A pivotal mediator of actin dynamics is the protein cofilin, which promotes filament severing and depolymerization, facilitating the breakdown of existing filaments, and the enhancement of filament growth from newly created barbed ends. It does so in concert with actin interacting protein 1 (Aip1), which serves to accelerate cofilin's activity. While progress has been made in understanding its biochemical functions, the physiologic processes the cofilin/Aip1 complex regulates, particularly in higher organisms, are yet to be determined. We have generated an allelic series for WD40 repeat protein 1 (Wdr1), the mammalian homolog of Aip1, and report that reductions in Wdr1 function produce a dramatic phenotype gradient. While severe loss of function at the Wdr1 locus causes embryonic lethality, macrothrombocytopenia and autoinflammatory disease develop in mice carrying hypomorphic alleles. Macrothrombocytopenia is the result of megakaryocyte maturation defects, which lead to a failure of normal platelet shedding. Autoinflammatory disease, which is bone marrow–derived yet nonlymphoid in origin, is characterized by a massive infiltration of neutrophils into inflammatory lesions. Cytoskeletal responses are impaired in Wdr1 mutant neutrophils. These studies establish an essential requirement for Wdr1 in megakaryocytes and neutrophils, indicating that cofilin-mediated actin dynamics are critically important to the development and function of both cell types.
Introduction
Cofilin is a pivotal regulator of the actin cytoskeleton in eukaryotes, mediating cytoskeletal responses during cellular organization,1 polarity,2 motility,3,4 endocytosis,5 morphogenesis,6 and cytokinesis.7,8 Functioning downstream of numerous signaling pathways, particularly those mediated by Rho-family GTPases, it promotes filament severing and depolymerization, facilitating both the breakdown of existing filaments and the enhancement of filament growth from newly created barbed ends. It is therefore a mediator of actin dynamics, promoting the rapid turnover of filament networks. Cofilin's activity is regulated at a number of different levels, by phosphoinositides,9 pH,10 and, in metazoans, the phosphorylation state of serine residue 3.11 Two families of phosphatases, slingshot12 and chronophin,13 are known to activate cofilin by dephosphorylating serine residue 3. Conversely, the Lin11, Isl-1, and Mec-3 (LIM) kinases and testicular protein kinases phosphorylate serine 3, inhibiting cofilin activity by preventing its binding to actin.14,,,,–19
Cofilin is also regulated by interactions with protein 14-3-3ζ20 and actin interacting protein 1 (Aip1).21 Aip1 binds the cofilin/actin complex, and extensive investigations have indicated that Aip1 enhances cofilin's capacity to sever actin filaments, and may also accelerate depolymerization by capping their barbed ends.21,,–24 Mutations in Aip1 can disrupt cytoskeletal behavior in yeast,21 Xenopus,22 Drosophila,25 Caenorhabditus elegans,26 Dictyostelium,27 and Arabidopsis.28 While progress has been made in understanding the biochemical and cellular functions of the cofilin/Aip1 complex, the physiologic processes it regulates, particularly in higher organisms, are largely unknown. Genetic deletion of one of the 3 murine cofilin family members (cofilin 1, or nonmuscle n-cofilin) provided the first in vivo confirmation of a role in mammals, establishing an essential requirement for cofilin 1 in neuronal growth and neural crest cell migration.6 The recent identification of mutations in the muscle-specific form, cofilin 2, in patients with Nemaline myopathy indicate that this member of the family is necessary for muscle development.29 Several studies have also implicated cofilin in platelet function. Thrombin stimulation can induce the dephosphorylation of cofilin in human platelets,30 and evidence suggests that activation of cofilin by outside-in signals might facilitate the dynamic cytoskeletal responses required for shape change, secretion, and aggregation.31,32 Roles for cofilin in T-cell activation33,34 and neutrophil cytoskeletal behavior have also been postulated.33,35,–37
To unravel the physiologic role of the cofilin/Aip1 complex in mammals, we have generated an allelic series for WD40-repeat protein 1 (Wdr1), the mouse homolog of Aip1. We report that incremental reductions in Wdr1 function result in a dramatic phenotype gradient. Deficiencies in Wdr1 disrupt megakaryocyte maturation and platelet shedding, provoke neutrophilic autoinflammatory disease, and cause embryonic lethality. This array of phenotypic perturbations is caused by the pleiotropic effects of Wdr1 in multiple cell types. Our findings establish an essential role for Wdr1 in megakaryocytes and neutrophils, and suggest that cofilin-mediated actin dynamics are critical to their development and function.
Materials and methods
Mice
All animal experiments conformed to the regulatory standards of, and were approved by, the Melbourne Health Research Directorate Animal Ethics Committee, or the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine. C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and 129S6/SvEv, Rag2-deficient, and Jh-deficient mice were from Taconic (Hudson, NY). All mice were housed in a specific pathogen-free (SPF) environment. The rd mutation was induced on an inbred C57BL/6 background, isolated on a mixed C57BL/6J:129S6/SvEv:C3HeB/FeJ background,38 and back-crossed 10 generations to C57BL/6. A 129-derived embryonic stem (ES) cell line (clone ID XN462) harboring a pGT2lxf gene trap insertion within Wdr1 was obtained from BayGenomics (San Francisco, CA).39 XN462 ES cells were injected into C57BL/6 blastocysts, and agouti offspring from the resulting male chimeras were tested for transmission of the gene trap insertion by diagnostic polymerase chain reaction (primer sequences available on request).
Mapping and nucleic acid sequencing
The rd was mapped by outcrossing the mutation on a C57BL/6 background to wild-type 129S6/SvEv animals for 2 generations, and collecting affected progeny from a subsequent F2 intercross. A genome-wide scan of 80 simple sequence length polymorphism markers (Research Genetics, Huntsville, AL) was performed. Products of additional meioses were analyzed using Massachusetts Institute of Technology (MIT) simple sequence length polymorphism, in-house cytosine/adenosine (CA) repeat and single nucleotide polymorphism markers. Genomic DNA was subjected to polymerase chain reaction amplification, treated with ExoSAP-IT (USB, Cleveland, OH) and directly sequenced using BigDye Terminators v3.0 (Applied Biosystems, Foster City, CA).
Antibodies and flow cytometry
Antibody to F4/80 (clone CI:A3–1) was obtained from Serotec (Kidlington, United Kingdom); all other antibodies were obtained from BD Pharmingen (San Jose, CA). Single-cell suspensions of blood, spleen, and thymus were subjected to red cell lysis with 0.15 M NH4Cl and preincubated with an antibody to CD16 and CD32 (clone 2.4G2; BD Pharmingen). Cells were stained with antibodies and analyzed using a Beckman Coulter (Fullerton, CA) EPICS XL-MCL flow cytometer. Thrombopoietin levels were measured by enzyme-linked immunosorbent assay using the Quantikine Mouse Tpo Immunoassay kit (R&D Systems, Minneapolis, MN).
Histologic, immunohistochemical, and immunofluorescence analyses
Tissues were fixed in 10% neutral-buffered formalin and paraffin-embedded. Sections were prepared by standard techniques and stained with hematoxylin and eosin. Tissues for immunohistochemistry and immunofluorescence were embedded in Tissue-tek OCT (Sakura Finetek, Zoeterwoude, The Netherlands) and frozen in liquid N2. Cryosections were fixed in ice-cold acetone and washed in phosphate-buffered saline. Sections were incubated at 4°C with antibodies, washed twice in phosphate-buffered saline, and incubated with the appropriate secondary. Images were captured by Axiocam attached to an Axioplan 2 microscope (Zeiss, Oberkochen, Germany) using Plan Fluor 10×/0.3 or 20×/0.5 objective lenses. Images were acquired with Axiovision software.
