phase 2 culture, consistent with previous protocols.
The modified Fibach 2-phase liquid culture conditions and HbF protein induction were optimized using different concentrations of FG-4497 with and without HU, measuring the percentage increase in HbF MFI. Flow cytometry was used to screen for the HbF induction potential of each PHI. In the absence of HU, PHI increases in HbF MFI ranged from 9.8% to 220% over vehicle control, with FG-4100 serving as a negative control in addition to the vehicle control. In the presence of HU, 5 PHIs exhibited further HbF induction compared with PHI treated alone (Figure 2). FG-4669 induced maximal induction alone or with HU. The PHIs with the highest capacity to induce HbF were FG-2216, FG-4497, FG-4515, FG-4667, and FG-4669. HPLC was not performed in this screening set of experiments.
HbF induction in vitro by PHIs. HbF expression in erythroid progenitors that were differentiated from primary human CD34+ bone marrow–derived cells. The percentage increase in HbF mean fluorescence intensity is derived by comparison to cells stimulated with vehicle control (0.2% DMSO) without PHI or HU. PHI (20 μM) and HU (5 μM) were added as described in “Differentiation of human erythroid progenitors ex vivo.” Error bars are SE.
HbF induction in vitro by PHIs. HbF expression in erythroid progenitors that were differentiated from primary human CD34+ bone marrow–derived cells. The percentage increase in HbF mean fluorescence intensity is derived by comparison to cells stimulated with vehicle control (0.2% DMSO) without PHI or HU. PHI (20 μM) and HU (5 μM) were added as described in “Differentiation of human erythroid progenitors ex vivo.” Error bars are SE.
FG-2216 reversibly induces endogenous Epo in rhesus macaques
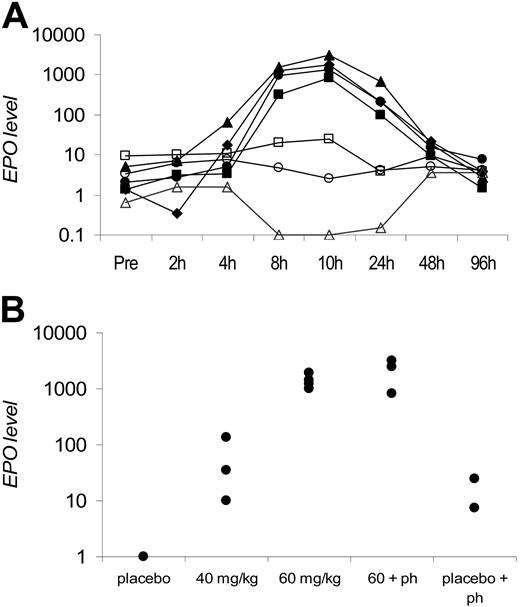
Due to its capacity to induce HbF ex vivo and epo in vivo and the availability of sufficient drug to conduct chronic dosing studies, FG-2216 was selected to test for induction of erythropoiesis and HbF expression in nonhuman primates. To establish a logical dosing regimen for the chronic multidose studies, serial plasma samples were obtained after a single oral dose to measure plasma drug and Epo levels, which were determined by human ELISA. Circulating plasma Epo levels increased as early as 4 hours after oral administration of FG-2216, peaked at approximately 8 to 10 hours, and returned to near baseline by 48 hours (Figure 3A). These findings are consistent with reversible inhibition of HIF-PHs and a transient activation of HIF by FG-2216. Additionally, varying single doses of FG-2216 were tested either alone or in combination with phlebotomy, and the maximal Epo levels induced are shown (Figure 3B).
Epo-induction in rhesus macques. (A) Circulating plasma Epo levels after a single oral dose of FG-2216. The 4 top lines (closed shapes) represent 4 animals receiving a single dose of 60 mg/kg, the middle 2 lines (open shapes) represent phlebotomized animals without drug, and the bottom line (open triangle) represents 1 animal without phlebotomy or drug. Epo levels (mIU/mL) in logarithmic scale are plotted against time points before and after drug dosing. (B) Maximal plasma Epo levels in rhesus macaques after a single oral dose of FG-2216. Each data point represents the peak plasma Epo value from 1 monkey. Treatment groups included placebo (n = 2), FG-2216 at 40 mg/kg (n = 3) or 60 mg/kg (n = 4), 60 mg/kg with phlebotomy (60 + ph; n = 3), or 15% to 20% blood volume phlebotomy alone (placebo + ph; n = 2). Epo levels (mIU/mL) in logarithmic scale are plotted against treatment cohorts.
