Abstract
Various pathologies are characterized by the accumulation of toxic iron in cell compartments. In anemia of chronic disease, iron is withheld by macrophages, leaving extracellular fluids iron-depleted. In Friedreich ataxia, iron levels rise in the mitochondria of excitable cells but decrease in the cytosol. We explored the possibility of using deferiprone, a membrane-permeant iron chelator in clinical use, to capture labile iron accumulated in specific organelles of cardiomyocytes and macrophages and convey it to other locations for physiologic reuse. Deferiprone's capacity for shuttling iron between cellular organelles was assessed with organelle-targeted fluorescent iron sensors in conjunction with time-lapse fluorescence microscopy imaging. Deferiprone facilitated transfer of iron from extracellular media into nuclei and mitochondria, from nuclei to mitochondria, from endosomes to nuclei, and from intracellular compartments to extracellular apotransferrin. Furthermore, it mobilized iron from iron-loaded cells and donated it to preerythroid cells for hemoglobin synthesis, both in the presence and in the absence of transferrin. These unique properties of deferiprone underlie mechanistically its capacity to alleviate iron accumulation in dentate nuclei of Friedreich ataxia patients and to donate tissue-chelated iron to plasma transferrin in thalassemia intermedia patients. Deferiprone's shuttling properties could be exploited clinically for treating diseases involving regional iron accumulation.
Introduction
The pathologic effects of iron accumulation in tissue are recognized in diseases of systemic iron overload, in which the liver, heart, and endocrine glands are the principal affected organs.1 At the cellular level, labile iron begins to rise once the intracellular capacity for iron storage is surpassed, leading to catalytic formation of reactive oxygen species (ROS) that ultimately overwhelm the cellular antioxidant defense mechanisms and lead to cell damage.2
In recent years, several pathologies have been shown to be associated with specific defects in cellular iron metabolism that do not give rise to conspicuous systemic iron overload.3 In the neuromuscular disorder Friedreich ataxia, the deficiency of the iron-chaperone protein frataxin is thought to lead to mitochondrial iron accumulation due to improper processing of iron for heme and iron-sulfur-cluster formation.4 In the group of neuromuscular disorders termed NBIA (neurodegeneration with brain iron accumulation), a deficiency in pantothenate kinase (PKAN-2), a key enzyme in coenzyme A synthesis,5 leads to iron deposition and ensuing brain damage by still-unresolved mechanisms.6 In hereditary X-linked sideroblastic anemia, impaired heme synthesis causes mitochondrial iron accumulation and siderosis in erythroid cells.3 However, an extreme example of systemic misdistribution of iron is encountered in anemia of chronic disease (ACD), where iron accumulates in reticuloendothelial cells responsible for recycling aged erythrocytes, presumably due to decreased activity of the iron-efflux protein ferroportin.7 The marked down-regulation of ferroportin in the plasma membranes of macrophages in ACD is thought to be triggered posttranslationally by the circulating iron-regulatory peptide hepcidin, which is induced by inflammation8 and transcriptionally by certain cytokines.7,8 Thus, macrophage iron accumulation in ACD not only coexists with anemia but also is largely responsible for it.9
Here we explore the concept of relocating iron from areas of accumulation to areas of deprivation using agents capable of permeating membranes, chelating cell labile iron and donating iron to physiologic acceptors. Most agents in clinical use for managing systemic iron overload1 were designed and/or are applied for correcting or preventing hepatic and cardiac siderosis, the major causes of premature death in thalassemia.2 Those chelators demonstrably reduce labile iron levels in plasma, but a few, by virtue of their membrane-crossing ability, also do so in cells.1,10 However, whether such agents could or should be used in diseases in which regional iron accumulation is not accompanied by hyperferremia is questionable. In the present case, the goal of chelation is not merely systemic depletion, but redistribution of iron.
The selection of the orally active chelator deferiprone (DFP) as a prototype iron-relocating agent is based on several observations. DFP has been shown to shuttle tissue iron into the circulation to higher-affinity chelators such as desferrioxamine (DFO)11 and to increase transferrin (Tf) saturation in individuals with unimpaired serum iron–binding capacity.12 Donation of iron by DFP to DFO and to transferrin was also observed in vitro.11 Recently, DFP was found to reduce iron accumulation in the dentate nuclei of Friedreich ataxia patients,13 indicating its ability to cross the blood-brain barrier in humans, which is consistent with previous findings in animals.14-16
In the present study we assess DFP's capacity to act as an iron-relocating agent at the cellular level. To trace labile iron mobilized by chelators, we used model cardiac and macrophage cells in culture and fluorescent metal sensors (FMS) targeted to cellular and extracellular compartments. We show that DFP can relocate iron accumulated in cell compartments within and across the cell, and we demonstrate that iron withdrawn from sites of cell accumulation can be safely transferred to extracellular transferrin for physiologic reuse.
Methods
Materials
Calcein green (CALG) 3,3′-bis[N,N-bis(Carboxymethyl) aminomethyl]fluorescein and its acetomethoxy (AM) precursors CALG-AM, lissamine rhodamine sulfonyl chloride, and sulforhodamine were from Molecular Probes (Eugene, OR). Human serum holotransferrins and apotransferrins (aTf) were from Kamada (Rehovot, Israel). Diethylene-triamine-pentaacetic acid (DTPA), nitrilotriacetate (NTA), EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide), ferric ammonium citrate (FAC), ferrous ammonium sulfate (FAS), succinyl acetone, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 4-morpholine ethanesulfonic acid (MES), core histone mixture from calf thymus (H), N,N′-hexamethylene-bis-acetamide, and octyl-glucoside were from Sigma-Aldrich (St Louis, MO). Rhodamine isothiocyanate was from Fluka (Buchs, Switzerland). DFP (1,2-dimethyl-3-hydroxypyridin-4-one) was from Apo-Pharma (Toronto, ON). DFO was from Novartis (Basel, Switzerland). The red fluorescent mitochondrial metal–sensor rhodamine B-[(1, 10-phenanthrolin-5-yl aminocarbonyl] benzyl ester (RPA), was a kind gift from U. Rauen and R. Sustmann, University of Duisburg-Essen, Essen, Germany. A summary of the probes used and their properties is given in Table 1.
Methods
Complexes of iron with NTA (Fe-NTA) were generated by mixing FAS and NTA (1:3 molar ratio) in water and allowing iron to oxidize in ambient conditions. The DFP-Fe(III) complexes were generated by mixing Fe-NTA with DFP in water; formation of fully substituted DFP-Fe complexes was confirmed by measuring absorbance at 455 nm.19
Cell-culture lines.
Rat H9C2 cardiomyocytes, J774 mouse macrophages, and erythroleukemia (MEL) were grown in 5% CO2 Dulbecco-modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 4.5 g/L D-glucose, glutamine, and antibiotics (Biological Industries, Kibbutz Bet Haemek, Israel). Cells were plated 1 day before experimentation onto 96-well plates, or onto microscopic slides attached to perforated tissue culture 3-cm plates.
Probe loading into cells.
