Wiskott-Aldrich syndrome (WAS) is a rare X-linked immunodeficiency with microthrombocytopenia, eczema, recurrent infections, autoimmune disorders, and malignancies that are life-threatening in the majority of patients. In this long-term, retrospective, multicenter study, we analyzed events that occurred in 96 WAS patients who received transplants between 1979 and 2001 who survived at least 2 years following hematopoietic stem-cell transplantation (HSCT). Events included chronic graft-versus-host disease (cGVHD), autoimmunity, infections, and sequelae of before or after HSCT complications. Three patients (3%) died 2.1 to 21 years following HSCT. Overall 7-year event-free survival rate was 75%. It was lower in recipients of mismatched related donors, also in relation with an older age at HSCT and disease severity. The most striking finding was the observation of cGVHD-independent autoimmunity in 20% of patients strongly associated with a mixed/split chimerism status (P < .001), suggesting that residual-host lymphocytes can mediate autoimmune disease despite the coexistence of donor lymphocytes. Infectious complications (6%) related to splenectomy were also significant and may warrant a more restrictive approach to performing splenectomy in WAS patients. Overall, this study provides the basis for a prospective, standardized, and more in-depth detailed analysis of chimerism and events in long-term follow-up of WAS patients who receive transplants to design better-adapted therapeutic strategies.
Introduction
Wiskott-Aldrich syndrome (WAS; OMIM 301000) is a rare X-linked recessive disorder (incidence, 1-10:1 million) characterized by bleeding secondary to micro-thrombocytopenia as well as platelet dysfunction, defective lymphocyte function associated with recurrent infections, eczema, autoimmune manifestations, and later in life an increased incidence of lymphoma.1,,,,–6 The classic WAS phenotype manifests itself as early as the neonatal period with petechiae, bruises, bloody diarrhea, and infections such as purulent otitis media, pneumonia, and eczema. In classic WAS, mean platelet volume is 3.8 to 5.0 fL compared with 7.0 to 10.5 fL in healthy subjects.4 In general, affected patients demonstrate both cellular and humoral immunodeficiency leading to recurrent bacterial, viral, and fungal infections. Immunologic abnormalities of WAS include T-cell lymphopenia, defective proliferative response to CD3 cross-linking, impaired antibody response to polysaccharide antigens, defective monocyte chemotaxis, abnormalities of stimulated dendritic cells, and an increased lymphocyte apoptosis with age.7,,,,–12 Initially, most affected infants have normal number of circulating lymphocytes, but lymphopenia usually develops by age 6 to 8 years or earlier, possibly due to increased apoptosis.9,11,12 The gene responsible for WAS, the WAS protein gene (WASP), was cloned and sequenced in 1994.5 The WASP gene has 12 exons and encodes a 502 amino-acid protein (WASP), which is predominantly expressed in nonerythroid hematopoietic cells.
WASP is a member of a family of proteins involved in regulating actin polymerization. The dynamic nature of cytoskeletal changes plays a key role in a variety of cellular processes, including lymphoid and myeloid development, platelet production, efficient recruitment to the immune synapse formed between T- and antigen-presenting cells, as well as cell adhesion and migration through tissues and endothelial membranes, thus explaining the complexity of the clinical phenotype.
