Abstract
The signal transduction pathways that lead activated natural killer (NK) cells to produce cytokines, releases cytotoxic granules, or do both, are not clearly dissected. For example, phosphoinositide 3-kinases (PI3Ks) are key players in the execution of both functions, but the relative contribution of each isoform is unknown. We show here that the catalytic isoform p110δ, not p110γ, was required for interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and granulocyte macrophage colony-stimulating factor (GM-CSF) secretion, whereas neither was necessary for cytotoxicity. Yet, when both p110δ and p110γ isoforms were inactivated by a combination of genetic and biochemical approaches, cytotoxicity was decreased. NK-cell numbers were also affected by the lack of p110δ but not p110γ and more severely so in mice lacking both subunits. These results provide genetic evidence that p110δ is the dominant PI3K isoform for cytokine secretion by NK cells and suggest that PI3Ks cooperate during NK-cell development and cytotoxicity.
Introduction
Cellular cytotoxicity and production of inflammatory cytokines are the 2 main functions of natural killer (NK) cells. Although distinct cell subpopulations can specialize in one function rather than the other,1-3 most NK cells develop the potential to do both. Multiple signal transduction pathways can activate NK cells, including some that are also involved in B and T lymphocyte stimulations.4 Some of the pathways are independent of Syk-family kinases and revolve around phosphoinositide 3-kinases (PI3Ks).5,6 PI3Ks are divided into 3 subclasses on the basis of their structure. Class I PI3Ks, which are the most studied in mammalian cell signaling, are further subdivided in 2 groups. Class IA consists of 5 isoforms of regulatory subunits (p85α, p50α, p55α, p85β, and p55γ) and 3 isoforms of catalytic subunits (p110α, p110β, and p110δ), which can interact in various combinations and are mostly activated downstream of tyrosine kinase–associated receptors for growth factors, antigens, cytokines, and costimulatory molecules.7 Class IB includes only one regulatory isoform (p101) and one catalytic isoform (p110γ), and they are mostly activated downstream of G-coupled protein receptors.8 P110γ and p110δ are expressed primarily in leukocytes, whereas p110α and p110β are expressed in all cells. The recruitment and activation of class I PI3Ks take place through adaptor molecules containing PI3K binding motif (YXXM), which interacts with SH2 domains of the regulatory subunits. Activated class I PI3Ks convert phosphatidylinositol-4,5-bisphospate (PIP2) to phosphatidylinositol-3,4,5-trisphospate (PIP3).9 PIP3 acts as a binding site for various intracellular molecules containing pleckstrin-homology (PH) domains that mediate crucial downstream effector functions. For example, AKT/PKB,10 Vav,11 BTK (Bruton agammaglobulinemia tyrosine kinase), ITK (IL-2–inducible T-cell kinase),12 and PLCγ (phospholipase C-γ) contain PH domains. Class II and III PI3Ks regulate vesicle trafficking,13 but little is known about their role in lymphocytes.
Class I PI3Ks regulate development, differentiation, and activation of T and B lymphocytes. The lack of p110α or p110β is incompatible with life,14,15 and therefore the precise assessment of the role of these isoforms in lymphocytes awaits conditional gene-targeting technology. The lack of p85α, p110γ, or p110δ disrupts various aspects of B- and T-cell development and functions.16-19 In addition, mice lacking both p110γ and p110δ had more severe defects in thymopoiesis.20,21
PI3Ks are key players in effector functions of human5,22,23 and mouse NK cells, including spontaneous and antibody-dependent cell cytotoxicity (ADCC),4,24,25 as well as IFN-γ production.26 That PI3Ks mediate cytokine production in NK cells is also highlighted by the increased production in mice lacking a negative regulator of PI3K, Src homology 2-containing inositol 5′-phosphatase 1 (SHIP-1).27 However, these findings have not yet been validated in vivo in PI3K mutant mice, nor has the issue of PI3K isoform specificity been addressed in NK cells, except for our recent work showing that p110δ is essential for NK cell immunity to transplanted lymphomas.28 The only other references to NK cell biology found in published studies of PI3K mutant mice reported no defect in NK-cell development or differentiation in p85α-deficient mice.16,29 Filling this gap in the knowledge of NK-cell biology is relevant to preclinical models, as selective PI3K inhibitors are emerging as a new generation of therapeutics.30-32 Therefore, we set out to assess the impact that the lack of p110γ, p110δ, or both had on NK cellularity, differentiation, cytotoxicity, and IFN-γ production in gene-targeted mice.
