Abstract
Upon recognition of their respective cellular partners, T and B cells acquire their antigens by a process of membrane capture called trogocytosis. Here, we report that various inhibitors of actin polymerization or of kinases involved in intracellular signaling partially or fully inhibited trogocytosis by CD8+ and CD4+ T cells, whereas they had no effect on trogocytosis by B cells. Similarly, trogocytosis by T cells was inhibited at 4°C, whereas in B cells it was independent of temperature, indicating that trogocytosis by B cells does not rely on active processes. By contrast, most inhibitors we tested impaired both T-cell and B-cell activation. The differential effect of inhibitors on T-cell and B-cell trogocytosis was not due to the higher affinity of the B-cell receptor for its cognate antigen compared with the affinity of the T-cell receptor for its own antigen, but it correlated tightly with the abilities of T cells and B cells to form conjugates with their target cells in the presence of inhibitors. Trogocytosis thus has different requirements in different cell types. Moreover, the capture of membrane antigen by B cells is identified as a novel signaling-independent event of B-cell biology.
Introduction
T lymphocytes and B lymphocytes are the 2 main cell types responsible for the adaptive immune response in vertebrates. Whereas B cells recognize native, unprocessed antigens using their B-cell receptor (BCR), T cells recognize antigenic peptides bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs) using their T-cell receptor (TCR). Antigen recognition results in activation of the lymphocytes, the acquisition of their effector functions, and their cooperation with other cell types in the course of the adaptive immune response.
Like many receptors on the cell surface, the antigen receptors on the surface of lymphocytes are taken up into the cell by endocytosis together with the antigens they bind.1,2 This is surprising because the antigens recognized by the TCR, the peptide-MHC complexes, are integral membrane proteins, which do not normally pass from one cell membrane to another. This observation, first reported for CD8+ cytotoxic T lymphocytes (CTLs),2 was confirmed by several other studies of the 2 major classes of T cell: CD8+ (CTL) and CD4+ (helper) T cells.3 Likewise, in an elegant system developed by Batista et al, B cells have also been reported to acquire antigens that are membrane-bound and to be able to introduce them, like soluble antigens, in the presentation pathway.4
Our group has demonstrated that peptides bound to MHC complexes translocate from the APC to the T cell in membrane fragments that contain both lipids and many other membrane-bound proteins.5 We coined the term trogocytosis to describe this process of unidirectional transfer of plasma membrane material from target cells to effector cells of the immune system.6 Initially, using well-characterized murine models of antigen-specific lymphocytes, we made this observation in CD8+ CTLs but, later, we showed that CD4+ T cells and B cells also perform trogocytosis (ie, they acquire membrane-anchored antigen in fragments of membrane).7,8 Trogocytosis has since also been reported for most other hematopoietic cells including natural killer (NK) cells (see Roda-Navarro and Reyburn9 for a review), dendritic cells,10 monocytes,11,12 and neutrophils,13 indicating that antigen recognition by antigen receptors is not the only molecular trigger for trogocytosis. Worthy of note, activated but not resting CD4+ and CD8+ T cells were shown to acquire membrane patches from target cells in the absence of antigen and independently of the TCR.14-16
Trogocytosis is now a well-recognized feature of T- and B-cell biology, and numerous hypotheses propose that the process is involved in the control of immune responses or in the spreading of pathogens.3,6,17,18 The lack of information about the molecular players involved in trogocytosis is a major obstacle to understanding the mechanism and the roles that the process may play in different cell types. In comparison with T cells5,8,15,19-21 and with NK cells (see Roda-Navarro and Reyburn9 for a review), much less is known on the parameters governing B-cell trogocytosis.
The acquisition of antigen by B cells is a central process of adaptive immunity that has been known for decades. Upon antigen recognition, the B cell internalizes the antigen, processes it into protein fragments, and presents these peptides bound to MHC class II molecules on its own surface. This peptide-MHC complex is then recognized by CD4+ helper T cells, which stimulate the B cell to secrete antibodies (Abs) of higher affinity for their antigens and of diversified biologic functions.1,22 To date, the acquisition of membrane-bound antigens by B cells constitutes the sole unequivocal role for trogocytosis. In the course of a previous study exploiting redirected trogocytosis to characterize what molecules could trigger the phenomenon, we observed certain differences between T and B lymphocytes. Here, we compared antigen-triggered trogocytosis in T and B lymphocytes with the goal to understand whether differences between these 2 cell types may help us to decipher the molecular mechanisms whereby fragments of plasma membrane can transfer from one cell to another one.
Methods
Cell lines and mice
Effector cells originated from OT-I mice (CD8+ T cells specific for OVA257-264 presented by H-2Kb), DO11.10 mice (CD4+ T cells specific for OVA323-339 presented by I-Ad), or MD4 mice (B cells specific for hen egg lysozyme [HEL]). Spleens from the 3-83 strain of transgenic mice (containing B cells specific for H-2Kk) were shipped to us by Dr Tybulewicz (London, United Kingdom). T cells (preactivated in vitro with the specific antigen) or naive B cells were exposed to target cells in various combinations as summarized in Table 1. The origin, characteristics, and culture of the target cells and effector cells are detailed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article.)
Reagents and antibodies
Reagents and antibodies used in this study are reported in Document S1.
Target cell staining
For their subsequent use in trogocytosis assays, target cells were either stained with a lipophilic dye (PKH67, PKH26, or DiI) or were cell surface biotinylated, as described previously in detail.23 For their subsequent use in conjugate formation assay, cells were sometimes labeled with carboxy-fluorescein succinimidyl ester (CFSE). For this, cells (10 × 106/mL) in complete culture medium were incubated for 5 minutes at 37°C with 2.5 μM CFSE and then washed 3 times in complete culture medium.