Because of the complex structure and multifunctional design of WASP and the variations in the clinical phenotype resulting from mutations of the WASP gene, the correlation between phenotype and genotype was examined by a number of investigators.5 Despite the initially contradictory reports, in-depth analysis of large cohorts of families with mutations leads to the conclusion that the clinical phenotype is strongly influenced by the effect of the mutation on the protein expression, correlating with a physician-graded scoring system (score from 1 to 5) of the severity of WAS-associated symptoms.13,,,,–18 The frequency and severity of infections, the extent of eczema, and the progression to autoimmune diseases and malignancies usually correlate with absence of WASP in patient lymphocytes (scores 4 and 5). In contrast, scores of 1 to 3 describe patients with milder forms of the disease.14,,,–18 While this approach can be useful in the clinical setting, caution is required because the protein expression/phenotype correlation is not absolute and patients with residual full-length protein may still develop severe manifestations.18,19 This relates presumably to the functionality of the residual protein and in part to secondary genetic influences and environmental factors. The prognosis of classic WAS with a complete absence of WASP expression in the absence of hematopoietic stem-cell transplantation (HSCT) is poor.3,19,20 Two major high-risk groups have been identified: patients with autoimmune manifestations and those with severe bleeding.19,20 HSCT is the only curative approach to WAS providing correction of the immunodeficiency and platelet disorder when appropriate myeloablative and immunoablative conditioning regimen is used.20,,,,,,,,–29
Patients with matched sibling or parent donors (MSD) and matched unrelated donors (URD) exhibit the highest survival rates up to 80%, especially if transplantation occurs at an early age with a URD.30,,–33 In the absence of a compatible donor, the use of a mismatched related donor (MMRD) is associated with a significantly lower survival rate.28,30,32,,,–36 Taking into account the complicated features of this disease, as well as the many different reasons for HSCT, we have addressed the question of the long-term outcome of these patients after transplantation in a multicenter retrospective study, particularly looking at the long-term outcome in patients surviving at least 2 years beyond HSCT, based on before/per/after HSCT events, such as donor compatibility, disease severity, and age at HSCT. The impact of splenectomy, relationship between the degree of chimerism and autoimmune manifestations following HSCT, immune reconstitution, as well as any other event seriously affecting the long-term outcome were also considered.
Methods
The institutional review boards of each of the 19 participating centers approved this study. For a list of the participating centers, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Informed consent was obtained in accordance with the Declaration of Helsinki.
Ninety-six WAS patients who underwent HSCT and were alive with a follow-up at least 2 years afterward were studied. The transplants were performed in 19 centers from 14 European countries between 1979 and 2001. In the same period of time, 49 other patients with WAS received transplants in these centers, but died within the first 2 years after HSCT. European centers known to have performed HSCT for this condition were identified from the Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation (EBMT) and from the European Society for Immunodeficiency (ESID) database on WAS. The collaborating centers completed a detailed questionnaire approved by the EBMT/ESID group for each patient. Data collection was completed in December 2004.
Patient characteristics
Patient characteristics are shown in Table 1. The diagnosis was confirmed by mutation analysis, family history, or by clinical and laboratory observations. Disease severity before transplantation was expressed as a score of 1 to 5 according to Ochs15 and Ochs and Thrasher.16 Splenectomy was performed before HSCT in 28 of 96 patients (29%). Age at transplantation ranged from 0.3 to 17.7 years (median, 2 years).
Procedures
Forty-five patients received an HSCT from an MSD. Of these, 42 were siblings and 3 were parents. Hematopoietic stem cell (HSC) source was bone marrow (BM) in 42 cases, or cord blood (CB) in 3 cases. Following failure of an MMRD HSCT, one patient was retransplanted with marrow from an MSD. Thirty-two patients received an HSCT from a URD. HSC source was BM (n = 24) or CD34 positively selected (n = 8) peripheral blood stem cells (PBSCs). Full compatibility data were available from 20 patients. Sixteen patients were 10/10 HLA antigen compatible, while 4 were HLA mismatched HLA at one locus. Nineteen patients received 22 HSCTs from an MMRD (BM n = 15, PBSC n = 5, BM + PBSC n = 2). All MMRDs were parents. Three patients had a second MMRD HSCT within the year following the first HSCT. Eighty-five patients received a conditioning regimen consisting of busulfan (16 or 20 mg/kg total dose) and cyclophosphamide (200 mg/kg total dose) in accordance with the EBMT guidelines.28,31,,,–35 In vivo immunosuppression (anti–LFA-1 monoclonal antibodies with or without anti-CD2), alemtuzumab monoclonal antibodies, or antithymocyte globulin was administered to 25 of 32 patients receiving a URD and 13 of 19 an MMRD HSCT, respectively. Other regimens included fludarabine (150 mg/m2 total dose) and melphalan (140 mg/m2 total dose). T-cell depletion was used in all of the MMRDs, in 5 of 32 URD, and in 2 of 45 MSD HSCTs. T-cell depletion methods included E-rosetting, monoclonal antibodies or, since 1996, positive selection of CD34+ cells. Methotrexate was given as graft-versus-host disease (GVHD) prophylaxis following HLA-identical HSCT and after 1983, cyclosporin A with or without a short course of methotrexate. Patients who received a T cell–depleted transplant using either monoclonal antibodies or E-rosetting received cyclosporin A. Recipients of CD34+ HSC were not given any further GVHD prophylaxis. GVHD was graded according to standard criteria. Overall, 18 of 45 recipients of MSD (40%), 13 of 32 recipients of URD (40%), and 7 of 19 recipients of MMRD (37%) HSCT, respectively, developed grade 2 or higher acute GVHD.