Materials and methods
Mice and cell lines
P110γ-deficient mice, p110δ-deficient mice, and p110γ and p110δ double-deficient mice were previously described.17,20,33 P110γ-deficient mice (C57BL/6J [B6] and B6 × 129/Sv), p110δ-deficient mice (B10.D2 and B6 × 129/Sv), and their relative wild-type (WT) controls were maintained under specific-pathogen-free conditions at the Babraham Institute (Cambridge, United Kingdom). All animal experiments were approved and performed in accordance with United Kingdom Home Office and institutional guidelines. The mice used in each experiment were sex and age matched. YAC-1 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in RPMI1640 (Gibco, Grand Island, NY) supplemented with 10% FCS (TCS Cellworks, Buckingham, United Kingdom), 10 μM 2-mercaptoethanol (Gibco), 100 U/mL penicillin (Gibco), and 100 U/mL streptomycin (Gibco), which we refer to as complete RPMI.
Flow cytometry
Antimouse NK1.1 (clone PK136), CD49b (DX5), CD3ϵ (145-2C1), Ly49CI (5E6), Ly49G2 (4D11), Ly49D (4E5), Ly49CFHI (14B11), CD16/32 (2.4G2), CD43 (S7), NKG2ACE (20d5), and NKG2D (CX5) monoclonal antibodies (mAbs), and isotype-matched controls were purchased from BD Bioscience (Oxford, United Kingdom) or eBioscience (San Diego, CA). The Abs were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), peridinin chlorophyll protein-cyanine 5.5 (PerCPCy5.5), or biotin. Fresh single-cell suspensions of spleens were prepared after lysing red blood cells by hypotonic ammonium chloride solution. Live splenocytes were counted by trypan blue exclusion, stained, and analyzed by flow cytometry. The numbers of T, NK, and NKT cells were determined by multiplying the percentage of gated cells by the total number of live leukocytes. Flow cytometry was performed with a FACSCalibur or an LSRII (BD Bioscience) and analyzed with FlowJo software (TreeStar, Ashland, OR).
Cytotoxicity assay
Chromium release assays were performed following a standard protocol. In brief, NK cells were enriched from spleen by anti-DX5 antibody microbeads or NK-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) after lysis of red blood cells by distilled water and either used for assays or cultured for 1 week in complete RPMI with 1000 U/mL hrIL-2 (Chiron, Berkshire, United Kingdom). In some experiments, 10 μM broad-spectrum PI3K inhibitor LY294002 (Calbiochem, San Diego, CA) or 0.05 to 30 μM p110γ-specific inhibitor AS252424 (Merck Serono, Geneva, Switzerland) was added to NK cells for 30 minutes at 37°C prior to the assay. DMSO was used as vehicle control in the media. As target cells, YAC-1 cells were labeled with 51Cr (Amersham, Freiburg, Germany) at 100 μCi (3.7 MBq)/1 × 106 cells and cocultured at 5000 cells/well with serially diluted NK cells for 4 hours. The γ-scintillation of supernatant was measured by TopCount.NXT (Packard, Schwadorf, Austria). Percentage lysis was calculated as follows: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Detection of intracellular IFN-γ
Fresh NK cells were enriched from spleens by anti-DX5 antibody microbeads using magnetic-activated cell sorting (MACS; Miltenyi Biotec) and stimulated in 96-well tissue culture plates that had been precoated with 10 μg/mL anti-NK1.1 mAb (clone PK136; BD Bioscience) or with isotype-matched control in complete RPMI with or without 1000 U/mL human recombinant IL-2 (hrIL-2; Chiron) for 5 hours. Brefeldin A (10 μ/mL; Sigma) was added for the last 4 hours and 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) and 1 μg/mL ionomycin (Sigma) were added as a positive control. In some experiments, 10 μM broad-spectrum PI3K inhibitor LY294002 (Calbiochem) or 1 μM p110γ-specific inhibitor AS252424 (Merck Serono) was added to NK cells for 5 hours. DMSO was used as the vehicle control in the media. Cells were fixed with 4% paraformaldehyde in PBS after cell surface staining and stained intracellularly with anti–IFN-γ antibody (clone XMG1.2; BD Bioscience) in 0.5% saponin in PBS. Fc blocking Ab (clone 2.4G2) was added immediately before every staining step. Flow cytometry was performed as described. Cells were analyzed by flow cytometry, and NK cells were gated as NKG2D+ and CD3−.