Trogocytosis assays
Trogocytosis experiments were performed as described previously.23 In brief, after staining with a membrane dye, target cells were placed in U-bottomed 96-well plates (0.5 × 106 cells/well in 200 μL final volume). When T cells were used as effectors, target cells were incubated with the indicated concentration of the appropriate peptide. After 1 hour at 37°C, cells were washed 3 times with 200 μL culture medium. After the last wash, cell pellets were resuspended in 100 μL medium containing 105 T or B cells, centrifuged for 30 seconds at 160g to promote conjugate formation, and then left at 37°C or, in some experiments, at 4°C for 1 hour. Conjugates were then dissociated by washing cells twice in phosphate-buffered saline containing 0.5 mM ethylenediaminetetraacetic acid (EDTA) and pipetting them up and down thoroughly, before staining with a monoclonal antibody (mAb) against CD8, CD4, or B220. Cells were then analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, Mountain View, CA). Effector cells were gated positively according to their staining with lineage-specific markers (CD8 for CTL, CD4 for T helper cells, and B220 for B cells). When PKH- or DiI-labeled target cells were used, trogocytosis was measured directly by detecting these fluorescent markers on the effectors. When biotinylated target cells were used, flow cytometric detection of trogocytosis was performed by staining with fluorescent streptavidin together with CD8+, CD4+, or B-cell markers. In some cases, trogocytosis with MD4 B cells was performed in the presence of the indicated concentrations of soluble hen egg lysozyme (sHEL). In experiments involving inhibitory drugs, T and B cells were pretreated at 37°C before coincubation with their targets. All these drugs were left throughout the assays. Conditions for the use of the different drugs are summarized in Table 2. Redirected trogocytosis16 was performed similarly except that P815 cells were used as targets and were mixed with effector cells that had been preincubated with 5 μg/mL of the different unlabeled mAbs for 30 minutes at 4°C. Values used for our analyses were median fluorescence intensities (mfi). Residual trogocytosis in the presence of the indicated inhibitors was calculated using the following formula: 100 × (mfi with antigen − mfi without antigen, with drug)/(mfi with antigen − mfi without antigen, without drug), where mfi is the median fluorescence intensity.
Conjugate formation
Conjugate formation was analyzed as described previously.24 Briefly, 5 × 105 CFSE-labeled target cells were incubated with 105 PKH26-labeled T cells, or with anti-B220 mAb-stained B cells, in 200 μL culture medium in 96-well U-bottom plates for 1 hour at 37°C. Cells were then fixed with 1% paraformaldehyde overnight at 4°C and analyzed by flow cytometry. Conjugates were identified as being CFSE+ PKH26+ for T cells and CFSE+ B220+ for B cells. Formation of conjugates by redirected trogocytosis was performed similarly except that CFSE-labeled P815 cells were used as targets and were incubated with effector cells in the presence of 5 μg/mL of the various unlabeled mAbs for 30 minutes at 4°C. In some assays, drugs were used as described in Table 2. Percentages of conjugated lymphocytes were calculated as 100 × (lymphocytes in conjugate/total number of lymphocytes). For B cells, the total number corresponds to the number of cells staining positively for B220, whereas for T cells, which are an almost pure population after in vitro stimulation, we simply used the number of PKH26-labeled cells.
Antigen presentation assays
In an initial step of antigen capture, 106 MD4 B cells, expressing an HEL-specific IgM BCR, were coincubated for 1 hour at 37°C with 5 × 106 J558L target cells (either stable J558L transfectants expressing membrane-bound HEL [mHEL], or untransfected J558L as negative control, or J558L in the presence of sHEL). All conditions were tested either with or without latrunculin B (25 μM). B cells were then labeled with anti–B220-phycoerythrin and selected magnetically using antiphycoerythrin microbeads (Miltenyi, Auburn, CA). Sorted cells (105) were then incubated at 37°C for 24 hours with 105 1H11-34 cells (T-cell hybridoma cells specific for HEL107-116 presented by I-Ed). The levels of interleukin-2 (IL-2) in the harvested culture supernatants were measured using an enzyme-linked immunosorbent assay (ELISA) kit (BD Bioscience, San Jose, CA).
Results
T cells require an active actin cytoskeleton and enzymatic activities to perform trogocytosis but B cells do not
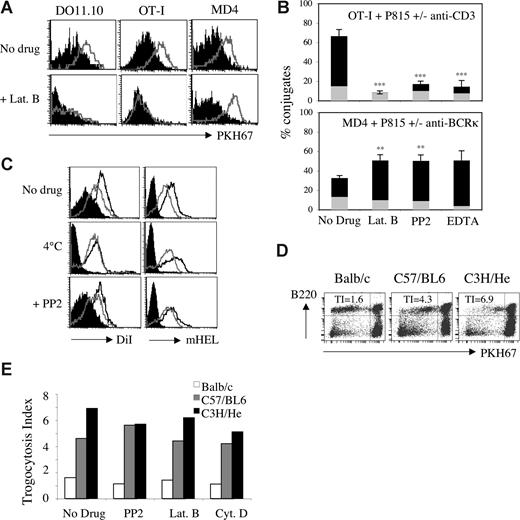
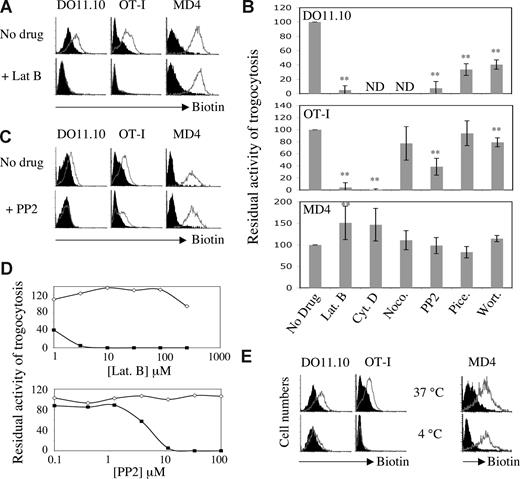
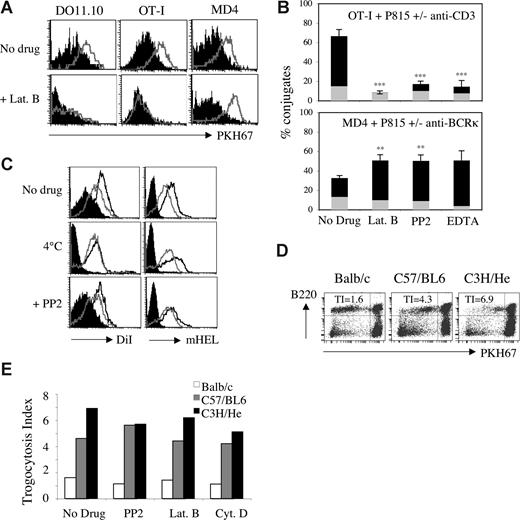
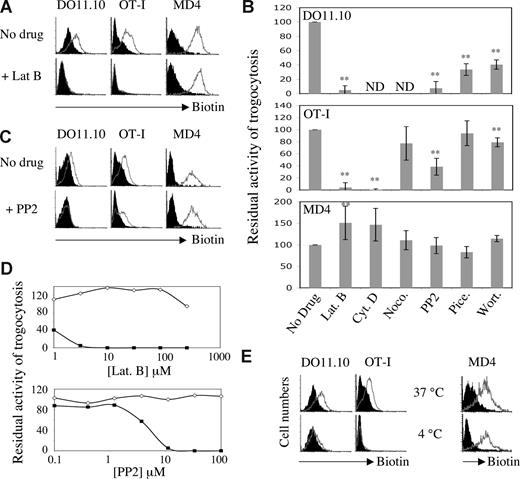
