Abstract
During early pregnancy, uterine mucosa decidualization is accompanied by a drastic enrichment of CD56highCD16− natural killer (NK) cells. Decidual NK (dNK) cells differ from peripheral blood NK (pbNK) cells in several ways, but their origin is still unclear. Our results demonstrate that chemokines present in the uterus can support pbNK cell migration through human endothelial and stromal decidual cells. Notably, we observed that pregnant women's pbNK cells are endowed with higher migratory ability compared with nonpregnant women's or male donors' pbNK cells. Moreover, NK cell migration through decidual stromal cells was increased when progesterone-cultured stromal cells were used as substrate, and this correlated with the ability of progesterone to up-regulate stromal cell chemokine expression. Furthermore, we demonstrate that dNK cells migrate through stromal cells using a distinct pattern of chemokines. Finally, we found that pbNK cells acquire a chemokine receptor pattern similar to that of dNK cells when they contact decidual stromal cells. Collectively these results strongly suggest that pbNK cell recruitment to the uterus contributes to the accumulation of NK cells during early pregnancy; that progesterone plays a crucial role in this event; and that pbNK cells undergo reprogramming of their chemokine receptor profile once exposed to uterine microenvironment.
Introduction
Natural killer (NK) cells represent a distinct population of circulating and tissue-resident lymphocytes that play an important role in the early phases of immune responses against microbial pathogens by exhibiting cytotoxic functions and secreting a number of cytokines and chemokines. NK cells develop from a lymphoid precursor resident in the bone marrow (BM), considered the main site of NK cell generation, however, the existence of a pathway of NK cell development in the thymus has been recently suggested and evidence also indicates that final maturation of NK cell precursors can occur in the periphery.1-3
During development and activation, NK cells acquire a multiple cell surface receptor system including both activating and inhibitory receptors that finely control their functional activation.4 Some of these receptors are oligoclonally distributed and/or are expressed at different densities on circulating NK cells. Based on cell surface density of these receptors, phenotypically distinct peripheral blood NK (pbNK) cell populations have been identified and suggested to represent specialized subsets capable of performing different functions and endowed with distinct migratory properties.5
Mature NK cells circulate mainly in the peripheral blood, but are also present in several lymphoid and nonlymphoid organs such as spleen, lymph nodes, tonsils, liver, lungs, intestine, and uterus.1,6-8 Interestingly, NK cells are the most abundant class of lymphocytes found in the mucosal tissues of maternal uterus where their number reaches 70% to 80% of the total leukocytes in the first trimester of pregnancy, then start to decline, and return to basal levels at the end of pregnancy.7-9 Much evidence indicates that decidual NK (dNK) cells are phenotypically and functionally distinct from pbNK cells. In this regard, data clearly demonstrate that human dNK cells expressing high levels of CD56 and lacking the expression of CD16, and thus resembling the peripheral blood CD56highCD16− NK cell subset, display a unique transcriptional profile.10
Although dNK cells express a number of activating receptors (such as NKp30, NKp44, NKp46, CD244) and are endowed with an intact cytolytic machinery, they are poorly cytotoxic and fail to polarize the microtubule-organizing center and the cytolytic granules toward the synapse.11,12
Numeric variations of utezine NK (uNK) cells have been also described during the menstrual cycle, with their number increasing in the proliferative phase and reaching the maximal level in the late secretory phase. These uNK cell numeric variations have been correlated to hormone-induced decidualization rather than to the embryo implantation and to changes in chemokine expression in decidual tissues.13-15 Inside the uterine compartment, NK cells are found as single cells or aggregates around endometrial glands and vessels where they might play a crucial role for normal development of placenta and/or its vasculature and uterine tissue remodeling, by producing cytokines, chemokines, and angiogenic factors.14,16,17
The origin of dNK cells is presently unknown, and it is still debated whether they arise from NK cell progenitors present in the uterus prior to pregnancy, or are recruited from other tissues and/or from NK cell populations recruited from blood.18,19
Studies aimed at understanding the molecules potentially involved in the control of NK cell accumulation in the uterus have shown that first-trimester human NK cells express a distinct pattern of adhesion molecules and chemokine receptors compared with both CD56high and CD56low peripheral blood counterparts. In particular, dNK cells exhibit high levels of αEβ7, α1β1, αXβ2, and αDβ2, whereas they do not express the laminin receptor α6β1. They also display the β5 integrin subunit and selectively express high levels of tetraspan 5, and CD151 and CD9 tetraspanins.10,17,20-22 In regard to the chemokine receptor profile, dNK cells differently from the pbNK cell counterpart exhibit higher levels of CXCR3, lower levels of CXCR4, and very low or undetectable levels of CXCR1 or CXCR2 and CX3CR1 or CCR1,2,3,5,6,7.23-25 Moreover, evidence indicating the ability of trophoblast or endometrial cells to produce chemokines acting on pbNK cells and dNK cells has been provided.23,24,26,27
Although all these findings indicate that dNK cells express several adhesion molecules and chemotactic receptors that might control their migration and localization into different uterine compartments, data showing the ability of NK cells to migrate through decidual tissues are still lacking, nor it is known whether the mild systemic inflammatory status already observed in early pregnancy results in an enhanced migratory ability of pbNK cells into the uterine compartment.
