Abstract
Antibody-dependent cellular cytotoxicity (ADCC) and complement fixation both appear to play a role in mediating antitumor effects of monoclonal antibodies (mAbs), including rituximab. We evaluated the relationship between rituximab-induced complement fixation, natural killer (NK)–cell activation, and NK cell–mediated ADCC. Down-modulation of NK- cell CD16 and NK-cell activation induced by rituximab-coated target cells was blocked by human serum but not heat-inactivated serum. This inhibition was also observed in the absence of viable target cells. C1q and C3 in the serum were required for these inhibitory effects, while C5 was not. An antibody that stabilizes C3b on the target cell surface enhanced the inhibition of NK-cell activation induced by rituximab-coated target cells. Binding of NK cells to rituximab-coated plates through CD16 was inhibited by the fixation of complement. C5-depleted serum blocked NK cell–mediated ADCC. These data suggest that C3b deposition induced by rituximab-coated target cells inhibits the interaction between the rituximab Fc and NK-cell CD16, thereby limiting the ability of rituximab-coated target cells to induce NK activation and ADCC. Further studies are needed to define in more detail the impact of complement fixation on ADCC, and whether mAbs that fail to fix complement will be more effective at mediating ADCC.
Introduction
Monoclonal antibodies (mAbs) are now a mainstay of therapy for a number of cancers. Rituximab was the first chimeric mAb to be approved for clinical use and remains the most extensively used mAb in cancer therapy. Rituximab binding of CD20 has been shown to signal apoptosis in a subset of lymphoma cell lines.1 However, there is little evidence that signaling plays an important role in clinical responses to rituximab. Growing evidence suggests multiple interacting mechanisms, including complement-mediated cytotoxicity (CMC) and antibody-dependent cellular cytotoxicity (ADCC), play a role in the antitumor response of rituximab and other mAbs
Evidence is conflicting related to the role CMC plays in mediating the antitumor effects of rituximab. Van Meerten et al used target cells that express varying amounts of CD20 on their surface, and concluded that rituximab-mediated CMC depends on CD20 expression level and acts in a complementary manner to ADCC.2 Target cell expression of the complement inhibitory proteins CD55 and CD59 correlates with the ability of rituximab to induce CMC in vitro,3 and CMC is enhanced when these proteins are blocked.4 However, no correlation was found between CD55/CD59 expression by lymphoma cells and clinical response to therapy.5 In mouse models using murine lymphomas expressing human CD20, Golay et al found that complement plays a key role in mediating rituximab's antitumor effects.6,7 Cragg and Glennie reached similar conclusions from studies of human B-cell lines in severe combined immunodeficiency (SCID) mice.8 Clinically, depletion of complement and evidence for complement fixation on target cells can be seen following rituximab therapy.9,10 Takami et al recently described a case where supplementation of rituximab with complement by infusion of serum in the cerebrospinal fluid promoted antitumor activity in the central nervous system,11 suggesting complement may mediate the antitumor activity of rituximab in the absence of cellular immune effectors. In addition, a case was reported by Klepfish et al in which the use of fresh-frozen plasma as a source of complement induced a response to rituximab in a patient previously refractory to treatment.12,13 Nevertheless, there is no definitive evidence that complement activation correlates with or is required for clinical responses.
ADCC is another mechanism that is likely to play a central role in the response to clinical mAb therapy. Clynes et al demonstrated that Fcγ-receptor knock-out mice have a limited antitumor response to mAb in several tumor models.14 Most convincingly, patients homozygous for the V158 (VV) polymorphism on CD16 have higher clinical response rates to rituximab than carriers for F158 (VF or FF).15-17 These results suggest that ADCC is a major mechanism, and that CD16 plays a key role in the antitumor effect of rituximab.
Traditional cytotoxicity assays allow for evaluation of antitumor activity, but fail to differentiate the mechanisms by which target cells are lysed. Although cytotoxicity assays are the gold standard for measuring mAb-induced cell lysis, chromium release can be the result of either CMC or ADCC. We previously reported a coculture assay that allows for precise measurement of mAb-induced natural killer (NK)–cell activation.18 In this system, peripheral blood mononuclear cells (PBMCs) and target cells are cocultured with the mAb. Response of NK cells to mAb-coated targets is determined by phenotypic evaluation of NK cells. NK- cell response is quantified by CD16 down-modulation, which follows interaction between CD16 and IgG,19,20 and up-regulation of CD69 and CD54, which serve as phenotypic markers of NK activation.18 We previously used this model to evaluate mAbs with varying affinities for CD16, and found that NK activation is a reasonable surrogate for ADCC.21 In the current studies, we used these assays to evaluate the relationship between complement fixation and the ability of mAb-coated target cells to induce NK activation. As outlined here, we found that complement fixation impedes NK activation induced by mAb-coated target cells through inhibiting the binding of CD16 to the mAb, and results in the inhibition of ADCC. These results indicate the relationship between complement fixation and the clinical efficacy of mAb may be more complex than previously assumed.
Methods
Antibodies and serum
Rituximab (Biogen-Idec, Cambridge, MA; and Genentech, South San Francisco, CA) was purchased commercially. The 3E7 mAb specific for C3b/iC3b was previously described.22 The anti-CD11b (Bear1) mAb was obtained from Biodesign (Saco, ME), and the anti-CD18 was obtained from BD Pharmingen (San Diego, CA). C1q-, C3-, and C5-depleted sera were obtained commercially from Complement Technology (Tyler, TX).