ELISA
NK cells were enriched from spleens by NK isolation kit (Miltenyi Biotec) after lysis of red blood cells by hypotonic ammonium chloride solution and cultured for 1 week in complete RPMI containing 1000 U/mL hrIL-2 (Chiron). After 1-week culture, the percentage of CD3−NK1.1+ NK cells was more than 95%. NK cells (5 × 104 cells/well) were stimulated with 1 μg/mL purified anti–mouse NK1.1, CD16, or Ly49D mAbs (BD Bioscience), and plated in 96-well tissue culture plates that had been precoated with 1 μg/mL goat F(ab)2 anti–mouse or 1 μg/mL goat F(ab)2 anti–rat IgG (Caltag). For the stimulation with 10 μg/mL purified anti–mouse NKG2D mAb (eBioscience), N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTAP; Sigma) was precoated.26 The cells were incubated in complete RPMI with 1000 U/mL hrIL-2 for 48 hours. Mouse IL-12 (Peprotech, Rocky Hill, NJ) at 0.2 μg/mL was added as a control. In some experiments, 100 ng/mL PMA and 1 μg/mL ionomycin were added for the last 4 hours. Sandwich enzyme-linked immunosorbent assay (ELISA) for IFN-γ was performed with purified antimouse IFN-γ (clone R4–6A2; BD Bioscience), biotin-conjugated antimouse IFN-γ (clone XMG1.2; BD Bioscience), and streptavidin-alkaline phosphatase (BD Bioscience) and developed with p-nitrophenyl phosphate substrate (Sigma). ELISA for TNF-α and granulocyte macrophage colony-stimulating factor (GM-CSF) was performed using DuoSet ELISA Development Systems (R&D Systems, Abington, United Kingdom) and TNB substrate (BD Bioscience). The amount of IFN-γ, TNF-α, or GM-SCF in the supernatants was calculated based on standard curves measured by MultiskanEX (Labsystems, Helsinki, Finland).
Detection of cell death
NK cells were enriched, cultured, and stimulated as described. After 40 hours of stimulation, the cells were stained with FITC-conjugated annexin V (BD Bioscience) and propidium iodide (PI; Sigma) following the manufacturer's instructions. Cell death was measured by flow cytometry immediately after staining.
Statistics
Statistical analysis was performed by 2-tailed Student t test using Excel software (Microsoft, Seattle, WA) or using SPSS software (SPSS 2005; SPSS, Chicago, IL).
Results
PI3K p110δ regulates NK-cell numbers and receptor expression
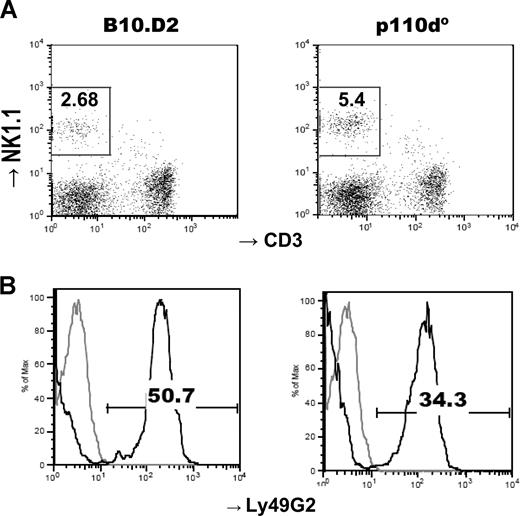
We first enumerated DX5+CD3− NK and DX5+CD3+ NKT cells in a set of B6 × 129 mice. The cellularity in p110γ-deficient mice was not significantly different from WT controls, but p110δ-deficient mice had about 40% fewer NK cells and about 60% fewer NKT cells (Table 1). The NK-cell reduction, but not the NKT-cell reduction, was more severe in p110γδ double-deficient mice. We next enumerated NK1.1+CD3− NK and NK1.1+CD3+ NKT cells in single gene-deficient mice on a B6 or B10.D2 genetic background. The results were in line with those obtained in mice on a mixed background. NK- and NKT-cell numbers were normal in p110γ-deficient mice, whereas p110δ-deficient mice displayed reduced numbers of NK and NKT cells, although only the decrease of NKT cells was statistically significant (Table 2). Collectively, these results show that p110δ is an important regulator of NK- and NKT-cell development. The data from the double-deficient mice also suggest possible redundancy between p110γ and p110δ, at least for NK-cell development. We next analyzed the expression of a panel of cell surface receptors. Although most of the receptors enumerated exhibited normal levels of expression, some were abnormally expressed, including Ly49G2, Ly49C/I, and the late differentiation markers CD11b/CD43 (Table 3; Figure 1; and data not shown). Although these results do not clearly identify a stage of development where PI3Ks may play key roles, they do suggest that p110γ and p110δ participate in NK-cell differentiation.