Assessment of HSCT outcome
Chimerism studies were performed by using several techniques, including karyotyping, assessment of red blood cell antigens, immunoglobulin allotypes, HLA-typing, Southern-blot hybridization, PCR analysis with microsatellite probes, FISH, allele-specific antibodies to HLA, and WASP protein staining. Full chimerism was defined by the presence of 95% or more donor blood cells. Mixed donor chimerism was defined as a variable percentage of donor cells ranging from 5% to 94% and split chimerism as the presence of donor T cells associated with host myeloid and B cells.
Immunologic investigations
T and B lymphocyte counts and function were analyzed by standard methods, ie, T- and B-cell enumeration using standard markers (CD3, CD4, and CD8), in vitro T-cell proliferation tests, serum immunoglobulin concentrations, and serum antibodies following immunization. T-cell recovery was defined as CD3 cell count of more than 1 G/L with CD4 cell count of more than 0.4 G/L, and positive mitogen and antigen-induced proliferations. B-cell function was deemed to be satisfactory if a patient was not receiving intravenous immunoglobulin. Ninety-six percent (43 of 45) in the MSD group, and 84% (27 of 32) in the URD groups had T- and B-cell recovery at 2 years after HSCT. In the MMRD group, 79% (15 of 19) had T-cell and 74% (14 of 19) B-cell recovery at 2 years following HSCT.
Event-free survival
Time to any adverse event seriously affecting the outcome was measured from 2 years after HSCT. For event-free survival (EFS), the following adverse events were taken into account: autoimmunity, active chronic GVHD (cGVHD), second HSCT, splenectomy after HSCT, death more than 2 years after HSCT, eczema lasting more than 2 years after transplant, severe infectious complications, and both disease- and transplant-related sequelae.
Statistics
Means were compared by the Student t test. Proportions were compared using the χ2 test for values of more than 5, Fisher exact test for those less than or equal to 5. The tests were 2-sided. The EFS was calculated using the product-limit method.37 Differences between the groups were assessed using the log-rank test.38 Confidence intervals (CIs) were calculated from standard errors (95% CI; 1.96 × SE). Multivariate analysis was performed using the Cox stepwise regression analysis to determine the independent contribution of each prognostic factor.39 Data analysis was performed using JMP version 6.0.3 statistical software (SAS Institute, Cary, NC).