By making use of a novel approach in which trogocytosis is triggered by Abs, we obtained data suggesting that, unlike T cells, B cells undergo trogocytosis in the presence of drugs that block the actin cytoskeleton.16 This approach, however, used nonphysiologic stimulation of T and B cells with Abs and therefore did not provide information regarding antigen-mediated trogocytosis. To explore possible differences in trogocytosis triggered by antigen recognition by T cells and B cells, we investigated the effect of various inhibitors on trogocytosis by murine T or B lymphocytes from well-characterized transgenic strains. Ovalbumin (OVA)–specific OT-I CD8+ T cells, DO11.10 CD4+ T cells, and hen egg lysozyme (HEL)–specific MD4 B cells efficiently trogocytosed membrane fragments from respective target cells (EL4, A20, and J558L cells) when these cells expressed their cognate antigen at the plasma membrane, as shown by the detection on lymphocytes of biotinylated determinants initially present in the membrane of target cells (Figure 1A). In the presence of 25 μM latrunculin B, an inhibitor of actin polymerization, trogocytosis by CD8+ and CD4+ T cells was fully inhibited, whereas trogocytosis by B cells was not (Figure 1A). Similar results were obtained with another actin-depolymerizing agent, cytochalasin D (Figure 1B). In most of our experiments, the extent of trogocytosis by B cells was in fact increased by the presence of these 2 inhibitors, although this effect was not statistically significant for cytochalasin D (Figure 1B). Nocodazole, an inhibitor of microtubule polymerization, had only a marginal effect on the extent of trogocytosis by CD8+ T cells (which was not statistically significant), and had no detectable effect on B-cell trogocytosis (Figure 1B).
The differential effect of inhibitors and temperature on T-cell and B-cell trogocytosis. (A) DO11.10 CD4+ T cells (left panels), OT-I CD8+ T cells (middle panels), and MD4 B cells (right panels) were exposed in the presence or absence of 25 μM latrunculin B (Lat. B) to their respective biotinylated target cells that either expressed, on their surface, the appropriate antigen (open histograms) or not (closed histograms). After 1 hour at 37°C, conjugates were dissociated and lymphocytes were stained with fluorescent streptavidin to detect capture of biotinylated components, and with lineage-specific mAbs. Graphs show the level of biotin staining on gated effector cells. (B) Quantification of trogocytosis efficiency performed by lymphocytes in the presence or absence of 25 μM latrunculin B, 10 μM cytochalasin D (Cyt. D), 100 nM nocodazole (Noco.), 10 μM PP2, 100 μM piceatannol (Pice.), and 100 nM wortmannin (Wort.). Residual trogocytosis in the presence of the indicated inhibitors was calculated using the formula described in Document S1. DO11.10 CD4+ T cells were not tested in presence of cytochalasin D and nocodazole. Bars represent the standard deviations; n = 5 for T CD4+ cells and n > 6 for T CD8+ cells and B cells. Levels of statistical significance were calculated using the Student t test; **P < .01. (C) As in panel A, except that 10 μM PP2 was used instead of latrunculin B. The data are from experiments in which all cells were treated in parallel with the same aliquots of the indicated inhibitors. (D) OT-I CD8+ T cells (squares) and MD4 B cells (diamonds) were analyzed as in panel A except that a range of concentrations of latrunculin B (top panel) or PP2 (bottom panel) was used. Residual trogocytosis was calculated as in panel B. Similar results were obtained in 4 independent experiments. (E) As in panel A except that cocultures were incubated at 37°C or at 4°C rather than with or without inhibitor. Note that for experiments performed at 4°C, we used an effector to target ratio of 15:1 to ensure that all B cells were in contact with a target cell.
The differential effect of inhibitors and temperature on T-cell and B-cell trogocytosis. (A) DO11.10 CD4+ T cells (left panels), OT-I CD8+ T cells (middle panels), and MD4 B cells (right panels) were exposed in the presence or absence of 25 μM latrunculin B (Lat. B) to their respective biotinylated target cells that either expressed, on their surface, the appropriate antigen (open histograms) or not (closed histograms). After 1 hour at 37°C, conjugates were dissociated and lymphocytes were stained with fluorescent streptavidin to detect capture of biotinylated components, and with lineage-specific mAbs. Graphs show the level of biotin staining on gated effector cells. (B) Quantification of trogocytosis efficiency performed by lymphocytes in the presence or absence of 25 μM latrunculin B, 10 μM cytochalasin D (Cyt. D), 100 nM nocodazole (Noco.), 10 μM PP2, 100 μM piceatannol (Pice.), and 100 nM wortmannin (Wort.). Residual trogocytosis in the presence of the indicated inhibitors was calculated using the formula described in Document S1. DO11.10 CD4+ T cells were not tested in presence of cytochalasin D and nocodazole. Bars represent the standard deviations; n = 5 for T CD4+ cells and n > 6 for T CD8+ cells and B cells. Levels of statistical significance were calculated using the Student t test; **P < .01. (C) As in panel A, except that 10 μM PP2 was used instead of latrunculin B. The data are from experiments in which all cells were treated in parallel with the same aliquots of the indicated inhibitors. (D) OT-I CD8+ T cells (squares) and MD4 B cells (diamonds) were analyzed as in panel A except that a range of concentrations of latrunculin B (top panel) or PP2 (bottom panel) was used. Residual trogocytosis was calculated as in panel B. Similar results were obtained in 4 independent experiments. (E) As in panel A except that cocultures were incubated at 37°C or at 4°C rather than with or without inhibitor. Note that for experiments performed at 4°C, we used an effector to target ratio of 15:1 to ensure that all B cells were in contact with a target cell.