Reduced Ly49G2 expression in NK cells in p110δ-deficient mice. (A) Lymphocytes were gated by forward and side scatters on freshly explanted splenocytes and further gated on NK1.1+CD3− (boxes) for analysis of NK-cell receptor expression. The numbers within the quadrants represent percentages of NK cells. (B) Ly49G2 receptor expression on NK cells (dark lines). Gray lines represent isotype control staining. One representative of 4 mice per group is shown. The numbers above the brackets represent percentages of NK cells positive for Ly49G2.
Reduced Ly49G2 expression in NK cells in p110δ-deficient mice. (A) Lymphocytes were gated by forward and side scatters on freshly explanted splenocytes and further gated on NK1.1+CD3− (boxes) for analysis of NK-cell receptor expression. The numbers within the quadrants represent percentages of NK cells. (B) Ly49G2 receptor expression on NK cells (dark lines). Gray lines represent isotype control staining. One representative of 4 mice per group is shown. The numbers above the brackets represent percentages of NK cells positive for Ly49G2.
Redundant functions for p110γ and p110δ during NK-cell cytotoxicity
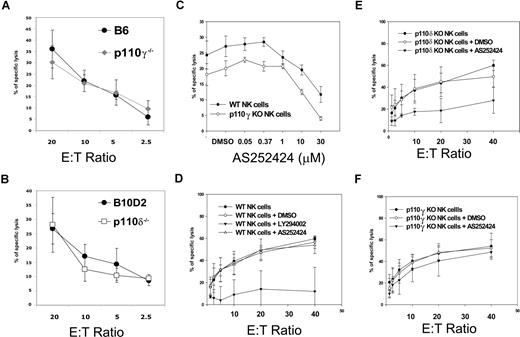
Fresh NK cells and IL-2–activated NK cells from p110γ- or p110δ-deficient mice did not show any defect in cytotoxicity against YAC-1 cells (Figure 2A,B and data not shown) or against RMA-S cells (data not shown), suggesting that either other class IA catalytic PI3K isoforms selectively control NK cytotoxicity or that the various isoforms are, in general, redundant for this function. To test for redundancy between p110γ and p110δ, we used a small-molecule inhibitor specific for p110γ, AS252424. Doses higher than 10 μM nonspecifically inhibited cytotoxicity in p110γ-deficient cells at a fixed E/T ratio, whereas 1 μM had a slight effect on WT NK cells (Figure 2C). Thus, we decided to use this low dose of AS252424 because of no obvious off-target effects in order to ask the question whether p110δ-deficient and WT NK cells were differentially sensitive to such a low dose of AS252424. The general PI3K inhibitor LY294002 reduced cytotoxicity in WT cells, confirming that PI3Ks are crucial in this process (Figure 2D), while 1 μM AS252424 had little effect across various E/T ratios. However the same dose of AS252424 did reduce cytotoxicity in p110δ-deficient NK cells (Figure 2E) and not in WT or p110γ-deficient NK cells (Figure 2F). These data suggest that p110γ and p110δ have redundant functions during NK-cell cytotoxicity.
