Abstract
Inflammatory responses by mast cells are characterized by massive exocytosis of prestored granular mediators followed by cytokine/chemokine release. The vesicular trafficking mechanisms involved remain poorly understood. Vesicular-associated membrane protein-8 (VAMP-8), a member of the soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE) family of fusion proteins initially characterized in endosomal and endosomal-lysosomal fusion, may also function in regulated exocytosis. Here we show that in bone marrow–derived mast cells (BMMCs) VAMP-8 partially colocalized with secretory granules and redistributed upon stimulation. This was associated with increased SNARE complex formation with the target t-SNAREs, SNAP-23 and syntaxin-4. VAMP-8–deficient BMMCs exhibited a markedly reduced degranulation response after IgE+ antigen-, thapsigargin-, or ionomycin-induced stimulation. VAMP-8–deficient mice also showed reduced plasma histamine levels in passive systemic anaphylaxis experiments, while cytokine/chemokine release was not affected. Unprocessed TNF accumulated at the plasma membrane where it colocalized with a VAMP-3–positive vesicular compartment but not with VAMP-8. The findings demonstrate that VAMP-8 segregates secretory lysosomal granule exocytosis in mast cells from cytokine/chemokine molecular trafficking pathways.
Introduction
Mast cells are granulated cells of hematopoietic origin localized to tissues that play a role in innate and adaptive defense to pathogens as well as in various inflammatory and immunoregulatory responses. They are also central effectors in anaphylaxis, allergy, and asthma.1-3 These functions depend on the release of proinflammatory mediators following activation through cell surface receptors such as the high-affinity IgE receptor (FcϵRI).4 The mast cell inflammatory response is characterized by an early phase with massive discharge of mediators stored in cytoplasmic secretory granules (SGs) through multigranular/compound exocytosis and a late phase that involves generation of arachidonic acid metabolites and de novo synthesis of cytokines/chemokines and growth factors released through vesicular carriers.2-4
The signaling events required for degranulation and cytokine/chemokine secretion, although involving common initial elements, are distinct and depend on particular regulatory requirements of each pathway. In agreement, deficiency in Bcl10 or Malt1, a signaling complex upstream of NF-κB, affected cytokine production but not mast cell degranulation.5 Both processes are also likely regulated at the level of intracellular trafficking and fusion. Cytokine release is still poorly characterized, but involves vesicular carriers along the secretory pathway. In macrophages, it has been shown that these vesicles fuse with recycling endosomes to discharge at specific sites at the phagocytic cup.6,7
Mast cell SGs are secretory lysosomes with an intimate connection between the secretory and endocytic pathway, as in other hematopoietic cells.8-11 The protein sorting to these compartments is regulated by synaptotagmins9,12 as well as Munc13-4 and rab27a as shown in cytotoxic T cells.11 Furthermore, constitution of an appropriate granular compartment and proper loading of prestored inflammatory mediators depend partly on proteoglycans expression. Genetic targeting of the heparin biosynthesis pathway or the serglycin core in mice revealed severe defects in SG maturation.13-15 Release from SGs in mast cells involves soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE) membrane fusion proteins. These are divided into vesicular or v-SNAREs including vesicular-associated membrane protein (VAMP) family members and target or t-SNAREs including syntaxins and soluble NSF attachment proteins (SNAP) family members. SNAREs contain an approximately 60-aa α-helical SNARE motif, 4 of which combine to form a stable tetrameric core complex that catalyzes membrane fusion.16 Previous studies demonstrated that mast cells use the t-SNAREs SNAP-23 and syntaxin-4 in IgE-dependent degranulation.17-19 Several VAMP proteins are expressed in mast cells including VAMP-2, VAMP-3, VAMP-7, and VAMP-8.18,20 The latter showed significant colocalization with SGs, while for the others colocalization was minor. However, evidence for the function of v-SNAREs in mast cell degranulation is limited. Similarly, data about involvement of SNARE proteins in cytokine secretion are nonexistent, with the exception that prestored TNF may be transported into this compartment after re-endocytosis of newly synthesized protein21 or involve an N-linked glycosylation-dependent sorting mechanism.22 In macrophages, TNF secretion involves a vesicular compartment containing syntaxin-6, syntaxin-7, and Vti1b (vesicle transport through interaction with t-SNARE homologue 1b) that fuses with VAMP-3–positive recycling endosomes to mediate specific delivery at the phagocytotic cup.6
The v-SNARE VAMP-8, also known as endobrevin, is expressed in many organs and tissues including lungs, kidney, heart, and salivary glands,23 but not in neurons.24 It was initially characterized as a v-SNARE involved in homotypic fusion of early and late endosomes24,25 and in the heterotypic fusion between late endosomes and lysosomes.26 More recently, it was demonstrated to play a role in regulated exocytosis in the pancreas and other glands of the exocrine system23,27 as well as in platelets.28 Based on previous data showing that mast cell secretory lysosomes contain VAMP-8,18 we examined whether this v-SNARE protein plays a role in mast cell degranulation and cytokine secretion by analyzing cells from VAMP-8–deficient mice. Our findings demonstrate that VAMP-8 is part of a trafficking pathway that distinguishes degranulation from cytokine release.
