Abstract
Endothelial cells (ECs) regulate the barrier function of blood vessels. Here we show that basal and angiopoietin-1 (Ang-1)–regulated control of EC permeability is mediated by 2 different functional states of sphingosine kinase-1 (SK-1). Mice depleted of SK-1 have increased vascular leakiness, whereas mice transgenic for SK-1 in ECs show attenuation of leakiness. Furthermore, Ang-1 rapidly and transiently stimulates SK-1 activity and phosphorylation, and induces an increase in intracellular sphingosine-1-phosphate (S1P) concentration. Overexpression of SK-1 resulted in inhibition of permeability similar to that seen for Ang-1, whereas knockdown of SK-1 by small interfering RNA blocked Ang-1-mediated inhibition of permeability. Transfection with SKS225A, a nonphosphorylatable mutant of SK-1, inhibited basal leakiness, and both SKS225A and a dominant-negative SK-1 mutant removed the capacity of Ang-1 to inhibit permeability. These effects were independent of extracellular S1P as knockdown or inhibition of S1P1, S1P2, or S1P3, did not affect the Ang-1 response. Thus, SK-1 levels in ECs powerfully regulate basal permeability in vitro and in vivo. In addition, the Ang-1–induced inhibition of leakiness is mediated through activation of SK-1, defining a new signaling pathway in the Ang-1 regulation of permeability.
Introduction
The control of vascular homeostasis is mediated through the maintenance of endothelial cell (EC) monolayer integrity, which is responsible for the tonic impermeable nature of blood vessels. Altered endothelial integrity underlies changes in vascular permeability, as seen in inflammatory responses and angiogenesis, and in diseases, such as rheumatoid arthritis and atherosclerosis. Stimuli, such as thrombin, histamine, and tumor necrosis factor (TNF), are powerful inducers of EC permeability,1 whereas the angiogenic factor angiopoietin-1 (Ang-1), acting through the tyrosine kinase receptor Tie2, and sphingosine-1-phosphate (S1P), acting through the G protein coupled receptors S1P1 or S1P3, inhibit the action of these stimulators and are potent protectors of barrier function.2-4
The Ang-1/Tie2 axis is essential for vascular development and, in the adult, for the formation and maintenance of EC integrity.2 In vivo, transgenic expression of Ang-1 in mice results in leakage resistant blood vessels,5 and systemic administration of Ang-1 inhibits vessel leak.6 In vitro, the antipermeability and anti-inflammatory effects of Ang-1 appear to function at 2 levels, one to inhibit the basal permeability of EC and the other to limit the activity of agents, such as thrombin and TNF.3 The signaling pathways regulated in response to ligand-induced phosphorylation of Tie2 are only now being elucidated.7 AKT is involved in the prosurvival and angiogenic activities of Ang-1.8 Interaction of ShcA with Tie2 and phospholipase D-dependent regulation of mitogen-activated protein kinase (MAPK) are involved in Ang-1-mediated cell migration9 and a Ras/MAPK cascade is responsible for Ang-1–induced cell proliferation.10 We have shown that Ang-1 inhibits thrombin-induced activation of Rho- and Ca2+-dependent pathways, together with a newly described protein kinase C (PKC) ζ-dependent signaling pathway.11 In addition, Jho et al12 have shown that Ang-1 inhibits vascular endothelial cell growth factor (VEGF)-induced phospholipase C-inositol-1,4,5-trisphosphate (PLC-IP3)-dependent Ca2+ influx to inhibit EC permeability. Because Ang-1 can regulate multiple pathways implicated in EC permeability, we sought to determine whether it affects other signaling pathways.
Sphingosine kinase (SK) regulates the conversion of sphingosine into its biologically active metabolite, S1P. SK-1 is activated by a variety of stimuli, including serum, VEGF, platelet-derived growth factor, and hyperglycemia,13 and is involved in promoting cell survival and proliferation. SK-1 is also involved in estrogen-dependent regulation of tumor cell growth and survival, and elevated levels of SK-1 are seen in some human tumors.14
Several effects of SK-1, for example, estrogen-dependent cancer growth, are the result of autocrine or paracrine effects of extracellular S1P,15 acting through G protein-coupled cell surface receptors that belong to the endothelial differentiation gene receptor family, and are now referred to as S1P1-5. These S1P receptors regulate proliferation, survival, and differentiation.16 S1P1 is essential for vascular maturation and in mature ECs, S1P regulates migration17 and inhibits permeability through S1P-receptor-dependent mechanisms.4 Interestingly, activated protein C also mediates its barrier protective effects through S1P receptors.18 FTY720, an analog of S1P, inhibits VEGF-induced permeability, angiogenesis, and tumor vascularization.19 Other effects of SK-1, such as the induction of a novel PECAM-1–dependent signaling pathway, are the result of intracellular effects of the signaling system,20 although the intracellular receptors for S1P are yet to be identified.
Here we investigate the role of the SK-1–S1P axis in regulating vascular permeability and describe 2 important findings. The first is that SK-1 controls the tonic impermeable nature of blood vessels. The second is that Ang-1 exerts its antipermeability effects through SK-1 independent of the action of exogenous S1P.
Methods
The experimental procedures were approved by the Animal Ethics Committee of the Institute of Medical and Veterinary Science and conform to the guidelines established by the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Reagents
Ang-1 was obtained from R&D Systems (Minneapolis, MN). n,n-Dimethylsphingosine (DMS) and S1P were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). [γ-32P]ATP was from Geneworks (Adelaide, Australia). JET-013 was from Cayman (Ann Arbor, MI) and VPC 23 019 was from Avanti Polar Lipids (Alabaster, AL). The kinase inhibitor U0126 was from Cell Signaling Technology (Danvers, MA).
Cells and cell culture
Umbilical cords were collected after consent was obtained in accordance with the Declaration of Helsinki. Human umbilical vein endothelial cells (HUVECs) were grown as described.21 Cells were used at passage 4 or less.
SK-1 and recombinant adenoviral constructs
Wild-type human SK-1, FLAG, and mutants possessing an alanine at position 225 (SK-1S225A) or an aspartate at position 82 (SK1G82D) were made as previously described.22,23 Recombinant adenoviruses were made by subcloning Kpn I/XhoI fragments from pcDNA3-SK-1 constructs into the pAdEasy-1 vector (Qbiogene, Irvine, CA). Virus was amplified in HEK293 cells and purified by CsCl gradient ultracentrifugation. Virus titers were determined using the TCID50 method, as recommended by Qbiogene. For infection with adenoviral constructs, HUVECs were grown to 80% confluence and exposed to one plaque forming unit/cell for 2 hours in M119 medium with 2% fetal calf serum (FCS) and a further 22 hours with medium containing 20% FCS.
SK-1 transfection with small interfering RNA
Small interfering RNA (siRNA) targeted to human SK-1 [r(GAGCUGCAAGGCCUUGCCC)d(TT) and r(GGGCAAGGCCUUGCAGCUC)d(tt)] and control nonsilencing siRNA [r(UUCUCCGAACGUGUCACGU)d(TT) and r(ACGUGACACGUUCGGAGAA)d(TT)] were synthesized by QIAGEN-Xeragon (Germantown, MD). siRNA targeted to human S1P1, GCGGACAAGGAGAACAGCAUUAAAC and GUUUAAUGCUGUUCUCCUUGUCCGC, and human S1P3, UAGAGGAUCACGAUGGUCACCAGGA and UCCUGGUGACCAUCGUGAUCCUCUA were synthesized by Invitrogen (Carlsbad, CA). The transfection of the siRNA into HUVECs by using the HiPerFect transfection reagent was done according to the manufacturer's protocol (QIAGEN).