Samples from human subjects
PBMCs were obtained from healthy volunteers after obtaining informed consent and were processed and analyzed as previously reported.18,21 Approval was obtained from the University of Iowa Institutional Review Board for these studies. Briefly, mononuclear cells were isolated and red blood cells were removed by resuspending cells in 5 mL red cell lysis buffer according to standard procedures. Autologus normal human serum (serum) was used in select experiments at the indicated concentrations. An aliquot of autologous serum was heated to 57°C for 30 minutes to produce heat-inactivated serum. Cryopreserved single-cell suspensions of primary follicular lymphoma (FL) cells were thawed using standard procedures.
NK-cell activation
Cocultures were performed as previously reported,18,21 using fresh PBMCs as a source of NK cells. Target cells included the standard lymphoma cell lines, Raji and Daudi, or FL cells. Media consisted of RPMI supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol. PBMCs and B cells were mixed at a 1:1 effector-target (E/T) ratio at a final concentration of 1 × 106 PBMCs and 1 × 106 B cells/mL and cultured for 20 hours at 37°C. Various concentrations of rituximab, autologous serum (from PBMC donors), heat-inactivated serum, or commercially purchased C1q-depleted serum, C3-depleted serum, or C5-depleted serum were added as indicated. C5-depleted serum induced a low degree of dose-dependent change in NK-cell phenotype in the absence of rituximab. Therefore, for samples containing C5-depleted serum, the phenotype seen in the presence of C5-depleted serum without rituximab was used as the background level when calculating phenotypic change induced by rituximab.
NK-cell phenotypic analysis
Immunofluorescent staining was performed on cocultured cells as previously reported.18,21 Briefly, cells were washed and stained with directly conjugated commercial antibodies, including anti-human CD56 AlexaFluor 647, CD54 PE (BD Pharmingen), CD16 FITC (Serotec, Raleigh, NC), and CD3 PE-Cy7 (Caltag Laboratories, Burlingame, CA) per the manufacturer's protocol for 15 minutes on ice. Cells were washed twice, fixed in 2% formaldehyde solution, and stored at 4°C for flow cytometry within 24 hours. Flow cytometric analysis (4-color) was performed on the LSR II (BD Immunocytometric Systems, San Jose, CA). Data were analyzed using FlowJo software (TreeStar, Ashland, OR). CD16 and CD54 expression of NK cells was determined by gating on CD3−, CD56+ lymphocytes. CD16 expression was reported as median fluorescence. In prior studies, we found that NK-cell expression of CD54 was bimodal, with activated NK cells expressing bright CD54.18,21 Therefore, NK-cell activation is reported as the percentage of NK cells that were CD54 bright.
Microtiter plate adhesion assay
A microplate adhesion assay, based on a similar assay used to measure the function of bifunctional antibodies,23 was developed to evaluate the avidity of NK cells for IgG. U-bottomed 96-well EIA/RIA microtiter plates (Corning, Corning, NY) were coated with various concentrations of rituximab overnight. Nonspecific binding was blocked by the addition of 10% heat-inactivated fetal calf serum. Media, 50% human serum, or heat-inactivated human serum was added for 30 minutes at 37°C. After washing, 5 × 105 purified human NK cells were added for 30 minutes at room temperature. Plates were then centrifuged for 5 minutes at 500g. Cell pellets formed when cells were not bound to rituximab-coated wells. When rituximab was present, NK cells bound to the walls of the well, and no pellet was noted. The presence or absence of cell pellets was determined by evaluating single-wavelength absorbance at 405 nm, which measures light scattering due to the pellet. Lower absorbance is seen when cells bind to the walls. Higher absorbance is seen when cell pellets form, indicating decreased binding.
Cytotoxicity assay
A total of 3 × 106 Raji cells were labeled with 7.4 MBq (200 μCi) of Na251CrO4 at 37°C for 1.5 hours. NK cells from healthy human donors were purified from PBMCs using the NK-cell isolation kit II (Miltenyi Biotec, Auburn, CA) per the manufacturer's instructions. 51Cr-labeled target cells were washed and dispensed into 96-well flat-bottomed plates (1 × 104 cells/well). Serial dilutions of effector cells (E/T ratio of 50:1 to 1:1), rituximab (5 μg/mL), and C5-depleted serum or heat-inactivated C5-depleted serum (20% final concentration) were added as indicated in triplicate. After 4 hours of incubation at 37°C, 51Cr activity in the supernatant was measured. The percentage of specific cytolysis was calculated from the counts of samples according to the following formula: percentage of specific lysis = 100 × (E − S) / (M − S). E represents the experimental release, S is the spontaneous release, and M is the maximum release.