We extended our study to inhibitors of the Src-, Syk-, and phosphatidylinositol-3-kinase (PI3K) pathways (PP2, piceatannol, and wortmannin, respectively), which target important enzymes in TCR and BCR signaling and are all frequently used to block activation of T and B cells. Confirming our previous studies,5,8,19 we found that PP2 markedly inhibited trogocytosis in both CD4+ and CD8+ T cells (Figure 1C). Wortmannin also inhibited trogocytosis by CD4 + T cells as well as by CD8+ T cells, albeit to a much lesser extent (Figure 1B). Piceatannol inhibited trogocytosis by CD4+ T cells and, in some but not all experiments, by CD8+ T cells (Figure 1B). When we tested B cells, however, we again found that none of these inhibitors had a significant effect on trogocytosis (Figure 1B,C). No inhibition of trogocytosis by B cells was observed even at concentrations of inhibitors that were much higher than those described commonly in the literature (Figure 1D). We can be sure that all the inhibitors were active because they all, except for nocodazole, had an effect on at least one type of cell among the CD4 T, CD8 T, and B cells we used; in the case of nocodazole, we checked the activity of the drug by its effect on the microtubule cytoskeleton on fibroblasts (not shown). In addition, when treated with PP2 (Figure S1) or latrunculin B (not shown), both T-cell activation and B-cell activation were inhibited, showing that B cells are not intrinsically resistant to these inhibitors. These data indicate that trogocytosis by T cells and by B cells has different requirements for signaling: T cells require the actin cytoskeleton and kinases such as Src-kinase, Syk-kinase, and PI3K (with some differences between CD8+ and CD4+ T cells), whereas these activities are all dispensable for B-cell trogocytosis. To explore whether B-cell trogocytosis requires any enzymatic or active processes, we performed the incubation between target and effector cells at 4°C. As shown in Figure 1E, we found that, at 4°C, trogocytosis by T cells was completely inhibited whereas, in B cells, it was not. Our observation that the capture of membrane fragments from APCs by B cells is not affected by a broad range of inhibitors was quite unexpected and suggests that B-cell trogocytosis can occur independently of signaling enzymes or the cytoskeleton. For T cells, our study also broadens the panel of inhibitors of trogocytosis described in previous studies.5,15,19,21
T cells, but not B cells, require active actin cytoskeleton and enzymatic activities to form conjugates with target cells
To investigate the underlying mechanisms accounting for the difference observed in T-cell and B-cell trogocytosis and since transfer of membrane material has been shown to require direct cell-cell contact, we measured the formation of cellular conjugates between target cells and lymphocytes in the absence or presence of inhibitors. We found that the percentage of OT-I CD8+ T cells conjugated to EL4 target cells increased from 8.5% to 87.2% of total OT-I cells when the antigen was added (Figure 2A). This increase was abolished by latrunculin B (Figure 2A,B) and markedly reduced by PP2 (Figure 2B). Similar results were obtained with CD4+ T cells (not shown). In marked contrast, the formation of conjugates between MD4 B cells and their target cells was not inhibited by latrunculin B or PP2 (Figure 2C,D); on the contrary, these inhibitors tended to increase conjugate formation, although the differences were not statistically significant. Somewhat surprisingly to us, even when we treated lymphocytes with 2 mM EDTA, the B cells continued to form conjugates with their target cells, whereas the formation of conjugates between T cells and their targets, which is known to depend critically upon divalent cations, was completely inhibited. The extent of the inhibitory effect of these various treatments on the formation of conjugates therefore correlates strongly with the degree of inhibition of trogocytosis documented in Figure 1, indicating that conjugate formation is a key step preceding trogocytosis and reinforcing the notion that trogocytosis occurs at the immunologic synapse formed between a lymphocyte and its cellular partner.
Formation of stable conjugates with target cells. (A) OT-I CD8+ T cells (labeled in red with the fluorescent lipophilic dye PKH26) were mixed at 37°C in the presence or absence of 25 μM latrunculin B with EL4 target cells (labeled in green with CFSE) that had either been pulsed (+pOVA) or not (−pOVA) with OVA257-264 peptide. After 1 hour, cells were fixed and analyzed by flow cytometry. The percentages of T cells found conjugated to target cells indicated in the dot plots were calculated as follows: 100 × [(PKH26+ CFSE+ events)/(total number of PKH26+ events)]. (B) Percentages of conjugates formed by OT-I cells with their targets in the presence of the indicated inhibitors, calculated as in panel A. Gray represents the “background” level of conjugates formed with target cells in the absence of antigen, and black represents the level of conjugates formed with target cells in the presence of antigen. Levels of statistical significance were calculated using the Student t test; ***P < .001. (C) As in panel A except that MD4 B cells were stained with the B cell–specific B220 mAb before mixing them with CFSE-labeled J558L or J558LmHEL cells. The percentages of B cells found in conjugates with target cells were calculated using the following formula: 100 × [(B220+ CFSE+ events)/(total number of B220+ events)]. (D) As in panel B but for MD4 B cells. Bars represent standard deviation; n = 4.
Formation of stable conjugates with target cells. (A) OT-I CD8+ T cells (labeled in red with the fluorescent lipophilic dye PKH26) were mixed at 37°C in the presence or absence of 25 μM latrunculin B with EL4 target cells (labeled in green with CFSE) that had either been pulsed (+pOVA) or not (−pOVA) with OVA257-264 peptide. After 1 hour, cells were fixed and analyzed by flow cytometry. The percentages of T cells found conjugated to target cells indicated in the dot plots were calculated as follows: 100 × [(PKH26+ CFSE+ events)/(total number of PKH26+ events)]. (B) Percentages of conjugates formed by OT-I cells with their targets in the presence of the indicated inhibitors, calculated as in panel A. Gray represents the “background” level of conjugates formed with target cells in the absence of antigen, and black represents the level of conjugates formed with target cells in the presence of antigen. Levels of statistical significance were calculated using the Student t test; ***P < .001. (C) As in panel A except that MD4 B cells were stained with the B cell–specific B220 mAb before mixing them with CFSE-labeled J558L or J558LmHEL cells. The percentages of B cells found in conjugates with target cells were calculated using the following formula: 100 × [(B220+ CFSE+ events)/(total number of B220+ events)]. (D) As in panel B but for MD4 B cells. Bars represent standard deviation; n = 4.
The differential sensitivity of trogocytosis in T cells and B cells to inhibitors is independent of the affinity of the stimulus received by their antigen receptors
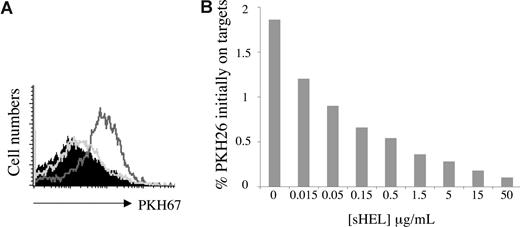
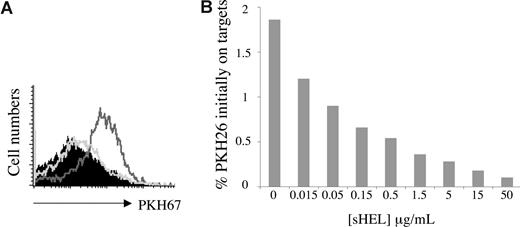
The presence of membrane antigen is the only critical requirement we have thus far identified for trogocytosis by B cells. This requirement for antigen was confirmed by the observation that sHEL inhibited, in a dose-dependent manner, the trogocytosis of membrane from J558LmHEL target cells by MD4 B cells (Figure 3A,B).