SK-1 activity assays
SK-1 activity was determined using d-erythro-sphingosine and [γ-32P]ATP as substrates, as described.24
S1P levels
S1P levels were determined after metabolic labeling of cells with [32P]orthophosphate as described.23
Endothelial permeability assays
Permeability assays were performed as described.11 HUVECs (105) were cultured in transwells (3 μm; Corning Life Sciences, Acton, MA) for 24 hours in complete medium and then in 2% FCS medium for an additional 24 hours. Adenovirus-infected ECs were plated onto transwells 24 hours after the infection, incubated in 20% FCS M119 medium for 24 hours, and then changed to 2% FCS M119 medium for another 24 hours before assay. siRNA-transfected cells were plated onto transwells 24 hours after transfection and incubated M119 medium with 20% FCS for another 24 hours before the assay. Cells were pretreated with reagents in combination as required [Ang-1 (0.2 μg/mL), S1P (1 μM), DMS (5 μM), VPC 23 019 (10 μM), and JET-013 (1 μM)]. Fluorescein isothiocyanate (FITC)-conjugated dextran (2 μg, molecular weight 40 000) was added to the upper chamber of all wells. The amount of FITC-dextran in the lower chambers of the transwells was measured over a 30-minute period and determined by using a LS 50B Luminescence Spectrometer (PerkinElmer, Beaconsfield, United Kingdom; excitation wavelength, 485 nm; emission wavelength, 530 nm). Permeability is given as the amount of FITC-dextran passing from the top chamber to the bottom chamber.
VE-cadherin staining
HUVECs were infected with empty vector (EV) or SK-1 in adenovirus. Forty-eight hours later, the cells were replated onto LabTek slides for 45 minutes and washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 10 minutes, and permeabilized with PBS containing 0.1% Triton X-100.
Transfected cells were plated onto LabTek slides 24 hours after the transfection and incubated in M119 medium containing 2% FCS for another 24 hours. Cells were treated with or without Ang-1 (0.2 μg/mL) for 1 hour then washed and fixed. The cells were then incubated with mouse monoclonal anti-VE-cadherin21 overnight at 4°C, followed by Alexa Fluor 488 goat antimouse IgG (Invitrogen). They were viewed using a 40× objective on an Olympus BX51 microscope (Olympus, Hamburg, Germany) equipped with excitation filters for fluorescein and acquired to a Photometrics Cool Snap FX charge-coupled device camera (Roper Scientific, Friedland, Germany). Images were adjusted for brightness and contrast using V++ software (Digital Optics, Auckland, New Zealand).
Miles assay for in vivo permeability
Miles assays were performed essentially as given in Horowitz et al.25 The SK-1 knockout (KO) mice26 had been backcrossed with C57BL6 mice for 7 generations. SK-1 transgenic mice on the C57BL6 background were generated by Ozgene (Bentley, Australia), where the human SK-1 gene was injected into the nucleus via a vector driven by the Tie-2 promoter, to give high levels of expression in the endothelial cell compartment. Mice were injected intravenously with 200 μL of 0.5% Evans Blue dye. After 10 minutes, intradermal injections of PBS, VEGF (10 ng), and histamine (1 μM) were given into the shaved back area (2 sites per agent). The mice were killed 30 minutes later, and permeability was measured as the extent of blue dye perfusion away from the injection site. For quantification, a biopsy of the affected area was taken, the dye eluted in formamide overnight at 37°C, and the absorbance read at 620 nm.
Immunoprecipitation and immunoblotting
Cells were grown to confluence and then changed to 2% FCS for 24 hours before treatment with Ang-1. Immunoblotting was performed essentially as given by Li et al.11 For each immunoprecipitation, 500 μg protein was used. A monoclonal mouse anti-PECAM-121 or anti-Tie2 (Upstate, Lake Placid, NY) antibody was used. Antibodies used for probing were a monoclonal antiphosphotyrosine antibody (Cell Signaling), a polyclonal rabbit antiphospho-extracellular signal-regulated kinase (ERK) 1/2 antibody (Promega, Madison, WI), and an antiphospho-SK-1 antibody.23 After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody and reactive bands were detected by chemiluminescence (ECL Western Blotting Detection Reagents, GE Healthcare, Little Chalfont, United Kingdom). Membranes were stripped using stripping buffer (Re-Blot Plus Western Blot Recycling Kit, Chemicon, Temecula, CA) and reprobed with rabbit anti-ERK1/2 (Promega), or anti-PECAM-1, anti-Tie2, or anti-Flag (Sigma-Aldrich, St Louis, MO) antibodies.
Statistical significance was determined using Student t test, with values of P less than .05 considered significant.
Results
Angiopoietin-1 increases SK-1 activity and phosphorylation in ECs
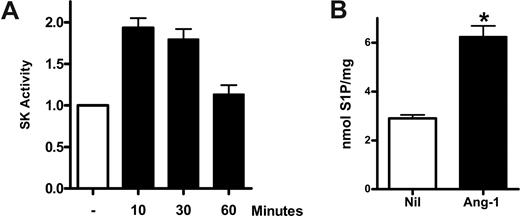
To determine the possibility that Ang-1 may signal through SK-1, we sought initially to determine whether Ang-1 stimulated SK-1 activity. HUVECs were grown to confluence, treated with Ang-1 at 0.2 μg/mL (previously shown to be optimal for effects on ECs3 ) for various times, and the SK-1 activity in the cell lysates determined. Figure 1A shows that stimulation with Ang-1 resulted in a transient increase in endogenous SK-1 activity, which was maximal at 10 to 30 minutes. In the 3 experiments performed, stimulation with Ang-1 for 10 minutes resulted in a 93% plus or minus 4% increase in SK-1 activity. Furthermore, such stimulation results in a 116% plus or minus 17% increase in intracellular S1P (Figure 1B).
Ang-1 induces SK-1 activation in HUVECs. (A) HUVECs were either untreated or treated with Ang-1 at 0.2 μg/mL for various times. Cells were lysed and SK-1 activity measured. Shown are pooled data from 3 experiments and expressed as fold change in relation to untreated group, where the SK-1 activity was normalized to 1.0 (*P < .01 vs untreated group). (B) HUVECs were untreated (Nil) or treated with Ang-1 at 0.2 μg/mL for 15 minutes. Intracellular S1P levels were determined. Data are mean plus or minus SEM from 3 independent experiments (*P < .001 vs untreated group).
Ang-1 induces SK-1 activation in HUVECs. (A) HUVECs were either untreated or treated with Ang-1 at 0.2 μg/mL for various times. Cells were lysed and SK-1 activity measured. Shown are pooled data from 3 experiments and expressed as fold change in relation to untreated group, where the SK-1 activity was normalized to 1.0 (*P < .01 vs untreated group). (B) HUVECs were untreated (Nil) or treated with Ang-1 at 0.2 μg/mL for 15 minutes. Intracellular S1P levels were determined. Data are mean plus or minus SEM from 3 independent experiments (*P < .001 vs untreated group).