For cytosolic loading of CALG, cells in DMEM plus 10mM Na-HEPES (DMEM-HEPES) at 37°C were exposed for 10 minutes to 0.25 μM CALG-AM, washed and incubated in DMEM-HEPES containing 0.5 mM probenecid (to minimize probe leakage). For CALG-Fe(III) (1:1) complexes and for sulforhodamine loading into endosomes, cells were exposed to 50 μM to 100 μM complex in DMEM-HEPES for 30 minutes at 37°C and washed extensively with the same probe-free medium and subsequently with HEPES-buffered saline (HBS; 20 mM HEPES, 135 mM NaCl; pH 7.4).20,21 RPA was loaded into mitochondria as described in earlier studies.21-23
Histone-CALG.
For loading into the nucleus, CALG was coupled to core histones from calf thymus (H) 7.2 mg/mL, in 50 mM Na-MES, 100 mM NaCl, pH 5.5, containing 1.25 mM CALG:Cobalt (Co protects CALG metal-binding groups). Coupling was initiated by adding 12 mM EDC, followed by incubation for 1 hour at room temperature and overnight at 5°C. After exhaustive dialysis, 11 mM DTPA was added (to remove Co(II)) and histone-CALG (H-CALG) was further dialyzed against HBS. CALG coupling to H was estimated at 1.1:1 by fluorescence (λ excitation 480 nm, λ emission 520 nm). H-CALG-Fe was prepared by titrating CALG with FAS. Loading 10μM H-CALG or H-CALG-Fe into cells was done in DMEM-HEPES containing 100 μM chloroquine (for improved FMS targeting to cell nuclei),24 followed by HBS washes.
Fluorescein-DFO-histone.
The probe was prepared by coupling of N-(fluorescein-5-thiocarbamoyl)-desferrioxamine (Evrogen, Moscow, Russia) to H using fluorescein-DFO (FlDFO)–Fe (1:1) complex generated by adding 1.1 mM FeSO4 to 1 mM FlDFO in HBS. To 2.64 mL of FlDFO-Fe complex was added 8.5 mg H, followed by 0.18 mL of 1 M MES (Na form), pH 6.5, and 15 mg of EDC. After overnight agitation at 5°C, the mixture was dialyzed (3.5 kDa cut-off tubing) against 10 mM acetic acid; 1 mM EDTA; 150 mM NaCl, pH 4.5 (to remove bound iron); and finally HBS. The fluorescein-DFO-histone (H-FlDFO) ratio was 1:1, based on stoichiometric iron-quenching of H-FlDFO fluorescence (λ excitation 494 nm, λ emission 520nm). H-FlDFO, like H-CALG, was loaded into cells in DMEM-HEPES.
Rhodamine-labeled apotransferrin.
Human apotransferrin (4 mg/mL in 25 mM Na2CO3, 75 mM NaHCO3; pH 9.8), was incubated at 5°C overnight with 1 mM lissamine rhodamine sulfonyl chloride and the labeled protein isolated by gel filtration on Sepharose G25 (Sigma-Aldrich) preequilibrated with 150 mM NaCl, 20 mM MES; pH 5.3.
Transfer of iron from DFP-Fe to apotransferrin.
DFP-Fe complexes were generated by incubating Fe-NTA (50:150 μM) with either 150 μM or 250 μM DFP in HBS for 20 minutes, and completion of complex formation was assessed by absorption at 455 nm. The complexes were mixed with 50 μM aTf and 25 mM NaHCO3 and incubated for 1.5 hours in a 5% CO2 incubator. Because of the large spectral overlap of DFP-Fe and transferrin-Fe, we added DTPA (10 mM) to selectively scavenge iron from DFP-Fe. Transferrin that was free of low-molecular components was obtained by either filtering 0.5 mL through 30-kDa cut-off spin filters (Pall, East Hills, NY) via centrifugation (2900g for 20 minutes), or by use of a 5-mL dry-spin–gel filtration-centrifugation at 1100g over precentrifuged Sephadex G-50 medium columns (Sigma-Aldrich). The complex-free transferrin was diluted in HBS, pH 7.4. Tf-Fe was analyzed by absorbance at 465 nm, and aTf by tryptophan fluorescence (280 nm excitation, 306 nm emission; Felix spectrofluorimeter station version 2.5; Photon Technology International, Lawrenceville, NJ).
Iron-loading of cells was done by overnight incubation of cells with 100 μM FAC in culture conditions followed by washing with HBS containing 100 μM DFO, and with HBS alone. To achieve selective accumulation of iron in mitochondria, cells were treated with 1 mM succinyl acetone for 3 hours at 37°C.
Cell hemoglobin synthesis.
MEL cells cultured for 2 days in media supplemented with 5mM hexamethylene-bis-acetamide were washed and lysed with octyl-glucoside (1.5%). After 2 minutes centrifugation at 12 000g, 100 μL of lysate supernatants was transferred to a 96-well microplate for estimating hemoglobin (by measuring absorbance at 410 nm). Alternatively, lysates were mixed with 100 μL freshly prepared tetramethylbenzidine (0.5 mg/mL in 10% acetic acid) and finally 8 μL of 30% H2O2. Absorption (604 nm) was read after 90 seconds.
Data acquisition and analysis.
Epifluorescence imaging (Nikon TE 2000 microscope; Melville, NY; and Orca-Era CCD camera; Hamamatsu, Bridgewater, NJ) coupled to a Volocity 4 system (Improvision, Coventry, United Kingdom) was used for image data acquisition and analysis.20 For high-throughput fluorescence monitoring we used fluorescence plate reading (Tecan-Safire; Neotec, Männedorf, Austria) essentially as described.20
Results
To follow DFP-mediated translocation of iron from the medium to cells, within cells, and from cells to the medium, we used fluorescent acceptors and donors of iron that undergo quenching or dequenching upon metal binding or removal. An additional feature of these probes was their localization in specific cell compartments or in the extracellular medium.
Transfer of iron from extracellular DFP-iron complexes to mitochondria
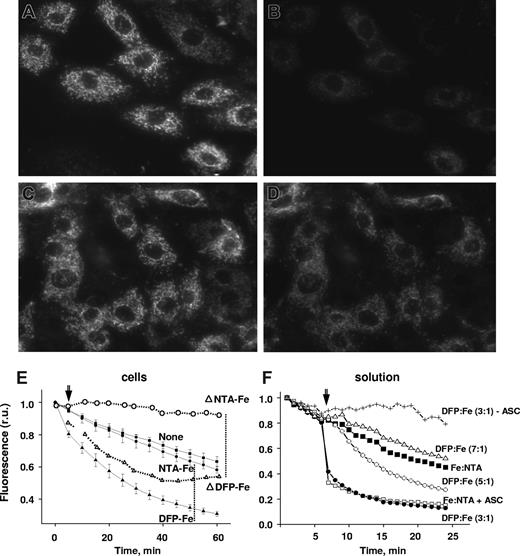
Ingress of ionic iron from the extracellular medium to the mitochondria of H9C2 cardiomyocytes was followed with the high-affinity Fe(II)–acceptor probe, RPA22,23 (Figure 1). The hydrophobic cationic rhodamine B is responsible for RPA's potentiometric partitioning in the mitochondria, and phenanthroline is responsible for detection of Fe(II).22 Exposure of cells to Fe(III)-NTA evoked no significant changes in RPA fluorescence relative to the spontaneous decrease (t½ 60 minutes), probably due to binding of endogenous labile iron and/or to probe photobleaching. On the other hand, addition of preformed DFP-Fe(III) complexes (DFP-Fe, ratio 3:1) evoked a time-dependent (t½ 15 minutes) quenching of mitochondrial RPA fluorescence (Figure 1E). Consistent with RPA's selectivity for Fe(II), no quenching of RPA by Fe-NTA occurred in solution unless ascorbate was added. Transfer of iron from DFP-Fe complexes to RPA in the presence of ascorbate was detected over a wide range of DFP to Fe ratios (1:1 to 7:1).
