Because nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) express CD20, rituximab may be used as a nonmutagenic treatment option to avoid late toxicities in this rather indolent entity. Between 1999 and 2004, the German Hodgkin Study Group (GHSG) investigated the activity of rituximab (375 mg/m2 in 4 doses) in a phase 2 trial in 21 relapsed or refractory NLPHL patients. The initial diagnosis of NLPHL was confirmed in 15 of the 21 enrolled patients by reference pathology. The remaining cases were reclassified as Hodgkin lymphoma transformed to T-cell rich B-cell lymphoma (TCRBCL; n = 2) or CD20+ classical Hodgkin lymphoma (cHL; n = 4). In NLPHL patients the overall response rate was 94%, including 8 complete remission (CR) and 6 partial remission (PR). With a median follow-up of 63 months (range, 3-84), the median time to progression was 33 months, with the median overall survival (OS) not reached. Thus, rituximab is highly effective in relapsed and refractory NLPHL. This study is registered at http://www.klinisches-studienzentrum.de/trial/285.
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) accounts for approximately 5% of Hodgkin lymphoma (HL) and has a typical immunophenotype of the malignant cell po-pulation (CD30−CD15−CD20+) as compared with cHL (CD30+CD15+CD20−).1,2 NLPHL can resemble T-cell rich B-cell lymphoma (TCRBCL)3 and often presents in early stages with an indolent course and excellent prognosis.4,5 Early-stage NLPHL patients treated with chemotherapy are more likely to die from secondary malignancies and cardiovascular disease compared with primary neoplasm.1 The slow disease progression and the CD20+ tumor cells in NLPHL prompted us to evaluate rituximab in a phase 2 clinical trial.6 Here we report the 7-year follow-up of this trial showing that single agent rituximab has substantial and long-lasting antitumor effects in NLPHL and CD20+ classical Hodgkin lymphoma (cHL) patients when given at standard doses.
Methods
Patients were enrolled between 1999 and 2004 at 17 different centers. Eligibility criteria included NLPHL at first or higher relapse or progressive disease after at least one standard regimen. In addition, at least 30% of tumor cells had to stain positive for CD20. All histologic slides were reviewed by an independent expert panel consisting of 6 reference pathologists. Patients received 4 weekly infusions of standard dose rituximab at 375 mg/m2. The study was approved by the institutional review board at each study site, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Remission status according to the International Workshop Criteria was checked 4 weeks after the end of treatment, and then every 3 months for 2 years, every 6 months until the fifth year, and once a year later on. Time-to-progression (TTP) and overall survival (OS) were analyzed using the Kaplan-Meier method. A complete description of the design of this phase 2 multicenter study was reported elsewhere.6
Results and discussion
Initially, 21 patients with NLPHL (19 males, one female) were included. The diagnosis of NLPHL was confirmed in 15 (71%) of these patients by expert reference pathology. The remaining patients were either reclassified as HL transformed to TCRBCL (n = 2) or CD20-positive cHL (n = 4) and excluded from the analysis of response, TTP, and OS. The characteristics of the confirmed NLPHL patients are listed in Table 1. Median time after first diagnosis was 12 years (range, 0.5-21 years). At the time of study entry all NLPHL patients were in first to third relapse (median = 2). Nine of 15 (60%) patients had stage I/II disease, and 6/15 (40%) had advanced stage III/IV disease.