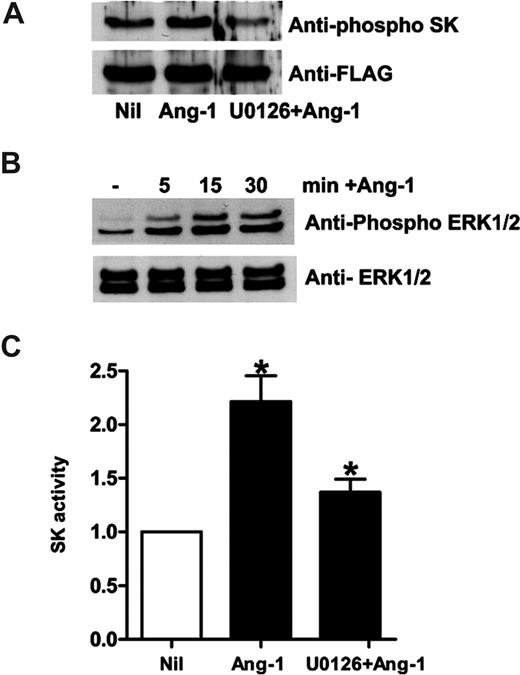
Ang-1 treatment induced an increase in the level of phosphorylation of SK-1, as detected by an antibody raised against the phosphorylated form of SK-123 (Figure 2A). The level of phosphorylation was maximal 15 minutes after Ang-1 stimulation (data not shown). SK-1 phosphorylation by agents, such as phorbol 12-myristate 13-acetate and TNF, is mediated by the mitogen-activated serine/threonine protein kinases, ERK1/2.23 Ang-1 activation of its receptor, Tie2, is also known to activate ERK1/2 to promote EC survival and migration.27 Ang-1 treatment of ECs phosphorylates ERK1/2, which was maximal by 15 minutes, with declining levels seen 30 minutes after stimulation (Figure 2B). The ERK1/2 pathway inhibitor U0126 blocked the Ang-1–mediated increase in SK-1 activity (Figure 2C) and its phosphorylation (Figure 2A) demonstrating that Ang-1, acting through an ERK1/2-dependent pathway, activates SK-1.
Ang-1 induces SK-1 activation in HUVECs through ERK1/2. (A) HUVECs were infected with adenovirus carrying hSK-1-FLAG. Cells were lysed after no treatment (Nil), treatment with Ang-1 at 0.2 μg/mL for 30 minutes (Ang-1), or pretreatment with U0126 (2 μM for 20 minutes) followed by treatment with Ang-1 (U0126 + Ang-1). Phospho-SK-1 in the top panel and total SK-1 in the bottom panel are shown. (B) HUVECs were untreated (−), or treated with Ang-1 for various times. Phospho-ERK is shown in the top panel and total ERK in the bottom panel. (C) HUVECs were untreated (Nil), treated with Ang-1 at 0.2 μg/mL for 15 minutes (Ang-1), or pretreated with U0126 (2 μM) for 20 minutes and then treated with Ang-1 for 15 minutes (UO126 + Ang-1). The SK-1 activity was measured. Pooled data from 3 experiments are expressed as in Figure 1A (mean ± SEM; *P < .05 vs untreated cells).
Ang-1 induces SK-1 activation in HUVECs through ERK1/2. (A) HUVECs were infected with adenovirus carrying hSK-1-FLAG. Cells were lysed after no treatment (Nil), treatment with Ang-1 at 0.2 μg/mL for 30 minutes (Ang-1), or pretreatment with U0126 (2 μM for 20 minutes) followed by treatment with Ang-1 (U0126 + Ang-1). Phospho-SK-1 in the top panel and total SK-1 in the bottom panel are shown. (B) HUVECs were untreated (−), or treated with Ang-1 for various times. Phospho-ERK is shown in the top panel and total ERK in the bottom panel. (C) HUVECs were untreated (Nil), treated with Ang-1 at 0.2 μg/mL for 15 minutes (Ang-1), or pretreated with U0126 (2 μM) for 20 minutes and then treated with Ang-1 for 15 minutes (UO126 + Ang-1). The SK-1 activity was measured. Pooled data from 3 experiments are expressed as in Figure 1A (mean ± SEM; *P < .05 vs untreated cells).
SK-1 regulates endothelial cell permeability and vascular leak in vivo
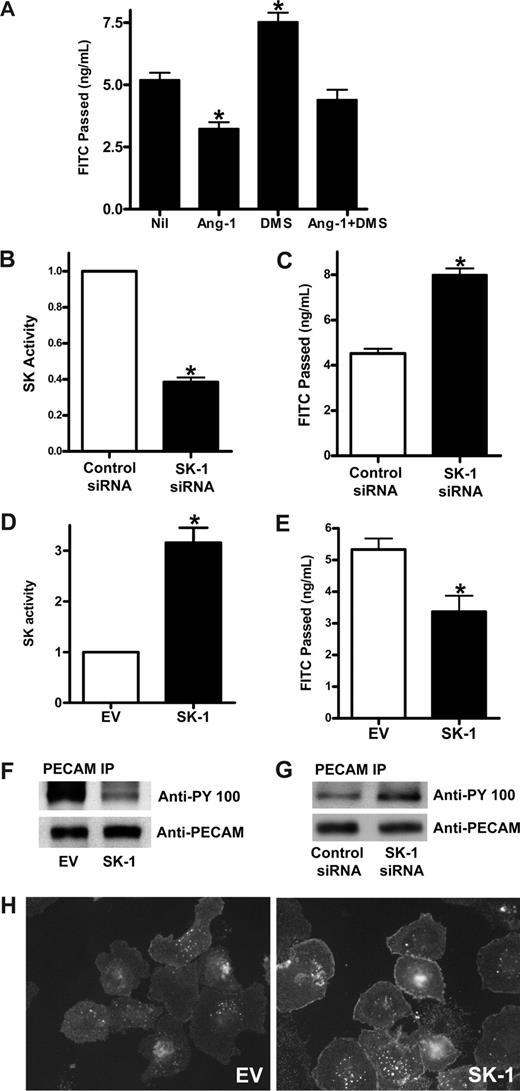
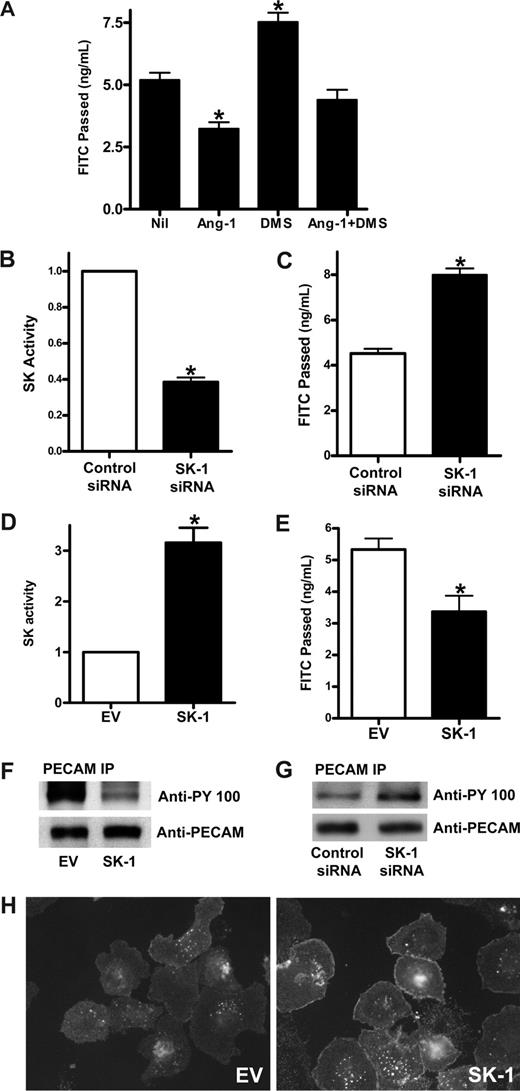
To determine whether Ang-1 acts through SK-1 to regulate permeability, the effects on permeability of DMS, a competitive inhibitor of SK, was examined. Pretreatment with DMS increased the baseline permeability of ECs (Figure 3A), highlighting a potential role of SK in regulation of permeability. In addition, Ang-1 inhibited permeability as we have reported previously,3 and this inhibition was partially removed by pretreatment with DMS. These results suggested 2 things: (1) that SK may be important in the regulation of basal EC permeability, and (2) that Ang-1 may signal through SK to inhibit permeability.