Epo-induction in rhesus macques. (A) Circulating plasma Epo levels after a single oral dose of FG-2216. The 4 top lines (closed shapes) represent 4 animals receiving a single dose of 60 mg/kg, the middle 2 lines (open shapes) represent phlebotomized animals without drug, and the bottom line (open triangle) represents 1 animal without phlebotomy or drug. Epo levels (mIU/mL) in logarithmic scale are plotted against time points before and after drug dosing. (B) Maximal plasma Epo levels in rhesus macaques after a single oral dose of FG-2216. Each data point represents the peak plasma Epo value from 1 monkey. Treatment groups included placebo (n = 2), FG-2216 at 40 mg/kg (n = 3) or 60 mg/kg (n = 4), 60 mg/kg with phlebotomy (60 + ph; n = 3), or 15% to 20% blood volume phlebotomy alone (placebo + ph; n = 2). Epo levels (mIU/mL) in logarithmic scale are plotted against treatment cohorts.
The Epo levels induced by FG-2216 were much higher than those from 2 phlebotomized rhesus macaques and 1 rhesus administered vehicle control (which showed no time-dependent increase). Oral administration of FG-2216 induced dose-dependent increases in Epo, with maximal Epo levels exceeding 1000 mIU/mL at 60 mg/kg (Figure 3A). Epo induction at 60 mg/kg did not appear to synergize with large-volume phlebotomy (Figure 3B). These data indicate that FG-2216 is a potent and reversible inducer of endogenous Epo in nonhuman primates.
Chronic administration of FG-2216 was well tolerated and induced erythropoiesis in rhesus macaques
For the chronic FG-2216 dosing over many months, an intermittent dosing regimen was tested that would transiently activate HIF yet induce sufficient Epo to prevent apoptosis of erythroid progenitors and promote erythroid maturation.25 Based on the magnitude and duration of Epo elevation after a single oral dose, a protocol was implemented whereby FG-2216 was administered orally on Mondays and Thursdays, which was previously shown to induce erythropoiesis in rodents where the FG-2216 half-life is shorter than in monkeys (Figure 4).
Chronic dosing treatment schema in rhesus macaques. The number of days for each phase of treatment is at least 6 to 8 weeks, except for 3 weeks in the recovery phase.
Chronic dosing treatment schema in rhesus macaques. The number of days for each phase of treatment is at least 6 to 8 weeks, except for 3 weeks in the recovery phase.
All animals tolerated chronic, intermittent oral administration of FG-2216 at 40 or 60 mg/kg. There were no significant changes in serum electrolytes, glucose, lactate dehydrogenase, aspartate amino-transferase (AST), alanine amino-transferase (ALT), albumin, or creatinine levels. There were also no significant changes in the percentages of white blood cell subsets or platelet counts (Table 1).
Within 1 to 2 weeks of FG-2216 dosing, animals began to exhibit reticulocytosis with an average 2- to 3-fold increase over baseline, which was sustained for the entire duration of drug treatment (Table 2). At the end of the study duration, treated animals had higher total hemoglobin levels compared with animals administered vehicle control: baseline was 120 (± 5 g/L; 12.0 ± 0.5 g/dL; 40 mg/kg was 132 (± 8 g/L; 13.2 ± 0.8 g/dL; P = .01), and 60 mg/kg was 148 (± 15 g/L; 14.8 ± 1.5 g/dL; P < .001); absolute hemoglobin increases ranged from 6.5 to 17 g/L (0.65 to 1.7 g/dL) at 40 mg/kg and 8.5 to 37 g/L (0.85 to 3.7 g/dL) at 60 mg/kg g/dL (Figure 5A,B). When weekly phlebotomy was initiated along with concomitant FG-2216 dosing, drug-treated animals maintained higher hemoglobin levels (140 ± 11 g/L [14.0 ± 1.1 g/dL], P < .001) compared with animals administered vehicle control (111 ± 11 g/L [11.1 ± 1.1 g/dL]). Two to 3 weeks after the cessation of drug dosing or phlebotomy, the total hemoglobin levels of all animals returned to baseline.