The role of the B-cell receptor–antigen interaction in B-cell trogocytosis. (A) MD4 B cells were incubated for 1 hour at 37°C with the following target cells labeled with the fluorescent lipophilic dye PKH26:J558L (filled histogram), J558LmHEL (black line histogram), or J558LmHEL in the presence of 100 μg/mL sHEL (gray line histogram). The B cells were then analyzed for PKH26 acquisition. Histograms show the levels of PKH26 fluorescence on B220+ B cells. (B) Coincubation of MD4 B cells with PKH26-labeled J558LmHEL cells in the presence of increasing concentrations of sHEL for 1 hour at 37°C. Ordinate values correspond to the percentage of staining due to the capture of PKH26 relative to the level of staining on the PKH26-labeled J558LmHEL target cells.
The role of the B-cell receptor–antigen interaction in B-cell trogocytosis. (A) MD4 B cells were incubated for 1 hour at 37°C with the following target cells labeled with the fluorescent lipophilic dye PKH26:J558L (filled histogram), J558LmHEL (black line histogram), or J558LmHEL in the presence of 100 μg/mL sHEL (gray line histogram). The B cells were then analyzed for PKH26 acquisition. Histograms show the levels of PKH26 fluorescence on B220+ B cells. (B) Coincubation of MD4 B cells with PKH26-labeled J558LmHEL cells in the presence of increasing concentrations of sHEL for 1 hour at 37°C. Ordinate values correspond to the percentage of staining due to the capture of PKH26 relative to the level of staining on the PKH26-labeled J558LmHEL target cells.
One major difference between antigen recognition by T cells and B cells is the affinity of binding between the antigen and its receptor, which is typically much higher for the BCR (10−9-10−11 M) than for the TCR (10−6-10−4 M). It is possible, therefore, that the higher interaction strength of B cells with their target cells, compared with T cells, allows B cells to capture membrane fragments even in the presence of inhibitors. To address the issue of the strength of interaction between BCR and antigen on the sensitivity of trogocytosis to signaling inhibitors, we used 3 different experimental systems.
First, we used a “redirected trogocytosis” assay,16 in which trogocytosis is triggered by a mAb either against the BCR or against the TCR/CD3 complex in the presence of target cells expressing Ab Fc domain receptors (FcRs). The mAb bridges between the T cell or B cell and the target cell by binding on the one hand to the BCR or TCR and on the other hand to the target cell FcRs. In this setup, the stimuli for both types of cells are conveyed by molecular scaffolds of similar stability involving a high-affinity interaction (that of either the anti-BCR or anti-TCR/CD3 mAb with their respective antigens) and one of weaker affinity (between the mAb and the FcR). Under these “redirected” conditions, trogocytosis by MD4 B cells was unaffected by latrunculin B, whereas trogocytosis by both DO11.10 CD4+ T cells and OT-I CD8+ T cells was inhibited by the drug (Figure 4A). Likewise, conjugate formation between T cells and target cells was inhibited by latrunculin B, PP2, or EDTA under these conditions, whereas these inhibitors had no effect on the formation of conjugates between B cells and target cells (Figure 4B). This reinforces our previous finding that, when the same anti–MHC class I mAb was used to trigger redirected trogocytosis in naive T and B cells, latrunculin B and cytochalasin D also inhibited trogocytosis only by T cells and not by B cells.16 A further advantage of using redirected trogocytosis is that it rules out a possible role of the APC in determining the passive or active nature of trogocytosis since the presenting cell is the same in both T-cell and B-cell assays.
The differential sensitivity of T- and B-cell trogocytosis to inhibitors is not due to differences in affinities of the TCR and BCR for their respective ligands. (A) DO11.10 CD4+ T cells (left panels), OT-I CD8+ T cells (middle panels), and MD4 B cells (right panels) were used in a redirected trogocytosis assay against PKH67-labeled P815 target cells triggered by either the 2C11 anti-CD3 mAb (open histograms for T cells, and closed for B cells) or the anti-BCR κ chain mAb (open histograms for B cells, and closed for T cells). The effector cells were treated with 25 μM latrunculin B before and during coculture with the P815 target cells (bottom panels) or left untreated (top panels). Graphs show the levels of PKH67 fluorescence on gated effector cells. Similar results were obtained in 3 independent experiments. (B) The percentages of OT-I CD8+ T cells (top panel) or MD4 B cells (bottom panel) found conjugated to P815 target cells in the absence of mAb (gray) or in the presence (black) of 2C11 anti-CD3 mAb (for OT-I T cells) or anti-BCR κ chain mAb (for MD4 B cells) was calculated as in Figure 2. Bars represent standard deviations; n = 4. Levels of statistical significance were calculated using the Student t test; **P < .01 and ***P < .001. (C) MD4 B cells were exposed to the following target cells labeled with the fluorescent lipophilic dye DiI: HEK (closed histograms), HEKmHELWT (open histograms), and HEKmHELK97A (gray line histograms). Effector cells were either left untreated (top panels), or treated for 20 minutes at 37°C with 10 μM PP2 (bottom panels) or placed at 4°C (middle, horizontal panels) before coculture with target cells. After 1 hour of coculture at 37°C (top and bottom panels) or at 4°C (middle panels), we measured the capture of membrane components (DiI; left panels) and of mHEL (biotinylated F10.6.6 mAb + fluorescent streptavidin; right, vertical panels). Similar results were obtained in 4 independent experiments. (D) Splenocytes from 3.83 mice were exposed for 1 hour at 37°C to PKH67-labeled splenocytes from Balb/c (no affinity, left panel), C57/BL6 (weak affinity, middle panel), or C3H/He (high affinity, right panel) before analysis by flow cytometry. These respective affinities for the indicated H-2 antigen were reported in.25 B220+ and B220− splenocytes (donor cells) (PKH67bright) fall within the right quadrants, while the B220+ and B220− 3.83 splenocytes (recipient cells) occupy the left quadrants. Numbers represent trogocytosis indexes (TIs) calculated as indicated below. (E) As in panel D except that the indicated inhibitors were added during trogocytosis. Trogocytosis indexes were calculated as follows: mfi on recipient B220+ cell in the upper left quadrant (B cells)/mfi on recipient B220− cells in the lower left quadrant (non-B cells). Similar results were obtained in 3 independent experiments.