SK-1 regulates EC permeability changes. (A) HUVECs were untreated (Nil) or treated with 5 μM DMS for 15 minutes (DMS), Ang-1 (0.2 μg/mL) for 30 minutes (Ang-1), or Ang-1 and DMS (Ang-1 + DMS). Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs untreated cells). (B) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were lysed and SK-1 activity measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to control cells where the SK-1 activity was set to 1.0 (*P < .001 vs control siRNA cells). (C) HUVECs were transfected with control siRNA or siRNA against hSK-1. Permeability was measured 48 hours later. Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs control siRNA cells). (D) HUVECs were infected with adenoviral carrying EV or human SK-1. After 48 hours, SK-1 activity was measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to EV cells, which was normalized to 1.0 (*P < .001 vs EV cells). (E) HUVECs were infected with EV or SK-1 in adenovirus. Permeability was measured 72 hours later. Permeability is given as the FITC-dextran passage in 30 minutes. Shown are pooled data from 3 experiments (*P < .01 vs EV cells). (F) HUVECs were infected with EV or SK-1 in adenovirus. After 48 hours, cell lysates were immunoprecipitated with an anti-PECAM-1 antibody. Western blots for phosphotyrosine (top panel) and PECAM-1 (bottom panel) are shown. (G) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were lysed and immunoprecipitated with an anti-PECAM-1 antibody. Western blots for phosphotyrosine (top panel) and PECAM-1 (bottom panel) are shown. (H) HUVECs were infected with EV (EV) or SK-1 (SK-1) in adenovirus. Forty-eight hours after infection, the cells were replated onto LabTek slides and washed and fixed 45 minutes after plating. Cells were stained with anti–VE-cadherin antibody. For imaging information, see “VE-cadherin staining” section in “Methods.” All data are mean plus or minus SEM.
SK-1 regulates EC permeability changes. (A) HUVECs were untreated (Nil) or treated with 5 μM DMS for 15 minutes (DMS), Ang-1 (0.2 μg/mL) for 30 minutes (Ang-1), or Ang-1 and DMS (Ang-1 + DMS). Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs untreated cells). (B) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were lysed and SK-1 activity measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to control cells where the SK-1 activity was set to 1.0 (*P < .001 vs control siRNA cells). (C) HUVECs were transfected with control siRNA or siRNA against hSK-1. Permeability was measured 48 hours later. Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs control siRNA cells). (D) HUVECs were infected with adenoviral carrying EV or human SK-1. After 48 hours, SK-1 activity was measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to EV cells, which was normalized to 1.0 (*P < .001 vs EV cells). (E) HUVECs were infected with EV or SK-1 in adenovirus. Permeability was measured 72 hours later. Permeability is given as the FITC-dextran passage in 30 minutes. Shown are pooled data from 3 experiments (*P < .01 vs EV cells). (F) HUVECs were infected with EV or SK-1 in adenovirus. After 48 hours, cell lysates were immunoprecipitated with an anti-PECAM-1 antibody. Western blots for phosphotyrosine (top panel) and PECAM-1 (bottom panel) are shown. (G) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were lysed and immunoprecipitated with an anti-PECAM-1 antibody. Western blots for phosphotyrosine (top panel) and PECAM-1 (bottom panel) are shown. (H) HUVECs were infected with EV (EV) or SK-1 (SK-1) in adenovirus. Forty-eight hours after infection, the cells were replated onto LabTek slides and washed and fixed 45 minutes after plating. Cells were stained with anti–VE-cadherin antibody. For imaging information, see “VE-cadherin staining” section in “Methods.” All data are mean plus or minus SEM.
To investigate further whether SK-1 is important in the control of basal EC barrier function, SK-1 levels and activity were varied. SK-1 was depleted using siRNA and RT-PCR showed that the mRNA level was consistently reduced by approximately 70%.20 The SK-1 activity in these cells was reduced by 62% plus or minus 5% (Figure 3B), and there was an increase in basal permeability (48% ± 7%) in SK-1 siRNA-transfected cells compared with control siRNA-transfected cells (Figure 3C). SK-1 levels were increased by infecting cells with adenovirus carrying human SK-1 cDNA. The dose of virus was adjusted to give a 2- to 5-fold increase above control (Figure 3D), similar to levels achieved with endogenous increases in SK activity when stimulated with agents, such as TNF28 and VEGF.29 In these SK-1 overexpressing cells, the permeability was significantly decreased (Figure 3E).
Changes in EC permeability occur through regulation of junctional integrity. The adhesion molecule PECAM-1 is a marker for junctional integrity and has been implicated in permeability regulation.30 Overexpression of SK-1 in HUVECs resulted in a significant decrease in the phosphorylation of PECAM-1 (Figure 3F) consistent with tightening of junctions and a decrease in permeability. Conversely, cells transfected with siRNA against SK-1 had increased tyrosine phosphorylation of PECAM-1 (Figure 3G) consistent with relaxation of barrier integrity and the increase in permeability. The SK-1 effects on tightening cell junctions were further substantiated by the relocalization of VE-cadherin to sites of cell-cell interactions in cells overexpressing SK-1 (Figure 3H).