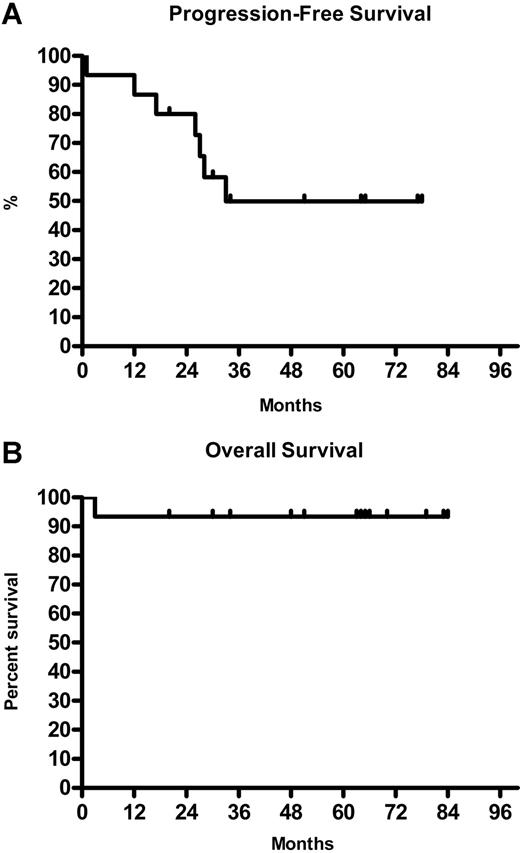
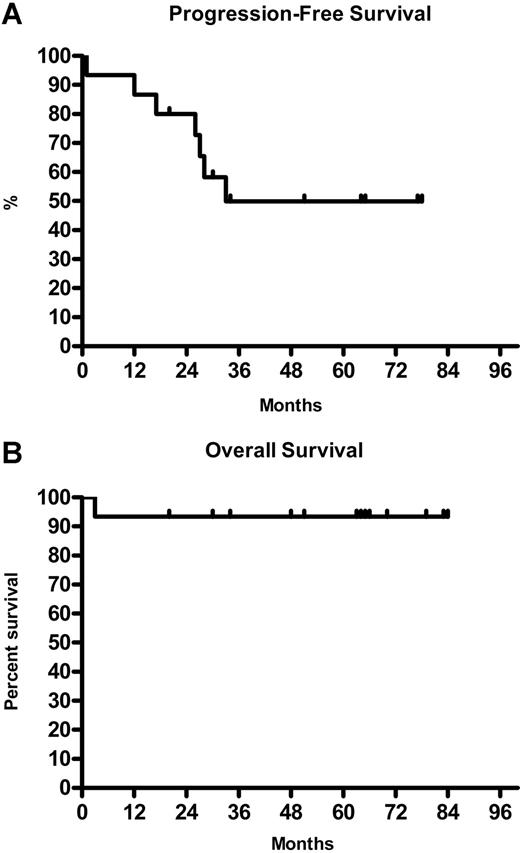
The overall response for 15 NLPHL patients was 94% (8 CR, 6 PR). All 9 patients with localized stage I/II responded to treatment (5 CR and 4 PR). In advanced stage III/IV the response rate was 83% (3 CR, 2 PR) (Table 1). The median TTP was 33 months with the median OS not reached (Figure 1A,B).
Time to progression and overall survival of NLPHL patients on rituximab. (A). Kaplan-Meier analysis of time to progression for 8 of 15 NLPHL patients. (B) Kaplan-Meier analysis of overall survival for 14 of 15 NLPHL patients.
Time to progression and overall survival of NLPHL patients on rituximab. (A). Kaplan-Meier analysis of time to progression for 8 of 15 NLPHL patients. (B) Kaplan-Meier analysis of overall survival for 14 of 15 NLPHL patients.
More patients with NLPHL die from late toxicities including cardiac failure and secondary neoplasms than from their underlying malignancy.7 Therefore, at least in early favorable NLPHL patients, several international groups have evaluated new treatment approaches with reduced toxicity to better avoid side-effects of standard treatment. Less toxic treatment options include watch and wait after complete lymphadenectomy in children and involved-field radiotherapy.8,9
The only other trial using rituximab in NLPHL reported 22 patients with either untreated or previously treated disease receiving standard dose rituximab.10 The ORR in this study was 100%. However, with a short median follow-up of 13 months, 9 patients had relapsed, resulting in a progression-free survival of 10.2 months compared with 33 months in our trial. In contrast to the present trial, more than half of the patients (12/22) in the trial by Ekstrand et al10 had been previously untreated.