Results
Survival, EFS, and chimerism
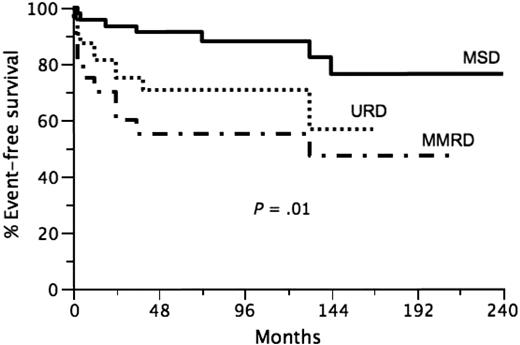
Of 96 patients who were alive at 2 years after HSCT, 93 are still alive with a median follow-up of 7 years (range, 2-25 years). Three late deaths were observed. One was due to fulminant meningococcal sepsis at 25 months after transplantation in an otherwise healthy but before HSCT splenectomized patient who was prescribed penicillin prophylaxis. Another patient received a second haploidentical transplantation 12 years after the first HSCT from the same donor and died of Nocardia and Actinomyces sepsis at day 75 after second HSCT. The third patient died from Addison disease at 21 years after HSCT because of poor compliance with treatment. The overall 7-year EFS was 75%(95% CI, 66%-84%). The 7-year EFS was significantly influenced by donor group: MSD HSCT 7-year EFS 88% (95% CI, 78%-98%), URD HSCT 7-year EFS 71% (95% CI, 55%-87%), and MMRD HSCT 7-year EFS 55% (95% CI, 33%-77%; Figure 1, P = .01). When each group is compared with the other, the P values are the following: MSD versus MMRD, P = .003; MSD versus URD, P = .03; URD versus MMRD, P = .28. Of note, the power of the latter comparison was low due to the more restricted number of patients in the MMRD group. The impact of age and disease-severity factors (age, disease severity, chimerism, autoimmunity, cGVHD, splenectomy) on the 7-year EFS in all patients and as a function of HSCT donor origin is presented in Tables 2 and 3. When considering all of the patients (Table 2), chimerism, autoimmunity, cGVHD and splenectomy had significant impact on 7-year EFS. However, when each group was analyzed separately, age at transplantation, disease severity, and splenectomy had a significant impact on EFS only in the MMRD group (Table 3). A multivariate analysis of impact of various factors on the 7-year EFS in all patients showed that chimerism and cGVHD were the 2 independent variables significantly affecting the EFS (Table 4). However, the nonsignificant impacts could be linked to a lack of power given the restricted number of patients in each group. Of the 96 patients analyzed, 78 had full chimerism and 18 had mixed or split chimerism at last report. No significant difference was observed in the percentages of full-donor chimerism among the 3 groups (Table 3).
Event-free survival in transplanted Wiskott-Aldrich syndrome patients according to donor group (P = .01). — indicates the MSD group; …, URD group; and  •
•  •, MMRD group.
•, MMRD group.
Event-free survival in transplanted Wiskott-Aldrich syndrome patients according to donor group (P = .01). — indicates the MSD group; …, URD group; and  •
•  •, MMRD group.
•, MMRD group.
GVHD
Seven patients (7%) had active cGVHD (MSD n = 2, URD n = 3, MMRD n = 2) at 2 years after HSCT. In the MSD group, one patient had limited cGVHD that resolved in the third year after HSCT, one patient had extensive cGVHD requiring immunosuppression until 7.5 years after HSCT. In the URD group, 2 patients had extensive cGVHD lasting 3 and 5 years, respectively, after HSCT. One patient had limited cGVHD resolving in the third year after transplantation. Finally, in the MMRD group, one patient had limited cGVHD, which resolved within 3 years after HSCT, and one had extensive cGVHD requiring immunosuppression until 4 years after HSCT.