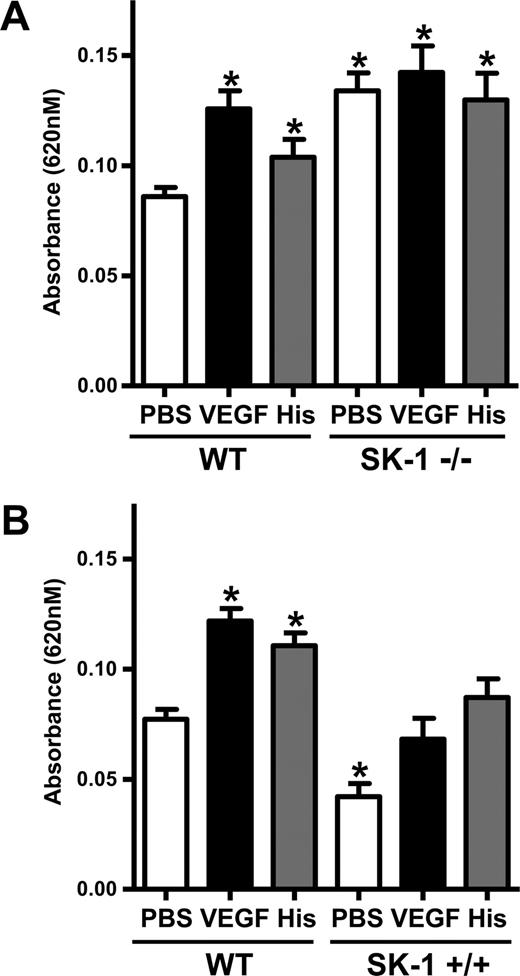
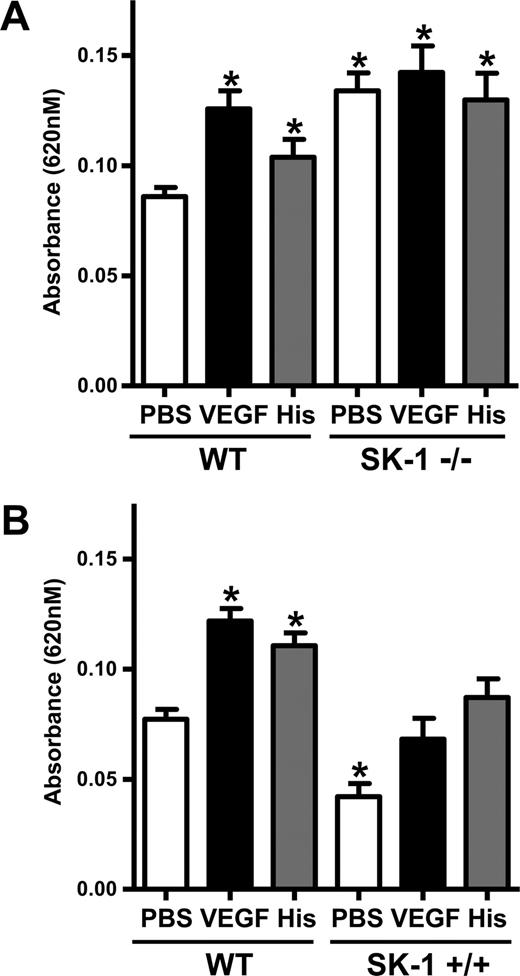
To determine whether SK-1 plays a significant role in the regulation of permeability in vivo, the classic Miles assay for measurement of vascular permeability was used in mice deficient in SK-1.26 We noted a significant increase in permeability in SK-1 KO mice injected with PBS alone compared with age- and sex-matched wild-type (WT) mice. No further increase in vascular leak was achieved after VEGF or histamine stimulation in these mice, although the WT controls responded to these stimuli (Figure 4A). Further confirmation of a role for SK-1 in regulation of basal permeability was obtained using transgenic mice overexpressing SK-1 in endothelial cells. These mice show a 2-fold increase in SK-1 activity in organs, such as spleen, lung, and heart (data not shown). Permeability measurements showed a decrease in Evans Blue dye with subcutaneous injection of PBS. When challenged with VEGF or histamine, WT mice responded, showing an increase in permeability, whereas the SK-1 transgenic mice showed a suppressed VEGF or histamine response (Figure 4B). Analysis of plasma VEGF (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and the number of blood vessels showed no significant differences between the WT, KO, and transgenic animals.
SK-1 regulates EC permeability and vascular leakage in vivo. (A) The absorbances of the dye eluted from the injected areas (PBS, VEGF, and histamine) of 10 age- and sex-matched WT mice and 10 SK-1 KO mice (SK-1−/−) were read at 620 nm. The pooled results (mean ± SEM) for each group of mice are shown (*P < .05 vs WT mice injected with PBS). (B) The absorbances of the dye eluted from the injected areas of 8 age- and sex-matched WT mice and 8 SK-1 transgenic mice (SK-1+/+) were read at 620 nm. The pooled results (mean ± SEM) for each group of mice are shown (*P < .05 vs the WT mice injected with PBS).
SK-1 regulates EC permeability and vascular leakage in vivo. (A) The absorbances of the dye eluted from the injected areas (PBS, VEGF, and histamine) of 10 age- and sex-matched WT mice and 10 SK-1 KO mice (SK-1−/−) were read at 620 nm. The pooled results (mean ± SEM) for each group of mice are shown (*P < .05 vs WT mice injected with PBS). (B) The absorbances of the dye eluted from the injected areas of 8 age- and sex-matched WT mice and 8 SK-1 transgenic mice (SK-1+/+) were read at 620 nm. The pooled results (mean ± SEM) for each group of mice are shown (*P < .05 vs the WT mice injected with PBS).
SK-1 mediates the Ang-1 effects on permeability
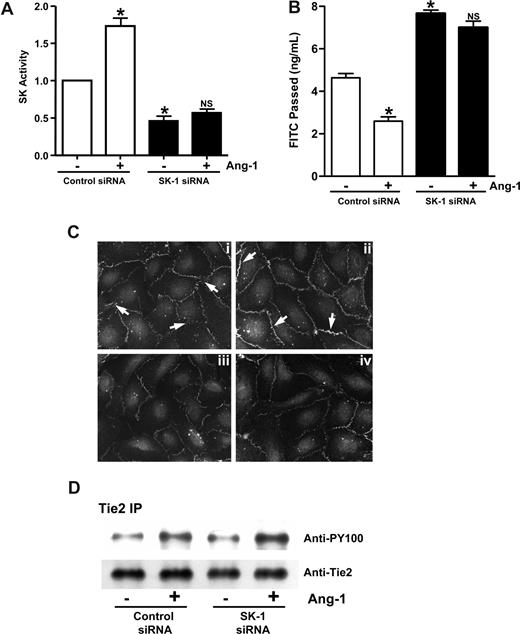
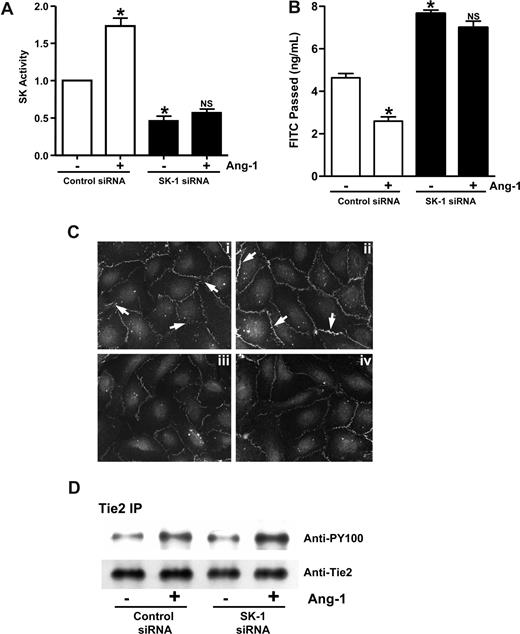
Ang-1 treatment of ECs inhibits their permeability. Treatment of the cells with both DMS and Ang-1 reversed the effect of DMS (Figure 3A), suggesting that Ang-1 and SK-1 may act through a common pathway. To investigate the possibility that SK-1 may be involved in Ang-1 signaling to inhibit permeability, cells were depleted of SK-1 by siRNA. These cells failed to show an increase in SK-1 activity after Ang-1 stimulation, whereas control siRNA-transfected cells behaved as normal, showing a 78% plus or minus 9% increase in SK-1 activity after Ang-1 stimulation (Figure 5A). Ang-1 was able to inhibit permeability in control siRNA-transfected cells (Figure 5B), similar to normal ECs.3 However, treatment with Ang-1 did not induce any significant inhibition of permeability in SK-1–depleted cells (Figure 5B). Increasing the concentration of Ang-1 used in these experiments did not have any further functional effects (data not shown). In control cells, Ang-1 treatment resulted in an increase in VE-cadherin staining and less diffuse staining of the cell-cell junctions (Figure 5Ci,ii). In cells where SK-1 was depleted by siRNA, the VE-cadherin at the cell junctions was significantly reduced and Ang-1 treatment had no effect (Figure 5Ciii,iv), demonstrating the requirement for SK-1 in Ang-1–mediated effects.