Results
Normal human serum inhibits NK-cell CD16 down-modulation and CD54 up-regulation induced by rituximab-coated target cells
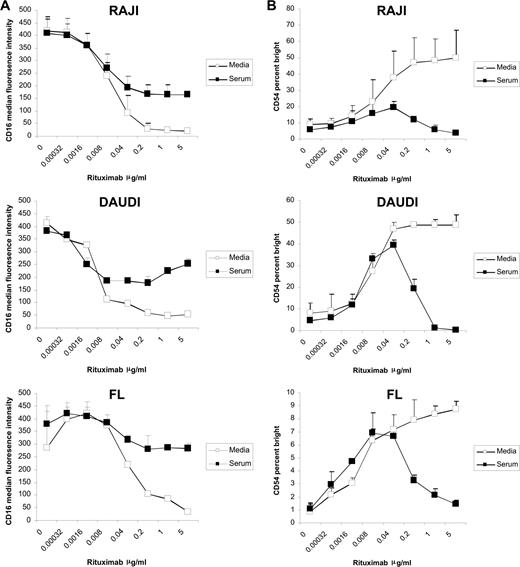
Previously, we demonstrated that the addition of rituximab to a mixture of target B cells and PBMCs from healthy donors in a coculture assay induces down-modulation of NK-cell CD16 and up-regulation of CD54 in a dose-dependent manner.18 In the current studies, we evaluated the effect of serum on this effect. Serum blocked down-modulation of CD16 induced by rituximab-coated target cells at rituximab concentrations greater than 0.04 μg/mL. This inhibition was observed in cultures with Raji and Daudi cell lines and FL (Figure 1A).
Serum inhibits rituximab-induced NK-cell CD16 down-modulation and CD54 up-regulation. PBMCs and Raji, Daudi, or FL cells were mixed at a 1:1 ratio for 20 hours in the presence or absence of 50% serum with varying concentrations of rituximab. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. (A) NK-cell CD16, expressed as median fluorescence, in the absence and presence of serum (n = 3). (B) NK-cell CD54, expressed as a percentage of CD54 bright, in the absence and presence of serum (n = 3). Error bars represent the standard deviation (SD) of the mean.
Serum inhibits rituximab-induced NK-cell CD16 down-modulation and CD54 up-regulation. PBMCs and Raji, Daudi, or FL cells were mixed at a 1:1 ratio for 20 hours in the presence or absence of 50% serum with varying concentrations of rituximab. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. (A) NK-cell CD16, expressed as median fluorescence, in the absence and presence of serum (n = 3). (B) NK-cell CD54, expressed as a percentage of CD54 bright, in the absence and presence of serum (n = 3). Error bars represent the standard deviation (SD) of the mean.
In the absence of serum, CD54 up-regulation was noted beginning at a rituximab concentration of 0.002 μg/mL, reached a plateau at a rituximab concentration of approximately 0.2 μg/mL, and remained stable up to 5 μg/mL, the highest concentration of rituximab tested. In the presence of serum, CD54 up-regulation was seen at the lower rituximab concentrations, but peaked at 0.04 μg/mL. CD54 up-regulation decreased at rituximab concentrations greater than 0.04 μg/mL when serum was present (Figure 1B). Similar results were seen when Raji, Daudi, or FL cells were used (Figure 1B); when CD69 and IFNγ were used as markers of NK activation; and when purified NK cells were used in the assay (data not shown).
The inhibitory effects of normal human serum are dose dependent and abrogated with heat inactivation
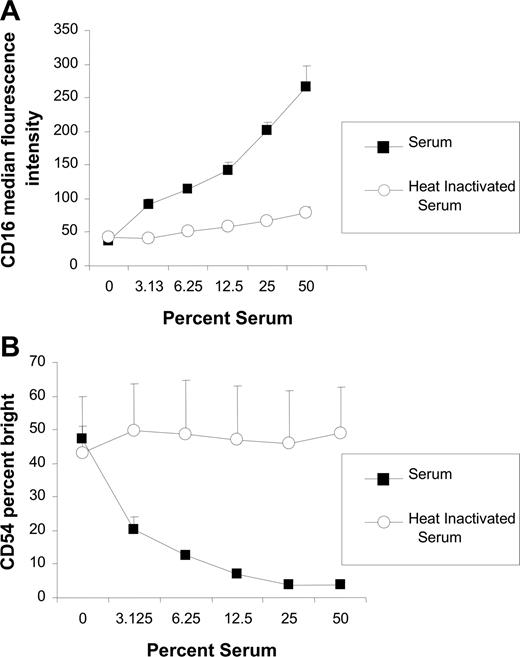
To investigate whether the inhibitory effects of serum on NK-cell activation was dose dependent, the effects of various concentrations of normal serum were assessed. The effect of serum was concentration dependent, with higher concentrations of serum having a greater effect on inhibition of both CD16 down-modulation (Figure 2A) and CD54 up-regulation (Figure 2B). When the serum was heat inactivated, the inhibitory effect of the serum was abrogated. These results demonstrate that the inhibitory properties of serum are dose dependent and heat labile, suggesting that the inhibition is due to complement activity.
Inhibitory effect of serum is dose dependent and abrogated by heat inactivation. PBMCs and Raji cells were mixed at a 1:1 ratio for 20 hours in varying concentrations of serum or heat-inactivated serum in the presence of 0.2 μg/mL rituximab. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. (A) NK-cell CD16, expressed as median fluorescence, after incubation with varying concentrations of serum and heat-inactivated serum (n = 3). (B) NK-cell CD54, expressed as a percentage of CD54 bright, after incubation with varying concentrations of serum and heat inactivated serum (n = 3). Error bars represent SD of the mean.