Here we analyzed whether pbNK cell recruitment to the uterus may contribute to the accumulation of NK cell number in this nonlymphoid organ during early pregnancy, by evaluating the ability of pbNK cells from both pregnant women and nonpregnant women or male donors to migrate throughout endothelial and stromal decidual cells, and the effect of progesterone in these events. We also studied the ability of stromal cells to support dNK cell migration that can be relevant for their specific localization inside the uterine compartment. Finally, we studied whether the chemokine receptor profile of pbNK cells may undergo tissue-specific modulation when these cells contact stromal or endothelial cells in the uterus.
Methods
Cells
Human pbNK and dNK cell purification.
Mononuclear cells were isolated from peripheral blood of women undergoing elective pregnancy termination (8-12 weeks of gestation), male donors, or women in the first week of cycle by Lymphoprep (Nycomed, Oslo, Norway) gradient centrifugation. Cells were then incubated with FITC-conjugated anti-CD3 plus anti-CD14 and PE-conjugated anti-CD19 mAbs (BD Biosciences, San Jose, CA) for 30 minutes at 4°C, and NK cells were negatively selected by a FACSAria cell sorter (BD Biosciences). The resulting NK cell population was assayed by 3-color immunofluorescence using PE-conjugated anti-CD56; PerCP-conjugated anti-CD3; and FITC-conjugated anti-CD16, anti-CD9, or anti-CD14 mAbs (BD Biosciences) and analyzed by flow cytometry using a FACSCalibur (BD Biosciences). The purity was routinely more than 95% CD56+CD16+CD3−CD9−CD14−.
Decidual samples from elective first-trimester pregnancy terminations were washed extensively in PBS before mincing with sterile scissor, and then digested with 1.5 μg type I DNase and 24 μg type IV collagenase (both from Sigma-Aldrich, St Louis, MO) in 5 mL RPMI medium for 30 minutes at 37°C. Cell suspensions were then purified by Lymphoprep density gradient centrifugation and immediately used for 3-color immunofluorescence and cytofluorimetric analysis. For migration assay, dNK cells were further purified through staining with PE-conjugated anti-CD56 and PerCP-conjugated anti-CD3 and positive selection by cell sorting. The purity of the resulting dNK cell population was more than 90% CD56+CD9+CD16−CD3−CD14−.
Decidual human endothelial cell and stromal cell purification.
Endothelial and stromal (ST) cells from decidual tissues of women undergoing elective pregnancy termination were purified as previously described with some modifications.28,29 Briefly, decidual tissues were digested overnight at 4°C with 0.25% trypsin (Sigma-Aldrich), 50 μg/mL DNase1 (Boehringer Mannheim, Mannheim, Germany) in PBS and then treated with collagenase type I (3 mg/mL; Worthington Biochemical, DBA, Milano, Italy) for 30 minutes at 37°C. Following Lymphoprep density gradient centrifugation, decidual human endothelial cells (DECs) were isolated by positive selection using Dynabeads M-450 (Dynal, Oslo, Norway) coated with lectin ulex europaeus 1 (Sigma-Aldrich). The purity of the resulting DEC population was more than 98% as verified by staining with antibodies to VWF, CD105, VE cadherin (Dako, Milano, Italy), and CD31/PECAM-1 kindly provided by M. R. Zocchi (San Raffaele Hospital, Milan, Italy; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). DECs were cultured using endothelial serum-free basal medium (GIBCO, Carlsbad, CA) supplemented with 20 ng/mL bFGF, 10 ng/mL EGF, and penicillin (50 U/mL)/streptomycin (50 μg/mL).
Decidual ST cells were obtained by culturing the endothelial-negative cell fraction in RPMI plus 10% fetal calf serum (FCS) without adding cytokines. Nonadherent cells were removed by extensive washing, and adherent cells were used only when the resulting cell population was negative for CD14, CD45 (BD Biosciences), CK8-18, VWF, or CD31 and positive for α-actin, vimentin, CD13, CD10, and CD105 (Dako) (Figure S2). ST cells were grown using RPMI plus 10% FCS either supplemented or not with 100 nM progesterone (Calbiochem, San Diego, CA) or estrogen (1 nM; Sigma-Aldrich in accordance with the Declaration of Helsinki). For all the experiments, primary cultures of DECs and ST cells between the third and sixth passage were used.
Informed consent was obtained from all donors providing peripheral blood and tissue specimens, and ethical approval was obtained from the Ethics Committee of University “La Sapienza” Rome, Rome, Italy, and the Ethics Committee of the Materal-Children Hospital (Istituto di Ricovero e Cura a Caraltere Scientifico [IRCCS] “Burlo Garofolo,” Trieste, Italy).
RNase protection assay and real-time quantitative polymerase chain reaction analysis
RNase protection assay.