SK-1 mediates the Ang-1 effects on permeability. (A) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were treated with or without Ang-1 for 30 minutes and SK-1 activity measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to untreated control cells where the SK-1 activity was set to 1.0 (*P < .001 vs control siRNA cells without treatment). NS indicates no significant difference versus SK-1 siRNA cells without treatment. (B) HUVECs were transfected with control siRNA or siRNA against hSK-1. Forty-eight hours later, cells were untreated or treated with Ang-1 (0.2 μg/mL) for 30 minutes, and permeability is measured. Permeability is given as the FITC-dextran passed after 30 minutes. Pooled data from 3 experiments are shown (mean ± SEM; *P < .01 vs control siRNA cells without treatment). NS, vs SK-1 siRNA cells without treatment. (C) HUVECs were transfected with control siRNA (Ci and Cii) or siRNA against hSK-1 (Ciii and Civ). Forty-eight hours later, cells were untreated (Ci and Ciii) or treated (Cii and Civ) with Ang-1 for 1 hour and then stained for VE-cadherin. In panel Ci, arrow shows diffuse, broad VE-cadherin staining with classic zipper-like pattern indicative of immature junctions. In panel Cii, arrow indicates increased VE-cadherin staining at the junctions, with a more linear staining pattern indicative of mature EC junctions. (D) HUVECs were transfected with control siRNA or siRNA against hSK-1. Forty-eight hours later, cells were untreated (−) or treated (+) with Ang-1 (0.2 μg/mL) for 30 minutes then were lysed and immunoprecipitated with an anti-Tie2 antibody. Western blots for phosphotyrosine (top panel) and Tie2 (bottom panel) are shown. For imaging information, see “VE-cadherin staining” section in “Methods.”
SK-1 mediates the Ang-1 effects on permeability. (A) HUVECs were transfected with control siRNA or siRNA against hSK-1. After 48 hours, cells were treated with or without Ang-1 for 30 minutes and SK-1 activity measured. Pooled data from 3 experiments are shown and are expressed as the fold change in relation to untreated control cells where the SK-1 activity was set to 1.0 (*P < .001 vs control siRNA cells without treatment). NS indicates no significant difference versus SK-1 siRNA cells without treatment. (B) HUVECs were transfected with control siRNA or siRNA against hSK-1. Forty-eight hours later, cells were untreated or treated with Ang-1 (0.2 μg/mL) for 30 minutes, and permeability is measured. Permeability is given as the FITC-dextran passed after 30 minutes. Pooled data from 3 experiments are shown (mean ± SEM; *P < .01 vs control siRNA cells without treatment). NS, vs SK-1 siRNA cells without treatment. (C) HUVECs were transfected with control siRNA (Ci and Cii) or siRNA against hSK-1 (Ciii and Civ). Forty-eight hours later, cells were untreated (Ci and Ciii) or treated (Cii and Civ) with Ang-1 for 1 hour and then stained for VE-cadherin. In panel Ci, arrow shows diffuse, broad VE-cadherin staining with classic zipper-like pattern indicative of immature junctions. In panel Cii, arrow indicates increased VE-cadherin staining at the junctions, with a more linear staining pattern indicative of mature EC junctions. (D) HUVECs were transfected with control siRNA or siRNA against hSK-1. Forty-eight hours later, cells were untreated (−) or treated (+) with Ang-1 (0.2 μg/mL) for 30 minutes then were lysed and immunoprecipitated with an anti-Tie2 antibody. Western blots for phosphotyrosine (top panel) and Tie2 (bottom panel) are shown. For imaging information, see “VE-cadherin staining” section in “Methods.”
We have previously shown that Ang-1 mediates its effects on permeability through the Tie2 receptor.3 The loss of Ang-1 response in the SK-1–depleted cells is not through alteration in Tie2 receptor expression as control cells and cells treated with siRNA to SK-1 showed similar levels of Tie2 expression. In addition, Ang-1 treatment resulted in similar degrees of phosphorylation of the receptor in both control and siRNA-treated cells (Figure 5D). Thus, SK-1 must act downstream of the initial Ang-1/Tie2 signaling event, and SK-1 activity is required for ECs to respond to Ang-1 to inhibit permeability.
Ang-1 regulation of permeability is independent of S1P receptors
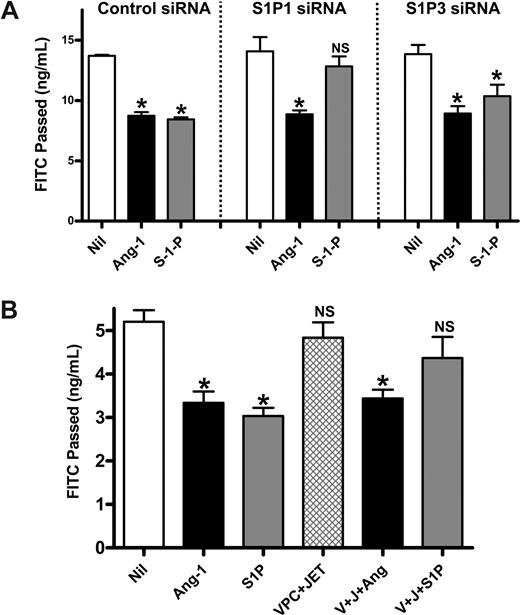
Extracellular S1P regulates permeability through S1P1 and S1P3.31 To determine whether extracellular S1P is involved in the ability of Ang-1 to inhibit permeability, cells were depleted of S1P1 and S1P3 using siRNA. RT-PCR showed a 70% to 80% decrease in mRNA level (Figure S2). Cells transfected with control siRNA responded to both Ang-1 and S1P showing an inhibition of permeability (Figure 6A). Knockdown of S1P1 with siRNA removes most of the S1P-mediated inhibition of permeability.31 However, the Ang-1 effect on permeability remains intact (Figure 6A). Knockdown of S1P3 did not alter the inhibitory effects of S1P or Ang-1 on permeability, indicating that in our system S1P predominantly signals through S1P1. Further, when S1P1, S1P2, and S1P3 receptors were inhibited using the inhibitors JET-013 and VPC23019, which are directed to S1P232 or S1P1 and S1P3,33 respectively, the S1P-mediated reduction in permeability was abrogated, but Ang-1 remained able to function (Figure 6B). Thus, Ang-1–mediated inhibition of permeability is independent of exogenous S1P activation of the S1P receptors, S1P1, S1P2, and S1P3.
Ang-1 inhibition of EC permeability is independent of the S1P receptor. (A) HUVECs were transfected with control siRNA, siRNA against S1P1 or against S1P3. Seventy-two hours after transfection, cells were untreated (Nil), treated with Ang-1 for 30 minutes (Ang-1), or treated with S1P at 1 μM for 15 minutes (S-1-P) and permeability measured. Permeability is given as the FITC-dextran passage in 30 minutes. The pooled data from 3 experiments are shown (*P < .01 vs untreated cells). NS indicates no significant difference versus untreated cells. (B) HUVECs were untreated (Nil), treated with Ang-1 (Ang-1) 0.2 μg/mL for 30 minutes, S1P (S1P) 1 μM for 15 minutes, JET-013 (JET) 1μM, and VPC 23 019 (VPC) 10 μM for 30 minutes (JET + VPC), or pretreated with JET-013 and VPC 23 019 for 30 minutes then treated with Ang-1 (J + V + Ang) or S1P (J + V + S1P). Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs untreated cells). All data are mean plus or minus SEM. NS indicates no significant difference versus untreated cells.