Transfer of iron from extracellular DFP-iron complexes to mitochondria. H9C2 cells loaded with the mitochondrial iron-sensor RPA were incubated with or without DFP-Fe (15:5 μM) and epifluorescence microscopic images were recorded every 5 minutes under settings for rhodamine. Representative fields of initial cell fluorescence at time 0 (A,C) and after incubation for 1 hour in the presence (B) or absence (D) of 5 μM DFP-Fe complex. Magnification was ×600; oil objective was a (Plan Apo) 60×/1.40 NA. (E) Mean fluorescence values in relative units (r.u.) plus or minus SD of 5 cells per field calculated for each time-point image and normalized to the initial fluorescence, representing cells incubated without (None) and with 5 μM Fe-complex (DFP-Fe or NTA-Fe); the lines denoted as ΔDFP-Fe and ΔNTA-Fe were corrected for spontaneous decay given by the control (None). Arrow indicates time the Fe complex was added. (F) Effect of various iron chelates on the fluorescence (f normalized to initial value, f0, in r.u.) of 0.5 μM RPA in solution (HBS buffer). The iron chelates all contained 5μM Fe complexed to NTA (1:3 ratio) in the absence (■) and presence (□) of 100 μM ascorbate (Fe:NTA, Fe:NTA + ASC) or to DFP, with 100 μM ascorbate, at DFP:Fe ratios of 3:1 (●), 5:1 (○), and 7:1 (▵). The values of fluorescence intensity are means of triplicate samples run in parallel, plus or minus SEM.
Transfer of iron from extracellular DFP-iron complexes to mitochondria. H9C2 cells loaded with the mitochondrial iron-sensor RPA were incubated with or without DFP-Fe (15:5 μM) and epifluorescence microscopic images were recorded every 5 minutes under settings for rhodamine. Representative fields of initial cell fluorescence at time 0 (A,C) and after incubation for 1 hour in the presence (B) or absence (D) of 5 μM DFP-Fe complex. Magnification was ×600; oil objective was a (Plan Apo) 60×/1.40 NA. (E) Mean fluorescence values in relative units (r.u.) plus or minus SD of 5 cells per field calculated for each time-point image and normalized to the initial fluorescence, representing cells incubated without (None) and with 5 μM Fe-complex (DFP-Fe or NTA-Fe); the lines denoted as ΔDFP-Fe and ΔNTA-Fe were corrected for spontaneous decay given by the control (None). Arrow indicates time the Fe complex was added. (F) Effect of various iron chelates on the fluorescence (f normalized to initial value, f0, in r.u.) of 0.5 μM RPA in solution (HBS buffer). The iron chelates all contained 5μM Fe complexed to NTA (1:3 ratio) in the absence (■) and presence (□) of 100 μM ascorbate (Fe:NTA, Fe:NTA + ASC) or to DFP, with 100 μM ascorbate, at DFP:Fe ratios of 3:1 (●), 5:1 (○), and 7:1 (▵). The values of fluorescence intensity are means of triplicate samples run in parallel, plus or minus SEM.
Transfer of iron from extracellular DFP-Fe complexes to nuclei.
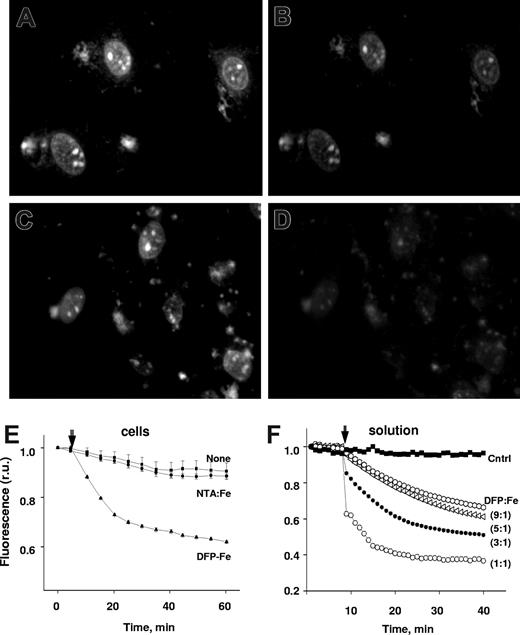
With the view of targeting a fluorescent iron sensor into the cell nucleus, we constructed a conjugate of H with the high-affinity iron chelator and iron sensor H-FlDFO.11 To minimize uptake via the endocytic pathway, incubation was carried out at room temperature and in the presence of chloroquine, as described in “Histone-CALG.” H-FlDFO within nuclei was rapidly (t½ ≤ 5 minutes) quenched by DFP-Fe (3:1) added to the extracellular medium (Figure 2E), consistent with the high cell permeability of DFP-Fe complexes shown in Figure 1. In solution, H-FlDFO is efficiently and rapidly (t½ < 1 minute) quenched by DFP-Fe complexes even at a 9:1 DFP:Fe ratio (Figure 2F), as expected from DFP's capacity to shuttle iron to DFO both in vivo and in vitro.11
Transfer of iron from extracellular DFP-iron complexes to nuclei. H9C2 cells loaded with the nuclear iron sensor H-FlDFO were incubated with or without 5μM DFP-Fe complex (DFP:Fe ratio 3:1) and epifluorescent microscopic images were recorded every 5 minutes under settings for fluorescein. Representative fields of initial cell fluorescence at time 0 (A,C) and after incubation for 1 hour in the absence (B) or presence (D) of 5μM DFP-Fe complex. (E) Mean fluorescence values in r.u. plus or minus SD of 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0), representing cells incubated without (None) and with 5μM DFP-Fe complex (DFP-Fe). Arrow indicates time the Fe complex was added. (F) Effect of DFP-Fe chelates (DFP:Fe at ratios of 1:1, 3:1, 5:1, 9:1) on the fluorescence (normalized to initial value, f0, in r.u.) of 0.5μM H-FlDFO in solution (HBS buffer). The values of fluorescence intensity are means of triplicate samples run in parallel, plus or minus SEM.
Transfer of iron from extracellular DFP-iron complexes to nuclei. H9C2 cells loaded with the nuclear iron sensor H-FlDFO were incubated with or without 5μM DFP-Fe complex (DFP:Fe ratio 3:1) and epifluorescent microscopic images were recorded every 5 minutes under settings for fluorescein. Representative fields of initial cell fluorescence at time 0 (A,C) and after incubation for 1 hour in the absence (B) or presence (D) of 5μM DFP-Fe complex. (E) Mean fluorescence values in r.u. plus or minus SD of 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0), representing cells incubated without (None) and with 5μM DFP-Fe complex (DFP-Fe). Arrow indicates time the Fe complex was added. (F) Effect of DFP-Fe chelates (DFP:Fe at ratios of 1:1, 3:1, 5:1, 9:1) on the fluorescence (normalized to initial value, f0, in r.u.) of 0.5μM H-FlDFO in solution (HBS buffer). The values of fluorescence intensity are means of triplicate samples run in parallel, plus or minus SEM.