RNase protection assay was performed using RiboQuant multiprobe kit (BD Biosciences Pharmingen, San Diego, CA) according to the manufacturer's instructions. Briefly, 32P-radiolabeled probe set hCK5 was hybridized with 8 μg RNA from DECs and ST decidual cells. Samples were then digested with RNase and the remaining “RNase-protected” probes were purified, resolved on a sequencing gel, and identified by size. Undigested probes were used as reference size marker and L32 and GAPDH transcripts as RNA loading control. As negative control, yeast RNA was used.
Real-time quantitative PCR analysis.
Human chemokine mRNA expression was analyzed by real-time quantitative polymerase chain reaction (RT-Q-PCR) using a commercial Taqman assay reagent (Applied Biosystems, Foster City, CA). The endogenous gene human β-actin was amplified using a commercial Taqman assay reagent. PCR reactions were performed on ABI Prism 7700 Sequence Detection System according to the manufacturer's instructions (Applied Biosystem). cDNA was amplified in triplicate with primers for CXCL12/SDF-1 (Hs00930455 m1), CX3CL1/fractalkine (Hs00171086 m1), CXCL10/IP-10 (Hs00171042 m1), and CCL2/MCP-1 (Hs00234140 m1), all conjugated with fluorochrome FAM, and β-actin (4326315E) conjugated with fluorochrome VIC (Applied Biosystems, Foster City, CA). For each amplification run, a standard curve was generated using 5 serial dilutions of total cDNA derived from PMA/ionomycin-activated peripheral blood mononuclear cells (PBMCs). Each cDNA tested was diluted 5-fold and 2.5 μL of the diluted cDNA was added to 22.5 μL of the reaction mixture for PCR amplification. The average of the threshold cycles was used to interpolate standard curves and to calculate the transcript amount in samples using SDS version 1.7a software (Applied Biosystem). The relative chemokine amount of each sample, normalized with β-actin, was expressed as arbitrary units.
The primer pairs used for RT-PCR analyses were as follows: CXCL10/IP-10, forward GGAACCTCCAGTCTCAGCACC and reverse CAGCCTCTGTGTGGTCCAATCC; CX3CL1/fractalkine, forward CCCAAAACTCTCCTCTGCTG and reverse AGGTGCTCTGCTGGTAAG; GAPDH, forward ACCACAGTCCATGCCATCAC and reverse TCCACCACCCTGTTGCTGTA (MWG-Biotech, High Point, NC).
Immunofluorescence and flow cytometric analysis
Chemokine receptor expression on freshly isolated pbNK cells or dNK cells was evaluated by performing a 3-color immunofluorescence staining. Cells were washed with PBS and incubated with chemokine receptor–specific mAbs against human CCR1, CCR5, CXCR1 (R&D System, Minneapolis, MN), CXCR4 (BD Biosciences) or purified rabbit anti–human CX3CR1 (Torrey Pines Biolabs, Houston, TX); with integrin-specific mouse mAbs against human CD18, CD11a, CD11b, CD11c, CD29, CD49d, CD49e; or with rat mAb against CD49f (Immunotech SA, Marseille, France) for 30 minutes on ice, followed by anti–mouse, anti–rabbit, or anti–rat Ig fluorochrome-conjugated secondary F(ab′)2 Ab (GAM, GARB, and GART, respectively; Cappel Laboratories, Cooper Biomedical, Malvern, PA). Cells were then incubated for 15 minutes with normal mouse serum, and then anti-CD3 and anti-CD56 fluorochrome-conjugated mAbs were simultaneously added for an additional 30 minutes at 4°C. Staining of CXCR3 was performed using PE-conjugated anti-CXCR3 mAb (R&D Systems) and using PE-conjugated mouse IgG (BD Biosciences) as control Ab. For fluorescence measurement, only data from 10 000 to 30 000 single cell events were collected using a standard FACScalibur flow cytometer and data were analyzed using CELLQuest (BD Biosciences). Histograms shown were obtained by applying a gate on CD56+CD3− NK cells. Treatment of PBMCs with type IV collagenase did not affect chemokine receptor expression on pbNK cells (data not shown).
Migration assay
Cell migration was measured using a Transwell migration chamber (diameter insert: 6.5 mm, pore size: 5 μm; Costar, Cambridge, MA). Highly purified NK cells derived from peripheral blood of either male donors, nonpregnant women, or women in the first trimester of pregnancy, or from decidual tissue, were assayed for their ability to migrate through a monolayer of DECs or ST cells grown or not with progesterone (100 ng/mL). As chemoattractant, different concentrations of CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 (all from R&D Systems) were added in the lower compartment. After 120 minutes at 37°C, the number of migrated cells was counted using an inverted microscope with 100× magnification. Data are expressed as the mean plus or minus SD of percentage of migrated cells obtained from 4 independent experiments. In some experiments, migration of CD56highCD16− NK cells was evaluated by recovering migrated cells and performing immunofluorescence and cytofluorimetric analysis.
Coculture of pbNK cells with stromal or endothelial decidual cells
pbNK cells from women in the first trimester of pregnancy were cocultured in 24-well plates precoated with DECs or ST cells grown in the presence of progesterone or medium (RPMI plus 10% FCS). Following 36 hours of incubation at 37°C, NK cells were recovered and analyzed for the chemokine receptor expression by 3-color immunofluorescence staining and fluorescence-activated cell sorting (FACS) analysis as described in “Immunofluorescence and flow cytometric analysis.”