Hemoglobin increases by PHI. (A) Hemoglobin (g/dL) values are presented with respect to number of days on treatment in 1 rhesus macaque treated with FG-2216 (●) and 1 control animal (○). (B) Hemoglobin levels in treated and control rhesus macaques. In each animal, 2 to 3 hemoglobin values from the last 2 weeks of the treatment period were averaged. The mean hemoglobin values (g/dL) and standard errors shown are derived from all animals in the same treatment cohort. The number of animals for the respective cohorts is as follows: no drug control (n = 2), 40 mg/kg (n = 3; *P = .01), 60 mg/kg (n = 4, **P < .001), and 60 mg/kg with phlebotomy (n = 4, **P < .001).
Hemoglobin increases by PHI. (A) Hemoglobin (g/dL) values are presented with respect to number of days on treatment in 1 rhesus macaque treated with FG-2216 (●) and 1 control animal (○). (B) Hemoglobin levels in treated and control rhesus macaques. In each animal, 2 to 3 hemoglobin values from the last 2 weeks of the treatment period were averaged. The mean hemoglobin values (g/dL) and standard errors shown are derived from all animals in the same treatment cohort. The number of animals for the respective cohorts is as follows: no drug control (n = 2), 40 mg/kg (n = 3; *P = .01), 60 mg/kg (n = 4, **P < .001), and 60 mg/kg with phlebotomy (n = 4, **P < .001).
Chronic dosing with FG-2216 induced a small elevation of HbF expression in vivo
Relative to animals administered vehicle control, FG-2216–treated animals exhibited increases in the percentage of HbF-containing reticulocytes (% F+ retics) 1 to 2 weeks after chronic dosing began (Table 2). These increased levels of F+ retics plateaued at the end of the treatment period. All animals in the same cohort were combined to derive the mean and standard deviation values at baseline, 40 mg/kg, 60 mg/kg, and 60 mg/kg with phlebotomy. When compared with similarly treated control animals, the P values were .098 for animals at 60 mg/kg and .034 for animals at 60 mg/kg with phlebotomy. Phlebotomy and FG-2216 dosing did not appreciably increase the percentage of F+ retics compared with FG-2216 dosing alone. While the increases in F+ retics were modest, the HbF content within red cells appeared to be heterocellular by flow cytometry. Approximately 2 weeks after cessation of FG-2216 dosing, the percentage of F+ retics returned to baseline, indicating the reversibility of its effect on HbF induction.
The percentage of HbF-containing mature red cells (F+ cells) was also examined (Table 2). Three to 4 weeks after initiation of dosing, FG-2216–treated animals showed an increase in the percentage of F+ cells, which also plateaued at the end of the treatment period. When compared with similarly treated control animals, the P values were .07 for animals at 60 mg/kg and .018 for animals at 60 mg/kg with phlebotomy. As in F+ retics, weekly phlebotomy concomitant with FG-2216 dosing did not appreciably increase the percentage of F+ cells compared with animals treated with FG-2216 alone. The absolute amount of hemoglobin subtypes was also analyzed weekly by HPLC. However, the absolute HbF protein was less than 1% at baseline and remained between 1% and 2% within all vehicle and drug-treated cohorts, which was confirmed by mass spectroscopy (data not shown).
Discussion
The role of the HIF pathway in protecting against the consequences of oxygen deprivation is well established, and new tools aimed at HIF-α stabilization and EPO induction are currently being developed for clinical application. Previously, other HIF PHIs18,26–28 have been shown to stabilize HIF-α both in vitro and in vivo, with induction of epo expression in murine liver,27 kidneys,27 and brain.28 In our current studies in 1G6 cells, the select panel of PHIs tested all showed increased in vitro epo expression in a linear and dose-dependent manner. Oral administration of the 7 active PHIs in mice confirmed significant epo induction in circulating plasma, although PHI potency in the 1G6 cell line did not necessarily predict a similar response in the mice, likely due to differences in pharmacokinetics, biodistribution, and metabolism. The effects of these PHIs most resemble the specific erythropoietic stimulus that high altitude would exert in vivo.