Methods
Reagents and antibodies
Rabbit Abs to syntaxin-2, syntaxin-3, syntaxin-4, SNAP-23, VAMP-4, VAMP-8, and Munc18-2 have been described.18,29,30 Mouse monoclonal anti–DNP-IgE18 was used either as ascites or after affinity purification. Mouse monoclonal VAMP-2 (clone Cl 69.1), rabbit SNAP-23 antibodies, and rabbit anti–VAMP-3 (for biochemistry) were from Synaptic Systems (Göttingen, Germany). Rabbit anti–VAMP-3 (for confocal imaging) was from Novus Biologicals (Littleton, CO). Monoclonal mouse antiserotonin (clone 5HT-H209) was from Dako (Glostrup, Denmark). Rat monoclonal antimouse mMCP-6 (tryptase) and rat monoclonal antiserotonin (cloneYC5/45) were purchased from R&D Systems (Lille, France) and AbDSerotec (Oxford, United Kingdom), respectively. Mouse anti–β-actin mAb and p-nitrophenyl N-acetyl-β-d-glucosaminide were from Sigma-Aldrich (Saint-Quentin Fallavier, France). Hamster antimouse FcϵRI, rat antimouse c-kit-biotin, SA-PE, and rat antimouse mAb TNF coupled to Cy5 antibodies were from eBioscience (San Diego, CA). Antirabbit alexa 488, antimouse alexa 568, and SA-647 were from Molecular Probes (Eugene, OR). Goat anti–rat IgG:Dylight549 (mouse adsorbed) was obtained from AbDSerotec. IL-3 and SCF were purchased from Peprotech (Levallois, France). Murine IL-1β was purchased from ProSpec Tany Technogene (Rehovot, Israel). Murine cytokines/chemokine detection Duoset enzyme-linked immunosorbent assay (ELISA) kits (IL-4, IL-6, TNF, and MIP-1α) were purchased from R&D Systems. The TNF-alpha–converting enzyme (TACE) inhibitor TAPI-1, ionomycin, thapsigargin, and PMA were all from Calbiochem (La Jolla, CA).
Mice
The generation of VAMP-8–deficient mice was reported previously.27 Mice were housed under specific pathogen-free conditions at Biological Resource Center (BRC), Institute of Molecular and Cell Biology, Singapore. Mice were maintained in the 129/SvJ background and genotyping was performed as before. All experiments were done in accordance with national guidelines and approved by an institutional ethics committee.
Histology
Tongue, abdominal skin, and ear tissues from 3 sets of WT and VAMP-8–deficient mice were collected and fixed in 10% formalin. After paraffin embedding, 5-μm sections were cut and stained with toluidine blue. Metachromatically stained mast cells were enumerated by counting 5 high-power fields (40×) per section using a Leica DFC320 microscope (Wien, Austria). Mast cells were also obtained from peritoneal cavity and were stained using toluidine blue.
Mast cell isolation and culture
Bone marrow cells isolated from the femurs of VAMP-8–deficient mice were washed in complete IMDM medium containing 15% FCS, 25 mM HEPES (pH 7.4), 1 mM sodium pyruvate, 1% nonessential amino acid, 54 μM β-mercaptoethanol with 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Frederick, MD), and were then cultured in complete medium containing 10 ng/mL IL-3 and 10 ng/mL SCF for 4 to 8 weeks to obtain bone marrow–derived mast cells (BMMCs). Every 5 days, medium was replaced. All cell cultures were grown at 37°C in a humidified atmosphere with 5% CO2. Rat peritoneal mast cells (RPMCs) were obtained by peritoneal lavage with 50 mL ice-cold PBS/0.1% BSA using Wistar male or female rats purchased from the animal facility of the University of Trieste. Rats were killed using CO2 inhalation. The cell suspension was centrifuged at 200g for 8 minutes at 4°C, and mast cells were purified over a one-step Percoll/0.1% BSA gradient (density at 20°C, 0.883 g/mL). Final population of mast cells in pellet was more than 98%.
Flow cytometric analysis of c-kit and FcϵRI expression
BMMCs were washed with ice-cold PBS, and indirect immunofluorescence staining was performed with rat antimouse c-kit-biotin followed by SA-PE and hamster antimouse FcϵRI followed by antihamster biotin and SA-PE.
Passive systemic anaphylaxis experiments
Mice were sensitized by intravenous injection using 30 μg purified anti-DNP IgE.18 After 24 hours they were challenged intravenously with 100 μg DNP-HSA (Sigma-Aldrich) in 200 μL PBS for 2 minutes, and blood was collected in ice-cold 5-μL heparin-containing tubes. Serum histamine concentration was determined using a histamine immunoassay kit (Beckman Coulter, Marseille, France) according to the manufacturer's instructions.