Redundant roles of p110γ and p110δ in spontaneous cytotoxicity. Standard chromium release assays with YAC-1 target cells. E/T ratios are based on actual NK- cell numbers as assessed by flow cytometry. (A) WT (●) and p110γ-deficient B6 IL-2–activated NK-cells ( ). (B) WT (●) and p110δ-deficient (□) B10.D2 IL-2–activated NK cells. Each data point is the mean of triplicates of 1 representative of 3 independent experiments. Error bars represent plus or minus SD (C) WT and p110γ-deficient B6 fresh NK cells were preincubated with the indicated concentrations of p110γ-specific inhibitor AS252424 and then coincubated with target cells at a 5:1 E/T ratio. The dose of 1 μM was chosen for further experiments because the upper dose of 10 μM inhibited cytotoxicity in p110γ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD. (D-F) Fresh NK cells of the indicated genotypes were preincubated with media, 10 μM LY294002, or 1 μM AS252424 and then coincubated with target cells at the indicated E/T ratios. LY294002 inhibited cytotoxicity in WT cells, while AS252424 inhibited cytotoxicity only in p110δ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD.
). (B) WT (●) and p110δ-deficient (□) B10.D2 IL-2–activated NK cells. Each data point is the mean of triplicates of 1 representative of 3 independent experiments. Error bars represent plus or minus SD (C) WT and p110γ-deficient B6 fresh NK cells were preincubated with the indicated concentrations of p110γ-specific inhibitor AS252424 and then coincubated with target cells at a 5:1 E/T ratio. The dose of 1 μM was chosen for further experiments because the upper dose of 10 μM inhibited cytotoxicity in p110γ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD. (D-F) Fresh NK cells of the indicated genotypes were preincubated with media, 10 μM LY294002, or 1 μM AS252424 and then coincubated with target cells at the indicated E/T ratios. LY294002 inhibited cytotoxicity in WT cells, while AS252424 inhibited cytotoxicity only in p110δ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD.
Redundant roles of p110γ and p110δ in spontaneous cytotoxicity. Standard chromium release assays with YAC-1 target cells. E/T ratios are based on actual NK- cell numbers as assessed by flow cytometry. (A) WT (●) and p110γ-deficient B6 IL-2–activated NK-cells ( ). (B) WT (●) and p110δ-deficient (□) B10.D2 IL-2–activated NK cells. Each data point is the mean of triplicates of 1 representative of 3 independent experiments. Error bars represent plus or minus SD (C) WT and p110γ-deficient B6 fresh NK cells were preincubated with the indicated concentrations of p110γ-specific inhibitor AS252424 and then coincubated with target cells at a 5:1 E/T ratio. The dose of 1 μM was chosen for further experiments because the upper dose of 10 μM inhibited cytotoxicity in p110γ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD. (D-F) Fresh NK cells of the indicated genotypes were preincubated with media, 10 μM LY294002, or 1 μM AS252424 and then coincubated with target cells at the indicated E/T ratios. LY294002 inhibited cytotoxicity in WT cells, while AS252424 inhibited cytotoxicity only in p110δ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD.
). (B) WT (●) and p110δ-deficient (□) B10.D2 IL-2–activated NK cells. Each data point is the mean of triplicates of 1 representative of 3 independent experiments. Error bars represent plus or minus SD (C) WT and p110γ-deficient B6 fresh NK cells were preincubated with the indicated concentrations of p110γ-specific inhibitor AS252424 and then coincubated with target cells at a 5:1 E/T ratio. The dose of 1 μM was chosen for further experiments because the upper dose of 10 μM inhibited cytotoxicity in p110γ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD. (D-F) Fresh NK cells of the indicated genotypes were preincubated with media, 10 μM LY294002, or 1 μM AS252424 and then coincubated with target cells at the indicated E/T ratios. LY294002 inhibited cytotoxicity in WT cells, while AS252424 inhibited cytotoxicity only in p110δ-deficient NK cells. Each data point is the mean of 3 independent experiments. Error bars represent plus or minus SD.