Hematopoietic cell analyses
Blood was drawn by retro-orbital puncture and collected in Microtainer brand tubes containing EDTA (Becton Dickinson, Franklin Lakes, NJ). Blood samples were analyzed using a Cell-Dyn 3500R automated veterinary hematology analyser (Abbott Diagnostics, Abbott Park, IL). Megakaryocyte counts were performed by manual counting from sections of sternum and spleen after staining with hematoxylin and eosin. A minimum of 10 high-power fields were scored. Clonal cultures of hematopoietic cells were performed as described.40 Cultures of 2.5 × 104 adult bone marrow cells or 5 × 104 spleen cells in 1 mL of 0.3% agar in Dulbecco modified Eagle medium (DMEM) supplemented with newborn calf serum (20%) were stimulated with 100 ng/mL murine stem cell factor (SCF), 10 ng/mL murine IL-3, and 4 U/mL human erythropoietin (EPO) and incubated for 7 days at 37°C in a fully humidified atmosphere of 10% (v/v) CO2 in air. Agar cultures were fixed, stained for acetylcholinesterase, Luxol Fast Blue, and hematoxylin, and the cellular composition of each colony was determined at 100× to 400× magnification. Colonies were scored as erythroid, myeloid or mixed-erythroid at 35× magnification. For bone marrow transplantations, recipient 6- to 12-week-old C57BL/6-Ly-5.1 mice were given 11 Gy of gamma-irradiation in a split dose; 500 × 103 nucleated donor bone marrow cells were injected retro-orbitally in a volume of 200 μL.
Neutrophil purification
For chemotaxis and actin polymerization assays, bone marrow neutrophils were isolated as described previously.41 For all other experiments, isolation of neutrophils was performed as described.42 Briefly, murine bone marrow was harvested into Hanks balanced saline solution without calcium, magnesium, phenol red, and sodium bicarbonate (pH 7.2) containing 15 mM EDTA (ethylenediaminetetraacetic acid) and 1% bovine serum albumin (Hanks balanced saline solution–EDTA). After centrifugation (350g for 10 minutes at room temperature), cells were resusupended in Hanks balanced saline solution–EDTA and layered onto 3-layered Percoll (Amersham Biosciences, Piscataway, NJ) gradients of 78%, 69%, and 52% Percoll and centrifuged at 1500g for 30 minutes at room temperature without braking. Neutrophils were harvested from the 69%/78% interface, with sample purity, as determined by flow cytometry and cytospin preparations, between 93% and 98%.
Chemotaxis and actin polymerization assays
Neutrophils were plated in 3-μm Transwells (Fisher Scientific, Pittsburgh, PA; 106 cells/Transwell) in the absence or presence of 250 ng/mL MIP-2 (R&D Systems) in the lower chamber and incubated 3 hours at 37°C before enumeration. To measure filamentous actin content, bone marrow cells were labeled with phycoerythrin (PE)-conjugated anti-Gr-1, treated with MIP-2 (250 ng/mL), then simultaneously fixed and stained with FITC-conjugated phalloidin (Molecular Probes, Eugene, OR). Mean fluorescence intensity of the phalloidin-FITC signal was determined for the Gr-1-PE positive population by flow cytometry. Relative filamentous actin content (%filamentous actin) was determined by normalizing mean fluorescence intensity to the value for the untreated control animal.
Neutrophil immunofluorescence microscopy
Neutrophils were allowed to adher to poly-L-Lysine-coated coverslips, fixed with 4% paraformaldehyde, permeabilized with 0.5% TritonX-100, and blocked with 2% goat serum for 1 hour. Cells were incubated with either rabbit anticofilin antibody (1:400) followed by FITC-conjugated goat antirabbit secondary antibody (1:1000), or with Alexa-546–conjugated phalloidin before DAPI. Images were acquired with a 12-bit cooled CCD camera (SensiCam) using a Leica TCS4 SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany) and the 63×/1.32 objective lens, controlled by Axiovision 4.1 software.
Platelet half-life
In vivo platelet clearance assays were performed as described.43 Mice were injected via the retro-orbital venous plexus with 30 mg/kg biotin N-hydroxysuccinimide-biotin (Calbiochem, La Jolla, CA) diluted in 10% DMSO in phosphate-buffered saline. For analysis, 2 μL of tail blood was added to 1 mL of buffered saline glucose citrate (BSGC) buffer (116 mM NaCl, 13.6 mM trisodium citrate, 8.6 mM Na2HPO4, 1.6 mM KH2PO4, 0.9 mM Na2EDTA, 11.1 mM glucose, 1 μg/mL prostaglandin I2); 500 μL of cell suspension was incubated with FITC-conjugated anti-CD41 antibody and streptavidin-PE, washed with BSGC, and analyzed.
Flow cytometric analysis of megakaryocytes
Bone marrow was resuspended in 2 mL CATCH medium (0.38% sodium citrate, 1 mM adenosine, 2 mM theophylline, 3 μg/mL prostaglandin I2 in calcium- and magnesium-free Hanks balanced saline solution), filtered through a 100-μm cell strainer and centrifuged at 400g for 4 minutes at room temperature. After resuspension in 1 mL CATCH diluted 1:1 with phosphate-buffered saline/5% fetal bovine serum, and preincubation with antibodies to CD16 and CD32, cells were labeled with FITC-conjugated anti-CD41 and washed with CATCH diluted 1:1 with phosphate-buffered saline/5% fetal bovine serum. After centrifugation at 400g for 4 minutes at room temperature, cells were resuspended in 1 mL propidium iodide (50 μg/mL in 0.1% sodium citrate), RNAse A was added to 50 μg/mL, and after incubation at room temperature for 30 minutes, the cells were filtered and analyzed. Before analysis, a manual count was performed to compare total nucleated cell numbers before and after preparation. In 3 separate experiments, each comprising 4 mice of each genotype, recovery of nucleated cells was 67% plus or minus 8.3%.
Electron microscopy
Platelet pellets, whole bone marrow, or red cell–depleted peripheral blood leukocytes were resuspended in 10 volumes of 2.5% glutaraldehyde, 2.0% formaldehyde in 0.1 M cacodylate buffer, with 2 mM CaCl. Samples were washed with 0.1 M cacodylate buffer, and postfixed in 1% osmium tetroxide/0.1 M cacodylate buffer. After washing, dehydration, and Glauert EMBED resin infiltration, samples were cured at 60°C for 2 days. Then 60-nm sections were cut and stained with 1% aqueous uranyl acetate and Reynold lead citrate. Grids were viewed using a Hitachi (Tokyo, Japan) H7500 transmission electron microscope, and images acquired by a Gatan 2kx2k CCD camera (Pleasonton, CA).