Degranulation measurements
Release of prestored granular mediators was determined by measuring the release of β-hexosaminidase or histamine.18 Briefly, 4- to 6-week-old BMMCs (2 × 106/mL) were sensitized overnight with anti-DNP IgE in IL-3 and SCF-containing complete medium. To measure release, sensitized cells were washed 2 times with Tyrode buffer and stimulated with indicated concentrations of DNP-HSA, ionomycin/PMA, or thapsigargin for indicated times. Following stimulations, cells were placed on ice for 10 minutes and centrifuged at 300g for 10 minutes at 4°C. The enzymatic activities of β-hexosaminidase in supernatants and in cells solubilized with 0.5% Triton X-100 were determined as described earlier.18 Histamine concentration was determined as stated under “Passive systemic anaphylaxis experiments.”
Determination of cytokine production
BMMCs were sensitized with anti-DNP IgE overnight. After washing, BMMCs were resuspended in growth factor containing complete medium at 106 cells/mL in 24-well tissue culture plates. Cells were stimulated with DNP-HSA (10 ng/mL) over different time points at 37°C. Supernatants were collected and TNF, IL-6, IL-4, or MIP-1α was quantified using Duoset cytokine ELISA kits according to the manufacturer's instructions. MIP-1α secretion was also determined after stimulation with ionomycin (1 μM)/PMA (20 nM) or IL-1β (25 ng/mL).
Immunoblotting and immunoprecipitation
Two million cells (in 1 mL complete growth factor–containing medium) were sensitized overnight with anti-DNP IgE. After washing, cells were resuspended in Tyrode buffer and challenged with DNP-HSA (10 ng/mL). Stimulation was arrested using ice-cold PBS. Cell lysates were prepared in 50 mM HEPES (pH 7.2) containing 1% Triton X-100, 0.1% SDS, 50 mM NaCl, 50 mM NaF, 1 mM sodium orthovanadate, and protease inhibitors aprotinin 1000 U/mL (Sigma-Aldrich), pepstatin 10 μg/mL, leupeptin 20 μg/mL, and AEBSF 2 μM (Alexis, Carlsbad, CA). For immunoprecipitation, cells were lysed in buffer containing 0.5% Triton X-100 and 0.5% octylglucoside for 30 minutes at 4°C. In some experiments, cells were also treated with 1 mM N-ethylmaleimide (NEM) before lysis as described.18 Proteins were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) or PVDF (Sigma-Aldrich). Membranes were blocked with 4% BSA for 1 hour followed by incubation with primary antibodies (1 hour at room temperature [RT]). After several washes, blots were incubated with donkey anti–rabbit IgG HRP (1/30 000) or goat anti–mouse IgG HRP (1/20 000) (Jackson Immunoresearch, Newmarket, United Kingdom) or protein A HRP (1/40 000) (Sigma-Aldrich) for 45 minutes and were developed by enhanced chemiluminescence (ECL; GE, Paris, France). Quantification analysis of coimmunoprecipitated complexes was performed using Image J software (National Institutes of Health, Bethesda, MD).
Confocal microscopy
For confocal immunofluorescence microscopy, BMMCs were seeded in 24-well plates on coverslips coated with 0.025% (wt/vol) poly-l-lysine (Sigma) for 4 hours at 37°C in a humidified atmosphere with 5% CO2. After stimulation, cells were washed twice with ice-cold PBS and fixed for 20 minutes on ice in 10 mM PIPES (pH 6.8), 150 mM NaCl, 5 mM EGTA, 5 mM MgCl2, 5 mM glucose containing 4% paraformaldehyde (IF buffer) followed by 2 washes in IF buffer for 2 minutes. Fixed cells were permeabilized in IF buffer containing 0.025% saponin for 20 minutes at room temperature, followed by blocking in IF buffer containing 0.012% saponin and 7% horse serum (Invitrogen) for 30 minutes at RT. Staining with primary antibodies was performed in IF buffer containing 0.012% saponin and 5% horse serum either overnight at 4°C or for 2 hours at RT followed by incubation with secondary antibodies for 60 minutes at RT. After washing, cells were mounted in Prolong-Gold antifading reagent (Molecular Probes) and were analyzed using confocal laser-scanning microscope LSM 510 (Zeiss, Oberkochen, Germany). Images were taken using a 63× oil-immersion objective lens. Quantitative analysis of the degree of the colocalization/overlay was measured using Carl Zeiss LSM 510 Image examiner Software between 2 channels.