Normal intracellular IFN-γ by IL-2–activated mutant NK cells
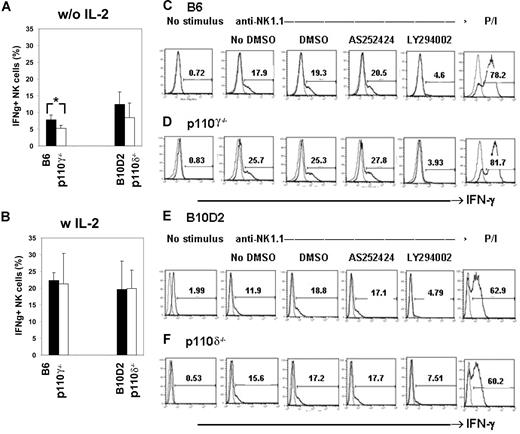
To investigate the role of p110γ and p110δ in cytokine production, we visualized intracellular IFN-γ by flow cytometry. When p110γ- or p110δ-deficient NK cells were stimulated by anti-NK1.1 mAb in the absence of IL-2, the percentage of IFN-γ+ cells was reduced in both cases, although such reduction was statistically significant only for p110γ-deficient cells (Figure 3A). Nevertheless, the percentage of IFN-γ+ NK cells and the mean fluorescence intensity were normal in both mutant populations stimulated in the presence of IL-2 (Figure 3B and data not shown). To test the possibility of redundancy between the 2 isoforms in this function, the same dose of p110γ-specific inhibitor AS252424 that inhibited cytotoxicity in p110δ-deficient NK cells (Figure 2E) was used in this assay (1 μM), and compared with 10 μM LY294002. The latter caused a strong reduction in the frequency of IFN-γ+ NK cells in all genotypes (Figure 3C-F). On the contrary, the p110γ-specific inhibitor AS252424 did not have an effect, at this dose, on any of the genotypes (Figure 3C-F). Thus, although simultaneous inactivation of both p110γ and p110δ had a reproducibly significant effect on cytotoxicity (Figure 2E), it did not appear to reduce IFN-γ production upon receptor stimulation and IL-2 costimulation. However these results do not address the question whether secretion of cytokine proceeds normally in mutant cells.
Intracellular IFN-γ production. (A) The graph shows the summary of intracellular IFNγ in p110γ- or p110δ-deficient NK cells in the absence of IL-2. (B) The graph shows the summary of intracellular IFNγ in p110γ- or p110δ-deficient NK cells in the presence of IL-2. Means and SDs are shown (n = 4-8). The ordinates indicate IFN-γ+ NK-cell population (%). *P = .016. (C-F) NK cells of the indicated genotypes were treated with DMSO, 10 μM LY294002, or 1 μM AS252424 and stimulated with anti-NK1.1 mAb for 5 hours in the presence of IL-2. The figures indicate the percentage of IFN-γ+ cells (measured against an isotype control) within gated NKG2D+CD3− NK cells. One representative of 3 independent experiments for each genotype is shown.
Intracellular IFN-γ production. (A) The graph shows the summary of intracellular IFNγ in p110γ- or p110δ-deficient NK cells in the absence of IL-2. (B) The graph shows the summary of intracellular IFNγ in p110γ- or p110δ-deficient NK cells in the presence of IL-2. Means and SDs are shown (n = 4-8). The ordinates indicate IFN-γ+ NK-cell population (%). *P = .016. (C-F) NK cells of the indicated genotypes were treated with DMSO, 10 μM LY294002, or 1 μM AS252424 and stimulated with anti-NK1.1 mAb for 5 hours in the presence of IL-2. The figures indicate the percentage of IFN-γ+ cells (measured against an isotype control) within gated NKG2D+CD3− NK cells. One representative of 3 independent experiments for each genotype is shown.
Reduced cytokines in supernatant of p110δ-deficient NK cells
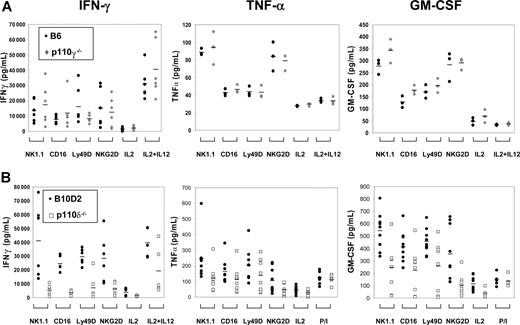
We quantified the amount of IFN-γ, TNF-α, and GM-CSF in culture supernatants upon various stimulations over a 48-hour incubation period. The amount of cytokine secreted by p110γ-deficient NK cells was normal (Figure 4A). In contrast, cytokine secretion by p110δ-deficient NK cells was remarkably decreased, despite culture with IL-2 (Figure 4B). Upon stimulation through NK1.1, CD16, Ly49D and NKG2D, IFN-γ and GM-CSF in the supernatants collected from p110δ-deficient NK-cell cultures were significantly reduced (P < .05 in all cases). We detected a reduction in TNF-α secretion in all stimulation conditions tested; however, the reduction was statistically significant only when cells were stimulated with anti-NK1.1. These results show that p110δ selectively regulates important steps that lead to secretion of cytokines.