Results
Generation of an allelic series for murine Wdr1
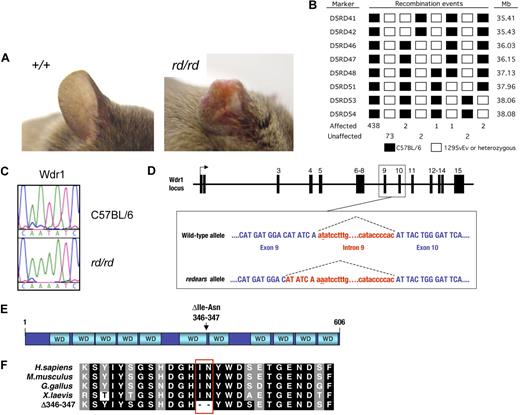
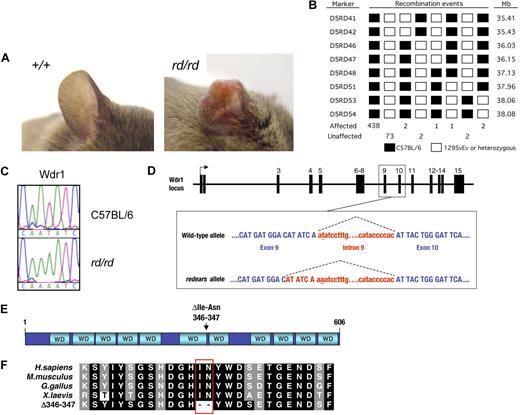
In an N-ethyl-N-nitrosourea mutagenesis screen for mutations affecting development and hematopoiesis,38 we isolated a pedigree called redears (rd), which exhibited reduced blood platelet levels (thrombocytopenia) and spontaneous inflammatory lesions on the ears and tail (Figure 1A). The rd was mapped by a standard positional cloning approach to a candidate interval of 1.7 Mb on the proximal end of mouse chromosome 5 (Figure 1B). Sequencing the coding region and intron/exon boundaries of candidate genes revealed a mutation in the gene encoding the WD40 repeat protein Wdr1 (Figure 1C). The mutation in Wdr1 is a T>A transversion in the second dinucleotide of the intron 9 splice donor (Figure 1D). The effect of this mutation is to reduce normal splicing to 20% to 30% of that seen in wild-type cells, in favor of cryptic splicing from an upstream donor (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.). The latter event produces a mutant transcript containing a 6-bp in-frame deletion that is predicted to cause the loss of 2 amino acids from the carboxyl-terminus of the sixth WD40 repeat of Wdr1 (Figure 1E,F). In vitro studies suggest the mutant protein has a reduced half-life and is incorrectly folded, and is therefore probably nonfunctional (Figure S2). Some wild-type protein is still produced from the rd allele, because of the small amount of normal splicing that occurs. This indicates that the rd allele of Wdr1 is hypomorphic, and not a complete loss of function.
Identification of an N-ethyl-N-nitrosourea–induced mutation in Wdr1 causing spontaneous inflammation and thrombocytopenia. (A) Typical ear lesion observed in mice homozygous for the redears mutation at 6 months of age. A full histopathologic survey of major organs detected no other gross abnormalities. (B) Haplotype panel summarizing 521 meiotic events generated by outcrossing rd to 129S6/SvEv and collecting progeny from subsequent intercross and backcross matings. The final candidate interval for rd, defined by D5RD47 and D5RD51, was 1.81 Mb. (C) Electropherograms of DNA sequence from the Wdr1 exon 9/intron 9 splice boundary showing the presence of a T-to-A transversion in the second nucleotide of the splice donor in homozygous rd mice. (D) Schematic of the Wdr1 locus, with splicing of intron 9 indicated by dashed lines. The mutated nucleotide in intron 9 is underlined. Wdr1 intron 9 is a noncanonical AT–AC intron, a rare (0.036%) class of intron whose intron splice donor and acceptor dinucleotide sequences are AT and AC, respectively. AT–AC intron splice donors possess a highly conserved consensus sequence, ATATCCT, and the rd mutation affects the second nucleotide of this consensus. This causes activation of a cryptic AT–AC splice donor consensus, ATATCAA, present 6 bp upstream in exon 9, producing a transcript containing a 6-bp in-frame deletion. This transcript was identified in the bone marrow and spleen of Wdr1rd/rd mice. (E) Domain structure of Wdr1. The protein comprises 11 WD40 repeats, which, from studies of Aip1 in yeast and C. elegans, are predicted to form 2 beta-propellers. The location of the 2 residues deleted in the mutant rd Wdr1 protein is indicated. (F) Amino acid sequence of Wdr1/Aip1 at the carboxy terminus of the sixth WD40 repeat. The sequence of the mutant rd Wdr1 protein, which lacks the highly conserved isoleucine and asparagine residues, is shown.
Identification of an N-ethyl-N-nitrosourea–induced mutation in Wdr1 causing spontaneous inflammation and thrombocytopenia. (A) Typical ear lesion observed in mice homozygous for the redears mutation at 6 months of age. A full histopathologic survey of major organs detected no other gross abnormalities. (B) Haplotype panel summarizing 521 meiotic events generated by outcrossing rd to 129S6/SvEv and collecting progeny from subsequent intercross and backcross matings. The final candidate interval for rd, defined by D5RD47 and D5RD51, was 1.81 Mb. (C) Electropherograms of DNA sequence from the Wdr1 exon 9/intron 9 splice boundary showing the presence of a T-to-A transversion in the second nucleotide of the splice donor in homozygous rd mice. (D) Schematic of the Wdr1 locus, with splicing of intron 9 indicated by dashed lines. The mutated nucleotide in intron 9 is underlined. Wdr1 intron 9 is a noncanonical AT–AC intron, a rare (0.036%) class of intron whose intron splice donor and acceptor dinucleotide sequences are AT and AC, respectively. AT–AC intron splice donors possess a highly conserved consensus sequence, ATATCCT, and the rd mutation affects the second nucleotide of this consensus. This causes activation of a cryptic AT–AC splice donor consensus, ATATCAA, present 6 bp upstream in exon 9, producing a transcript containing a 6-bp in-frame deletion. This transcript was identified in the bone marrow and spleen of Wdr1rd/rd mice. (E) Domain structure of Wdr1. The protein comprises 11 WD40 repeats, which, from studies of Aip1 in yeast and C. elegans, are predicted to form 2 beta-propellers. The location of the 2 residues deleted in the mutant rd Wdr1 protein is indicated. (F) Amino acid sequence of Wdr1/Aip1 at the carboxy terminus of the sixth WD40 repeat. The sequence of the mutant rd Wdr1 protein, which lacks the highly conserved isoleucine and asparagine residues, is shown.
To confirm this possibility, we obtained an independent allele (Wdr1gt) created by the insertion of a pGT2lxf gene trap vector into intron 2, 6.61 kb downstream of exon 2. Similar to Wdr1rd/+ mice, Wdr1gt/+ heterozygotes have no obvious phenotype. A complementation test was performed by inter-crossing Wdr1gt/+ and Wdr1rd/+ mice. Compound heterozygous male Wdr1rd/gt mice exhibit runting, thrombocytopenia (210 ± 103 × 109/L platelets compared with 1294 ± 148 × 109/L platelets for wild-type littermates at 3 weeks of age, n = 5), and inflammation of the ears and tail reminiscent of, but more severe than in, Wdr1rd/rd mice. These animals died at 3 to 6 weeks of age (Figure S3). This confirmed that the thrombocytopenia and inflammatory disease seen in the redears pedigree was caused by the mutation in Wdr1, and that the Wdr1gt allele produces less functional protein than Wdr1rd. No viable homozygous Wdr1gt/gt offspring were observed from Wdr1gt/+ intercrosses, indicating that these mice were dying in utero (Table 1). Genotyping demonstrated that although Wdr1gt/gt embryos were present in approximately normal Mendelian ratios at embryonic day 3.5, they were absent by embryonic day 10.5, establishing a fundamental requirement for Wdr1 in mammalian embryogenesis.