Electron microscopy
BMMCs were fixed in 1.5% glutaraldehyde (Serva, Heidelberg, Germany), diluted in 0.1 M cacodylate buffer (pH 7.4), stored for 20 minutes at room temperature, postfixed in 1% OsO4 for 60 minutes at 4°C, dehydrated in ethanol, and finally embedded in Dow epoxy resin (DER 332; Unione Chimica Europea, Milano, Italy). For double immunogold labeling of RPMCs, ultrathin sections, cut by an ultramicrotome (Ultracut UCT; Leica), were mounted on nickel grids etched for 1 minute with 1% periodic acid and rinsed in distilled water. Grids with the section sides facing downward were incubated in 20 mM Tris-HCl (pH 8.2), containing 2% BSA, 1% goat serum, 0.05% Tween-20, 0.1% Triton X-100, and exposed overnight at 4°C to rabbit anti–VAMP-8 diluted 1:20 in the same buffer. Grids were washed in 20 mM Tris-HCl (pH 8.2) containing 225 mM NaCl, 2 mM NaN3, 0.05% Tween-20, 0.5% BSA, 0.1% Triton X-100, and 0.5% goat serum and were incubated thereafter for 1 hour at room temperature with 10 nm gold-conjugated goat antirabbit (British Biocell International, Cardiff, United Kingdom) diluted 1:50 in Tris-HCl-BSA-Triton. Grids were rinsed, turned over with the section sides facing upward, according to Bendayan,31 and exposed to undiluted rabbit antiserotonin. The procedure followed thereafter was as described with the exception that 20 nm gold-conjugated protein A-G (British Biocell International) diluted 1:50 in Tris-HCl-BSA-Triton was used as revealing system. Sections were analyzed by transmission electron microscope (EM208; Philips, Eindhoven, The Netherlands). Micrographs were taken with a Morada camera (Olympus Soft Imaging Solutions, Muenster, Germany). Control experiments using normal rabbit and mouse IgG were performed in parallel. Background of gold particles found on the granule surface was 2.8% and within the granule matrix was 4.8% (SGs counted: n = 426 and 602 for specific and control staining, respectively)
Statistical analysis
Statistical analysis was performed with Origin Pro 7.5 Software (OriginLab, Northampton, MA). For in vivo data, a paired Student t test was used. For comparing responses of the mutant cells, a one-way Anova test was used, as indicated.
Results
Mast cell development is unaffected in VAMP-8–deficient mice
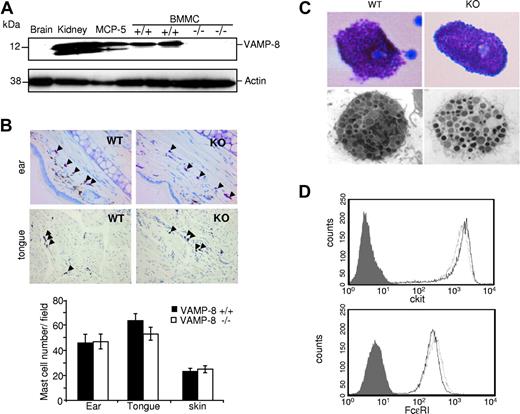
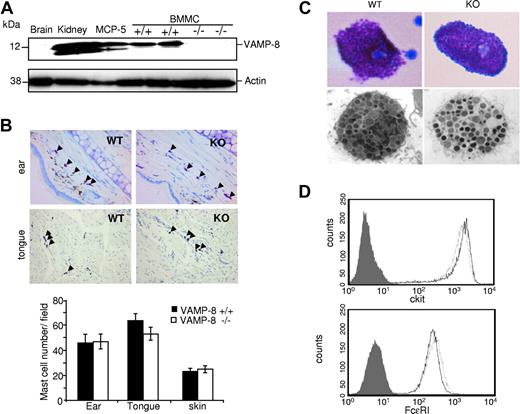
The role of VAMP-8 in mast cell secretory events was examined using VAMP-8–deficient mice generated via a gene knockout approach.27 We first verified the absence and presence of VAMP-8 protein in BMMCs derived from VAMP-8–deficient and WT mice, respectively. Figure 1A shows that VAMP-8 is highly expressed in WT BMMCs like in the MCP-5 murine mast cell line and in kidney extracts used as a control,23 while VAMP-8–deficient BMMCs lack expression as in brain.24 We also examined whether deficiency of VAMP-8 affects mast cell development and tissue distribution. Analysis of toluidine blue–stained sections from ear, skin, and tongue revealed no significant differences in mast cell numbers between WT and VAMP-8–deficient mice (Figure 1B). Light and electron microscopy examination of cell morphology and granular appearance did not show any differences in mast cells isolated from the peritoneum (Figure 1C). In cultured BMMCs, we also did not find any evidence of gross morphologic alterations as well as differences in the number of intracellular granules, although WT BMMCs contain a slight but significantly higher proportion of immature granules containing no or little electron-dense material (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; Table 1). BMMCs did, however, contain similar amounts of intragranular histamine on a per cell basis (32.6 ± 3.4 pM/cell and 29.1 ± 5.3 pM/cell) in WT versus VAMP-8–deficient cells and they expressed similar levels of the mast cell–specific surface markers c-kit and FcϵRI (Figure 1D).
Development of mast cells is not affected in VAMP-8–deficient mice. (A) VAMP-8 expression levels in tissue homogenates of brain, kidney, murine MCP-5 mast cells, and VAMP-8–deficient and WT BMMCs using anti–VAMP-8. Blots were stripped and reprobed with antiactin. (B) Representative toluidine blue staining of ear and tongue cross-sections (top panel) showing the distribution of tissue mast cells (arrowheads) in VAMP-8–deficient and WT mice (40× objective). (Bottom panel) Corresponding quantitative enumeration of mast cells in ear, tongue, and abdominal skin sections (3 mice/genotype). Data are means plus or minus SEM derived from 5 high-power fields counted/mouse. No significant difference among VAMP-8–deficient and WT mice was observed. (C) Representative toluidine blue staining (top panel) and ultrastructural analysis (bottom panel) by electron microscopy (original magnification ×13 500) of mouse peritoneal mast cells from VAMP-8–deficient and WT mice. (D) Expression of FcϵRI and c-kit on BMMCs from VAMP-8–deficient and WT mice. Continuous and dotted lines represent VAMP-8–deficient and WT BMMCs, respectively, compared with isotype control (dark area).