The differential sensitivity of T- and B-cell trogocytosis to inhibitors is not due to differences in affinities of the TCR and BCR for their respective ligands. (A) DO11.10 CD4+ T cells (left panels), OT-I CD8+ T cells (middle panels), and MD4 B cells (right panels) were used in a redirected trogocytosis assay against PKH67-labeled P815 target cells triggered by either the 2C11 anti-CD3 mAb (open histograms for T cells, and closed for B cells) or the anti-BCR κ chain mAb (open histograms for B cells, and closed for T cells). The effector cells were treated with 25 μM latrunculin B before and during coculture with the P815 target cells (bottom panels) or left untreated (top panels). Graphs show the levels of PKH67 fluorescence on gated effector cells. Similar results were obtained in 3 independent experiments. (B) The percentages of OT-I CD8+ T cells (top panel) or MD4 B cells (bottom panel) found conjugated to P815 target cells in the absence of mAb (gray) or in the presence (black) of 2C11 anti-CD3 mAb (for OT-I T cells) or anti-BCR κ chain mAb (for MD4 B cells) was calculated as in Figure 2. Bars represent standard deviations; n = 4. Levels of statistical significance were calculated using the Student t test; **P < .01 and ***P < .001. (C) MD4 B cells were exposed to the following target cells labeled with the fluorescent lipophilic dye DiI: HEK (closed histograms), HEKmHELWT (open histograms), and HEKmHELK97A (gray line histograms). Effector cells were either left untreated (top panels), or treated for 20 minutes at 37°C with 10 μM PP2 (bottom panels) or placed at 4°C (middle, horizontal panels) before coculture with target cells. After 1 hour of coculture at 37°C (top and bottom panels) or at 4°C (middle panels), we measured the capture of membrane components (DiI; left panels) and of mHEL (biotinylated F10.6.6 mAb + fluorescent streptavidin; right, vertical panels). Similar results were obtained in 4 independent experiments. (D) Splenocytes from 3.83 mice were exposed for 1 hour at 37°C to PKH67-labeled splenocytes from Balb/c (no affinity, left panel), C57/BL6 (weak affinity, middle panel), or C3H/He (high affinity, right panel) before analysis by flow cytometry. These respective affinities for the indicated H-2 antigen were reported in.25 B220+ and B220− splenocytes (donor cells) (PKH67bright) fall within the right quadrants, while the B220+ and B220− 3.83 splenocytes (recipient cells) occupy the left quadrants. Numbers represent trogocytosis indexes (TIs) calculated as indicated below. (E) As in panel D except that the indicated inhibitors were added during trogocytosis. Trogocytosis indexes were calculated as follows: mfi on recipient B220+ cell in the upper left quadrant (B cells)/mfi on recipient B220− cells in the lower left quadrant (non-B cells). Similar results were obtained in 3 independent experiments.
Second, we mutated the antigen recognized by MD4 B cells, in order to decrease its affinity for the BCR, by introducing a Lys97 for Ala (mHELK97A) mutation in the mHEL antigen, which is known to decrease of the affinity of HEL for the MD4 BCR from Kd = 50 pM to Kd = 0.1 μM.26 Membrane capture triggered by either the WT or mutant HEL, expressed at similar levels at the surface of J558L transfectants (Figure S2), was unaffected by treatment with PP2, by performing the incubation at 4°C (Figure 4C) or by treatment with latrunculin B (not shown).
Third, we used the 3-83 strain of mice, in which B cells express a transgenic BCR that recognizes various MHC class I molecules on target cells with either high affinity (H-2k), very low affinity (H-2b), or no detectable reactivity (H-2d).25 As with mutated HEL, the 3-83 B cells trogocytosed membrane from both low-affinity H-2b and high-affinity H-2k target splenocytes, and this capture was insensitive to PP2, latrunculin B, or cytochalasin D (Figure 4D,E). Experiments using HEK cells transiently transfected with plasmids driving the expression of either H-2Kk or H-2Kb gave results similar to those obtained with splenocytes (not shown). This and the preceding 2 experiments all support the conclusion that the differential sensitivity of T-cell and B-cell trogocytosis to inhibitors is not due to the difference in affinity of their respective antigen receptors for their ligands.
Of note, B-cell early activation, as indicated by the up-regulation of CD69 on the cell surface, was triggered with similar efficiency by both forms of HEL, but fully inhibited by PP2 or incubation at 4°C (not shown).
In both the MD4 and 3-83 systems, the amount of membrane capture by B cells (assessed by capture of the fluorescent lipophilic dye DiI) was noticeably decreased when target cells expressed the low-affinity antigen compared with the high-affinity one (Figure 4C-E), in line with our previous study on T cells showing that affinity does impact on the efficiency of trogocytosis.5
Antigen captured by B-cell trogocytosis in the presence of enzyme inhibitors is available for subsequent processing and T-cell activation
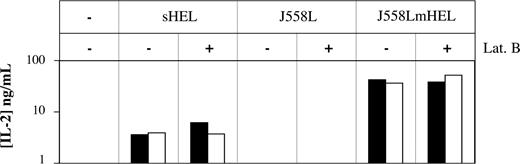
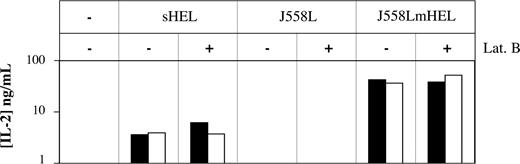
Finally, we evaluated the functional consequences of antigen capture by trogocytosis realized in the presence of signaling inhibitors. Indeed, although B cells captured similar quantities of membrane material in the presence and in the absence of signaling inhibitors, the mechanisms of capture were not necessarily the same. If different mechanisms operate under the different conditions, we might expect that the acquired materials had different fates within the cell. The capture of antigen by B cells is usually followed by internalization, proteolytic processing, and presentation of the antigen, in association with MHC class II molecules, to T cells.22 We used this assay to test whether the antigen-processing pathway was also followed by antigen acquired in the presence of the actin inhibitor latrunculin B (Figure 5). We first cocultured MD4 B cells with target cells expressing (J558LmHEL) or not expressing (J558L) membrane-bound HEL (mHEL) to trigger the capture of membrane. The B cells were then incubated with a T-cell hybridoma line 1H11-34, which produces IL-2 upon recognition of the HEL107-116 peptide processed from mHEL and presented by the I-Ed MHC class II molecule on the surface of the MD4 B cells. Although the J558LmHEL target cells, which express MHC molecules of the H-2b haplotype and lack MHC class II, could not directly activate 1H11-34 T cells (not shown), the MD4 B cells were separated magnetically from the J558LmHEL target cells before incubation with the 1H11-34 T cells. This prevented antigen acquisition from the target cells by B cells and its presentation to T cells during the secondary coculture. In negative controls, no IL-2 production was detected when T cells were cultured with MD4 B cells that had been exposed to J588L cells that did not express mHEL (Figure 5). In positive controls, T cells recognized the antigen and produced IL-2 when cultured with MD4 B cells that were incubated with sHEL or that were exposed to J558LmHEL. When latrunculin B was present during the coculture of MD4 B cells with J558LmHEL, IL-2 was produced with comparable efficiency by T cells in the secondary coculture, indicating that antigenic material captured by MD4 B cells in the presence of latrunculin B was processed and presented to T cells. As a control for the action of latrunculin B, we found that IL-2 secretion by T cells was fully inhibited when the drug was present during both the first and second cocultures (not shown). Thus, material captured by B-cell trogocytosis in the presence of latrunculin B remains available for subsequent processing and presentation to T cells, suggesting that the mechanisms involved in the capture of the antigen in the presence of this inhibitor are not dramatically different from those that occur in untreated cells.