Mutations in Wdr1 cause a spontaneous autoinflammatory disease
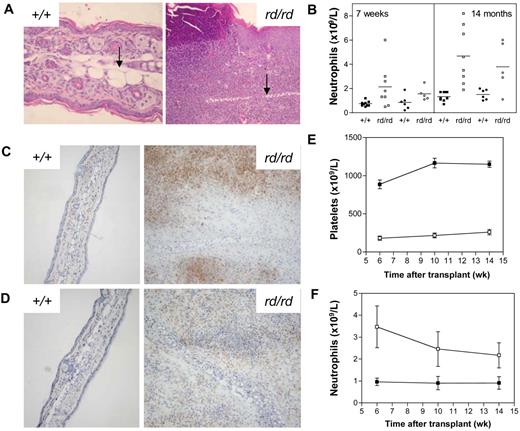
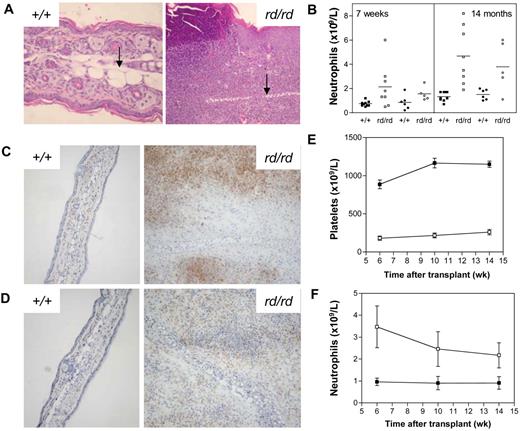
Inflammatory lesions developed on the ears and tails of Wdr1rd/rd mice at 3-6 weeks of age, with lesions progressing in severity over time to involve the limbs and paws. The defining feature of the inflammatory lesions was leukocytic infiltration, accompanied by epidermal hyperplasia, necrosis, edema, and cartilage destruction (Figure 2A). In addition, there was increased osteoclast number and activity associated with bone damage in the limbs and paws. Despite the progressive inflammatory disease, Wdr1rd/rd mice were healthy, fertile, and survived to at least 14 months of age. An examination of the hematopoietic compartment of Wdr1rd/rd mice revealed a number of changes. Peripheral blood neutrophil numbers were elevated, with a significant correlation between the severity of inflammation and neutrophil number (Figure 2B). Granulopoiesis was expanded in the bone marrow, with a concomitant decrease in erythropoiesis observed, a finding that was more pronounced in older animals, which were mildly anemic (data not shown). Both granulopoietic and erythropoietic activities were expanded in the spleen. However, lymphocyte numbers and major subset populations were normal (data not shown).
Spontaneous inflammatory disease in Wdr1 mutant mice. (A) Hematoxylin and eosin–stained ear sections showing the epidermal hyperplasia and leukocytic infiltration characteristic of inflamed ears, with the cartilaginous scaffold of the ear indicated (black arrow). To illustrate the scale of the ongoing inflammatory process, wild-type and rd/rd images were taken at 200 × and 100 × magnification, respectively. (B) Peripheral blood neutrophil numbers are elevated in Wdr1rd/rd mice compared with wild-type littermates. Individual male (■+/+, □ rd/rd) and female (● +/+, ○ rd/rd) mice are shown. (C) Anti-Gr1 immunohistochemistry on frozen sections of ear demonstrating the presence of large numbers of neutrophils within the lesion. (D) Anti-F4/80 immunohistochemistry demonstrating that macrophages are also present within the inflammatory lesion. Isotype controls for both Gr1 and F4/80 exhibited no staining (data not shown). (E,F) Peripheral blood platelet (E) and neutrophil (F) numbers in lethally irradiated recipients of wild-type or Wdr1rd/rd bone marrow (■+/+, □ rd/rd). The CD45.1/CD45.2 leukocyte polymorphism system was used to distinguish donor from recipient hematopoiesis. Donor engraftment levels, as measured by contribution to peripheral blood leukocytes, were more than 90% in each recipient; n = 8 recipient mice per donor genotype. Data shown in (E,F) represent the mean plus or minus a standard deviation.
Spontaneous inflammatory disease in Wdr1 mutant mice. (A) Hematoxylin and eosin–stained ear sections showing the epidermal hyperplasia and leukocytic infiltration characteristic of inflamed ears, with the cartilaginous scaffold of the ear indicated (black arrow). To illustrate the scale of the ongoing inflammatory process, wild-type and rd/rd images were taken at 200 × and 100 × magnification, respectively. (B) Peripheral blood neutrophil numbers are elevated in Wdr1rd/rd mice compared with wild-type littermates. Individual male (■+/+, □ rd/rd) and female (● +/+, ○ rd/rd) mice are shown. (C) Anti-Gr1 immunohistochemistry on frozen sections of ear demonstrating the presence of large numbers of neutrophils within the lesion. (D) Anti-F4/80 immunohistochemistry demonstrating that macrophages are also present within the inflammatory lesion. Isotype controls for both Gr1 and F4/80 exhibited no staining (data not shown). (E,F) Peripheral blood platelet (E) and neutrophil (F) numbers in lethally irradiated recipients of wild-type or Wdr1rd/rd bone marrow (■+/+, □ rd/rd). The CD45.1/CD45.2 leukocyte polymorphism system was used to distinguish donor from recipient hematopoiesis. Donor engraftment levels, as measured by contribution to peripheral blood leukocytes, were more than 90% in each recipient; n = 8 recipient mice per donor genotype. Data shown in (E,F) represent the mean plus or minus a standard deviation.
The phenotype of Wdr1rd/rd mice resembled some aspects of the human condition relapsing polychondritis. Although the pathogenesis of relapsing polychondritis is unclear, it has been suggested to be autoimmune in origin.44 Thrombocytopenia and inflammatory skin lesions are also characteristic of autoimmune conditions such as systemic lupus erythematosus and psoriasis, raising the possibility that the phenotype might be the result of an autoimmune reaction. Two hallmarks of autoimmune disease are circulating antinuclear antibodies and autoreactive T cells; however, screening by indirect immunofluorescent microscopy on HepG2 cells did not detect the presence of antinuclear antibodies in Wdr1rd/rd serum (data not shown). Similarly, immunohistochemistry on skin lesions demonstrated that the infiltrating leukocytes are primarily neutrophils, and to a lesser extent macrophages, rather than lymphocytes (Figure 2C,D). To examine directly whether lymphocytes are required for inflammatory disease, we crossed the Wdr1rd mutation onto the Rag2−/− background, which lacks functional B cells or T cells. Thrombocytopenia and spontaneous inflammatory lesions of the same nature and at the same rate as Wdr1rd/rd Rag2+/+ littermates developed in Wdr1rd/rd Rag2−/− animals, with disease onset or progression being unaffected by the absence of lymphocytes (Figure S4). It is therefore clear that the inflammatory disease seen in Wdr1rd/rd mice is not autoimmune in origin.