Autoimmune manifestations after HSCT
A striking observation is that 19 of 96 patients (20%) developed autoimmune manifestations after HSCT independently of cGVHD (Tables 5, 6). Two other patients of the MSD group had autoimmunity related to active cGVHD in the form of scleroderma and vitiligo. None in the other groups had autoimmunity related to cGVHD. Some patients had more than one manifestation. Autoimmune manifestations consisted of autoimmune thrombocytopenia (n = 10), autoimmune hemolytic anemia (n = 3), neutropenia (n = 1), vasculitis (n = 2), inflammatory bowel disease (n = 1), pericarditis (n = 1), Addison disease (n = 1), and autoimmune hypothyroidy (n = 4). Autoimmune manifestations appeared at a median of 1.5 years after HSCT (4 months to 10 years). The median duration of autoimmunity was 4 years (range, 1-20 years). Eight (8%) patients had ongoing autoimmune manifestations at the time of analysis. Autoimmune manifestations were more frequent in recipients of URD (9 of 32; 28%) and MMRD (5 of 19; 26%) than MSD HSCT (5 of 45; 11%; P = .04). We investigated whether autoimmunity after HSCT correlated with occurrence of autoimmunity before HSCT as a manifestation of WAS. Overall, 17 patients had autoimmune manifestations before transplantation of which 7 persisted thereafter (P = .06). In the MSD group, 7 patients had autoimmunity before HSCT of which only 1 persisted after HSCT. In the URD group, of the 6 patients presenting autoimmunity before transplantation, 3 (50%) had persistence of autoimmune manifestations. Finally, in the MMRD group, of the 4 patients with autoimmunity before transplantation, 3 (75%) had persistence of autoimmunity. Conversely, autoimmunity occurred de novo in 4 of 38 (11%) patients from the MSD, in 6 of 26 (23%) patients from the URD, and in 2 of 15 (13%) patients from the MMRD groups. The most striking observation was the strong correlation between autoimmunity occurrence and the chimerism pattern (Table 5). Overall, incidence of autoimmunity was 6 of 78 (8%) in patients with full chimerism and 13 of 18 (72%) in patients with mixed/split chimerism (P < .001). Of note, 2 of 4 patients (MSD, n = 2; URD, n = 1; MMRD, n = 1) who had autoimmunity and had to be splenectomized at 7, 17, 19, and 20 months after HSCT developed severe infections (Table 7). A long-lasting T- and B-cell immunodeficiency was observed in 12 patients from all groups. In the MSD group, 4% (2 of 45) patients and in the URD group 16% (5 of 32) patients had late T- and B-cell recoveries at more than 2 years after HSCT. In the MMRD group, 21% (4 of 19) patients had late T-cell recovery and 26% (5 of 19) had late B-cell recovery. Late T- and B-cell recovery was found to be respectively related to long-term immunosuppression for autoimmunity in 2 patients in the URD and 3 patients in the MMRD groups.
Other WAS-associated manifestations after HSCT
Two patients had developed non-Hodgkin lymphomas before transplantation; they were in complete remission at the time of HSCT. No malignancy occurred in the 96 patients following HSCT. Only 2 patients had transient eczema and 1 patient asthma for 2, 2.9, and 3 years after HSCT, respectively. In all groups, none of the nonsplenectomized patients developed severe infections after HSCT. In contrast, 6 of 28 (21%) splenectomized patients developed severe infections after HSCT (P = .001). In the MSD group, 2 of 7 splenectomized patients (29%), in the URD group 1 of 12 (8%) and in the MMRD group 3 of 9 (33%) had severe infections including a fatal meningococcal sepsis.
Sequelae
Sequelae (Table 7) resulted either from irreversible tissue damage that had taken place before HSCT or because of HSCT complications such as cGVHD, autoimmunity, or severe infections in splenectomized patients. Sequelae were largely due to damage before HSCT. Their frequency was highest in patients who underwent an HSCT from a URD or MMRD donor, possibly because a more risky HSCT was only undertaken in patients with more severe WAS disease. Overall, 20 of 96 (20%) patients had sequelae. There was a correlation between age at HSCT and risk of sequelae since only 5 of 45 (11%) patients transplanted at 2 years of age or younger had long-term sequelae compared with 15 of 51 (29%) transplanted after 2 years of age (P = .03). The risk of sequelae was highly significantly correlated to the severity of disease before HSCT, since none of the patients with score 2 or 3 developed sequelae, whereas 16 of 55 (29%) of patients with score 4 or 5 had sequelae (P = .002). The 5 patients younger than 2 years at HSCT with sequelae were all severely ill at time of transplant. They all underwent splenectomy either before or after HSCT.