Inhibitory effect of serum is dose dependent and abrogated by heat inactivation. PBMCs and Raji cells were mixed at a 1:1 ratio for 20 hours in varying concentrations of serum or heat-inactivated serum in the presence of 0.2 μg/mL rituximab. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. (A) NK-cell CD16, expressed as median fluorescence, after incubation with varying concentrations of serum and heat-inactivated serum (n = 3). (B) NK-cell CD54, expressed as a percentage of CD54 bright, after incubation with varying concentrations of serum and heat inactivated serum (n = 3). Error bars represent SD of the mean.
Killed and fixed Raji cells coated with rituximab are able to induce NK-cell CD16 down-modulation
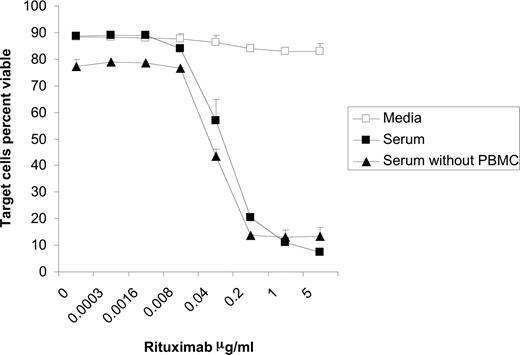
As would be expected, the addition of serum and rituximab to Raji cells induced lysis of the targets both in the presence and absence of PBMCs (Figure 3). This cell death, most likely due to CMC, was observed at rituximab concentrations beginning at 0.04 μg/mL. As illustrated in Figure 1B, NK-cell CD54 peaks and begins to decrease at 0.04 μg/mL rituximab when the cocultures are performed in the presence of serum. This suggests that lysis of rituximab-coated target cells through CMC could be inhibiting the ability of these targets to activate the NK cells. In order to evaluate this possibility, we assessed whether target cell viability affects the ability of rituximab to induce NK-cell CD16 down-modulation. Raji cells were fixed in 1% formaldehyde and washed before being used in the coculture assay. Results with fixed Raji cells were similar to those seen with viable target cells. In the absence of serum, surface CD16 on NK cells was modulated by rituximab-coated fixed Raji cells. This down-modulation was blocked when serum was added (Figure 4A). To confirm that the inhibition observed was independent of target cell viability, PBMCs were added to culture plates coated directly with rituximab in the absence of target cells. Under these conditions, NK-cell CD16 was down-modulated in the media alone, while the addition of serum blocked this down-modulation (Figure 4B). These studies using systems free of viable target cells indicate that the inhibitory effect of serum on rituximab-induced CD16 down-modulation is not due to lysis of the target cells by CMC.
Target cells are lysed in the presence of serum and rituximab irrespective of presence of effector cells. Raji cells were incubated for 20 hours in media, 50% serum, or 50% serum plus PBMCs with varying concentrations of rituximab. The percent of viable target cells were determined using flow cytometry by counting annexin V and propidium iodide–negative target cells (n = 3). Error bars represent SD of the mean.
Target cells are lysed in the presence of serum and rituximab irrespective of presence of effector cells. Raji cells were incubated for 20 hours in media, 50% serum, or 50% serum plus PBMCs with varying concentrations of rituximab. The percent of viable target cells were determined using flow cytometry by counting annexin V and propidium iodide–negative target cells (n = 3). Error bars represent SD of the mean.
Serum inhibits changes in CD16 expression in the absence of viable target cells. (A) Raji cells were fixed in 1% formaldehyde and washed extensively. Fixed cells were mixed with PBMCs at a 1:1 ratio for 20 hours with 5 μg/mL rituximab (n = 3). (B) Flat-bottom plates were coated with 10 μg/mL of rituximab. Plates were washed, and PBMCs were added and cultured for 20 hours. Incubations with fixed Raji cells and rituximab or rituximab-coated plates were preformed in the presence or absence of 50% serum and heat-inactivated serum (n = 5). Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD16, expressed as median fluorescence, was measured after incubation with media, serum, and heat-inactivated serum in the presence of fixed Raji cells (A) or flat-bottom plates coated with rituximab (B). Error bars represent SD of the mean.
Serum inhibits changes in CD16 expression in the absence of viable target cells. (A) Raji cells were fixed in 1% formaldehyde and washed extensively. Fixed cells were mixed with PBMCs at a 1:1 ratio for 20 hours with 5 μg/mL rituximab (n = 3). (B) Flat-bottom plates were coated with 10 μg/mL of rituximab. Plates were washed, and PBMCs were added and cultured for 20 hours. Incubations with fixed Raji cells and rituximab or rituximab-coated plates were preformed in the presence or absence of 50% serum and heat-inactivated serum (n = 5). Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD16, expressed as median fluorescence, was measured after incubation with media, serum, and heat-inactivated serum in the presence of fixed Raji cells (A) or flat-bottom plates coated with rituximab (B). Error bars represent SD of the mean.