Cofilin mislocalization and functional defects in Wdr1rd/rd neutrophils
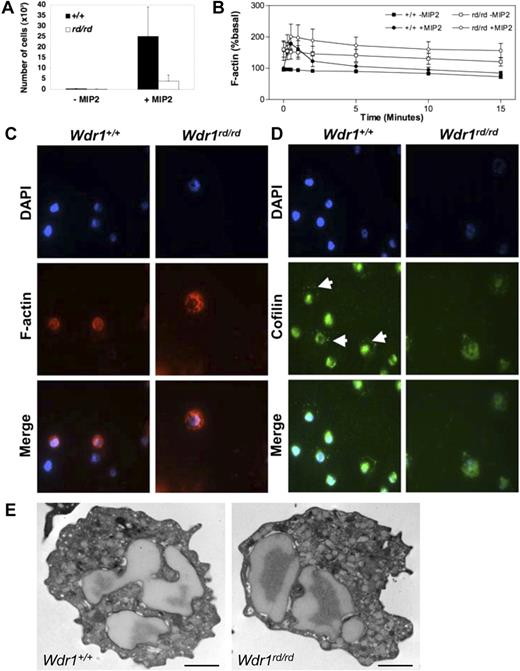
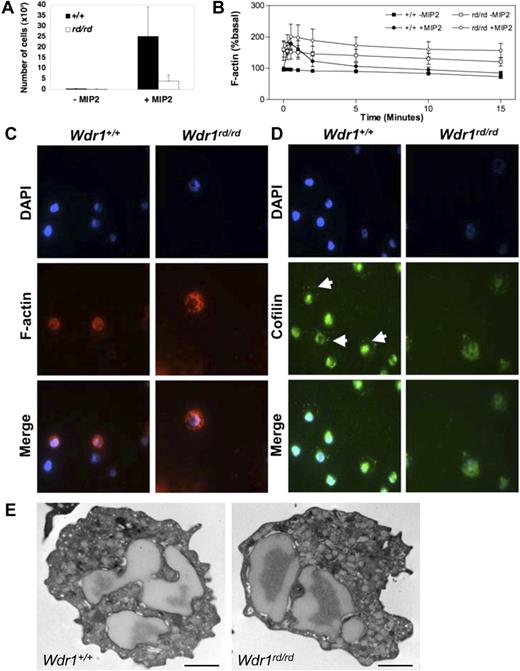
To examine whether the autoinflammatory disease observed in Wdr1rd/rd mice is caused by bone marrow–derived cells, reciprocal transplants of unfractionated Wdr1+/+ or Wdr1rd/rd bone marrow into lethally irradiated recipients were performed. Thrombocytopenia, neutrophilia, and severe inflammatory skin lesions were observed in wild-type recipients of bone marrow from Wdr1rd/rd but not Wdr1+/+ donors (Figure 2E,F). When bone marrow from wild-type donors was transplanted into 8-week-old Wdr1rd/rd recipients with pronounced inflammatory lesions, a clear reduction in the disease process was observed; redness and swelling ceased over the course of 4 weeks, and the lesions failed to progress (data not shown). However, the lesions were not completely resolved, presumably because of the irreparable tissue and cartilage damage that had already occurred. Given the results of these experiments, and the phenotype of Wdr1rd/rd Rag2−/− mice, nonlymphoid bone marrow–derived cells therefore must be responsible for initiating and maintaining inflammation. With their prominent role in the lesions, neutrophils are the primary candidates, particularly because neutrophil dysfunction is known to underlie certain inflammatory disorders. The functional response of Wdr1rd/rd neutrophils was examined by in vitro assays to measure chemokine responses that require actin reorganization. Wdr1rd/rd neutrophils mounted an aberrant cytoskeletal response to chemokine stimulation, characterized by failure to depolymerize filamentous actin at normal rates. Resting Wdr1rd/rd neutrophils showed elevated levels of filamentous actin and exhibit reduced rates of directed migration (Figure 3A,B). This was confirmed by immunofluorescence studies showing that filamentous actin distribution was abnormal (Figure 3C). Importantly, cofilin localization was perturbed in Wdr1rd/rd neutrophils (Figure 3D), consistent with data from C. elegans indicating that Aip1 is required for correct cellular distribution of cofilin.26 We next examined peripheral blood leukocytes by transmission electron microscopy and found no significant ultrastructural defects in Wdr1rd/rd neutrophils (Figure 3E). Together, these results indicate that while Wdr1 is not required for neutrophil maturation and release to the peripheral circulation, it is essential for normal motility and actin depolymerization kinetics, functions that are presumably mediated via interaction with cofilin.
Wdr1 deficiency causes cytoskeletal defects in neutrophils. (A) Neutrophil chemotaxis toward MIP-2 in 3-micron transwells, demonstrating defective migration of Wdr1rd/rd neutrophils. (B) Basal and MIP-2–stimulated actin polymerization, showing elevated resting filamentous actin levels and impaired actin depolymerization rates in Wdr1rd/rd neutrophils. Data shown represent the means plus or minus a standard deviation; n equals 6 mice per genotype. (C) Fluorescence microscopy showing the cellular distribution of filamentous actin in neutrophils. In wild-type cells, filamentous actin is distributed throughout the cytosol and is more concentrated at the cell cortex. In Wdr1rd/rd neutrophils, the formation of filamentous actin is enhanced with no obvious cortical actin. (D) Cellular localization of cofilin in neutrophils. In wild-type cells, cofilin is localized to the nucleus and to the periphery (white arrows). In contrast, in Wdr1rd/rd neutrophils cofilin is delocalized from the cell cortex and becomes diffused throughout the cytosol. (E) Transmission electron microscopy of peripheral blood neutrophils. No gross morphologic changes were seen in Wdr1rd/rd neutrophils. Scale bar indicates 1 μm.
Wdr1 deficiency causes cytoskeletal defects in neutrophils. (A) Neutrophil chemotaxis toward MIP-2 in 3-micron transwells, demonstrating defective migration of Wdr1rd/rd neutrophils. (B) Basal and MIP-2–stimulated actin polymerization, showing elevated resting filamentous actin levels and impaired actin depolymerization rates in Wdr1rd/rd neutrophils. Data shown represent the means plus or minus a standard deviation; n equals 6 mice per genotype. (C) Fluorescence microscopy showing the cellular distribution of filamentous actin in neutrophils. In wild-type cells, filamentous actin is distributed throughout the cytosol and is more concentrated at the cell cortex. In Wdr1rd/rd neutrophils, the formation of filamentous actin is enhanced with no obvious cortical actin. (D) Cellular localization of cofilin in neutrophils. In wild-type cells, cofilin is localized to the nucleus and to the periphery (white arrows). In contrast, in Wdr1rd/rd neutrophils cofilin is delocalized from the cell cortex and becomes diffused throughout the cytosol. (E) Transmission electron microscopy of peripheral blood neutrophils. No gross morphologic changes were seen in Wdr1rd/rd neutrophils. Scale bar indicates 1 μm.