DFP-mediated redistribution of iron between cellular compartments: from nuclei to mitochondria.
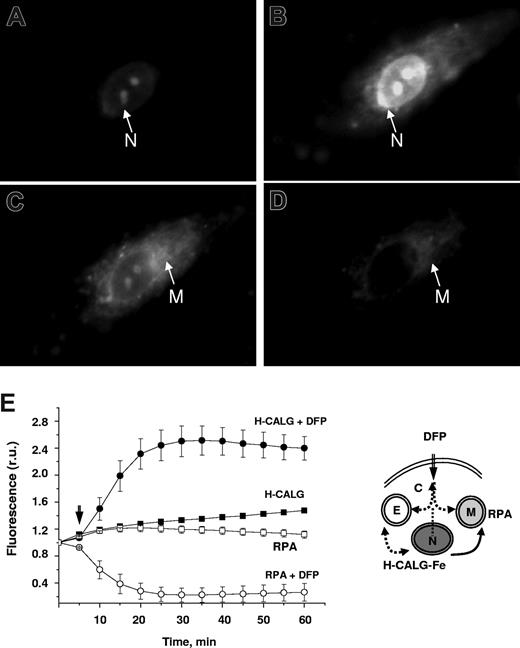
DFP's facilitation of iron movement between discrete cell compartments was followed in H9C2 cells sequentially labeled in cell nuclei with the iron-donor probe H-CALG-Fe(III) and in mitochondria with the Fe(II)-acceptor probe RPA. H-CALG-Fe penetrates the plasma membrane and accumulates initially in the cytosol and subsequently in the nucleus, particularly in nucleolus-like sites.21 H-CALG-Fe was used subsaturated with iron so that its accumulation in the nuclei could be followed via changes in the residual fluorescence of the unquenched fraction of the probe (revealed by chelation; Figure 3A). Loading of RPA into mitochondria was apparent from the characteristic perinuclear staining pattern (Figure 3C), from colocalization with other potentiometric probes and sensitivity to protonophores.22,23 Addition of 50μM DFP produced an increase in the fluorescence of H-CALG-Fe in the nuclei and a concomitant decrease in the fluorescence of RPA in the mitochondria, both of which occurred within 5 to 8 minutes and were complete within 20 minutes (Figure 3E). The changes are attributable to dequenching of H-CALG-Fe due to removal of iron by DFP and to quenching of RPA due to transfer of iron from DFP-Fe. Only minor changes in fluorescence were observed in untreated control cells. It is likely that nuclear H-CALG-Fe was not the only source of iron delivered to RPA by DFP, because the addition of DFP to RPA-loaded cells also led to quenching of mitochondrial probe (data not shown), although to a much lesser extent than in the presence of H-CALG-Fe.
DFP-mediated relocation of iron between cellular compartments: from nuclei to mitochondria. H9C2 cells were loaded with the nuclear iron sensor H-CALG that had been precomplexed to iron (H-CALG-Fe), followed by loading with the mitochondrial iron sensor RPA. The double-labeled cells were then exposed to 50μM DFP and epifluorescence images were recorded every 5 minutes. Representative fields of cell fluorescence observed under settings for fluorescein (A,B) and rhodamine (C,D). Images are shown at time 0 (A,C) and after incubation for 1 hour in the presence of 50μM DFP (B,D). (E) Mean fluorescence values plus or minus SD in r.u. of 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0), representing cells incubated with 50μM DFP (circles) and with no addition (squares). Fluorescence of H-CALG is indicated by filled symbols (● and ■), and fluorescence of RPA is indicated by open symbols (○ and □). The scheme illustrates entry of DFP into the cytosol (C), nuclei (N) containing H-CALG-Fe (iron donor) and mitochondria (M) containing RPA (iron acceptor), and transfer of iron from nuclei to mitochondria. E refers to endosomes.
DFP-mediated relocation of iron between cellular compartments: from nuclei to mitochondria. H9C2 cells were loaded with the nuclear iron sensor H-CALG that had been precomplexed to iron (H-CALG-Fe), followed by loading with the mitochondrial iron sensor RPA. The double-labeled cells were then exposed to 50μM DFP and epifluorescence images were recorded every 5 minutes. Representative fields of cell fluorescence observed under settings for fluorescein (A,B) and rhodamine (C,D). Images are shown at time 0 (A,C) and after incubation for 1 hour in the presence of 50μM DFP (B,D). (E) Mean fluorescence values plus or minus SD in r.u. of 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0), representing cells incubated with 50μM DFP (circles) and with no addition (squares). Fluorescence of H-CALG is indicated by filled symbols (● and ■), and fluorescence of RPA is indicated by open symbols (○ and □). The scheme illustrates entry of DFP into the cytosol (C), nuclei (N) containing H-CALG-Fe (iron donor) and mitochondria (M) containing RPA (iron acceptor), and transfer of iron from nuclei to mitochondria. E refers to endosomes.
DFP-mediated relocation of iron between cellular compartments: from endosomes to nuclei.
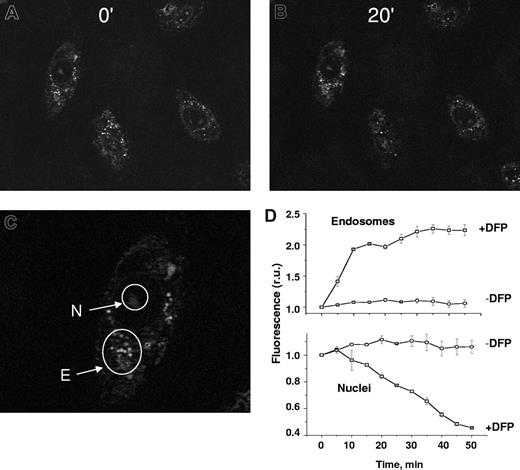
H9C2 cells were labeled sequentially in the nuclei with the iron-acceptor probe H-FlDFO and in the endosomes with the iron-donor probe CALG-Fe (Figure 4). We showed the bulk-phase pinocytic uptake of the iron-quenched probe CALG-Fe into endosome/pinosomes and its accessibility to DFP, as evidenced by dequenching, in a previous study.20 Moreover, CALG-Fe coendocytosed with the classical fluid phase markers sulforhodamine and rhodaminated dextran, both of which showed less than 65% cell colocalization as obtained with the Volocity program (not shown). H-FlDFO was detectable in the nuclear area, while CALG-Fe was concentrated in punctuate, perinuclear spots, attributable to endosome-trapped CALG-Fe (Figure 4). However, after incubation with 50 μM DFP, the fluorescence in the endosomes increased significantly, while that in the nuclei decreased relative to the respective untreated controls. The kinetics of the changes in the 2 compartments followed similar patterns, although in opposite directions. To ascertain whether the iron acquired by DFP was exclusively intracellular, the cells were washed with 0.1 mM DFO to remove all extracellular iron before the addition of DFP. Furthermore, as the addition of DFP to cells loaded with H-FlDFO alone failed to produce a decrease in its fluorescence (data not shown), the quenching of H-FlDFO in cells coloaded with CALG-Fe is most likely due to DFP-mediated mobilization of iron from endosomes. However, the possibility that in the course of the experiment some iron might have spontaneously leaked from endosomes to cytosol and rendered it transferable to mitochondria by added DFP cannot be ignored.