Development of mast cells is not affected in VAMP-8–deficient mice. (A) VAMP-8 expression levels in tissue homogenates of brain, kidney, murine MCP-5 mast cells, and VAMP-8–deficient and WT BMMCs using anti–VAMP-8. Blots were stripped and reprobed with antiactin. (B) Representative toluidine blue staining of ear and tongue cross-sections (top panel) showing the distribution of tissue mast cells (arrowheads) in VAMP-8–deficient and WT mice (40× objective). (Bottom panel) Corresponding quantitative enumeration of mast cells in ear, tongue, and abdominal skin sections (3 mice/genotype). Data are means plus or minus SEM derived from 5 high-power fields counted/mouse. No significant difference among VAMP-8–deficient and WT mice was observed. (C) Representative toluidine blue staining (top panel) and ultrastructural analysis (bottom panel) by electron microscopy (original magnification ×13 500) of mouse peritoneal mast cells from VAMP-8–deficient and WT mice. (D) Expression of FcϵRI and c-kit on BMMCs from VAMP-8–deficient and WT mice. Continuous and dotted lines represent VAMP-8–deficient and WT BMMCs, respectively, compared with isotype control (dark area).
VAMP-8–deficient mast cells show impaired degranulation responses in vitro and in vivo
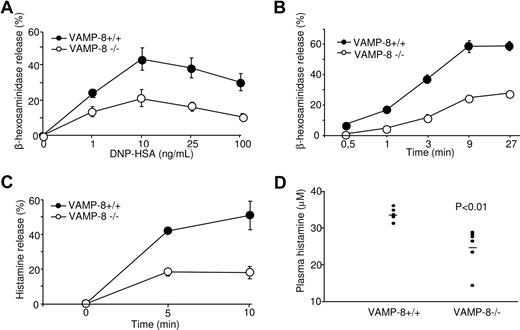
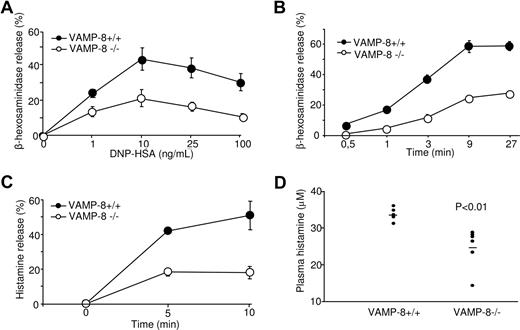
We have reported earlier that in the RBL mast cell line VAMP-8 colocalized with SGs.18 This suggested that VAMP-8 might be involved in the fusion events during degranulation. To examine this issue directly, we measured release of the granular-stored enzyme β-hexosaminidase in VAMP-8–deficient BMMCs after stimulation through FcϵRI. Cells were sensitized with anti-DNP IgE and subsequently stimulated with DNP-HSA in dose response (Figure 2A) and kinetic (Figure 2B) experiments. Release of β-hexosaminidase was optimal at a dose of antigen around 10 ng/mL, reaching maximal levels by 9 minutes. Relative to WT BMMCs, the degranulation of VAMP-8–deficient BMMCs was markedly inhibited. We also determined release of histamine, a major mediator of allergic inflammation and vasodilation that is specifically stored in mast cell SGs.2,4 Figure 2C shows that similar to β-hexosaminidase, the kinetics of histamine release was decreased in VAMP-8–deficient BMMCs compared with WT BMMCs. These data indicated that VAMP-8–deficient mast cells show a marked deficit in their capacity to release prestored mediators from their SGs.
Defective degranulation and passive systemic anaphylactic responses in VAMP-8–deficient BMMCs and mice. VAMP-8–deficient and WT BMMCs sensitized overnight with anti-DNP IgE were stimulated for 30 minutes with the indicated concentrations of DNP-HSA (A) or for indicated time points using 10 ng/mL (B), and release of β-hexosaminidase was determined. (C) VAMP-8–deficient and WT BMMCs were sensitized with IgE before stimulation with 10 ng/mL DNP-HSA and release of histamine was determined. Data (percentage release ± SEM) are from 3 individual experiments and are representative of 4, 5, and 2 in panels A, B, and C, respectively. All differences were significant (P < .01) and were determined by one-way ANOVA. (D) In vivo passive systemic anaphylactic challenge of VAMP-8–deficient and WT mice. Mice (n = 7) were passively sensitized intravenously with 30 μg purified IgE-anti-DNP and were challenged 24 hours later with 100 μg DNP-HSA. After 2 minutes, blood plasma was collected and plasma histamine concentrations were determined by ELISA. Horizontal bars represent the means.