Previous reports have suggested a relationship between hypoxia and HbF. Since the PHIs tested in this work increase HIF transcriptional activity and Epo induction, it was logical to screen them for HbF induction in primary human bone marrow CD34+ cells. This panel of PHIs showed a wide range of potency in increasing HbF-containing red cells. When hydroxyurea was added, the HbF effect was augmented in some (FG-2216 and FG-4649) but not in others (FG-4667 and FG-4669). Although these 7 PHIs induced Epo variably, the HbF effect was not directly correlated with Epo induction. PHIs and HU were added in the last 2 days of the phase-2 Fibach culture system (which already contains Epo), thus the PHIs likely do not induce additional Epo. These observations suggest that HbF induction is by 1 or more mechanisms: alternate (ie, non-Epo) pathways, HIF-α stabilization providing additional signals or factors that induce HbF in an Epo-primed erythropoietic environment, or by a hydroxyurea-like effect, since these PHIs have varying degrees of antiproliferative effects on human CD34+ cells. Nevertheless, increasing both Epo and HbF is an important clinical goal because while Epo induction alone is sufficient for renal diseases or myelodysplasia, Epo induction without HbF would lead to rapid accumulation of sickle erythrocytes and precipitate vasoocclusive crises in sickle cell disease and would likely have little therapeutic benefit in β-thalassemia.
Since in vivo HbF induction cannot be sufficiently tested in the murine models, we evaluated the ability of FG-2216 to induce erythropoiesis and HbF expression in rhesus macaques. The animals tolerated single dose and multiple chronic dosing over several months, with no adverse side effects attributable to the drug. The induction of endogenous Epo at both the 40-mg/kg and 60-mg/kg dose were sufficient to elevate total hemoglobin levels with repeated dosing, with Epo induced from 82- to 308-fold by a 60-mg/kg dose over animals administered vehicle control. There was no evidence of Epo desensitization with repeated administration, as evidenced by similarly high Epo levels after the first dose or after subsequent doses administered months later, and repeated administration resulted in sustained and significant increases in reticulocytosis and total hemoglobin level increases ranging from 6.5 to 37 g/L (0.65 to 3.7 g/dL).
The hemoglobin increment achieved by twice weekly oral administration of FG-2216 was robust. When the degree of Epo induced by FG-2216 is compared with a standard dose of recombinant human erythropoietin (rHuEpo; at 100 U/kg of epoetin-α administered intravenously 3 times per week to dialysis patients), the peak Epo levels induced by FG-2216 are below that achieved by rHuEpo.29,30 The increase of up to 37 g/L (3.7 g/dL) in Hb obtained with chronic dosing at the 60-mg/kg dose is consistent with the observed endogenous Epo induction. Additionally, chronic dosing protected against the development of anemia in animals undergoing weekly large-volume phlebotomy.
The safety of chronic PHI administration is of theoretical concern, since long-term activation of HIF-α may have untoward neoplastic effects and several cancers have been shown to express Epo receptor. These small molecules have varying specificity of prolyl or asparaginyl hydroxylase inhibition and biodistribution, and HIF-dependent genes are differentially expressed after various insults (eg, phlebotomy compared with acute renal failure, stroke, or myocardial infarction). Thus selectivity within the HIF transcriptome can be achieved. In this work, PHIs with erythropoietic potential were chosen; other investigators have tested PHIs to reduce fibrosis in chronic heart failure,31 stimulate angiogenesis in lung disease of prematurity,32 and mitigate ischemic injury following acute renal failure.33 To address safety, a full panel of serum chemistries was analyzed over the dosing period and was not significantly changed by chronic FG-2216 dosing, including electrolytes, glucose level, LDH level, liver enzymes, albumin level, and creatinine level, arguing against broad, nonspecific effects. Additionally, the peripheral blood effects were confined to the erythroid component of hematopoiesis, as the other hematopoietic lineages were unchanged. In fact, no adverse effects were observed throughout the entire 6-month dosing period and no interruptions of drug dosing were required during the study. As neither high altitude nor hypoxia in utero is sufficient to induce carcinogenesis, there are likely additional acquired defects necessary for cancer development related to HIF-α expression. Finally, HIF-α stabilization through PHIs is transient when given in a pulsatile fashion, and this contrasts with constitutively active HIF-α expression in many cancers. These results provide evidence that FG-2216 is safe, has a wide therapeutic window, and has broad implications in the treatment of anemia from various disorders. Nevertheless the study of any PHI administered chronically requires close monitoring for adverse effects.