Statistical analysis
Student t test was used for statistical evaluations.
Results
Chemokine mRNA expression pattern on DECs and ST cells, and chemokine receptor profile of pbNK and dNK cells
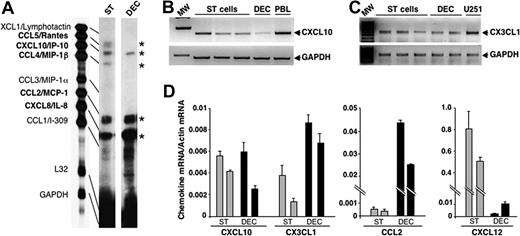
NK cell migration through endothelial cells and their tissue localization are orchestrated mainly by chemokines; thus, we first assayed the expression of chemokines able to support pbNK cell migration in DECs and ST cells. To this purpose, primary cultures of DECs and ST cells obtained from first-trimester decidual tissues were assayed for CCL, CXCL, and CX3CL1 chemokine mRNA expression by RNase protection assay or PCR analysis. The results obtained indicate that both DECs and ST cells express significant levels of mRNA for CCL2/MCP-1, CXCL8/IL-8, CXCL10/IP-10, CX3CL1/fractalkine, and CXCL12/SDF-1 (Figure 1A-D), while only ST cells express detectable levels of CCL5/Rantes and CCL4/MIP-1β (Figure 1A). By performing real-time quantitative PCR analysis on primary cultures of DECs and ST cells derived from the same donor, we found that CXCL10/IP-10 mRNA was expressed at similar levels on both DECs and ST cells, while CX3CL1/fractalkine and CCL2/MCP-1 were mainly expressed by DECs and CXCL12/SDF-1 by ST cells (Figure 1D).
Chemokine mRNA expression in DECs and decidual ST cells. (A) mRNA isolated from in vitro–cultured DECs or ST cells derived from the same donor were analyzed by RNase protection assay using hCK-5 multiprobe set. Data are representative of 3 independent experiments. (B,C) mRNA isolated from 3 different primary cultures of decidual ST cells and 2 different primary cultures of DECs were analyzed by RT-PCR for the expression of CXCL10/IP-10 or CX3CL1/fractalkine. RNA isolated from PMA/ionomycin-activated peripheral blood leukocytes (PBLs) was used as positive control for CXCL10/IP-10 and that from U251 glioblastoma cell line as positive control for CX3CL1/fractalkine (Giovanni Bernardini, Giuseppe Sciumé, A. Soriani, and A. Santoni, unpublished observation, June 2003). MW represents the molecular markers. β-actin and GAPDH are shown as mRNA loading control. These results are representative of 3 independent experiments. (D) mRNA isolated from primary cultures of DECs or ST cells obtained from the same donor was analyzed by real-time quantitative PCR assay for the expression of CXCL10/IP-10, CX3CL1/fractalkine, CCL2/MCP-1, and CXCL12/SDF-1. The relative chemokine amount of each sample was normalized with β-actin and expressed as arbitrary units plus SD. Results obtained from 2 different donors are shown.
Chemokine mRNA expression in DECs and decidual ST cells. (A) mRNA isolated from in vitro–cultured DECs or ST cells derived from the same donor were analyzed by RNase protection assay using hCK-5 multiprobe set. Data are representative of 3 independent experiments. (B,C) mRNA isolated from 3 different primary cultures of decidual ST cells and 2 different primary cultures of DECs were analyzed by RT-PCR for the expression of CXCL10/IP-10 or CX3CL1/fractalkine. RNA isolated from PMA/ionomycin-activated peripheral blood leukocytes (PBLs) was used as positive control for CXCL10/IP-10 and that from U251 glioblastoma cell line as positive control for CX3CL1/fractalkine (Giovanni Bernardini, Giuseppe Sciumé, A. Soriani, and A. Santoni, unpublished observation, June 2003). MW represents the molecular markers. β-actin and GAPDH are shown as mRNA loading control. These results are representative of 3 independent experiments. (D) mRNA isolated from primary cultures of DECs or ST cells obtained from the same donor was analyzed by real-time quantitative PCR assay for the expression of CXCL10/IP-10, CX3CL1/fractalkine, CCL2/MCP-1, and CXCL12/SDF-1. The relative chemokine amount of each sample was normalized with β-actin and expressed as arbitrary units plus SD. Results obtained from 2 different donors are shown.