Defective degranulation and passive systemic anaphylactic responses in VAMP-8–deficient BMMCs and mice. VAMP-8–deficient and WT BMMCs sensitized overnight with anti-DNP IgE were stimulated for 30 minutes with the indicated concentrations of DNP-HSA (A) or for indicated time points using 10 ng/mL (B), and release of β-hexosaminidase was determined. (C) VAMP-8–deficient and WT BMMCs were sensitized with IgE before stimulation with 10 ng/mL DNP-HSA and release of histamine was determined. Data (percentage release ± SEM) are from 3 individual experiments and are representative of 4, 5, and 2 in panels A, B, and C, respectively. All differences were significant (P < .01) and were determined by one-way ANOVA. (D) In vivo passive systemic anaphylactic challenge of VAMP-8–deficient and WT mice. Mice (n = 7) were passively sensitized intravenously with 30 μg purified IgE-anti-DNP and were challenged 24 hours later with 100 μg DNP-HSA. After 2 minutes, blood plasma was collected and plasma histamine concentrations were determined by ELISA. Horizontal bars represent the means.
To test the impact of VAMP-8 deficiency on the allergic response, in vivo, we analyzed the responsiveness of WT and VAMP-8–deficient mice in a passive systemic anaphylaxis experiment. Passive systemic anaphylaxis by IgE antibodies largely depends on mast cells, which rapidly release histamine and serotonin resulting in locally increased blood vessel permeability.2,32,33 Mice were primed by intravenous injection of purified monoclonal anti-DNP IgE antibody, and 24 hours later animals were challenged intravenously with DNP-HSA. The level of blood plasma histamine was determined 2 minutes after antigen challenge. VAMP-8–deficient mice showed reduced levels of histamine compared with WT mice (Figure 2D). A similar inhibition was confirmed in another set of experiments where histamine levels were determined 60 minutes after antigen challenge (P < .001, n = 9). No significant amounts of histamine were observed in mice challenged with vehicle (not shown).
VAMP-8–deficient mast cells are not impaired in cytokine/chemokine production
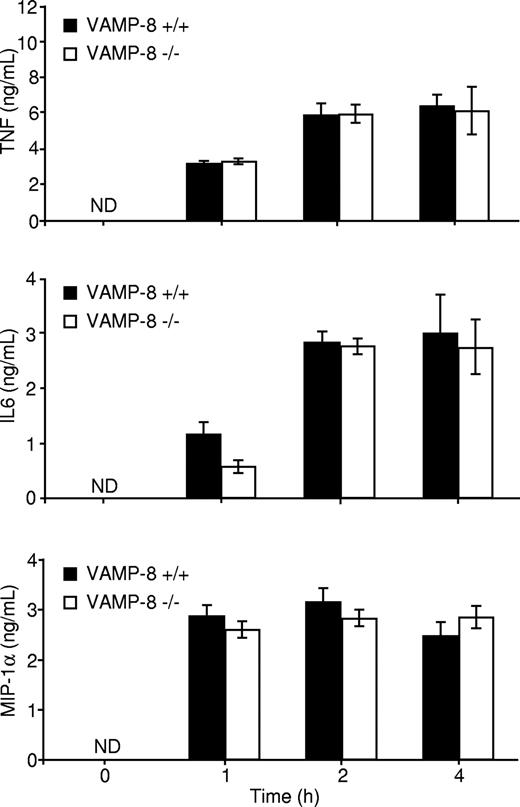
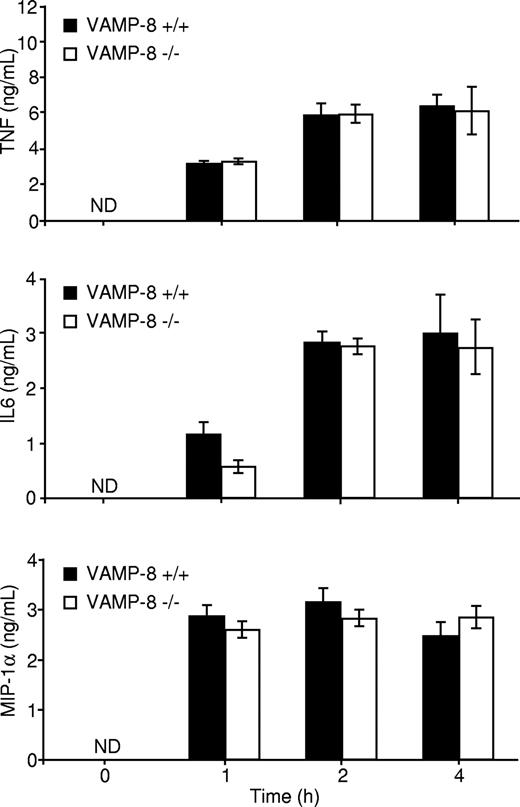
Another consequence of FcϵRI engagement is the production of proinflammatory cytokines such as IL-6, TNF, and the chemokine macrophage inflammatory protein 1α (MIP-1α).2 We analyzed FcϵRI-induced secretion of TNF, IL-6, and MIP-1α into the medium in WT and VAMP-8–deficient BMMCs. Our analysis revealed that no significant amounts of TNF, IL-6, and MIP-1α were released by short-term stimulation (< 30 minutes), indicating that little or undetectable amounts of these cytokines are contained in rapidly mobilized SGs under our culture conditions. We also could not detect cytokine production in the absence of antigen stimulation, demonstrating that the cells did not release spontaneously the cytokines tested within an 8-hour period (not shown). Cross-linking the receptor induced, however, the secretion of significant amounts of TNF, IL-6, and MIP-1α into the culture medium in a time-dependent manner. Maximal secretion was achieved between 1 to 2 hours, and no significant differences in cytokine release were observed between WT and VAMP-8–deficient BMMCs (Figure 3). These BMMCs also released IL-4 upon stimulation, albeit at very low levels (∼ 60 pg/mL), but no differences were seen between WT and VAMP-8–deficient BMMCs (not shown). Therefore, VAMP-8 deficiency impairs FcϵRI-induced degranulation but not cytokine and chemokine secretion.