Wdr1 is required for megakaryocyte maturation and platelet shedding
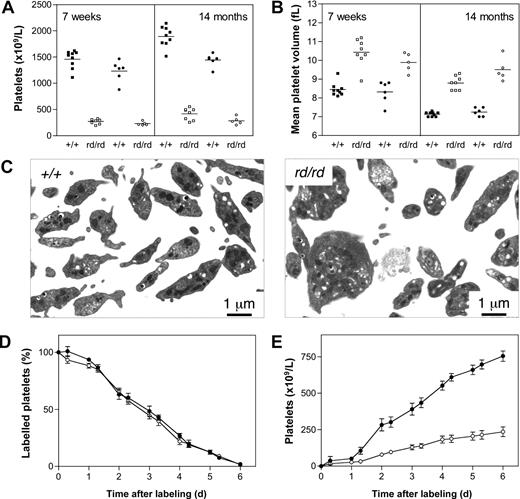
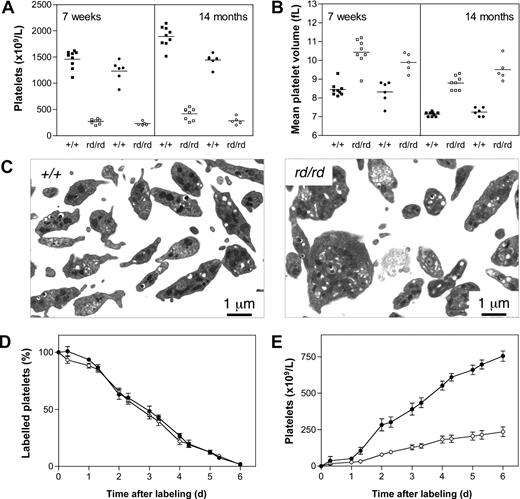
Circulating blood platelet levels in Wdr1rd/rd mice were approximately 20% that of wild-type littermates (Figure 4A). This profound thrombocytopenia was stable and did not change signifi-cantly over the life of the animals. Tail bleeding assays demonstrated a significant increase in time to cessation of bleeding after clipping of 2-mm tail sections and incubation in room temperature saline (3.9 ± 1.2 minutes, Wdr1+/+; 10.0 ± 0 minutes, Wdr1rd/rd, bleeding stopped by cauterization at 10 minutes after sectioning; data not shown). Wdr1rd/rd mice exhibited an increase in mean platelet volume (MPV; Figure 4B), and transmission electron microscopy studies revealed that Wdr1rd/rd platelets were characterized by decreased uniformity, microtubule coil disturbances, and aberrant distribution of α-granules and dense bodies (Figure 4C). In vivo biotinylation assays demonstrated that these platelets were cleared from the circulation at a normal rate (Figure 4D), and splenectomy did not result in an increased platelet count (Figure S5), ruling out splenic sequestration as the primary cause of thrombocytopenia. In contrast, the rate at which new platelets appeared in the circulation was significantly lower (Figure 4E), indicating that Wdr1rd/rd mice suffer a defect in platelet production. This is not caused by a deficiency in thrombopoietin, the major cytokine regulating platelet production, as serum levels measured by enzyme-linked immunosorbent assay were normal (Figure 5D). An explanation for the latter finding might involve the increase in mean platelet volume. Thrombopoietin is constitutively produced by the liver, and circulating levels are directly determined by the platelet and megakaryocyte mass.45,–47 Given the increased MPV observed in Wdr1-deficient mice, and the accompanying megakaryocytosis (next paragraph), it might be that the platelet and megakaryocyte mass in these animals is approximately equivalent to that in wild-type counterparts, and that despite the morphologic abnormalities, the thrombopoietin/platelet/megakaryocyte loop is in equilibrium.
Defective platelet production in Wdr1rd/rd mice. (A) Thrombocytopenia in Wdr1rd/rd mice at 7 weeks and 14 months of age. Individual male (■+/+, □ rd/rd) and female (●+/+, ○ rd/rd) mice are shown. Platelet counts for males at 7 weeks of age were Wdr1+/+ 1460 (± 172) vs. Wdr1rd/rd 271 (± 45); at 14 months of age Wdr1+/+ 1892 (± 194) vs. Wdr1rd/rd 417 (± 113). Platelet counts for females at 7 weeks of age were Wdr1+/+ 1232 (± 202) vs. Wdr1rd/rd 230 (± 37); at 14 months of age Wdr1+/+ 1442 (± 123) vs. Wdr1rd/rd 283 (± 73). Horizontal bars indicate the mean. (B) Elevated mean platelet volume in Wdr1rd/rd mice. The platelet counts and MPV values shown in (A,B) were calculated in the same cohort of mice, aged specifically for the purpose. Data from unrelated C57BL/6 control animals bled on the same days as these mice indicate that machine drift over the intervening 12 months was primarily responsible for the decrease in baseline MPV observed. Horizontal bars indicate the mean. (C) Transmission electron microscopy of blood platelets. Platelets from Wdr1rd/rd mice exhibit dramatic morphologic abnormalities characterized by increased size, loss of discoid shape, and the irregular distribution of granules and microtubule coil. (D) Platelet clearance rates are unaffected in Wdr1rd/rd mice. N-hydroxysuccinimide-biotin was injected intravenously and the disappearance of labeled platelets and emergence of labeled platelets were measured twice daily. (E) Platelet production rates are markedly reduced in Wdr1rd/rd mice. Absolute number of unlabeled platelets was calculated by multiplying % unlabeled platelets by total circulating platelet count at each time point. Data shown in panels D and E represent mean (± standard deviation); n equals 8 female mice per genotype (● +/+, ○ rd/rd). Subsequent studies with male mice produced similar results (data not shown). The ordinate of panel E refers to the number of unlabeled platelets in the circulation. Because biotin labeling levels of between 72% and 96% were achieved, the data shown have been normalized to reflect the fact that a population of unlabeled platelets existed at time 0. Hence, the normalized mean platelet count shown at day 6 for wild-type mice is 754 (± 87) × 109/L. Actual mean platelet counts at day 6 were 953 (± 81) × 109/L, Wdr1+/+; 287 (± 97) × 109/L, Wdr1rd/rd.
Defective platelet production in Wdr1rd/rd mice. (A) Thrombocytopenia in Wdr1rd/rd mice at 7 weeks and 14 months of age. Individual male (■+/+, □ rd/rd) and female (●+/+, ○ rd/rd) mice are shown. Platelet counts for males at 7 weeks of age were Wdr1+/+ 1460 (± 172) vs. Wdr1rd/rd 271 (± 45); at 14 months of age Wdr1+/+ 1892 (± 194) vs. Wdr1rd/rd 417 (± 113). Platelet counts for females at 7 weeks of age were Wdr1+/+ 1232 (± 202) vs. Wdr1rd/rd 230 (± 37); at 14 months of age Wdr1+/+ 1442 (± 123) vs. Wdr1rd/rd 283 (± 73). Horizontal bars indicate the mean. (B) Elevated mean platelet volume in Wdr1rd/rd mice. The platelet counts and MPV values shown in (A,B) were calculated in the same cohort of mice, aged specifically for the purpose. Data from unrelated C57BL/6 control animals bled on the same days as these mice indicate that machine drift over the intervening 12 months was primarily responsible for the decrease in baseline MPV observed. Horizontal bars indicate the mean. (C) Transmission electron microscopy of blood platelets. Platelets from Wdr1rd/rd mice exhibit dramatic morphologic abnormalities characterized by increased size, loss of discoid shape, and the irregular distribution of granules and microtubule coil. (D) Platelet clearance rates are unaffected in Wdr1rd/rd mice. N-hydroxysuccinimide-biotin was injected intravenously and the disappearance of labeled platelets and emergence of labeled platelets were measured twice daily. (E) Platelet production rates are markedly reduced in Wdr1rd/rd mice. Absolute number of unlabeled platelets was calculated by multiplying % unlabeled platelets by total circulating platelet count at each time point. Data shown in panels D and E represent mean (± standard deviation); n equals 8 female mice per genotype (● +/+, ○ rd/rd). Subsequent studies with male mice produced similar results (data not shown). The ordinate of panel E refers to the number of unlabeled platelets in the circulation. Because biotin labeling levels of between 72% and 96% were achieved, the data shown have been normalized to reflect the fact that a population of unlabeled platelets existed at time 0. Hence, the normalized mean platelet count shown at day 6 for wild-type mice is 754 (± 87) × 109/L. Actual mean platelet counts at day 6 were 953 (± 81) × 109/L, Wdr1+/+; 287 (± 97) × 109/L, Wdr1rd/rd.