Based on the evidence indicating a crucial role for sex hormones in the regulation of dNK cell accumulation, we evaluated the effect of progesterone on the expression of chemokines on DECs and ST cells, as both bear the progesterone receptor (Figure S3). As shown in Figure 2, we found that treatment of ST cells or DECs with progesterone significantly enhanced CXL10/IP-10, CX3CL1/fractalkine, and CCL2/MCP-1 mRNA levels without affecting those of CXCL12/SDF-1. In addition, up-regulation of CXL10/IP-10 and CX3CL1/fractalkine, but not CCL2/MCP-1 and CXCL12/SDF-1, was also observed following the exposure of ST cells to estrogen (Figure S4) as previously described.25
Progesterone enhances chemokine mRNA expression in DECs and ST cells. mRNA isolated from primary cultures of decidual ST cells and DECs grown with or without progesterone was analyzed for the expression of CXCL10/IP-10, CX3CL1/fractalkine, CCL2/MCP-1, and CXCL12/SDF-1 by real-time quantitative PCR assay as described in Figure 1D. Similar results were observed in 3 of 4 independent experiments. *P < .05, as evaluated by performing statistical analysis between progesterone versus nonprogesterone-grown cells using Student t test. Error bars represent SD.
Progesterone enhances chemokine mRNA expression in DECs and ST cells. mRNA isolated from primary cultures of decidual ST cells and DECs grown with or without progesterone was analyzed for the expression of CXCL10/IP-10, CX3CL1/fractalkine, CCL2/MCP-1, and CXCL12/SDF-1 by real-time quantitative PCR assay as described in Figure 1D. Similar results were observed in 3 of 4 independent experiments. *P < .05, as evaluated by performing statistical analysis between progesterone versus nonprogesterone-grown cells using Student t test. Error bars represent SD.
These results indicate that decidual endothelial and stromal tissues display a qualitatively and/or quantitatively different chemokine mRNA profile, and that progesterone and estrogen can positively modulate their chemokine expression, thus potentially affecting the migratory ability of NK cells.
We also investigated the expression of the receptors able to bind the chemokines produced by DECs and ST cells on pbNK cells with respect to their uterine counterpart. To this aim, pbNK and dNK cells were isolated from the same donor and freshly purified CD56+CD3− NK cell populations were assayed for the expression of some CC, CXC, and CX3C chemokine receptors by 3-color immunofluorescence. Similarly to pbNK cells, dNK cells express very low levels of CCR1 and CCR5, but unlike pbNK cells exhibit higher levels of CXCR3, lower levels of CXCR4, and undetectable levels of both CXCR1 and CX3CR1 (Figure S5).
Thus, pbNK cells express the receptors for all the chemokines we found produced by DECs or ST cells, while dNK cells preferentially bear CXCR3, the receptor for CXCL10/IP-10, CXCL9/Mig, and CXCL11/I-TAC, and low levels of CXCR4 the receptor for CXCL12/SDF-1.
Migration through DECs and ST cells of pbNK cells from pregnant women, nonpregnant women, and male donors
To evaluate whether the early pregnant status can affect the ability of pbNK cells to migrate through DECs or ST cells, freshly isolated highly purified pbNK cells derived from first-trimester pregnant women undergoing elective pregnancy termination were allowed to migrate through either DECs or ST cells using CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 as chemoattractants. As control, we used highly purified NK cells isolated from healthy male donors as well as from women in the first week of cycle exhibiting low sex hormone levels.
As shown in Figure 3, we found that pbNK cells from first-trimester pregnant women have a higher ability to migrate through DECs (Figure 3A) and ST cells (Figure 4A) with respect to the NK cells derived from male donors (Figures 3,4B) or from nonpregnant women (Figures 3,4C). Moreover, CXCL12/SDF-1, CX3CL1/fractalkine, and CXCL10/IP-10 are all able to significantly support the migration of pregnant female, male donor, and nonpregnant female NK cells through DECs and ST cells although the degree of NK cell migration through decidual tissues supported by CXCL12/SDF-1 is higher than that induced by different concentrations of CX3CL1/fractalkine or CXCL10/IP-10.
Migration of pbNK cells from pregnant women, nonpregnant women, or male donors through DECs. Highly purified pbNK cells isolated either from first-trimester pregnant women (A) or male donors (B) or nonpregnant women (C) were assayed for their ability to migrate through a monolayer of primary cultures of DECs using different concentrations of CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 as chemoattractants. Data are expressed as the mean plus SD of the percentage of migrated cells obtained from 4 independent experiments. *P < .05, as evaluated by comparing chemoattractant- versus control medium (C)–induced migration by Student t test.
Migration of pbNK cells from pregnant women, nonpregnant women, or male donors through DECs. Highly purified pbNK cells isolated either from first-trimester pregnant women (A) or male donors (B) or nonpregnant women (C) were assayed for their ability to migrate through a monolayer of primary cultures of DECs using different concentrations of CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 as chemoattractants. Data are expressed as the mean plus SD of the percentage of migrated cells obtained from 4 independent experiments. *P < .05, as evaluated by comparing chemoattractant- versus control medium (C)–induced migration by Student t test.
Migration of pbNK cells from pregnant and nonpregnant women or male donors through decidual ST cells: effect of progesterone. Highly purified pbNK cells isolated either from first-trimester pregnant women (A) or male donors (B) or nonpregnant women (C) were assayed for their ability to migrate through a monolayer of primary cultures of ST cells grown with or without progesterone (100 nM) using different concentrations of CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 as chemoattractants. All data are expressed as the mean plus SD of the percentage of migrated cells obtained from 4 independent experiments. * and **, P < .05, as evaluated by comparing chemoattractant- versus control medium (C)–induced migration or between migration through progesterone versus nonprogesterone-grown cells using Student t test, respectively.