Cytokine release is not affected in VAMP-8–deficient BMMCs. VAMP-8+/+ and VAMP-8−/− BMMCs were sensitized overnight with anti-DNP IgE and stimulated with 10 ng/mL DNP-HSA for the indicated times. Supernatants were collected, and TNF, IL-6, and MIP-1α protein concentrations were determined by ELISA. Data are means plus or minus SEM from triplicate samples derived from 3 different BMMC cultures and are representative of 5 independent experiments.
Cytokine release is not affected in VAMP-8–deficient BMMCs. VAMP-8+/+ and VAMP-8−/− BMMCs were sensitized overnight with anti-DNP IgE and stimulated with 10 ng/mL DNP-HSA for the indicated times. Supernatants were collected, and TNF, IL-6, and MIP-1α protein concentrations were determined by ELISA. Data are means plus or minus SEM from triplicate samples derived from 3 different BMMC cultures and are representative of 5 independent experiments.
Role of VAMP-8 in membrane fusion
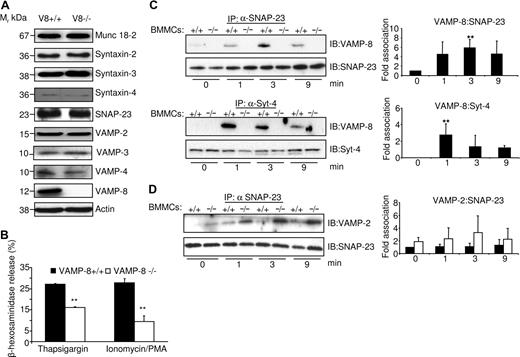
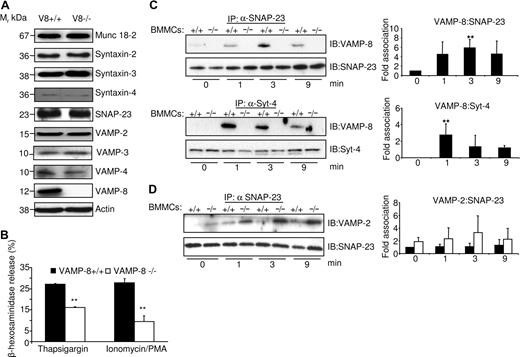
To further examine the role of VAMP-8 in the molecular events leading to mast cell degranulation, we explored whether deficiency of VAMP-8 alters the expression of other SNAREs that could be involved in granule fusion. Figure 4A shows that no significant differences were observed for several membrane trafficking proteins including syntaxin-2, syntaxin-3, syntaxin-4, SNAP-23, VAMP-2, VAMP-3, VAMP-4, and the accessory protein Munc18-2.
VAMP-8 acts at a late fusion step in the mast cell degranulation response. (A) The levels of SNARE and SNARE-related proteins in VAMP-8–deficient and WT BMMCs were analyzed. An equivalent of 2 × 105 BMMCs per lane was migrated on SDS-PAGE and analyzed by Western blotting using indicated specific Abs. (B) VAMP-8–deficient and WT BMMCs were stimulated with either thapsigargin (1 μM) or ionomycin/PMA (500 nM/20 nM) for 10 minutes, and percentage release of β-hexosaminidase was determined. Data are means plus or minus SEM from triplicate samples derived from 3 different BMMC cultures and are representative of 3 independent experiments. **P < .05 by one-way ANOVA. (C) Enhanced association of SNAP-23 and syntaxin-4 with VAMP-8 after stimulation. IgE-sensitized indicated BMMCs (107) were either left unstimulated (NS) or were stimulated (S) with 10 ng/mL DNP-HSA for indicated times followed by NEM treatment as described in “Immunoblotting and immunoprecipitation.” Immunoprecipitation was performed with indicated Abs followed by immunoblotting with anti–VAMP-8. Blots were then stripped and reprobed with Abs to either syntaxin-4 or SNAP-23 as indicated. Corresponding quantitative analysis is shown to the right (n = 3, **P < .05). (D) VAMP-8–deficient BMMCs show a tendency to increased complex formation with SNAP-23 in stimulated BMMCs. IgE-sensitized indicated BMMCs (107) were treated as described in panel C. Immunoprecipitation was performed with SNAP-23 Abs followed by immunoblotting with anti–VAMP-2. For loading control, blots were cut into 2 parts and probed with SNAP-23 as indicated. Corresponding quantitative analysis is shown to the right (n = 3).