Serum-depleted of C1q and C3 fails to inhibit activation of NK cells as indicated by up-regulation of CD54 induced by rituximab-coated Raji cells, while serum depleted of C5 inhibits NK activation
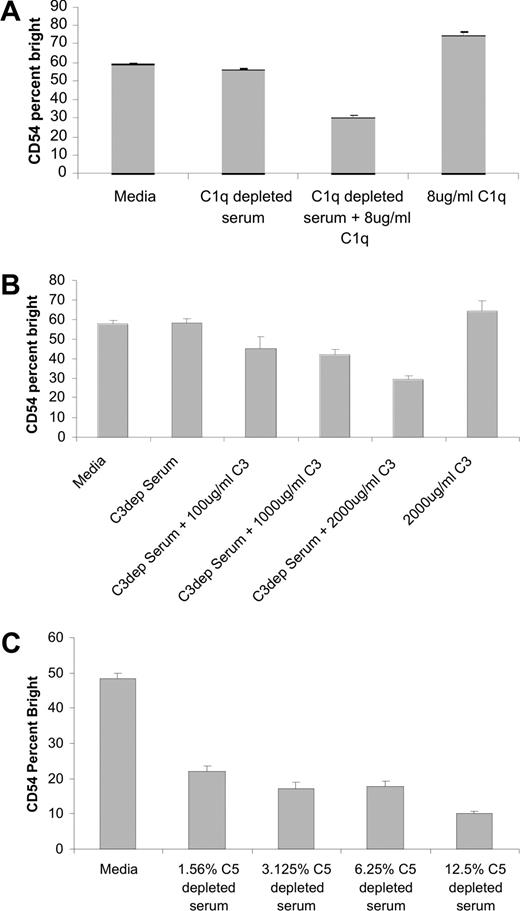
To confirm that the inhibitory activity of serum is due to complement, activation of NK cells induced by rituximab-coated target cells was assessed in the presence of serum depleted of C1q, C3, or C5. The addition of C1q-depleted serum to the coculture failed to inhibit NK-cell CD54 up-regulation induced by rituximab-coated targets (Figure 5A). The inhibition of NK-cell CD54 up-regulation was restored when purified C1q was added to the C1q-depleted serum. Purified C1q in the absence of serum had no effect on NK-cell CD54 expression. Similar results were observed in experiments using C3-depleted serum. Serum-depleted C3 failed to inhibit NK-cell CD54 up-regulation. The inhibitory effect of serum was restored when purified C3 was added to the C3 depleted serum (Figure 5B). Purified C3 also had no effect on NK-cell CD54 expression in the absence of serum. In contrast, serum depleted of C5 successfully blocked NK-cell CD54 up-regulation induced by rituximab-coated Raji cells (Figure 5C). In addition, there was no evidence of target-cell lysis through CMC in the presence of C5-depleted serum (data not shown). The finding that C3, but not C5, is necessary to block rituximab-induced NK activation suggests that C3b deposition on the rituximab-coated targets is the key component in the observed inhibitory effect.
Serum-depleted C5 inhibits NK-cell CD54 up-regulation, while serum-depleted C1q or C3 does not. PBMCs were mixed with Raji cells at a 1:1 ratio for 20 hours with 5 μg/mL of rituximab in the presence or absence of C1q-, C3-, or C5-depleted serum. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD54, expressed as a percentage of CD54 bright, was determined. (A) Evaluation in C1q-depleted serum, C1q-depleted serum with purified C1q added back, or purified C1q alone (n = 3). (B) Evaluation in C3-depleted serum, C3-depleted serum with purified C3 added back, or purified C3 alone (n = 3). (C) Evaluation in various concentrations of C5-depleted serum (n = 3). Error bars represent SD of the mean.
Serum-depleted C5 inhibits NK-cell CD54 up-regulation, while serum-depleted C1q or C3 does not. PBMCs were mixed with Raji cells at a 1:1 ratio for 20 hours with 5 μg/mL of rituximab in the presence or absence of C1q-, C3-, or C5-depleted serum. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD54, expressed as a percentage of CD54 bright, was determined. (A) Evaluation in C1q-depleted serum, C1q-depleted serum with purified C1q added back, or purified C1q alone (n = 3). (B) Evaluation in C3-depleted serum, C3-depleted serum with purified C3 added back, or purified C3 alone (n = 3). (C) Evaluation in various concentrations of C5-depleted serum (n = 3). Error bars represent SD of the mean.
C3b-stabilizing antibody enhances the inhibition of NK-cell CD54 up-regulation induced by rituximab-coated Raji cells
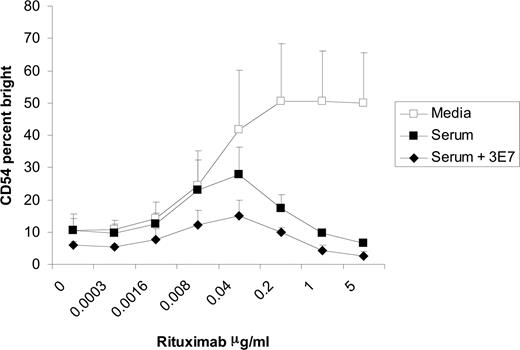
It was previously reported that mAb 3E7 prevents the degradation of C3b by competing with factor H and factor I, thereby increasing the amount of C3b present on the surface of target cells.24 We used this C3b-stabilizing mAb to further correlate C3 with the inhibition of NK-cell activation. The addition of 3E7 to serum in the coculture assay enhanced the degree of inhibition of NK-cell CD54 up-regulation when compared with human serum alone (Figure 6). These results suggest increased amounts of C3b on the surface of targets enhance the inhibitory effects of serum and provide evidence that C3b is responsible for the inhibition of NK-cell activation induced by rituximab-coated Raji cells.