Ang-1 inhibition of EC permeability is independent of the S1P receptor. (A) HUVECs were transfected with control siRNA, siRNA against S1P1 or against S1P3. Seventy-two hours after transfection, cells were untreated (Nil), treated with Ang-1 for 30 minutes (Ang-1), or treated with S1P at 1 μM for 15 minutes (S-1-P) and permeability measured. Permeability is given as the FITC-dextran passage in 30 minutes. The pooled data from 3 experiments are shown (*P < .01 vs untreated cells). NS indicates no significant difference versus untreated cells. (B) HUVECs were untreated (Nil), treated with Ang-1 (Ang-1) 0.2 μg/mL for 30 minutes, S1P (S1P) 1 μM for 15 minutes, JET-013 (JET) 1μM, and VPC 23 019 (VPC) 10 μM for 30 minutes (JET + VPC), or pretreated with JET-013 and VPC 23 019 for 30 minutes then treated with Ang-1 (J + V + Ang) or S1P (J + V + S1P). Permeability is given as the FITC-dextran passage in 30 minutes. Pooled data from 3 experiments are shown (*P < .01 vs untreated cells). All data are mean plus or minus SEM. NS indicates no significant difference versus untreated cells.
SK-1 activation is necessary for Ang-1–mediated EC permeability
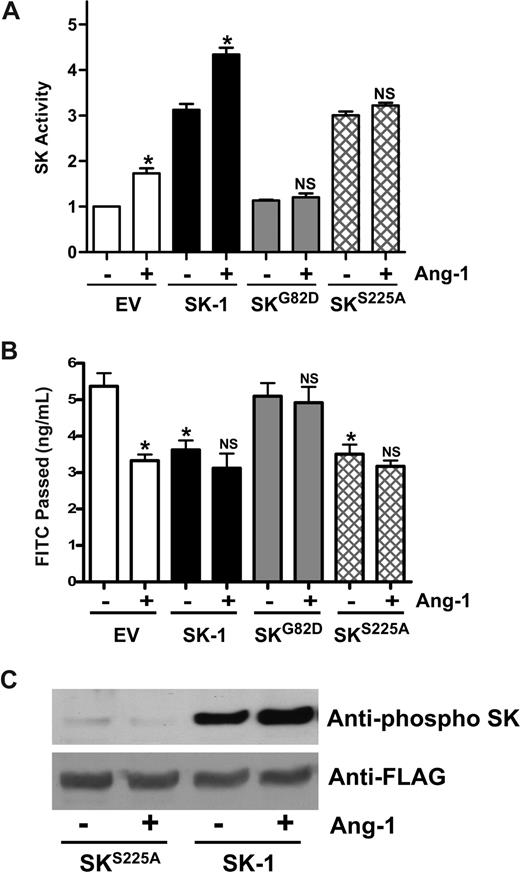
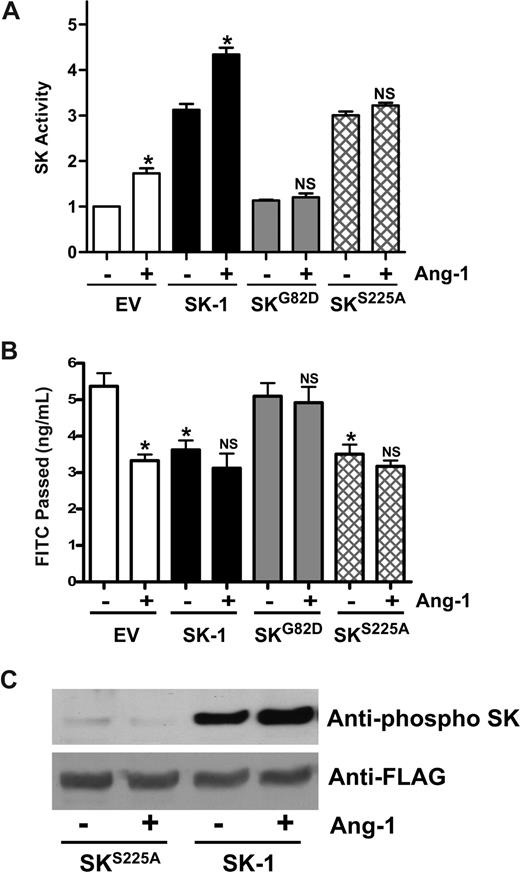
SK-1 possesses both intrinsic catalytic activity and activity that can be activated. We have previously used 2 mutants to dissect out these activities. The first is a catalytically inactive version of SK-1, SKG82D, which acts in a dominant-negative manner to specifically inhibit SK-1 activation without altering basal SK-1 activity.22 HUVECs expressing the SKG82D mutant show no detectable change in basal SK-1 activity, although overexpression of WT SK-1 results in a 2- to 3-fold increase in activity (Figure 7A). Ang-1 was able to promote SK-1 activity in both control and WT SK-1–transfected cells but failed to affect SK-1 activity levels in SKG82D-transfected cells (Figure 7A), WT SK-1–transfected cells inhibited permeability and Ang-1 treatment failed to exert any further inhibition. The SKG82D-transfected cells showed no change in basal permeability compared with control cells and failed to respond to Ang-1 (Figure 7B). Activation of SK-1 in response to TNF and PMA occurs via ERK1/2-mediated phosphorylation of SK-1 at serine 225.23 Mutation of this serine results in a nonphosphorylatable protein that, although having full intrinsic catalytic activity, cannot be activated and fails to translocate to the plasma membrane in an agonist-dependent manner.23 In HUVECs, expression of the SKS225A mutant increased the cellular SK-1 activity (Figure 7A), similar to the increases seen in other cell types.23,34 Consistent with this enhanced cellular SK-1 activity, permeability was decreased in these SKS225A-transfected cells (Figure 7B). However, Ang-1 did not increase SK-1 activity in SKS225A-transfected cells (Figure 7A) and did not inhibit further their permeability (Figure 7B), consistent with the inability of this mutant to be phosphorylated in response to Ang-1 (Figure 7C). Taken together, these results show that the Ang-1 inhibition of EC permeability is mediated in part through SK-1 and requires the activation of SK-1.
Mutation of SK-1 blocks the Ang-1 effects on permeability change. (A) HUVECs were infected with adenoviral carrying EV, SK-1, SKG82D, or SKS225A. After 48 hours, SK-1 activity was measured from cells that were either untreated (−) or treated with Ang-1 (+). Pooled data from 3 experiments are shown, expressed as the fold change in relation to EV untreated cells, normalized to 1.0 (*P < .01 versus untreated EV-infected cells or untreated SK-1-infected cells). NS indicates no significant difference versus untreated SKG82D or untreated SKS225A cells. (B) HUVECs were infected with EV, SK-1, SKG82D, or SKS225A in adenovirus. After 72 hours, cells were either untreated (−) or treated with Ang-1 (+) for 30 minutes and permeability measured. Permeability is given as the FITC-dextran passage in 30 minutes. Shown are the pooled data from 3 experiments (*P < .01 vs nontreated EV-infected cells). NS indicates no significant difference versus untreated SK-1, SKG82D, or untreated SKS225A-infected cells. All data are mean plus or minus SEM. (C) HUVECs were infected with SK-1 or SKS225A in adenovirus. Forty-eight hours after infection, cells were lysed after either no treatment (−) or treatment with Ang-1 (+). Phospho-SK-1 is shown in the top and total SK-1 in the bottom panel.