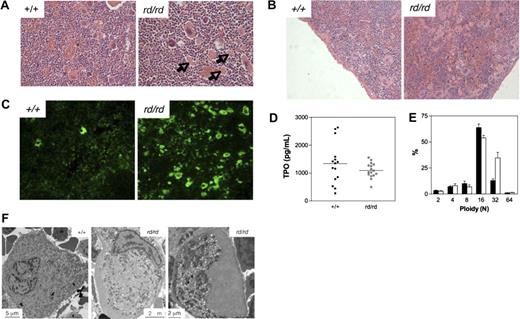
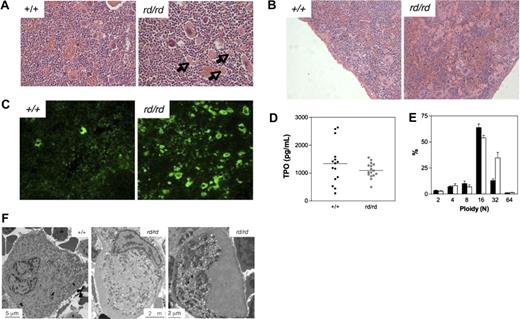
Defective megakaryocytopoiesis in Wdr1rd/rd mice. (A,B) Hematoxylin and eosin stained spleen sections illustrating megakaryocytosis in the bone marrow (A) and spleen (B) of Wdr1rd/rd mice. Arrows indicate examples of the fragments of megakaryocyte cytoplasm observed. (C) Anti-CD41 immunofluorescence of frozen sections, demonstrating the presence of many fragments of megakaryocyte cytoplasm in the spleen of Wdr1rd/rd mice. (D) Serum thrombopoietin levels as measured by enzyme-linked immunosorbent assay in 12-week-old male mice. Horizontal bars indicate the mean. (E) Ploidy distribution of bone marrow megakaryocytes demonstrating a shift toward 32N in Wdr1rd/rd mice; n equals 12 male mice per genotype. Solid bars represent the mean; errors bars, standard deviation. (F) Transmission electron microscopy studies of bone marrow megakaryocytes. A typical mature wild-type megakaryocyte is shown at left. In contrast, Wdr1rd/rd megakaryocytes are smaller and exhibit gross abnormalities, particularly failure of the demarcation membrane system to develop, and large peripheral zones devoid of organelles and granules. The Wdr1rd/rd megakaryocyte shown in the right panel contains no nucleus; this class of cell constituted approximately 25% of the more than 60 individual Wdr1rd/rd megakaryocytes that were viewed. It may be representative of the abundant cytoplasmic fragments seen in the bone marrow and spleen of Wdr1rd/rd mice.
Defective megakaryocytopoiesis in Wdr1rd/rd mice. (A,B) Hematoxylin and eosin stained spleen sections illustrating megakaryocytosis in the bone marrow (A) and spleen (B) of Wdr1rd/rd mice. Arrows indicate examples of the fragments of megakaryocyte cytoplasm observed. (C) Anti-CD41 immunofluorescence of frozen sections, demonstrating the presence of many fragments of megakaryocyte cytoplasm in the spleen of Wdr1rd/rd mice. (D) Serum thrombopoietin levels as measured by enzyme-linked immunosorbent assay in 12-week-old male mice. Horizontal bars indicate the mean. (E) Ploidy distribution of bone marrow megakaryocytes demonstrating a shift toward 32N in Wdr1rd/rd mice; n equals 12 male mice per genotype. Solid bars represent the mean; errors bars, standard deviation. (F) Transmission electron microscopy studies of bone marrow megakaryocytes. A typical mature wild-type megakaryocyte is shown at left. In contrast, Wdr1rd/rd megakaryocytes are smaller and exhibit gross abnormalities, particularly failure of the demarcation membrane system to develop, and large peripheral zones devoid of organelles and granules. The Wdr1rd/rd megakaryocyte shown in the right panel contains no nucleus; this class of cell constituted approximately 25% of the more than 60 individual Wdr1rd/rd megakaryocytes that were viewed. It may be representative of the abundant cytoplasmic fragments seen in the bone marrow and spleen of Wdr1rd/rd mice.
Having established that Wdr1rd/rd mice are unable to generate normal numbers of platelets, it was surprising that megakaryocytes were increased, to a modest degree in the bone marrow (Figure 5A), but quite dramatically in the spleen (Figure 5B). Hematoxylin and eosin-stained sections demonstrated an approximately 4-fold increase in nucleated megakaryocytes (117 ± 42 megakaryocytes for Wdr1rd/rd, 29 ± 13 megakaryocytes for Wdr1+/+, n = 3 mice per genotype) in the spleen, and colony assays confirmed a significant increase in megakaryocyte progenitors (17.7 ± 6.4 colonies for Wdr1rd/rd, 5.9 ± 0.8 colonies for Wdr1+/+, per 5 × 104 spleen cells plated, n = 4 mice per genotype). Megakaryocyte progenitor numbers in the bone marrow were normal (25.4 ± 7.3 colonies Wdr1rd/rd, 23.4 ± 2.1 colonies Wdr1+/+, per 2.5 × 104 bone marrow cells plated, n = 4 mice per genotype). This is consistent with previous studies demonstrating that, in mice, there is a preferential expansion of splenic versus bone marrow megakaryocytopoiesis in response to thrombocytopenia.48,49
In addition, large cellular fragments of what appeared to be enucleated megakaryocyte cytoplasm were found throughout the spleen and bone marrow (Figure 5A,B). Anti-CD41 immunofluorescence (Figure 5C) and flow cytometry (data not shown) confirmed that these fragments were megakaryocytic in origin, suggesting a breakdown in the platelet shedding process. The nucleated megakaryocyte population was characterized by a shift toward higher ploidy, with an increase in the n = 32 subset (Figure 5E). Whether this reflects a reactive response by megakaryocytes to chronic thrombocytopenia50 or indicates an intrinsic defect is unclear, but it demonstrates that the decrease in platelet production does not result from a failure of polyploidization. Transmission electron microscopy studies revealed striking morphologic abnormalities in Wdr1rd/rd megakaryocytes, which exhibited an immature, malformed demarcation membrane system (DMS), aberrant granule distribution, and large cytoplasmic regions that were entirely devoid of both DMS and granules. The latter appeared to be a continuation and enlargement of the actin-rich cortical zone (Figure 5F).
Discussion
The allelic series we have generated demonstrates that incremental reductions in Wdr1 function produce a phenotypic gradient. Deletion of cofilin 1 in mice causes death at embryonic day 9.56 and, as might be expected for a key regulator of cofilin activity, Wdr1 is also required for mammalian embryogenesis. The fact that Wdr1gt/gt embryos die before embryonic day 10.5 suggests that loss of Wdr1 might affect the ability of all 3 mammalian cofilins to function, resulting in earlier death than that caused by the loss of cofilin 1 alone.