C3b-stabilizing antibody (3E7) enhances the inhibition of NK-cell CD16 down-modulation and CD54 up-regulation. PBMCs and Raji cells were mixed at a 1:1 ratio for 20 hours in the presence or absence of 50% serum with varying concentrations of rituximab and 10 μg/mL of 3E7. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD54, expressed as a percentage of CD54 bright, was cultured in media, serum, or serum plus 3E7 (n = 3). Error bars represent SD of the mean.
C3b-stabilizing antibody (3E7) enhances the inhibition of NK-cell CD16 down-modulation and CD54 up-regulation. PBMCs and Raji cells were mixed at a 1:1 ratio for 20 hours in the presence or absence of 50% serum with varying concentrations of rituximab and 10 μg/mL of 3E7. Surface marker expression was determined using flow cytometry with gating on CD3−, CD56+ lymphocytes. NK-cell CD54, expressed as a percentage of CD54 bright, was cultured in media, serum, or serum plus 3E7 (n = 3). Error bars represent SD of the mean.
Blocking CR3 does not affect inhibition of NK activation
C3b is a ligand for complement receptor 3 (CR3; Mac-1, CD11b/CD18), which is expressed by NK cells. One explanation for the findings is that C3b induces an NK cell–inhibitory signal via CR3, or alternatively that it blocks a CR3 activation signal from a different ligand. CR3-blocking mAbs have been shown to inhibit CR3-mediated signaling and phagocytosis of effector cells.25-27 We used blocking mAbs to CD11b and CD18 to investigate whether the ability of complement to inhibit NK activation is through CR3. The inhibitory effects of human serum on rituximab-induced NK-cell CD16 down-modulation and CD54 up-regulation continued to be observed in the presence of anti-CD11b and anti-CD18 antibodies (data not shown). This suggests the inhibition of NK activation mediated by complement is not mediated through CR3.
NK-cell CD16 binding to rituximab is blocked by complement fixation
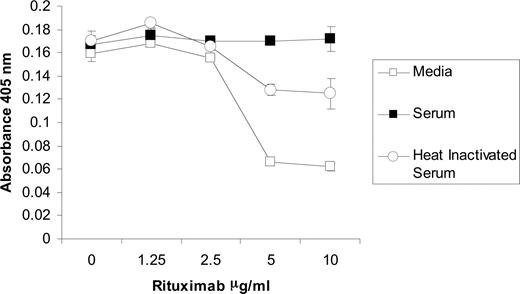
Because C3b deposits on human Fc regions that interact with CD16, it is possible that the activation of complement affects the binding of NK cells to rituximab. In order to further explore the mechanism in which complement fixation inhibits NK-cell activation, a cell adhesion assay was developed to analyze the effect of complement on the avidity between CD16 of intact NK cells and human IgG. Purified NK cells were applied to U-bottomed plates, centrifuged after incubation, and analyzed for absorbance at 405 nm. In the absence of rituximab, the cells pelleted at the bottom of the wells as shown by a high absorbance. When the plate was coated with rituximab, NK cells adhered to the walls and prevented the formation of a pellet, resulting in lower absorbance. An anti-CD16 mAb blocked this adhesion while an isotype control did not, indicating that the NK-cell adherence is through its CD16 binding to rituximab (data not shown). When the rituximab-coated plates were incubated with serum prior to application of NK cells, the adherence was blocked (Figure 7). Heat-inactivated serum was less effective at blocking the binding of NK cells to the walls of the plate. The residual inhibition of the heat-inactivated serum is likely due to the IgG within the serum binding to Fc receptors on the NK cells. These results provide further evidence that complement fixation blocks the binding of CD16 on NK cells to rituximab.
NK cell CD16 binding to rituximab is blocked by complement fixation. U-bottomed 96-well microtiter plates were coated with varying concentrations of rituximab. Wells were incubated with media, 50% serum, or 50% heat-inactivated serum. After washing, NK cells were allowed to sit on plate for 30 minutes at room temperature. High absorbance resulted from NK-cell pelleting that occurred in the absence of binding to rituximab. All samples were run in triplicate (n = 3). Data are representative of 3 independent experiments. Error bars represent SD of the mean.
NK cell CD16 binding to rituximab is blocked by complement fixation. U-bottomed 96-well microtiter plates were coated with varying concentrations of rituximab. Wells were incubated with media, 50% serum, or 50% heat-inactivated serum. After washing, NK cells were allowed to sit on plate for 30 minutes at room temperature. High absorbance resulted from NK-cell pelleting that occurred in the absence of binding to rituximab. All samples were run in triplicate (n = 3). Data are representative of 3 independent experiments. Error bars represent SD of the mean.