The ability to promote the expression of endogenous erythropoietin through the use of an orally available agent represents a significant advance over current methodologies. Following the cloning of human EPO,34 rHuEpo has been demonstrated to correct the anemia of end-stage renal disease35 and has become the standard of care for this indication,29 and the recent availability of an extended half-life preparation renders this approach less cumbersome. The induction of Epo through HIF PHI has several potential advantages over rHuEpo, including ease of administration, lack of immunogenicity,36–38 and the potential coordinate induction of other genes important for erythropoiesis. Several gene-transfer vector systems have also used Epo as a proof of principle for delivery of a therapeutic protein,39–43 but these systems are cumbersome for clinical application. Recently, a nonspecific GATA inhibitor, K-11706, was shown to induce epo in Hep3B cells and elevate epo in mice after oral administration.44 While HIF activity by DNA binding assays appeared to increase, HIF-α protein levels did not, indicating that epo induction by K-11706 is distinctly different from the PHIs studied here.
Several agents have been reported to induce HbF in baboons and rhesus macaques, and some of these agents have eventually been applied to the thalassemias and hemoglobinopathies with clinical benefit.45 Thus, the nonhuman primate represents an informative model for testing agents for their ability to induce HbF. Here we showed that FG-2216 increased the percentage of red cells and reticulocytes containing HbF by flow cytometry. When HbF was quantitated by HPLC, there were no differences between baseline, FG-2216 treatment, and phlebotomy. This is consistent with previous reports where other compounds have been shown to increase HbF production among cultured erythroid cells or among erythroid cells in vivo by flow cytometry alone.46–49 Three factors determine the ultimate level of fetal hemoglobin: F-cell production, as measured by the percentage of F+ retics in the total reticulocytes; the quantity of HbF per F+ cell; and the preferential survival of F+ cells compared with red cells that lack fetal hemoglobin.50 Here we present evidence that FG-2216 is associated with an increase in F+ retics and F+ cells, though the HbF distribution was heterocellular. This rise in F+ cells without a significant change by HPLC suggests that there were more HbF-containing cells without an increase in the overall HbF concentration per F+ cells. The low overall levels of HbF by HPLC do not permit an accurate assessment of F+/F cells. In contrast to what would be expected in the context of an underlying thalassemia or hemoglobinopathy phenotype, we observed no preferential survival or enrichment in the peripheral blood of F+ cells. Additionally, the choice of the rhesus macaque, a model that is generally considered to have a less robust HbF induction potential under stress phlebotomy, may also contribute to the low level of HbF protein produced. Indeed, the baboon has previously been shown to exhibit an exaggerated HbF response to phlebotomy, which may not model the human well.12 These factors may in part explain why a significant sustained increase in HbF levels as determined by HPLC was not observed in this model.
The combination of FG-2216 and hydroxyurea might be expected to produce a synergistic effect on HbF in the hemoglobinopathies. The potent induction of Epo demonstrated here could support the combination of FG-2216 and hydroxyurea as a viable potential treatment strategy for individuals with sickle cell anemia. We have previously demonstrated augmentation of the HbF response in such individuals when recombinant Epo was administered exogenously.51 Additionally, the increase in total hemoglobin level with FG-2216 was gradual, reducing concern for vasoocclusive crisis. Finally, the induction of nitric oxide synthase and other hypoxia-inducible genes may prove advantageous in sickle cell anemia.52 Nonetheless, these encouraging results support our plan to further screen additional HIF PHIs for their ability to maximally induce HbF at the protein level in vitro and in the nonhuman primate model prior to testing the effects of combination approaches.
Finally, HIF prolyl hydroxylase inhibitors represent an exciting new class of compounds with therapeutic utility. They can be administered orally, significantly elevate endogenous Epo, and potentially induce HbF and other factors that are important in the treatment of congenital and acquired anemias.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This research was supported in part by the Intramural Research Program at the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases.
National Institutes of Health
Authorship
Contribution: M.M.H., S.J.K., and J.F.T. designed and performed research experiments, analyzed data, and wrote the paper. N.S.L., A.W., M.M., C.W., I.L., A.L., and R.S. performed experiments and analyzed data. C.W., I.L., A.L., and S.J.K. contributed vital reagents. R.E.D. and G.P.R. designed experiments and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F Tisdale, Molecular and Clinical Hematology Branch (MCHB), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 9000 Rockville Pike, Building 10, 9N 116, Bethesda, MD 20892; e-mail: johntis@mail.nih.gov.