DFP-mediated relocation of iron between cellular compartments: from endosomes to nuclei. H9C2 cells were loaded with the nuclear iron-sensor H-FlDFO and subsequently exposed to preformed complexes of CALG and iron (CALG-Fe, 1:1 ratio) that were taken up by adsorptive pinocytosis into endosomes. The cells containing both labels were then exposed to 50 μM DFP and epifluorescent images were recorded every 5 minutes under fluorescein settings. Representative fields of cell fluorescence are shown at time 0 (A) and after incubation for 20 minutes (B) in the presence of 50 μM DFP. (C) The nuclei (N) and endosomes (E) are indicated by arrows. (D) Mean fluorescence values in r.u. within selected subcellular areas corresponding to endosomes (Di) and nuclei (Dii), using 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0).
DFP-mediated relocation of iron between cellular compartments: from endosomes to nuclei. H9C2 cells were loaded with the nuclear iron-sensor H-FlDFO and subsequently exposed to preformed complexes of CALG and iron (CALG-Fe, 1:1 ratio) that were taken up by adsorptive pinocytosis into endosomes. The cells containing both labels were then exposed to 50 μM DFP and epifluorescent images were recorded every 5 minutes under fluorescein settings. Representative fields of cell fluorescence are shown at time 0 (A) and after incubation for 20 minutes (B) in the presence of 50 μM DFP. (C) The nuclei (N) and endosomes (E) are indicated by arrows. (D) Mean fluorescence values in r.u. within selected subcellular areas corresponding to endosomes (Di) and nuclei (Dii), using 5 cells per field, calculated for each time-point image and normalized to the initial fluorescence (f/f0).
Transfer of iron from DFP-Fe complexes to transferrin.
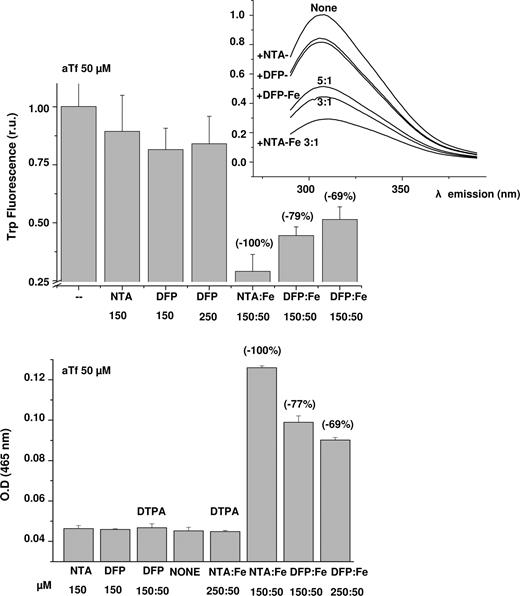
We followed iron complexation by transferrin using DFP-Fe complexes (3:1 and 5:1, 50 μM in Fe) and NTA-Fe complexes (3:1, 50 μM in Fe) as iron sources and apotransferrin (50 μM) as acceptor. To remove from the reaction mixture any traces of iron associated with low molecular weight complexes or free ligands, we added DTPA after 1 hour of reaction and followed it by size filtration. Filtration and differential chelation allowed the assessment of DFP-Fe transfer of iron to aTf both by light absorption of Tf-Fe complexes at 465 nm (Figure 5) and by tryptophan fluorescence of aTf that is quenched after specific binding of iron25 (Figure 5). While the 2 analytical methods monitored binding of iron to transferrin, the optical changes are mirror images of each other. Transfer of iron from DFP-Fe to transferrin ensued at both 3:1 and 5:1 stoichiometries of DFP:Fe and was of a stable nature: it persisted after addition of chelators that differentially dissociate DFP-Fe complexes (but not Fe-transferrin). On the other hand, DFP alone and NTA alone evoked only minor changes that were eliminated by addition of DTPA before the reaction with aTf, indicating that the changes were associated with traces of iron present in the medium. Essentially similar results were obtained by assessing the transfer of iron to apotransferrin using fluorescein-labeled aTf (not shown). However, with lissamine rhodamine-labeled aTf (R-aTf), the acquisition of iron evoked insignificant changes in fluorescence.
Transfer of iron from DFP-Fe complexes to transferrin. DFP-Fe complexes (1:3 and 1:5 ratios; 50 μM Fe(III)) were mixed with apotransferrin aTf (50 μM) and incubated for 1.5 hours in a humidified 5% CO2 incubator at 37°C. At the conclusion of the reaction, 10 mM DTPA was added and the low–molecular weight material was removed by filtration as described in “Histone-CALG.” The tryptophan (trp) fluorescence at 280 nm excitation/306 nm emission (top graph) was obtained from the emission spectra (inset). The high–molecular weight fractions were diluted in HBS, pH 7.4, and the absorbance was read at 465nm (bottom graph). Data are given as means plus or minus SD of 3 independent experiments. The labels below the bars indicate the complexes tested and their concentrations. The values above the bars represent the percentage change in absorbance or fluorescence.
Transfer of iron from DFP-Fe complexes to transferrin. DFP-Fe complexes (1:3 and 1:5 ratios; 50 μM Fe(III)) were mixed with apotransferrin aTf (50 μM) and incubated for 1.5 hours in a humidified 5% CO2 incubator at 37°C. At the conclusion of the reaction, 10 mM DTPA was added and the low–molecular weight material was removed by filtration as described in “Histone-CALG.” The tryptophan (trp) fluorescence at 280 nm excitation/306 nm emission (top graph) was obtained from the emission spectra (inset). The high–molecular weight fractions were diluted in HBS, pH 7.4, and the absorbance was read at 465nm (bottom graph). Data are given as means plus or minus SD of 3 independent experiments. The labels below the bars indicate the complexes tested and their concentrations. The values above the bars represent the percentage change in absorbance or fluorescence.
DFP-mediated mobilization of iron from H9C2 cells to extracellular transferrin: receptor-mediated uptake of holotransferrin as an index of iron shuttling
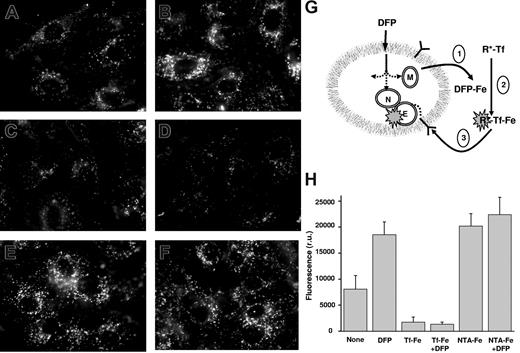
Mobilization of cellular iron by DFP and its transfer to extracellular transferrin are critical features of DFP's potential function as an iron shuttle. This capability was assessed using H9C2 cells incubated with lissamine R-aTf in the presence of DFP (Figure 6). The rationale was that intracellular iron mobilized to the extracellular medium by DFP would be transferred to the fluorescent Tf, which, upon binding iron, would then bind to cell-surface transferrin, and after receptor-mediated endocytosis, would become concentrated in endosomes. To increase the level of mitochondrial labile iron, the cells were pretreated with the heme-synthesis inhibitor succinylacetone, which causes selective accumulation of unused, chelatable iron in the mitochondria.22 After 1 hour of incubation with DFP, the level of cell-bound fluorescent aTf rose by 2.3-fold over controls without DFP (Figure 6A,B). The binding was specific and iron dependent, as it was fully blocked by excess holotransferrin (Figure 6C,D) and enhanced by addition of saturating concentrations of Fe-NTA (Figure 6E,F), both in the presence and in the absence of DFP. In control cells not treated with succinylacetone, DFP failed to enhance fluorescent-Tf binding (data not shown), indicating that under normal conditions where iron incorporation into heme is not blocked, the concentration of DFP-mobilized iron is insufficient to be detectable in this system.