Serum-depleted C5 inhibits NK cell–mediated ADCC
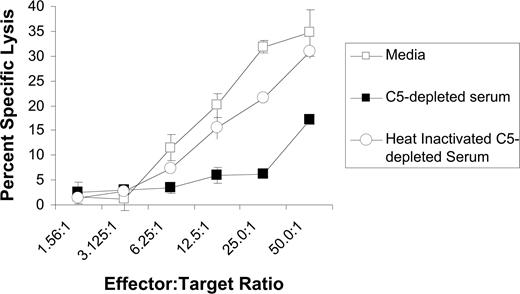
We next evaluated whether complement impairs rituximab-mediated ADCC using purified NK cells as effector cells. These studies were done using C5-depleted serum because such serum allows for activation of the earlier steps in the complement cascade, including C3b deposition, but does not induce development of the membrane attack complex or CMC of the target cell. As expected, C5-depleted serum was unable to induce CMC of rituximab-coated Raji cells (data not shown). Also as expected, purified NK cells induced ADCC of rituximab-coated Raji cells in the absence of serum. ADCC was inhibited by C5-depleted serum, but not by heat-inactivated, C5-depleted serum (Figure 8). These studies demonstrate that complement fixation upstream of C5 activation impairs the lysis of rituximab-coated target cells by NK cells.
C5-depleted serum inhibits rituximab-mediated ADCC. Raji cells were labeled with 51Cr and incubated with purified NK cells in various E/T ratios, 5 μg/mL rituximab, and either C5-depleted serum, heat-inactivated C5-depleted serum, or media. Percentage of specific lysis was measured based on 51Cr release. All samples were run in triplicate (n = 3). Data are representative of 3 independent experiments. Error bars represent SD of the mean.
C5-depleted serum inhibits rituximab-mediated ADCC. Raji cells were labeled with 51Cr and incubated with purified NK cells in various E/T ratios, 5 μg/mL rituximab, and either C5-depleted serum, heat-inactivated C5-depleted serum, or media. Percentage of specific lysis was measured based on 51Cr release. All samples were run in triplicate (n = 3). Data are representative of 3 independent experiments. Error bars represent SD of the mean.
Discussion
Most in vitro studies on mAb mechanisms of action involve the use of cytotoxicity assays. Measurements of cell lysis, when mAbs are added to target cells in the absence of immune effector mechanisms, are widely used to study signaling induced apoptosis. Serum is included as a source of complement in cytotoxicity assays involving mAbs and target cells to study CMC. ADCC is usually studied in the absence of human serum through the quantitation of cell lysis by the addition of effector cells to mAbs and target cells. These assays have proven to be quite informative when individual mechanisms are under evaluation. However, measuring target lysis alone makes it difficult to assess the relative role of each mechanism under physiologic conditions in which target cells, effector cells, complement, and the mAb are all present. We evaluated how complement impacts on NK-cell activation induced by mAb-coated target cells. Malignant B-cell lines served as the target cell, while rituximab was used as the mAb. This assay allowed us to quantitate NK activation separately from mAb-induced target lysis, which results from a variety of mechanisms.
These studies demonstrate that serum, but not heat-inactivated serum, blocks activation of NK cells by rituximab-coated target cells. The effect of serum on CD16 down-modulation was seen with nonviable target cells and in a target cell–free system, indicating that the inhibition of NK activation was not due to rapid CMC of target cells. Instead, it appears blocking of rituximab-mediated signaling via CD16 is likely responsible for this effect. C1 and C3 were required for this inhibition, but not C5. The ability of a C3b-stabilizing mAb to enhance the blocking effect points toward C3b deposition as being responsible. An adhesion assay demonstrated that serum effectively interferes with the binding of NK-cell CD16 to rituximab. C5-depleted serum blocked NK-mediated ADCC, indicating early steps in the complement cascade, not only inhibit NK activation, but also inhibit NK-mediated lysis of mAb-coated targets.
These results provide further evidence that CMC and ADCC are not completely independent mechanisms. Complement activation can deposit C3b (and its subsequent breakdown products iC3b and C3dg) on mAb-opsonized cells. Both C3b and iC3b can act as opsonins to enhance cell-mediated killing, even if the cells are not directly lysed by the membrane attack complex.28 Moreover, activation of complement by IgG-opsonized substrates can generate C5a, which can serve as a chemotactic factor to attract inflammatory cells. C5a also acts to up-regulate both CR329,30 and activating FcγRIII on monocytes and macrophages, thus enhancing local inflammation and ADCC at the tumor site.31-34
Activation of NK cells via CD16 may be mediated, at least in part, by interactions involving the CD11b/CD18 (CR3) molecules on the surface of the NK cell.35 C3b binding to CR3 could interfere with this interaction. As outlined here, serum inhibited NK activation even in the presence of antibodies that block CD11b and CD18, suggesting the observed effects on rituximab-induced NK activation are not due solely to a CR3-mediated pathway. Hong et al have reported that beta1,3-glucans can interact with CR3 and enhance sensitivity of antibody-coated target cells to immune effectors.36,37 Indeed, the relationship between CD16, IgG Fc, C3b, and CR3 appears to be quite complex, and the blocking studies outlined here do not totally exclude the possibility that CR3 is playing a role in the observed inhibition of NK activation by complement. We are currently exploring how these interactions affect CR3 pathway signaling.