Defective cytokine secretion by p110δ-deficient NK cells. Purified NK cells were cultured for 1 week in IL-2, and thereafter stimulated, in the presence of IL-2 with anti-NK1.1, CD16, Ly49D, and NKG2D mAbs, or IL-12 for 48 hours. Secreted IFN-γ, TNF-α, and GM-CSF proteins were measured by ELISA. (A) Cytokines secreted by p110γ-deficient ( ) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 1 experiment for TNF-α and GM-CSF (n = 3). Bars indicate the mean values of each group. (B) Cytokines secreted by p110δ-deficient (□) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 3 independent experiments for TNF-α and GM-CSF (n = 9). Bars indicate the mean values of each group.
) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 1 experiment for TNF-α and GM-CSF (n = 3). Bars indicate the mean values of each group. (B) Cytokines secreted by p110δ-deficient (□) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 3 independent experiments for TNF-α and GM-CSF (n = 9). Bars indicate the mean values of each group.
Defective cytokine secretion by p110δ-deficient NK cells. Purified NK cells were cultured for 1 week in IL-2, and thereafter stimulated, in the presence of IL-2 with anti-NK1.1, CD16, Ly49D, and NKG2D mAbs, or IL-12 for 48 hours. Secreted IFN-γ, TNF-α, and GM-CSF proteins were measured by ELISA. (A) Cytokines secreted by p110γ-deficient ( ) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 1 experiment for TNF-α and GM-CSF (n = 3). Bars indicate the mean values of each group. (B) Cytokines secreted by p110δ-deficient (□) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 3 independent experiments for TNF-α and GM-CSF (n = 9). Bars indicate the mean values of each group.
) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 1 experiment for TNF-α and GM-CSF (n = 3). Bars indicate the mean values of each group. (B) Cytokines secreted by p110δ-deficient (□) and WT NK cells (●). Data points represent individual samples pooled from 2 independent experiments for IFN-γ (n = 6), and 3 independent experiments for TNF-α and GM-CSF (n = 9). Bars indicate the mean values of each group.
Discussion
Knowledge of the cellular functions regulated by each PI3K isoform in leukocytes is a prerequisite for effective and safe therapeutic use of newly developed PI3K isoform-specific inhibitors.30-32 NK cells are central players in health and disease as they participate in transplantation, reproduction, infection, and cancer.3,34 The PI3K family is undoubtedly involved in NK-cell cytotoxicity and cytokine production, as shown by the use of broad-range inhibitors.4,5,23 However no data are available to elucidate the role of specific PI3K isoforms in these key NK functions in vivo. We show here that the class IA PI3K catalytic isoform p110δ is a key player in 3 aspects of NK-cell biology: development of normal cell numbers, differentiation, and secretion of cytokines.
The reduced cellularity in the NK and NKT compartments is reminiscent of the reduced cellularity in B and T cells in p110δ mutant mice.17,19 Although cell death and proliferation of p110δ-deficient NK cells was not grossly impaired in cultures (data not shown), reduced cellularity might be due to defective lymphocyte homeostasis in p110δ-deficient NK cells.
The establishment of the Ly49 receptor repertoire depends on the genetic background, and in particular, on the host MHC.35 Our results suggest that PI3Ks regulate NK differentiation but were not conclusive as to what specific stage. We are currently establishing lines of single- and double-gene mutant mice on a B6 background. This will allow us to elucidate the role of p110γ and p110δ in the establishment of the Ly49 repertoire.
The most striking result was the contrast between normal cytotoxicity and reduced IFN-γ secretion in p110δ-deficient NK cells. Given the reproducible reduction of cytotoxicity caused by broad-spectrum inhibitors across several human and murine systems,4,5,23 the lack of significant defects in YAC-1 or RMA-S killing by mutant NK cells was surprising. One possibility is that multiple PI3K isoforms can mediate cytotoxicity. Our data obtained by combining genetic and pharmacological inactivation of p110γ and p110δ support this notion and suggest that these 2 isoforms play redundant roles during cytotoxicity.