Migration of pbNK cells from pregnant and nonpregnant women or male donors through decidual ST cells: effect of progesterone. Highly purified pbNK cells isolated either from first-trimester pregnant women (A) or male donors (B) or nonpregnant women (C) were assayed for their ability to migrate through a monolayer of primary cultures of ST cells grown with or without progesterone (100 nM) using different concentrations of CXCL12/SDF-1, CX3CL1/fractalkine, or CXCL10/IP-10 as chemoattractants. All data are expressed as the mean plus SD of the percentage of migrated cells obtained from 4 independent experiments. * and **, P < .05, as evaluated by comparing chemoattractant- versus control medium (C)–induced migration or between migration through progesterone versus nonprogesterone-grown cells using Student t test, respectively.
As we found that progesterone up-regulates chemokine expression on DECs and ST cells, and since it has been reported that NK cells exhibit enhanced adhesion to pregnant tissues,30,31 we assayed the ability of the pbNK cells of pregnant and nonpregnant women and male donors to migrate through decidual ST cells and DECs grown in the presence or absence of progesterone. The results obtained indicate that progesterone treatment of ST cells significantly enhanced only the migration of male donor and nonpregnant women NK cells (Figure 4B,C) without affecting that of NK cells isolated from first-trimester pregnant women (Figure 4A). By contrast, progesterone treatment of DECs did not change the migratory behavior of all the pbNK cell populations assayed (Figure S6).
Since the majority of uterine NK cells are CD56highCD16−, we also investigated whether the CD56highCD16− pbNK cells have a preferential ability to migrate through DECs and ST cells. The results obtained indicate that CD56highCD16− pbNK cells can migrate through both DECs and ST cells, and interestingly, in accordance with their chemokine receptor profile this migration was significantly enhanced only when CXCL-10/IP10 was added as chemoattractant, while CXCL12/SDF-1 and CX3CL1/fractalkine preferentially attracted CD16+ pbNK cells. Similar results were observed when DECs and ST cells treated with progesterone were used as substrates (Figure S7,S8). No differences in the percentage of CD56highCD16− pbNK cell population were found between pregnant versus nonpregnant or male donors.
These data show, for the first time, that pbNK cells are able to migrate through both DECs and ST tissues, and this capacity is strongly enhanced during early pregnancy; they also suggest that fluctuations of progesterone levels occurring during early pregnancy tightly control this process by acting on ST cells.
dNK cell migration through decidual ST cells
To investigate the ability of ST cells to support also dNK cell migration, freshly isolated highly purified dNK and pbNK cells derived from the same donor were allowed to migrate through progesterone-cultured ST cells. As shown in Figure 5, dNK cells migrated through ST cells but unlike pbNK cells they migrated only in response to CXCL10/IP-10 and CXCL12/SDF-1 but not to CX3CL1/fractalkine. CXCL12/SDF-1–supported dNK cell migration was lower than that observed for the peripheral blood counterpart, while similar levels of migration were observed in response to CXCL10/IP-10. Moreover, unlike the peripheral blood counterpart, dNK cell migration through ST cells was significantly enhanced when progesterone-treated ST cells were used as substrate (Figure S9), thus suggesting that decidua-resident and blood-circulating NK cells from pregnant women display a different migratory behavior.
Chemokines differently support migration of pbNK and dNK cells through progesterone-treated decidual ST cells. Highly purified pbNK cells or dNK cells from the same women in the first trimester of pregnancy were assayed for their ability to migrate through a monolayer of primary cultures of ST decidual cells grown in the presence of progesterone (100 nM) as above. All data are expressed as the mean plus SD of the percentage of migrated cells obtained from 3 independent experiments. *P < .05, as evaluated by comparing chemoattractant-induced migration versus control medium by Student t test.
Chemokines differently support migration of pbNK and dNK cells through progesterone-treated decidual ST cells. Highly purified pbNK cells or dNK cells from the same women in the first trimester of pregnancy were assayed for their ability to migrate through a monolayer of primary cultures of ST decidual cells grown in the presence of progesterone (100 nM) as above. All data are expressed as the mean plus SD of the percentage of migrated cells obtained from 3 independent experiments. *P < .05, as evaluated by comparing chemoattractant-induced migration versus control medium by Student t test.
These data indicate that dNK and pbNK cells use a distinct pattern of chemokines to migrate through ST cells; this result strongly correlates with the levels of CXCR4, CX3CR1, and CXCR3 expression found on dNK versus pbNK cells (Figure S5).