Less severe mutations in Wdr1, however, reveal cellular weak spots in the process of actin filament control that result in 2 major disease phenotypes: autoinflammatory disease and macrothrombocytopenia. Transplantation confirmed that both are bone marrow intrinsic, and neither is dependent on the presence of functional B- or T-cells. Our data suggest that they are independent pathologic processes resulting from major functional defects in 2 very different and highly specialized cell types: neutrophils and megakaryocytes.
Several studies have implicated cofilin in the regulation of neutrophil actin dynamics,36,37 and because the only known role of Wdr1/Aip1 is to interact with cofilin and promote its activity, this would suggest that it is the cofilin/Wdr1 complex that plays a central role in the cytoskeletal behavior of the neutrophil. The mislocalization of cofilin and the impaired ability to depolymerize actin that we observed in Wdr1rd/rd neutrophils support this notion. How defects in neutrophil function might contribute to autoinflammatory disease is less clear. Despite impaired chemotaxis, Wdr1rd/rd neutrophils accumulate at sites of inflammation in vivo, suggesting that the cytoskeletal defects they exhibit in vitro are symptomatic of a broader functional deficit, one with a complex etiology and profound in vivo consequences. We hypothesize that Wdr1rd/rd neutrophils are intimately involved in the initiation and amplification of an abnormal inflammatory signal in which their malfunction promotes tissue damage and an inflammatory response that is unable to be resolved. Failure of Wdr1rd/rd neutrophils to undergo constitutive apoptosis may play a role in this process. Delayed neutrophil apoptosis has been observed in several neutrophilic inflammatory disorders,51,52 and it is becoming increasingly clear that the actin cytoskeleton plays a significant role in apoptosis (reviewed in53 ). It was recently demonstrated that mitochondrial translocation of cofilin is an early step in the induction of apoptosis, and that overexpression of constitutively active cofilin can induce apoptosis.54 Whether neutrophils fail entirely to undergo apoptosis, or instead proceed though an incomplete or abnormal apoptotic process, the release of pro-inflammatory mediators may be responsible for driving the chronic autoinflammatory disease observed in Wdr1rd/rd mice.
The other major cell type clearly affected by subtle reductions in Wdr1 levels is the megakaryocyte. Our histologic and ultrastructural studies demonstrate a profound megakaryocytosis in Wdr1rd/rd, characterized by gross morphologic abnormalities and the presence of cytoplasmic fragments throughout the bone marrow and spleen. Taken together, our data suggest that Wdr1rd/rd megakaryocytes undergo a protracted, disturbed maturation, and eventually proceed through an abortive platelet shedding process. The result is macrothrombocytopenia. In humans, it is well-recognized that MPV is elevated in patients with increased platelet production, usually in the context of accelerated destruction, such as in chronic immune thrombocytopenia, or in myeloproliferation. However, the data in mice are less clear-cut. There is good evidence that MPV is increased under conditions of emergency thrombopoiesis.55,56 We have observed this in wild-type mice stressed via treatment with antiplatelet serum (data not shown). Nonetheless, steady-state thrombocytopenia is not always linked to an increase in MPV.57 Given the profound megakaryocyte cytoplasmic maturation defects observed in Wdr1rd/rd mice, and the equally profound morphologic defects seen in Wdr1rd/rd platelets, the most likely explanation for the increase in MPV is the production of morphologically irregular platelets by grossly abnormal megakaryocytes.
The impaired cytoplasmic maturation of Wdr1rd/rd megakaryocytes is striking, and although there exist several other mouse models in which irregular maturation results in thrombocytopenia, the morphologic defects observed in Wdr1rd/rd mice are unique. Disorganized and malformed demarcation membranes have previously been described in megakaryocytes from mice with mutations in the transcription factors GATA-1,49 Fli-1,58 and NF-E2.59 The DMS defects seen in Wdr1rd/rd megakaryocytes are probably most reminiscent of those described in GATA-1 mutant animals, which exhibit a profound immaturity of the DMS and its absence from parts of the cytoplasm. Most remarkable, however, are the large cytoplasmic regions in Wdr1rd/rd megakaryocytes that are devoid not only of DMS but also of all granules, organelles, and membranes. The GATA-1 mice also exhibit wide regions of organelle-depleted peripheral zone, but not on the scale of that seen in Wdr1rd/rd megakaryocytes, in which, in several instances, up to half the cytoplasm was observed to form these cytoplasmic voids. We hypothesize that the large megakaryocytic cytoplasmic fragments present in the spleen and bone marrow represent these voids after shedding, because they are presumably unable to proceed through the complex cytoskeletal rearrangements required to produce platelets. These processes require a massive cytoplasmic reorganization,60,61 and the actin cytoskeleton has long been assumed to play a key role in their execution. However, the exact nature of its contribution is still unknown. Drugs such as the cytochalasins that interfere with actin polymerization have been shown to inhibit proplatelet extension,60 and recent evidence suggests that actin filament assembly adjacent to the DMS is required for proplatelet formation.62 Our results demonstrate that in the absence of Wdr1 and normal actin cytoskeleton function, this membrane structure is unable to develop properly at all. Presumably, in Wdr1rd/rd mice, the subsequent formation of proplatelet extensions, which is thought to be independent of actin, is impaired and aberrant platelet shedding occurs. Thus, while recent studies implicate cofilin in platelet function,30,–32,63 our data demonstrate an essential role for the cofilin/Wdr1 axis much earlier in their genesis. These results identify Wdr1 as a pivotal mediator of terminal stages in megakaryocyte development, and suggest cofilin is a key downstream effector of the signaling pathways that control their maturation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. Woodward, D. Skapura, M. Ross, C. Mason-Garrison, and M. White for excellent technical assistance; S. Ross, T. Carle, C. Evans, and M. Salzone for expert animal husbandry; J. Scott for flow cytometry assistance; C. Haueter for electron microscopy; and R. Walden for histology. The authors gratefully acknowledge D. Hilton for insightful discussions, and J. Bamburg and A. Nurden for comments on the manuscript.
This work was supported by grants from the National Institutes of Health (U01 HD39372 to M.J.J.; T32-CA-09598–16 to A.D.P.), the Australian Research Council (Queen Elizabeth II Fellowship to B.T.K.), the Leukemia Research Foundation (Postdoctoral Fellowship to B.T.K.), the AHA Texas Affiliate (0255899Y, 0455143Y to S.S.W.), the M.D. Anderson Cancer Center (Institutional Grants Program to S.S.W.), the American Legion (Auxiliary Award to A.D.P.), and the Australian Department of Education, Science and Training (Australian Postgraduate Award to R.A.S.).
National Institutes of Health
Authorship
Contribution: B.T.K. designed and performed research, analyzed data, and wrote the paper. M.J.J. designed research, obtained funding, analyzed data, and wrote the paper. S.S.W. designed research and analyzed data. I.P.W. and P.N. analyzed data. A.D.P., R.A.S., D.F.F., L.H.T., W.S.S., and J.Z. performed research and analyzed data. T.A.W., L.A.M., and K.J.H. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benjamin T. Kile, Division of Molecular Medicine, The Walter and Eliza Hall Institute, Parkville, Victoria 3050, Australia; e-mail:kile@wehi.edu.au.