Antigenic material captured by B cells in the presence of enzyme inhibitors is available for subsequent processing and T-cell activation. MD4 B cells were incubated at 37°C for 1 hour with either culture medium, sHEL, J558L, or J558LmHEL target cells, and in the presence or absence of 25 μM latrunculin B. MD4 B cells were then selected magnetically (purity > 85%) before an overnight incubation at 37°C with 1H11-34 T-cell hybridoma. T-cell activation was measured by determining concentrations of IL-2 in the supernatants by ELISA. Black and white bars correspond to 2 independent experiments.
Antigenic material captured by B cells in the presence of enzyme inhibitors is available for subsequent processing and T-cell activation. MD4 B cells were incubated at 37°C for 1 hour with either culture medium, sHEL, J558L, or J558LmHEL target cells, and in the presence or absence of 25 μM latrunculin B. MD4 B cells were then selected magnetically (purity > 85%) before an overnight incubation at 37°C with 1H11-34 T-cell hybridoma. T-cell activation was measured by determining concentrations of IL-2 in the supernatants by ELISA. Black and white bars correspond to 2 independent experiments.
Discussion
The main conclusion we draw from the data presented here is that trogocytosis has different requirements in T cells and in B cells. Trogocytosis occurs in both cell types in response to stimulation by antigen, yet it relies on an active process in T cells but not in B cells. A secondary conclusion is that in B cells the initial BCR-dependent events, such as capture of membrane-bound antigen, do not depend upon signaling, whereas later events, such as CD69 up-regulation, do require signaling.
We found that, in the case of B cells, trogocytosis clearly depends upon the presence of antigen on the APC, but it came as a surprise to find that trogocytosis occurs in the presence of inhibitors of all the biologic pathways we tested (Figure 1). Perhaps our most surprising observation was that, unlike T cells, B cells perform trogocytosis even at 4°C. B-cell activation clearly requires signaling,27 as we confirmed by our finding that some of the inhibitors (and low temperature) markedly inhibited or blocked CD69 up-regulation while not affecting trogocytosis (Figure S1). Therefore, in B cells, antigen capture and activation can be dissociated, whereas, in T cells, both events are sensitive to the same inhibitory agents.
A potential explanation to this difference lies with our observation that the formation of conjugates was dramatically affected by signaling inhibitors in T cells but not in B cells (Figure 2), indicating a marked difference in the way B cells and T cells interact with their targets. This conclusion is supported by the fact that B cells seem much less dependent than T cells upon key actors of the actin cytoskeleton mobilization28 or of the adhesion process such as LFA-1.29 Although the BCR/antigen interaction is of much higher strength than between a TCR and a peptide-MHC complex, our results in 3 separate experimental systems clearly ruled out that the differential sensitivity of trogocytosis by T cells and B cells to signaling inhibitors could be simply explained by this factor (Figure 4).
Our observation that the capture of membrane fragments from APCs by B cells is not affected by several different enzyme inhibitors was quite unexpected, yet, a careful review of the literature identifies BCR signaling and antigen internalization as mutually exclusive,30 a notion supported by the dissociation we observed between trogocytosis and activation in B cells. In addition, the literature provides several examples of signaling-independent events of B-cell biology including BCR endocytosis with antigen (see Caballero et al31 and references therein), BCR recruitment into lipid rafts,32 antigen capture by B cells,33 and, in one study,33 but not in another one,26 the formation of immunologic synapses between APCs and B cells. Our data now extend this list of signaling-independent events in B-cell biology to include membrane capture by trogocytosis. Thus, BCR signaling, although critical for many events of B-cell biology, might not be necessary for some of the earliest steps, including trogocytosis.
B cells acquire membrane-bound antigen by trogocytosis; they process the antigen and present it to CD4+ T cells to subsequently benefit from T-cell help.1,22 We demonstrated clearly that antigenic materials acquired by B cells in the presence of latrunculin B are available for processing and presentation to T cells (Figure 5), strongly suggesting that the molecular mechanisms involved in trogocytosis are not dramatically altered by the presence of this inhibitor.
How can we explain the occurrence of trogocytosis in the absence of signaling in B cells? Recent evidence indicates that dramatic morphologic and dynamic changes in the plasma membrane can be triggered in the absence of any signaling following protein-lipid interactions.34 Similarly, in a system where no source of energy is involved, lipid-lipid interactions between vesicles (a potential vector of membrane fragments during trogocytosis) and supported bilayers induce membrane lipid exchange that impacts on the lipid symmetry in the recipient membranes as well as on the adhesion/migration properties of the vesicle.35 Conceivably, recognition of either soluble or membrane-bound antigens (a unique property of B cells) might trigger morphologic changes similar to those reported in the in vitro systems mentioned above, thus accounting for the few early, signaling-insensitive events of B-cell biology. Conceptually, this property of B cells could relate to the B cell–like system recently identified in lamprey where lymphocytes capture particulate materials using glycophosphatidyl inositol–anchored VLRB receptors that are not connected to any known signaling pathway.36
Our study provides a foundation from which to explore the mechanism(s) of trogocytosis further. Because trogocytosis is performed not only by lymphocytes but also by most hematopoietic cells9-13 and is proposed to have important physiopathologic6,17 and possibly therapeutic12 consequences, exploring the mechanisms of trogocytosis is of a crucial importance and may open new ways to manipulate the immune response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof:
In a very recent paper, Quah et al report that activated B cells can share their BCR with other B cells via membrane transfer, and that this phenomenon is also insensitive to a broad range of inhibitors, including incubation at 4°C (Quah BJ, Barlow VP, McPhun V, Matthaei KI, Hulett MD, Parish CR. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci U S A. 2008;105:4259-4264).