In our trial the initial diagnosis of NLPHL was confirmed in 71% of cases by a panel of hematopathologists using conventionally and immunohistologically stained sections. In an international collaborative project on NLPHL this rate was even lower, with 219 of the 388 investigated cases confirmed (57%). This low rate underscores the relevance of reference pathology in this entity in order to differerentiate variants of cHL and TCRLBCL.11 The lack of expert reference pathology might, at least in part, explain the difference between this trial and the Stanford trial as there was no mandatory reference histology at study entry in the latter.
Thus, rituximab is an effective treatment option for NLPHL patients who do not respond to conventional therapy. Taking into account that patients with early-stage NLPHL have an excellent prognosis with a higher chance of dying from treatment-related mortality than from NLPHL, rituximab should be evaluated as frontline treatment in this patient group. It is important to note that a recent analysis of NLPHL and cHL patients treated in 3 GHSG study generations showed significantly better freedom from treatment failure (FFTF) and OS rates for NLPHL compared with cHL patients (92% vs 84%, P = .009; 96% vs 92%, P = .017).12 In addition, NLPHL patients also showed a tendency to better survival after relapse compared with cHL patients (P = .05) in the European Task Force on Lymphoma (ETFL) study.1
Four patients with relapsed or refractory CD20+ cHL were also treated in this trial. Two patients had stage II and 2 patients had advanced stage IV cHL. Three of the four patients achieved CR. Although only a minority of patients with cHL stain positive for CD20, rituximab might be considered as treatment option in patients having CD20+ malignant cells who relapse after high-dose chemotherapy or are not eligible for intensive chemotherapy. Recently, Younes et al observed efficacy of rituximab even in CD20-negative cHL suggesting an effect on the tumor microenvironment.13
Two of 21 patients had NLPHL transformed to TCRBCL. TCRBCL is a variant of diffuse large cell lymphoma with specific characteristic morphologic features, and transformation seems to occur in some NLPHL patients.5,14 The lymphoma cells resemble the lymphocytic and histiocytic (L&H) cells of NLPHL and share an abundance of reactive cells. These histologic similarities suggest a possible relationship between the 2 entities.15,–17 Both patients with advanced stage IV TCRBCL benefited from rituximab treatment, with ongoing CR at 73 and 70 months.
Four of 21 patients (1 NLPHL, 3 cHL) have died since enrollment. One patient with stage II cHL died in CR 16 months after treatment with rituximab due to an adenocarcinoma of the lung. Three patients with stage IV and stage II cHL and one patient with stage IV NLPHL died related to progressive disease.
In conclusion, therapy with rituximab is highly effective in relapsed or refractory NLPHL patients with respect to ORR, TTP, and OS. Rituximab is also efficient in CD20+ cHL and TCRBCL. Therefore, rituximab should be considered as a therapeutic alternative to aggressive salvage regimens in patients with relapsed and refractory NLPHL and in other CD20+ Hodgkin lymphoma. Based on the results of the present study, the GHSG is conducting a phase 2 study with rituximab as first-line treatment in stage I, A NLPHL patients without risk factors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Deutsche Krebshilfe grant KZ 70-2368 Di 8.
Authorship
Contribution: H.S. wrote the manuscript, collected data, and performed statistical analysis; U.R., F.M., T.E., C.D., R.S., and P.B. performed research; T.R. performed reference pathology; V.D. supervised the project, A.E. supervised the project and wrote the manuscript; M.R. performed research, wrote the manuscript, and supervised statistical analyses.
Conflict-of-interest disclosure: Roche supported the research and provided the drug supply.
Participants in the German Hodgkin Lymphoma Study Group (GHSG) may be found in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Correspondence: Marcel Reiser, MD, Department of Internal Medicine, University of Cologne, Cologne 50924, Germany; e-mail: Marcel.Reiser@uni-koeln.de.