Cytotoxicity and cytokine production may be regulated by independent signal transduction pathways, even downstream of the same receptor. For example, Vav-1 was required for cytotoxicity but not IFN-γ production.36 On the contrary, DAP12 was required for IFN-γ secretion but not for cytotoxicity.25 CD45 was also required for cytokine production but not for cytotoxicity.37 Our results provide yet another example that cytotoxicity and cytokine production are differentially sensitive to perturbation of signaling pathways. Whether these are qualitative or quantitative differences is still unclear.
In a previous study, the broad-range PI3K inhibitor LY294002 blocked IFN-γ secretion upon NK1.1 stimulation.26 Our data show that both p110γ- and p110δ-deficient NK cells produced less intracellular IFN-γ upon NK1.1 stimulation. However, this reduction was corrected by the addition of IL-2. LY294002 caused a much greater reduction than that seen in the mutant cells, but the simultaneous inactivation of p110γ and p110δ did not perturb IFN-γ production. The simplest interpretation of these results is that both p110γ and p110δ participate in the production of IFN-γ downstream of NK1.1. IL-2 may restore normal IFN-γ production by activating multiple PI3Ks, thus recruiting compensatory mechanisms. LY294002 blocks all these mechanisms and causes severe reduction in IFN-γ secretion.
When the amount of IFN-γ protein in the supernatants was measured, a marked reduction emerged only in p110δ-deficient NK-cell cultures. In contrast to the intracellular IFN-γ production, this defect was not reversed by IL-2. Secretion of other cytokines, including TNF-α and GM-CSF, was also reduced, suggesting a more general defect. How do we explain the discrepancy between normal intracellular protein synthesis and decreased secretion? We propose 3 possibilities. First, p110δ may directly regulate de novo synthesis. Second, it may control the secretion process itself. Third, it may sustain cell survival, a variable that could be relevant to the longer incubation time of the ELISA. The third possibility was formally excluded, because no increased cell death was detected in either p110γ- or p110δ-deficient NK cells, as measured by annexin V and propidium iodide staining (data not shown). Initial experiments suggest that IFN-γ transcription may occur normally despite the absence of p110δ (data not shown), thus we favor the second possibility that is p110δ may regulate secretion.
PI3Ks are known to be involved in IFN-γ transcription and translation downstream of IL-238,39 and downstream of IL-12 in mice,40 but not in humans.41 P110δ plays a central role in T-cell cytokine production,42 as well as in secretion of TNF-α and IL-6 by mast cells.43 A role for PI3Ks as crucial mediators of cytokine production is highlighted also by the increased production of RNA and proteins reported in T cells lacking one copy of the genes encoding for the phosphatases that antagonize PI3K activity, that is, Pten and SHIP.44 Similarly, SHIP1-deficient mouse NK cells produced greater amount of IFN-γ upon stimulation with Ab-coated tumor cells plus IL-12.27
In conclusion, the discrepancy we report here between normal intracellular production and reduced secretion of IFN-γ and other cytokines by p110δ-deficient NK cells suggests that PI3K isoforms control signaling checkpoints during the progression from cytokine gene transcription to secretion in mouse NK cells. PI3K p110δ emerges as the dominant isoform for cytokine secretion by NK cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a BBSRC Core Strategic Fund and Cancer Research-UK Project Grant (F.C.).
We appreciate Anne Segonds-Pichon's insights for statistical analysis of the data, Geoff Morgan for flow cytometry, and the staff at the Small Animal Facilities in the Babraham Institute for mouse husbandry. We are also grateful to Simona Zompi for the initial studies on PI3K p110δ-deficient mice; to Karen Anderson, Phil Hawkins, and Len Stephens for advice; and to Klaus Okkenhaug and Christian Rommel for helpful discussions and for critically reading the paper.
Authorship
Contribution: N.K., A.S., and F.C. designed the research; N.K., A.S., and L.W. conducted the research; M.C. and T.R. discovered and characterized the PI3Kγ-inhibitor; E.H. produced the p110γ-deficient mice; M.T. produced the p110δ-deficient mice and gave insights; and N.K. and F.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Colucci, Laboratory of Lymphocyte Signalling and Development, Babraham Institute, Cambridge CB22 3AT, United Kingdom; e-mail: francesco.colucci@bbsrc.ac.uk.