Mutation of SK-1 blocks the Ang-1 effects on permeability change. (A) HUVECs were infected with adenoviral carrying EV, SK-1, SKG82D, or SKS225A. After 48 hours, SK-1 activity was measured from cells that were either untreated (−) or treated with Ang-1 (+). Pooled data from 3 experiments are shown, expressed as the fold change in relation to EV untreated cells, normalized to 1.0 (*P < .01 versus untreated EV-infected cells or untreated SK-1-infected cells). NS indicates no significant difference versus untreated SKG82D or untreated SKS225A cells. (B) HUVECs were infected with EV, SK-1, SKG82D, or SKS225A in adenovirus. After 72 hours, cells were either untreated (−) or treated with Ang-1 (+) for 30 minutes and permeability measured. Permeability is given as the FITC-dextran passage in 30 minutes. Shown are the pooled data from 3 experiments (*P < .01 vs nontreated EV-infected cells). NS indicates no significant difference versus untreated SK-1, SKG82D, or untreated SKS225A-infected cells. All data are mean plus or minus SEM. (C) HUVECs were infected with SK-1 or SKS225A in adenovirus. Forty-eight hours after infection, cells were lysed after either no treatment (−) or treatment with Ang-1 (+). Phospho-SK-1 is shown in the top and total SK-1 in the bottom panel.
Discussion
This study has demonstrated distinct and critical roles for 2 important regulators of endothelial cell function in the control of vascular permeability. First, we show that basal levels of SK-1 are, in part, responsible for the impermeable basal state of ECs and the vascular system in vivo. Second, we define a novel signaling pathway that mediates the antipermeability effects of Ang-1, involving the activation of SK-1.
The SK-1 enzyme has 2 functional states. One is intrinsic catalytic activity independent of post-translational modifications, which is probably responsible for housekeeping roles. The other is agonist-induced activation resulting from phosphorylation of serine 225, which increases its Vmax by 14-fold and is responsible for its oncogenic function.23
Our results here support a new role for the intrinsic activity of SK-1, that of regulation of the basal impermeable nature of ECs. The key experiments supporting this contention were the increased permeability in cells depleted of SK-1 and the inhibition of basal permeability by not only WT SK-1, a molecule that increases basal levels and is also activatable, but also by SKS225A, which only increases basal levels as it is incapable of being activated.23 Importantly, using SK-1 KO and transgenic mice, we also showed that SK-1 is a regulator of permeability in vivo. Our in vitro data thus suggest that the intrinsic catalytic activity of SK-1 is the critical determinant of basal permeability.
Ang-1 is a powerful inhibitor of vascular permeability, as demonstrated in in vitro and in vivo studies.3,5 The pathways downstream of the Ang-1 receptor and Tie2-mediated regulation of permeability involve PLC-IP3 and PKCζ11,12 and, now as shown here, SK-1 activity. Activation of SK-1 by Ang-1 was demonstrated by changes in activation, namely, rapid increases in SK-1 activity levels leading to increases in intracellular S1P and increased SK-1 phosphorylation. Thus, Ang-1 can be added to the growing list of agonists activating the SK-1 pathway, all of which also activate ERK, the kinase involved in SK-1 phosphorylation.23 In ECs, these include inflammatory mediators, such as TNF28 and high glucose concentrations,35 angiogenic agents, such as VEGF,29 and, as we show here, the antipermeability agent, Ang-1. Interestingly, in contrast to Ang-1, VEGF and TNF are inducers of vascular leakage. Thus, it would appear that SK-1 could act as a fulcrum for both inflammatory and anti-inflammatory effects, similar to actions observed for agents, such as transforming growth factor-β. The mechanism whereby the one signaling molecule can mediate such opposing effects is not understood at present but could lie in unique ligand-specific signals being generated downstream of SK-1 activation, perhaps mediated through the ability of SK-1 to interact with multiple binding partners.
Support for the role of SK-1 activation mediating the inhibitory action of Ang-1 on permeability came from experiments using 2 mutants of SK-1. SKG82D is a catalytically inactive mutant that acts in a dominant-negative manner to block activation of SK-1, but not basal (intrinsic) SK-1 activity.22 Overexpression of SKG82D in ECs did not alter basal permeability but inhibited the Ang-1 stimulation of SK-1 activity levels and inhibited the effect of Ang-1 on permeability. SKS225A has full intrinsic catalytic activity but is incapable of ligand-mediated phosphorylation, membrane translocation, and activation.23 The oncogenic signaling potential of SK-1, as demonstrated in in vitro and in vivo experiments, is dependent on phosphorylation-induced translocation of SK-1 to the membrane and not on the increase in catalytic activity of SK-1.34 In ECs overexpressing SKS225A, although there was a decrease in basal permeability, no change in SK-1 activity was elicited by Ang-1 and no decrease in permeability was seen after Ang-1 stimulation. These results are again consistent with the inability of this mutant to be activated by agonists. These results also suggest that, although Ang-1 can target SK-1 to the plasma membrane (Figure S3A,B), this translocation of SK-1 is not essential in mediating basal permeability changes as the SKS225A mutant, which is not able to localize to the membrane,23 does show inhibition of basal permeability. Taken together, these experiments using the 2 SK-1 mutants confirm the involvement of SK-1 in the regulation of EC permeability and demonstrate that Ang-1–mediated changes in permeability require activation of SK-1, which results in an increase in the levels of intracellular S1P. However, the action of Ang-1 to inhibit endothelial cell permeability is independent of exogenous S1P, as knockdown or inhibition of the S1P1-3 receptors ablates the S1P-mediated inhibition while leaving the Ang-1 effects intact. Thus, the effects of Ang-1 are generated intracellularly rather than through the extracellular actions of S1P.
Extracellular S1P inhibits EC permeability through an S1P receptor-dependent mechanism,31 resulting in Rac activation and the strengthening of adherens junctions by increasing the formation of the VE-cadherin and β-catenin complexes,36 and by actions on the tight junction proteins ZO-1 and α-catenin.37 Our results suggest that the SK-1–dependent Ang-1–mediated signaling pathway is not produced through extracellular S1P. First, the Ang-1–mediated responses are independent of the S1P1-3 receptors; and second, alteration in SK-1 levels has no effect on Rac activity, even though we see changes in the actin cytoskeleton (Figure S4). Although we and others have previously reported that Ang-1 is able to regulate many of the pathways involved in permeability changes,11,12 which of these or perhaps other pathways is accessed through the SK-1 signaling cascade remains to be determined.
In summary, we propose that the SK-1 regulation of permeability falls into 2 categories: (1) the control of basal tonic permeability mediated by the intrinsic catalytic activity of SK-1, and (2) that after an activation event. In this case, Ang-1, acting through multiple pathways, including via intracellular SK-1, will act to dampen inflammatory responses, inhibit the action of the stimulators of vascular leakage, and return the vessel to its normal impermeable nature. Thus, the generation of inhibitors of intracellular SK-1 that are being contemplated for diseases, such as cancers and atherosclerosis, will have to take into account the multiple facets of this signaling pathway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jenny Drew and Anna Sapa for excellent technical assistance and the staff at the Women's & Children's Hospital and Burnside War Memorial Hospital for collection of umbilical cords.
This work was supported by grants from the National Health & Medical Research Council of Australia and the National Heart Foundation of Australia. J.R.G. is a Medical Foundation Fellow, University of Sydney.
National Institutes of Health
Authorship
Contribution: X.L. and M.S. performed research; M.P. and C.S.B. performed animal studies; X.L., C.N.H., M.A.V., and J.R.G. designed research; R.L.P. kindly provided SK-1 knockout mice; S.M.P. and P.X. contributed expert guidance; X.L. and J.R.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer R. Gamble, Centenary Institute of Cancer Medicine and Cell Biology, Locked Bag #6, Newtown, NSW 2042, Australia; e-mail: j.gamble@centenary.org.au.