Chemokine receptor expression on human pbNK cells is modulated upon coculture with ST decidual cells
The differences found between the chemokine receptor profile of pbNK cells versus dNK cells prompted us to hypothesize that the chemokine receptor repertoire of human pbNK cells undergoes tissue-specific modulation when these cells are recruited to the uterus. To verify this hypothesis, pbNK cells isolated from first-trimester pregnant women were cocultured with uterine-derived DECs or ST cells for 36 hours, and then assayed for the expression of chemokine receptors by 3-color immunofluorescence and cytofluorimetric analysis. As shown in Figure 6, coculture of pbNK cells with ST cells resulted in significant down-regulation of CCR1, CCR5, CXCR1, CXCR4, and CX3CR1, while CXCR3 was up-regulated, thus acquiring a chemokine receptor profile closely resembling that of dNK cells.
Chemokine receptor expression on pbNK cells cocultured with ST decidual cells. pbNK cells from first-trimester pregnant women were cocultured with ST cells grown in the presence of progesterone (100 nM) (C) or with control medium (B) for 36 hours at 37°C. After incubation, chemokine receptor expression on gated CD56+CD3− NK cells was analyzed. Control represents staining with FITC-conjugated GAM or GARB Abs. (A) Staining for the chemokine receptor expression on NK cells at the start of the experiment. Data are representative of 4 independent experiments. Percentages of total cells within the quadrant and the mean fluorescent intensity (m) are shown on the plots.
Chemokine receptor expression on pbNK cells cocultured with ST decidual cells. pbNK cells from first-trimester pregnant women were cocultured with ST cells grown in the presence of progesterone (100 nM) (C) or with control medium (B) for 36 hours at 37°C. After incubation, chemokine receptor expression on gated CD56+CD3− NK cells was analyzed. Control represents staining with FITC-conjugated GAM or GARB Abs. (A) Staining for the chemokine receptor expression on NK cells at the start of the experiment. Data are representative of 4 independent experiments. Percentages of total cells within the quadrant and the mean fluorescent intensity (m) are shown on the plots.
Similar data were obtained when pbNK cells were cocultured with the autologous adherent decidual cells (data not shown).
We observed a certain degree of modulation of chemokine receptor expression also when NK cells were cultured with control medium alone, but the variations observed were much lower and in some cases opposite to those mediated by ST cells (ie, down-modulation of CCR5 and CXCR4 instead of the up-regulation induced by control medium alone).
Moreover, when pbNK cells were cocultured with DECs grown or not in the presence of progesterone, modulation of the chemokine receptor profile of pbNK cells was not observed (Figure S10).
Collectively, these results suggest that in response to signals delivered by ST cells but not DECs, pbNK cells acquire a chemokine receptor profile that resembles that of uNK cells.
Discussion
During early pregnancy, maternal-fetal interaction creates a state of mild systemic inflammation, as revealed by the presence of activated vascular endothelium, leukocytosis, increased functions of cells of innate immunity such as monocytes, as well as increased plasmatic levels of inflammatory cytokines and chemokines such as IL-18, IL-12, TNFα, and IL-8.32
NK cell recruitment from blood to the tissues, as for other leukocytes, is a spatially and temporally integrated multistep process regulated by a number of chemoattractants and adhesive molecules.33-38 Chemokines are a superfamily of inflammatory mediators that properly guide leukocyte recruitment and positioning into healthy or diseased tissues by interacting with 7-transmembrane-domain receptors.38-40
In agreement with previous observations,25,41,42 our results revealed that both DECs and ST cells express significant but different levels of the mRNA coding CCL2/MCP-1, CXCL8/IL-8, CX3CL1/fractalkine, CXCL10/IP-10, and CXCL12/SDF-1, with CXCL10/IP-10 expressed at similar levels on both DECs and ST cells, CX3CL1/fractalkine and CCL2/MCP-1 expressed mainly by DECs, and CXCL12/SDF-1 expressed by ST cells. All chemokines produced by these decidual tissues have at least one functional counter receptor on pbNK cells and thus can support NK cell recruitment and/or retention to the uterine compartment.
In this regard, the ability of chemokines secreted by decidual cells or trophoblastic cells (such as CXCL9/Mig, CXCL10/IP-10, CXCL12/SDF-1, or CCL3/MIP-1α) to mediate pbNK or dNK cell chemotaxis in vitro has been documented.23-27 Among the chemokines present on decidual tissues, CX3CL1/fractalkine, one of the major chemoattractants for the NK cells and other cytotoxic lymphocytes, was found to be the most abundant and localized mainly to glandular epithelial, DECs, and ST cells. Based on the observation that CX3CL1/fractalkine expression on decidual tissues is maximal during the secretory phase and early pregnancy, its involvement in the recruitment of different leukocyte subsets in the decidua has been suggested.42 However, no evidence is yet available on the ability of CX3CL1/fractalkine as well as CXCL12/SDF-1 and CXCL10/IP-10 to drive pbNK or dNK cell migration across DECs or ST cells in pregnant women. The results presented here, by showing that CXCL12/SDF-1, CXCL10/IP-10, or CX3CL1/fractalkine can support pbNK cell migration through both DECs and ST cells, provide novel information and strengthen previous observations.
Among factors involved in the control of the good outcome of pregnancy, crucial actors are sex hormones and evidence indicates that numeric variations of NK cells in the decidua or in the late secretory phase of menstrual cycle parallel progesterone levels.13,14 However, as NK cells do not express the progesterone receptors, the effect of this hormone in the control of NK cell accumulation in the uterus has been attributed mainly to its ability to induce endometrial decidualization.