DFP-mediated mobilization of iron from H9C2 cells to extracellular apotransferrin: receptor-mediated uptake of holotransferrin as an index of iron transfer. H9C2 cells were pretreated with succinylacetone as described in “Methods” to increase mitochondrial labile iron levels. They were then incubated at 37°C in DMEM-HEPES medium containing 20 μM lissamine R-aTf with various additions, and epifluorescence microscopy images were obtained under rhodamine settings after 60 minutes of incubation. Representative images of cells with no addition (A), 30 μM DFP (B), 100 μM unlabeled holotransferrin (C), 100 μM unlabeled holotransferrin with 30 μM DFP (D), 20 μM Fe-NTA, 1:3 ratio (E), and 20 μM Fe-NTA with 30 μM DFP (F). (G) The illustration represents entry of DFP into cells, mobilization of iron from the cytosol (C), nuclei (N) and mitochondria (M), followed by exit of DFP-Fe complexes from the cells (step 1). This is followed by transfer of iron from DFP-Fe to R*-aTf to form R*-Tf-Fe (step 2), which then binds to transferrin receptors on the cell surface (step 3), concentrates in the endosomes, and is detected by fluorescence microscopy as punctuate fluorescence typical of microvesicles. (H) Mean cell-associated fluorescence values in r.u. of 5 cells per field (± SD, from 3 separate experiments), calculated from snapshots such as shown in panels A through F, obtained after 1 hour of incubation.
DFP-mediated mobilization of iron from H9C2 cells to extracellular apotransferrin: receptor-mediated uptake of holotransferrin as an index of iron transfer. H9C2 cells were pretreated with succinylacetone as described in “Methods” to increase mitochondrial labile iron levels. They were then incubated at 37°C in DMEM-HEPES medium containing 20 μM lissamine R-aTf with various additions, and epifluorescence microscopy images were obtained under rhodamine settings after 60 minutes of incubation. Representative images of cells with no addition (A), 30 μM DFP (B), 100 μM unlabeled holotransferrin (C), 100 μM unlabeled holotransferrin with 30 μM DFP (D), 20 μM Fe-NTA, 1:3 ratio (E), and 20 μM Fe-NTA with 30 μM DFP (F). (G) The illustration represents entry of DFP into cells, mobilization of iron from the cytosol (C), nuclei (N) and mitochondria (M), followed by exit of DFP-Fe complexes from the cells (step 1). This is followed by transfer of iron from DFP-Fe to R*-aTf to form R*-Tf-Fe (step 2), which then binds to transferrin receptors on the cell surface (step 3), concentrates in the endosomes, and is detected by fluorescence microscopy as punctuate fluorescence typical of microvesicles. (H) Mean cell-associated fluorescence values in r.u. of 5 cells per field (± SD, from 3 separate experiments), calculated from snapshots such as shown in panels A through F, obtained after 1 hour of incubation.
DFP-mediated iron delivery for hemoglobin synthesis.
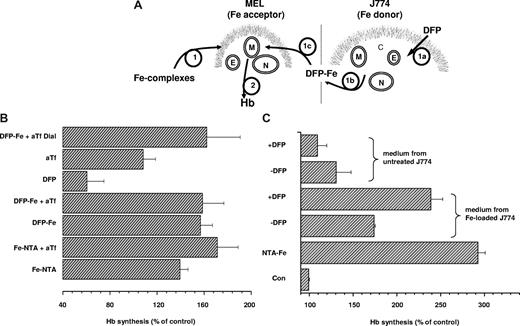
The potential of DFP-Fe complexes as donors of iron for physiologic use was assessed using a model system of cellular iron incorporation, synthesis of hemoglobin (Hb) by differentiating preerythroid cells. The well-documented induction of Hb synthesis in MEL cells by hexamethylene bisacetamide (HMBA) is highly dependent on the supply of iron.26 It is repressed in serum-free medium lacking transferrin, but it is significantly enhanced by the addition of Fe-NTA irrespective of the presence of Tf (Figure 7). DFP-Fe (3:1) supported Hb synthesis to a degree similar to that seen with Fe-NTA, both in the absence and presence of added Tf. Fully saturated holotransferrin (15 μM) was equally effective as Fe-NTA and DFP-Fe (data not shown), indicating that various forms of iron can equally support Hb synthesis in this experimental system. On the other hand, DFP without added iron depressed Hb synthesis below control levels, indicating that the chelator can have an iron-withdrawing effect under conditions of limited iron supply. In the serum-free medium used in these experiments, DFP (> 100 μM) without added iron, as well as DFO (100 μM), prevented differentiation and ultimately caused massive cell death (data not shown), an effect similar to DFO-induced iron deprivation in other hematopoietic cells.27 To demonstrate that transfer of iron from DFP-Fe to aTf gave rise to functionally active holotransferrin, DFP-Fe was allowed to interact with aTf for 1 hour, and then the mixture was exhaustively dialyzed (cut-off 12 kDa) against buffer containing 80 mg/L aTf to remove DFP-Fe without gaining contaminant iron from the solution. When added to differentiating MEL cells, this preparation, generated from DFP-Fe and aTf, fully supported Hb synthesis (Figure 7B).
Stimulation of Hb synthesis in murine erythroleukemia cells. (A) DFP-mediated iron delivery to murine erythroleukemia (MEL) cells synthesizing hemoglobin is schematically depicted. (B) MEL cells suspended in serum-free DMEM containing 1.25 mg/mL bovine serum albumin and 5 mM HMBA were supplemented with various iron complexes (step 1 in panel A) and cultured for 48 hours. Hemoglobin synthesis (step 2 in panel A) was assessed in terms of hemoglobin content in cell lysates as described in “Cell hemoglobin synthesis” and is expressed relative to the hemoglobin content in control cells with no additions (set as 100%). The additions included 10 μM Fe complexed to 30 μM NTA without (Fe-NTA) and with 15 μM human apotransferrin (Fe:NTA + aTf); 10 μM Fe complexed to 30 μM DFP without (DFP-Fe) and with 15 μM human apotransferrin (DFP-Fe + aTf); 30 μM DFP alone (DFP); 15 μM human apotransferrin alone (aTf). Generation of holotransferrin from DFP and apotransferrin (DFP-Fe + aTf Dial.): 10 μM Fe:30 μM DFP complex was preincubated with 15 μM human for 1 hour and dialyzed. Results shown are averages of 3 separate experiments plus or minus SD; the average Abs604 of control cells was 0.39. (C) DFP-mediated mobilization of iron from J774 (Fe donor; step 1a in panel A) cells to the extracellular medium (step 1b in panel A), followed by entry of DFP-Fe complexes into MEL (Fe acceptor) cells (step 1c in panel A) and intracellular donation of iron for hemoglobin (Hb) synthesis (step 2 in panel A). MEL cells were cultured for 48 hours in DMEM containing 1.25 mg/mL bovine serum albumin and 5 mM HMBA without (Con) or with 10 μM Fe-NTA (NTA-Fe), or with various supernatants of J774 cell lysates that were supplemented with 5 mM HMBA, and lysates were assayed for hemoglobin content. To obtain J774 mouse macrophage supernatants, J774 cells were cultured overnight without (untreated J774) or with 100 μM FAC (Fe-loaded J774), washed with 100 μM DFO to remove all extracellular iron, and incubated for 2 hours at 37°C in serum-free DMEM containing 1.25 mg/mL bovine serum albumin, without (−DFP) or with 30 μM DFP (+ DFP). The cell supernatants were collected and centrifuged to remove detached cells, and HMBA (5 mM) was added to MEL cells. Shown are values of hemoglobin (Hb; from a representative experiment) obtained in MEL cells exposed for 48 hours to the various conditions.