Acknowledgments
We thank members of the I3 club at IPBS for their kind gift of reagents and helpful suggestions on our work. We also thank Facundo Batista for the kind gift of the J558L cells and the mHEL transfectant, Victor Tybulewicz for providing us with 3-83 mouse spleens, Gunter Haemmerling for the H-2Kk cDNA, and Jean-Charles Guery for the 1H11-34 hybridoma. We thank Carol Featherstone for scientific editing of our paper.
This work was supported by funds from the Agence Nationale de Recherche/Recherche et Innovation en Biotechnologies (TAAVAC project; E.J.), Institut des Technologies Avancées en Sciences du Vivant (E.J.), Région Midi-Pyrénées (D.H.), and CNRS.
Authorship
Contribution: A.A. designed and performed most experiments, analyzed the data, made the figures, and wrote the paper; E.M. prepared molecular biology reagents; E.J. and D.H. coordinated the project, participated in the design of the experiments, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denis Hudrisier, Institut de Pharmacologie et de Biologie Structurale, Centre National de Recherche Scientifique, Unité Mixe de Recherche 5089, 205 route de Narbonne, 31077 Toulouse cedex 4; e-mail: denis.hudrisier@ipbs.fr; or Etienne Joly, Institut de Pharmacologie et de Biologie Structurale, Centre National de Recherche Scientifique, Unité Mixe de Recherche 5089, 205 route de Narbonne, 31077 Toulouse cedex 4; e-mail: atn@cict.fr.

![Figure 2. Formation of stable conjugates with target cells. (A) OT-I CD8+ T cells (labeled in red with the fluorescent lipophilic dye PKH26) were mixed at 37°C in the presence or absence of 25 μM latrunculin B with EL4 target cells (labeled in green with CFSE) that had either been pulsed (+pOVA) or not (−pOVA) with OVA257-264 peptide. After 1 hour, cells were fixed and analyzed by flow cytometry. The percentages of T cells found conjugated to target cells indicated in the dot plots were calculated as follows: 100 × [(PKH26+ CFSE+ events)/(total number of PKH26+ events)]. (B) Percentages of conjugates formed by OT-I cells with their targets in the presence of the indicated inhibitors, calculated as in panel A. Gray represents the “background” level of conjugates formed with target cells in the absence of antigen, and black represents the level of conjugates formed with target cells in the presence of antigen. Levels of statistical significance were calculated using the Student t test; ***P < .001. (C) As in panel A except that MD4 B cells were stained with the B cell–specific B220 mAb before mixing them with CFSE-labeled J558L or J558LmHEL cells. The percentages of B cells found in conjugates with target cells were calculated using the following formula: 100 × [(B220+ CFSE+ events)/(total number of B220+ events)]. (D) As in panel B but for MD4 B cells. Bars represent standard deviation; n = 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2008-01-134155/6/m_zh80120820300002.jpeg?Expires=1766360311&Signature=kZrCDj047OZv7KLlQ7JFyB1c~eobnv6BoHG13e11U9ZuyeQG8dBRvyUMTe-9TsiVYIKIeCcxR~feqxkJ8SnyqOgXThMmjgDNt1dHFIBfM~oHXY-KlJqoXNWGBG263ZhfQ3mtZPMM4BedPyC9IMi3B-5pflBRNqUTrQ-loxdZOnqjJxeJ767D5-AaEELUeoMtsgMY1rZA6CmTVYEV0CoUY4uHPa0-7yZQ1I6zL-hAQJTUqXKtss8XQnK98LtBUp0UH15ZjMKBOeGZUk2ROpCj7g66RPZqBFilRSxoGJqx7F2Dmbd09eOyY3yByaH5T36YhGaWgPYdUKoeDL23ckbEiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Formation of stable conjugates with target cells. (A) OT-I CD8+ T cells (labeled in red with the fluorescent lipophilic dye PKH26) were mixed at 37°C in the presence or absence of 25 μM latrunculin B with EL4 target cells (labeled in green with CFSE) that had either been pulsed (+pOVA) or not (−pOVA) with OVA257-264 peptide. After 1 hour, cells were fixed and analyzed by flow cytometry. The percentages of T cells found conjugated to target cells indicated in the dot plots were calculated as follows: 100 × [(PKH26+ CFSE+ events)/(total number of PKH26+ events)]. (B) Percentages of conjugates formed by OT-I cells with their targets in the presence of the indicated inhibitors, calculated as in panel A. Gray represents the “background” level of conjugates formed with target cells in the absence of antigen, and black represents the level of conjugates formed with target cells in the presence of antigen. Levels of statistical significance were calculated using the Student t test; ***P < .001. (C) As in panel A except that MD4 B cells were stained with the B cell–specific B220 mAb before mixing them with CFSE-labeled J558L or J558LmHEL cells. The percentages of B cells found in conjugates with target cells were calculated using the following formula: 100 × [(B220+ CFSE+ events)/(total number of B220+ events)]. (D) As in panel B but for MD4 B cells. Bars represent standard deviation; n = 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/12/10.1182_blood-2008-01-134155/6/m_zh80120820300002.jpeg?Expires=1765892884&Signature=BGc4G-jzJ5DsrAHAwy5RnyjP3QIL-vXuEe2cShCKaQ0etlLSFt6l85oVF5kdetbFBDijUGu5v2s5XIpd4roRjf~JDQyO1qy-e8HS5TDiS~8c~WsD01WqIkZ6koY35EflV2b8iRvaQOEoEu8pt9yyoUpXaWNlyJQ4VmKd7Y5HLq~5HynBWcKmSHZyBn4KE-svQA1gQCr77oN4SstiPyMpLl821xH0kMmbSqPBiq5asEZbhW4CYKEripOEtf06ibrihhFsVHgFLXf-aNZizTPAh-GSALihK6ADmhAeTPWfutGOONbUShS8YNXkXDw962Ws5XJ7TxFITDAjvY4Vsr6K-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)