Endometrial decidualization is a process associated with many functional and phenotypic modifications and an enhanced expression of CXCL9/MIg, CXCL10/IP-10, and CX3CL1/fractalkine has been described.25,41,42 Moreover, a cyclic variability in the expression levels of chemokines found in the endometrium during endometrial breakdown, repair, or embryo implantation as well as changes in the functional adhesiveness of pbNK cells to decidual vascular endothelial cells associated with early pregnancy have been described.15,30,31
Notably, we observed that pbNK cells from first-trimester pregnant women display higher migratory capacity compared with pbNK cells of nonpregnant women or male donors even if they express comparable levels of chemokine receptors or integrin subunits (Table S1), thus suggesting that pregnancy-associated factors acting at systemic level, including hormones (such as prolactin, chorionic gonadotrophin, and estrogens) and/or inflammatory cytokines (such as IL-12 and IL-18), can modulate the migratory behavior of pbNK cells without affecting their integrin and chemokine receptor profile.
The finding that migration of NK cells from pregnant women, differently from that of nonpregnant women or male donors, is not enhanced when progesterone-treated ST cells were used, may be attributable to their higher migratory ability that probably does not allow to observe a further increase dependent on progesterone treatment of ST cells. However, based on the evidence that NK cells from nonpregnant women or male donors exhibit an enhanced migration across progesterone-treated ST cells, and on the observation that progesterone increases chemokine expression on ST cells (this study; Sentman et al25 ; Kitaya et al41 ), it is likely that progesterone, by exerting local effects on ST cells, might favor recruitment of NK cells in the decidua before 8 to 9 weeks of pregnancy.
Moreover, we found that uNK cells can migrate through decidual ST cells using a pattern of chemokines distinct from that of pbNK cells, and this strongly correlates with their chemokine receptor profile (this study, Hanna et al23 ; Wu et al24 ; Sentman et al25 ).
Based on the multistep migration model formulated by Foxman et al,43 we would speculate that in response to a particular combination of chemokines coexpressed at specific sites of decidual tissue, NK cells use more than one receptor-ligand pair at any given step to be correctly targeted inside the uterus; these chemokines may act sequentially or in combination to contribute to both exit of NK cells from the circulation as well as to finely tune their correct position, organization, and retention into specific focal areas of the decidual tissues.
Finally, the demonstration herein reported, that human pbNK cells acquire a chemokine receptor repertoire similar to that of decidual NK cells when they contact ST cells, is, to the best of our knowledge, the first evidence indicating that communication between NK cells and ST cells results in modulation of NK cell migratory phenotype. It is presently unknown whether the NK cell–stimulating signals delivered by decidualized ST cells are membrane associated and/or soluble factors. Our finding, however, is in line with accumulating evidence indicating that signals provided by decidualized ST cells, including IL-15 and IL-11, critically control uNK development and/or functional differentiation.44-46 In this regard, it has been shown that incubation of CD16− pbNK cells with IL-15 results in the induction of a chemokine receptor pattern similar to that of uNK cells.23 Moreover, it has been recently reported that culture of CD16+ pbNK cells with conditioned medium derived from decidual ST cell results in conversion of CD16+ pbNK cells into CD16− cells as well as in the up-regulation of CD9 expression, and these effects are clearly dependent on TGFβ released by ST cells, suggesting an important role for TGFβ in influencing the uNK phenotype.47
In summary, our results strongly suggest that during early pregnancy, recruitment of peripheral blood human NK cells to the uterus contributes to the enhancement of NK cell number in this nonlymphoid organ. Once in the uterus, NK cells undergo reprogramming of their chemokine receptor profile thus acquiring a specific phenotypic and functional profile to ensure a good pregnancy outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr C. Tripodo (University of Palermo, Italy) for the immunohistochemical analysis of progesterone receptor expression on DECs and ST cells.
This work was supported by grants from Istituto Pasteur Fondazione Cenci Bolognetti, Ministero Istruzione Universita Ricerca–Programma di Ricerca di Rilevante Interesse Nazionale (MIUR-PRIN), Centro di Eccellenza Biologia e Medicina Moleculare (BEMM), and the European Network of Excellence (NoE) on Embryo Implantation Control (EMBIC) within Sixth Framework Programme (FP6) (contract number LSHN-CT-2004-512040).
Authorship
Contribution: C.C., H.S., S.M., R.B., and A.S. performed experiments; C.C. and A.G. analyzed results; C.A., F.B., C.M., and F.S. contributed new reagents/analytic tools; F.T. designed the research; A.S. and A.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Gismondi, Department of Experimental Medicine University “La Sapienza,” Viale Regina Elena 324, Rome, 00161 Italy; e-mail: angela.gismondi@uniroma1.it; and Angela Santoni, Department of Experimental Medicine University “La Sapienza,” Viale Regina Elena 324, Rome, 00161 Italy; e-mail: angela.santoni@uniroma1.it.