Stimulation of Hb synthesis in murine erythroleukemia cells. (A) DFP-mediated iron delivery to murine erythroleukemia (MEL) cells synthesizing hemoglobin is schematically depicted. (B) MEL cells suspended in serum-free DMEM containing 1.25 mg/mL bovine serum albumin and 5 mM HMBA were supplemented with various iron complexes (step 1 in panel A) and cultured for 48 hours. Hemoglobin synthesis (step 2 in panel A) was assessed in terms of hemoglobin content in cell lysates as described in “Cell hemoglobin synthesis” and is expressed relative to the hemoglobin content in control cells with no additions (set as 100%). The additions included 10 μM Fe complexed to 30 μM NTA without (Fe-NTA) and with 15 μM human apotransferrin (Fe:NTA + aTf); 10 μM Fe complexed to 30 μM DFP without (DFP-Fe) and with 15 μM human apotransferrin (DFP-Fe + aTf); 30 μM DFP alone (DFP); 15 μM human apotransferrin alone (aTf). Generation of holotransferrin from DFP and apotransferrin (DFP-Fe + aTf Dial.): 10 μM Fe:30 μM DFP complex was preincubated with 15 μM human for 1 hour and dialyzed. Results shown are averages of 3 separate experiments plus or minus SD; the average Abs604 of control cells was 0.39. (C) DFP-mediated mobilization of iron from J774 (Fe donor; step 1a in panel A) cells to the extracellular medium (step 1b in panel A), followed by entry of DFP-Fe complexes into MEL (Fe acceptor) cells (step 1c in panel A) and intracellular donation of iron for hemoglobin (Hb) synthesis (step 2 in panel A). MEL cells were cultured for 48 hours in DMEM containing 1.25 mg/mL bovine serum albumin and 5 mM HMBA without (Con) or with 10 μM Fe-NTA (NTA-Fe), or with various supernatants of J774 cell lysates that were supplemented with 5 mM HMBA, and lysates were assayed for hemoglobin content. To obtain J774 mouse macrophage supernatants, J774 cells were cultured overnight without (untreated J774) or with 100 μM FAC (Fe-loaded J774), washed with 100 μM DFO to remove all extracellular iron, and incubated for 2 hours at 37°C in serum-free DMEM containing 1.25 mg/mL bovine serum albumin, without (−DFP) or with 30 μM DFP (+ DFP). The cell supernatants were collected and centrifuged to remove detached cells, and HMBA (5 mM) was added to MEL cells. Shown are values of hemoglobin (Hb; from a representative experiment) obtained in MEL cells exposed for 48 hours to the various conditions.
Finally, we assessed DFP's ability to shuttle iron from cell to cell (Figure 7C). In vivo, direct intercellular transfer of iron via DFP is not likely, due to the presence of more than 50 μM due to the presence of more than 25 μM transferrin in plasma.28 However, intercellular transfer of iron via DFP could occur in the brain where the iron-binding capacity of transferrin in cerebrospinal fluid (CSF) is estimated to be in the submicromolar range.29,30 In the experiment illustrated in Figure 7A, J774 mouse macrophages were chosen as the iron-donating cells because of their high capacity for iron accumulation.31 DFP at a concentration of 30 μM, achieved in vivo with moderate oral doses,1,32 mobilized sufficient iron from iron-loaded J774 cells, but not from untreated ones, to significantly enhance Hb synthesis in MEL cells (Figure 7C). Spontaneous efflux of iron from iron-loaded J774 cells took place, as evidenced by the increased MEL cell Hb levels even in the absence of DFP (although increased, these levels were significantly lower in the absence of DFP than in its presence). This result provides support for the proposed use of DFP as an iron shuttle even without the mediation of transferrin, such as might occur in the central nervous system.
Discussion
The recognition that intracellular iron accumulation is one of the underlying causes of some clinical syndromes has led to the suggestion that it might be amenable to treatment with iron chelators.3,33,34 These syndromes include Parkinson disease, neurodegeneration with NBIA, and Friedreich ataxia, where iron deposits in specific areas of the brain have been identified by histologic or imaging techniques34 and are thought to be a central factor in the development of the diseases.35 Similarly, in anemia of chronic disease, iron is retained within erythrocyte-phagocytosing macrophages, presumably to prevent access by invading pathogens to iron from the circulation.7-9,31 While regional iron mobilization might be essential for stabilizing or reversing the toxic effects of labile iron, in some pathologies it might be equally important to render the mobilized iron available for metabolic reuse. This is especially important in those conditions in which regional iron accumulation is accompanied by regional or systemic deprivation. All the chelators in clinical use have been designed for massive removal and excretion of iron from organs of iron-overloaded patients.2,10,33 Here we assessed the potential application of an iron chelator in clinical use (DFP) as a shuttling agent capable of redistributing iron within and among cells, while satisfying certain basic requirements.
Permeability across cell membranes in the free and iron-bound form.
The high membrane permeability of the iron-free form of DFP is well documented, as shown by its capacity to rapidly access and deplete intracellular labile iron pools.36,37 On the other hand, the ability of DFP-Fe complexes to traverse intracellular and plasma membranes of cells has not been studied in detail.20,21,36,37 One indication is the capacity of orally administered DFP to increase serum transferrin saturation12 and labile iron11 within an hour after intake in thalassemia patients, which is attributable to the rapid exit of DFP-Fe complexes from cells into the circulation. At the intracellular level, it is shown in this work that DFP can facilitate the removal of labile iron from nuclei (Figure 3), from endosomes (Figure 4), and from mitochondria (Figure 6), and can transfer iron to acceptors in the mitochondria (Figure 3), nuclei (Figure 4), and extracellular medium (Figure 6). The transfer might involve dissociation of the DFP-Fe complexes and/or formation of ternary complexes with other ligands.
Ability to compete effectively for intracellular labile iron with other intermediate affinity species.