Discussion
In this long-term, multicenter, retrospective study on WAS patients surviving 2 years or more following HSCT, we analyzed the various events affecting quality of life. Our study is comprehensive in that it includes a large cohort of WAS patients treated with HSCT more than 22 years. The long time span is also the weakness of the study because HSCT has improved dramatically between 1979 and 2001 in terms of patient and donor selection, the timing of HSCT, conditioning regimens, immunosuppression, and infectious prophylaxis, among other factors. Another inevitable weakness when gathering long-term data from such a rare disease is the variations in experience between different centers. Despite these shortcomings, this retrospective study clearly confirms that for most patients HSCT resolves and prevents the long-term, life-threatening complications associated with WAS, regardless of donor matching, although as expected, long-term EFS is more likely to be better in recipients of MSD HSCT or URD HSCT. Nevertheless, serious events such as autoimmunity, infections, and sequelae can dramatically affect the quality of life among some survivors.
The most striking finding not previously reported is the occurrence of significant autoimmune manifestations independently of cGVHD. Autoimmunity was significantly related to mixed/split chimerism following HSCT, but not to pretransplant autoimmunity. Autoimmune manifestations mostly targeted blood cells and were antibody-mediated as is seen in WAS.17 Autoimmunity led to prolonged immunosuppression and significant infectious complications, particularly in patients who had splenectomy. The strong association found between autoimmunity and mixed/split chimerism as well as the WAS-like occurrence of autoimmune manifestations after HSCT suggests that these complications were mediated by residual-host lymphocytes. This finding could be specific to WAS since autoimmunity after HSCT independent of cGVHD is uncommon in other immune deficiencies.31 A more detailed analysis of chimerism performed on different lymphocyte lineages based primarily on WASP expression should provide further insight into which host-cell lineages could provoke autoimmunity. Because autoimmunity in WAS is complex, it will be of particular importance to see whether the presence of host B cells is sufficient to cause antibody-mediated autoimmunity and whether a deficiency in donor regulatory T cells might account for emergence of autoimmunity, as abnormal development/function of regulatory T cells has been suggested to take place in WAS.40,–42 The data from this retrospective study suggest that quantification of the minimum number/proportion of donor cells in each lymphocyte lineage needed to prevent autoimmunity would be a useful aim for future studies. Results of these studies will be critical to improve strategies for HSCT in WAS patients as well as in developing potential gene therapy trials.
Serious and even fatal infections following HSCT remain a long-term risk in WAS patients, even 2 years after transplant. According to our results, severe infections are clearly associated with splenectomy, causing death in 2 patients and serious sequelae in others. Therefore, in our view, splenectomy should not be performed in patients with WAS who are candidates for HSCT or who have already been treated with HSCT. Sequelae following HSCT were strongly associated with very severe disease before HSCT or with severe infection in splenectomized patients. In view of these complications, it is important to transplant these patients early. Patients younger than 2 years of age, including those receiving a transplant from an MMRD, have a better chance of long-term survival with a better quality of life than older patients at HSCT. This work provides the basis for more detailed analysis of consequences of chimerism on the precise outcome, and should be used to define the indications and modalities for HSCT and determine criteria for gene therapy.43 In the future, the latter would likely need to achieve a high frequency of cell transduction combined with adequate immunosuppression/myeloablation to control autoimmunity. Autoimmune manifestations after HSCT warrant further investigations in future prospective studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank Mahmut Ozahim, MD for his help with the statistical analysis and Chantale Marguet for her expert technical assistance.
Authorship
Contribution: H.O. designed research, performed research, analyzed data, and drafted the paper; A. Fischer designed and supervised research and revised the manuscript critically for important intellectual content; M.C.-C., L.D.N., A.J.C., and W.F. contributed to the interpretation of data and revised the manuscript; and A.S., A.J.T, E.M., M.A.S., F.L.D, S.B., P.V., A. Fasth, R.B., P.S., N.W., J.O., C.H., A.O.M., J.W., K.K., S.M.M., T.G., A.I., and P.L. submitted data and contributed to interpretation of data. All the authors participated sufficiently in the work to take public responsibility for appropriate portions of the content, and gave final approval of the version to be published.
A list of the participating centers appears online.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hulya Ozsahin, University Children's Hospital, Hematology/Oncology Unit, 6 rue Willy-Donzé, 1211 Geneva 14, Switzerland; e-mail: ayse.h.ozsahin@hcuge.ch.