An adherence assay was developed to assess the effect of complement on the avidity between CD16 on intact cells and IgG. The cell-adhesion approach was used because it allowed us to use live NK cells to study CD16 binding in its natural conformation in the membrane. Importantly, this assay was done in the absence of target cells. Therefore, it only measured interactions of the NK cell with the IgG, and would not be influenced by adhesion interactions between NK cells and target cells. Using this assay, we demonstrated that complement fixation blocks the binding of CD16 on the NK cells to rituximab. This finding is consistent with the known structures of these molecules. Crystal structures demonstrate that CD16 binds to human IgG1 Fc in the area of the Cγ2 and hinge region.38 The Cγ2 and hinge region has also been shown to be where C3b binds to human IgG1 Fc.39 Given that C3b is a large (177 kDa) protein, C3b deposited on the Fc portion of IgG1 could well hinder the interaction between mAb and CD16. These results provide further evidence that a primary mechanism involved in the observations outlined here is the ability of C3b to block interaction between CD16 and the Fc portion of human IgG1.
The ability to induce complement fixation varies depending on the mAb. Different anti-CD20 mAbs have been shown to vary in their ability to mediate CMC.40-42 In addition, trastuzumab is not efficient at fixing complement, and the data on the ability of alemtuzumab, an anti-CD52 mAb, to induce CMC is conflicting. We are currently exploring whether serum inhibits the ability of each of these mAbs to induce NK activation and mediate ADCC. Preliminary results indicate the inhibition of NK-cell activation by complement is not observed with all mAbs. For example, in contrast to the results with rituximab presented here, we have found that serum has no effect on the ability of trastuzumab on breast cancer cells to activate NK cells. Ongoing studies are exploring whether a correlation exists between the ability of a mAb to mediate CMC and the inhibition of its ability to activate NK cells and mediate ADCC in the presence of serum.
There is conflicting evidence as to whether levels of the complement regulatory proteins CD55 and CD59 play a role in clinical response to anticancer mAbs, including rituximab. In vitro studies demonstrate a correlation between low CD55 and CD59 and greater CMC-induced lysis of target cells.3,4 However, no correlation has been found between expression of these proteins by malignant B cells and response to rituximab therapy.5 Our findings suggest a possible explanation for this discrepancy. CD59 blocks the membrane attack complex, but has no influence on C3b opsonization. However, complement control protein CD55 suppresses complement activation at the C3b activation stage.43,44 Thus, higher CD55 expression could suppress C3b deposition, thereby paradoxically allowing for more effective NK activation and ADCC. In other words, enhanced CD55 activity could lead to a decrease in CMC, but allow for enhanced NK activation and ADCC. We are currently exploring this possibility by assessing whether enhanced CD55 expression leads to more effective NK activation in the assays outlined here.
NK activation in the absence of serum is first seen at mAb concentrations of approximately 0.002 μg/mL. In contrast, a concentration of approximately 0.04 μg/mL is required for CMC to occur. The concentration in which mAb begins to block NK activation is similar to the concentration of mAb that induces CMC (0.04 μg/mL). This is consistent with both CMC and blockade of NK activation being dependent on initiation of the complement cascade. NK activation is likely induced when multiple mAb molecules bridge between the target and effector cells in the immunologic synapse, and so cross-link CD16. This occurs at a relatively low mAb concentration because of the field effect of these interactions. In contrast, complement interacts with individual or a small number of mAb molecules on the surface of the target cell. As illustrated by our data, higher concentrations of mAb are needed to activate complement to induce either CMC or block NK activation. The observation that NK activation begins at a lower mAb concentration than does the inhibitory effect of complement fixation could have clinical implications. There appears to be a window of mAb concentrations in which NK activation is induced without fixation of complement. These concentrations are within the range of mAb concentrations one might expect to find clinically in tissues after rituximab therapy, and suggests that a more precise titration of mAb levels to fall within this window could lead to greater ADCC.
Our current understanding of the structure-function relationships of the mAb Fc allows us to engineer mAb with modified Fc function. Most effort in this regard, including our own, has been geared toward enhancing affinity of mAb Fc for Fcγ receptors.21 However, it is also possible to design a mAb that continues to recognize FcγR but no longer fixes complement. The results outlined here and the data supporting ADCC as a central mechanism of action suggest that an IgG which activates NK cells without fixing complement may widen the window and be more effective therapeutically by mediating ADCC at high mAb concentrations.
In conclusion, we have demonstrated that complement fixation resulting in C3b deposition inhibits the ability of mAb-coated targets to activate NK cells. This is most likely due to C3b blocking the interaction between the Fc portion of rituximab and CD16. We know CMC can serve as a mechanism by which target cells can be killed. Our studies suggest complement fixation can also have an inhibitory effect on NK activation and ultimately ADCC. Growing evidence indicates that ADCC is crucial to the antitumor effect of mAb therapy. If ADCC indeed serves as a major mechanism in mAb therapy, the ability of mAb to fix complement may actually limit its clinical efficacy. These findings, if confirmed, could have significant implications on how we use currently available mAbs, and how we prioritize selection of mAb with engineered function for future development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grant P50 CA97274 and Leukemia and Lymphoma Society grant no. 6100-07.
Authorship
Contribution: S.-Y.W. designed research, performed research, analyzed data, and wrote the manuscript; E.R. discussed and interpreted experimental results; R.P.T. discussed and interpreted experimental results and provided vital reagents; and G.J.W. generated the concept, designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George Weiner, 5970 JPP, Holden Comprehensive Cancer Center at the University of Iowa, 200 Hawkins Dr, Iowa City, IA 52242; e-mail: george-weiner@uiowa.edu.