Labile iron that is likely to be accessible to DFP is assumed to be bound to several possible ligands (eg, citrate, ATP) and to be in dynamic equilibrium between Fe(II) and Fe(III) as dictated by the redox status of the intracellular environment.38,39 DFP competes effectively for labile cell iron both in vitro and in vivo,32 and in individuals with normal iron balance it raises serum iron levels, indicating mobilization of tissue iron.12 This was also observed with isolated cells (Figures 3,4), where DFP readily chelated iron bound to the EDTA analog CALG in endosomes and nuclei and was indicated in other systems.40 An undesirable property of a chelating molecule would be interference with normal cellular iron metabolism by overchelation. In this respect, DFP ought to be used at restricted doses, as at high levels it appears to inhibit iron-requiring tyrosine and tryptophan hydroxylases.10,14
Ability to donate iron to physiologic acceptors.
The rationale behind DFP-mediated redistribution of iron leans on DFP's ability to transfer the metal to extracellular transferrin, thereby minimizing undesired iron loss by excretion via the urinary or biliary pathways. We surmised that under physiologic conditions iron transfer to aTf is likely to occur (1) spontaneously, as the pFe value of Fe(III) for transferrin is 3 orders of magnitude higher than for DFP41 (Table 1), and (2) directly as Fe(III), because the thermodynamically favored DFP-Fe complexes are (DFP)2-Fe(III) and (DFP)3-Fe(III).10,25,42 In vitro studies based on an indirect analytical method11 corroborated earlier observations that a single oral dose of 3 g DFP raised within 6 hours the transferrin saturation (determined by urea-gel electrophoresis) of a healthy individual from a normal level of 20% to 80%.12 In this work we provided direct demonstration of iron transfer from DFP-Fe to aTf at physiologic concentrations (Figure 5). Using spectrophotometric and fluorimetric methods, we showed that efficient transfer occurs at various DFP:Fe ratios and within 1 to 2 hours in culture conditions. The resulting holotransferrin formed from DFP-Fe was biologically active, as it was recognized by the transferrin receptor (Figure 6) and provided iron to cells for hemoglobin synthesis when presented together with DFP or after DFP's removal (Figure 7).
The direct bioavailability of DFP-bound iron was investigated using hemoglobin synthesis by cells in culture as a model. DFP-Fe supported hemoglobin synthesis even in the absence of transferrin (Figure 7), consistent with a similar activity of 1:1 complexes of iron with salicylaldehyde isonicotinoyl hydrazone.43 More important, DFP enhanced the transfer of iron from iron-loaded macrophages to preerythroid cells for hemoglobin synthesis, without the intermediary presence of transferrin (Figure 7). Such direct donation to intracellular iron-requiring or iron-metabolizing enzymes may not be a necessary feature if iron is efficiently transferred from the shuttle to circulating transferrin. However, it may be desirable or even essential in the brain, where the iron-binding capacity of transferrin in the CSF is negligible and has been estimated in the submicromolar range.29,30
Low toxic potential of iron-shuttling agent complexes.
A major concern in the use of chelators is the formation of iron complexes that undergo redox cycling and thereby catalyze ROS generation. This tendency is present in chelators with relatively low redox potentials, such as those based on amine- and carboxyl-liganding groups but not with the bidentate 3:1 complexing hydroxypyridone DFP.20,21,44,45 However, speciation studies10 and recent examination of ROS production42 indicated that complete abolition of labile iron by DFP is attained only at DFP:Fe(III) ratios close to 5:1. Whether DFP levels reached therapeutically are consistently high enough to avoid formation of substoichiometric redox-active complexes is difficult to predict. This is particularly important in susceptible, DNA-containing organelles such as nuclei and mitochondria. The same applies to intracellular signaling pathways activated by oxidative stress that could be generated by DFP-mediated redistribution of cell iron.
Permeability across the blood-brain barrier (BBB) for therapeutic applications to neurodegenerative diseases.
The capacity of an iron-shuttling agent to redistribute iron in the central nervous system (CNS) may be an essential requirement for iron redistribution in Parkinson disease, Friedreich ataxia, or NBIA. A high rate of BBB penetration is expected for free DFP, based on its low molecular weight (below 300) and lipophilicity. This was confirmed by direct measurement of DFP accumulation in the perfused rat brain.15,16 In rats given DFP doses several times higher than analogous doses given to thalassemia patients, brain tyrosine and tryptophan hydroxylase activities were significantly inhibited, as measured by the accumulation of 3,4-dihydroxyphenylalanine (DOPA) and 5-hydroxytryptophan, respectively, presumably via coordination to iron bound by these enzymes.14 Yet, despite DFP's brain accessibility, major effects on CNS function have not been reported.32,44
Whether DFP can alter the iron balance in the brain by shuttling iron across the BBB remains an open question. The absence of iron loading of the CNS in iron-overload conditions indicates that neither transferrin-bound nor non–transferrin-bound iron is able to freely cross the BBB.35 Because DFP treatment has not been reported to be associated with increased iron loading of the CNS in thalassemia patients, it is conceivable that DFP-Fe complexes do not readily permeate the BBB. The permeabilities of free and iron-bound DFP may differ due to differences in their molecular weights (473 for 3DFP:1Fe compared with 139 for DFP). In a recent study, 6 months of treatment with DFP was shown to result in a decrease in the iron-associated magnetic resonance imaging (MRI) transverse relaxation rate (R2*) signals in dentate nuclei of Friedreich ataxia patients.13 Whether this decrease was caused by egress of DFP-Fe complexes from the brain or by redistribution of iron from an area of accumulation to areas of lower concentration within the brain is unclear.
We show that redistribution of iron in multiple directions and to multiple recipients is experimentally feasible, particularly from areas of accumulation to areas of iron need, via donation to transferrin or by direct metal donation. A modality of iron chelation based on iron redistribution has several therapeutic implications. In the CNS, it could potentially be used to relocate local iron deposits that may be an important factor in the etiology of several neurodegenerative diseases. In ACD, it could be used to release iron trapped within macrophages. Current experimental approaches to alleviation of ACD tend to be directed at depressing the levels of hepcidin, the iron-regulator protein responsible for macrophage iron retention. However, recent evidence for hepcidin-independent down-regulation of the iron exporter ferroportin by the inflammatory mediators46 could obviate such an approach. As shown in this work, an alternate or complementary approach could be based on iron-shuttling agents that relocate misdistributed iron and safely bypass impaired internal routes of iron trafficking. Early pilot studies with iron chelators in rheumatoid arthritis patients with ACD47 provided clinical evidence that agents like DFP might be adopted as part of a short-term strategy of regional metal detoxification and systemic relocation that generates no significant urinary iron loss.48,49 Optimization of such strategies with novel chelators for treating the various conditions of regional iron accumulation demand further laboratory and clinical work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Association Française contre les Myopathies, the Israel Science Foundation (ISF), the EEC Framework 6 (LSHM-CT-2006-037296 Euroiron1) and French-Israeli Organization for Research in Neuroscience (AFIRN). Z.I.C. is the Sergio and Adelina Della Pergolla Professor of Life Sciences.
Authorship
Contribution: Y.-S.S. and W.B. performed experimentation and analysis; A.M. and Z.I.C. designed and supervised the research. All authors contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Z. I. Cabantchik, Alexander Silberman Institute of Life Sciences, Hebrew University of Jerusalem, Safra Campus at Givat Ram, Jerusalem, Israel 91904; e-mail: ioav@cc.huji.ac.il.