VAMP-8 acts at a late fusion step in the mast cell degranulation response. (A) The levels of SNARE and SNARE-related proteins in VAMP-8–deficient and WT BMMCs were analyzed. An equivalent of 2 × 105 BMMCs per lane was migrated on SDS-PAGE and analyzed by Western blotting using indicated specific Abs. (B) VAMP-8–deficient and WT BMMCs were stimulated with either thapsigargin (1 μM) or ionomycin/PMA (500 nM/20 nM) for 10 minutes, and percentage release of β-hexosaminidase was determined. Data are means plus or minus SEM from triplicate samples derived from 3 different BMMC cultures and are representative of 3 independent experiments. **P < .05 by one-way ANOVA. (C) Enhanced association of SNAP-23 and syntaxin-4 with VAMP-8 after stimulation. IgE-sensitized indicated BMMCs (107) were either left unstimulated (NS) or were stimulated (S) with 10 ng/mL DNP-HSA for indicated times followed by NEM treatment as described in “Immunoblotting and immunoprecipitation.” Immunoprecipitation was performed with indicated Abs followed by immunoblotting with anti–VAMP-8. Blots were then stripped and reprobed with Abs to either syntaxin-4 or SNAP-23 as indicated. Corresponding quantitative analysis is shown to the right (n = 3, **P < .05). (D) VAMP-8–deficient BMMCs show a tendency to increased complex formation with SNAP-23 in stimulated BMMCs. IgE-sensitized indicated BMMCs (107) were treated as described in panel C. Immunoprecipitation was performed with SNAP-23 Abs followed by immunoblotting with anti–VAMP-2. For loading control, blots were cut into 2 parts and probed with SNAP-23 as indicated. Corresponding quantitative analysis is shown to the right (n = 3).
Given that VAMPs are known to function in the fusion process of SGs, a presumed late step in mast cell activation, we investigated any possible alterations in signaling events upstream of degranulation and cytokine production in WT and VAMP-8–deficient BMMCs. Our results (Figure S2) revealed that early signaling events (phosphorylation of Syk, p38, p42-44ERK, and AKT) were not altered by VAMP-8 deficiency. The results differed, however, when the early receptor-stimulated signaling events were bypassed using PMA/ionomycin or thapsigargin. Figure 4B shows that both stimuli readily mobilized cytoplasmic granules for β-hexosaminidase release in WT cells. In contrast, as was the case for FcϵRI-induced stimulation, release was markedly inhibited in VAMP-8–deficient BMMCs compared with WT cells. Again, no differences were seen when PMA/ionomycin-induced MIP-1α production was examined (not shown). These data clearly demonstrate a role for VAMP-8 in a late step of SG exocytosis of preformed mediators, while early signaling events and other late events such as release of newly synthesized cytokines were not affected.
The collective findings above suggested that FcϵRI stimulation involved a VAMP-8–dependent fusion mechanism and SNARE complex formation during degranulation. To test this directly, we examined whether VAMP-8 formed complexes with other SNARE partners upon cell activation. IgE-sensitized BMMCs were either left unstimulated or were stimulated with DNP-HSA. To stabilize interactions among SNARE proteins, N-ethylmaleimide (NEM), a sulfhydryl-alkylating agent known to inactivate NSF and block SNARE disassembly and mast cell exocytosis, was added after stimulation.18,34 SNAP-23 and syntaxin-4 were then immunoprecipitated and associated VAMP-8 was evaluated by immunoblot analysis. Figure 4C (left panels) shows that little or no VAMP-8 was detected in a complex with SNAP-23 or syntaxin-4 in unstimulated cells. Following stimulation, an increase in the amount of VAMP-8 coprecipitating with SNAP-23 or syntaxin-4 was observed. Quantitative analysis (Figure 4C right panels) showed that complex formation peaked between 1 and 3 minutes, indicating that SNARE complex formation was modulated by stimulation. As expected, no association was observed in VAMP-8–deficient BMMCs (Figure 4C). We also analyzed SNARE complex formation of SNAP-23 with VAMP-2 (Figure 4D). Few SNAP-23–VAMP-2 complexes were observed in unstimulated cells. They did not change significantly in stimulated WT BMMCs, while in VAMP-8–deficient BMMCs they showed a tendency to increase, indicating some possible compensatory effects.
Analysis of intracellular compartments important in degranulation and TNF production
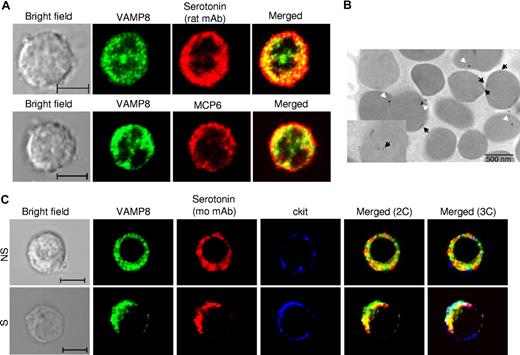
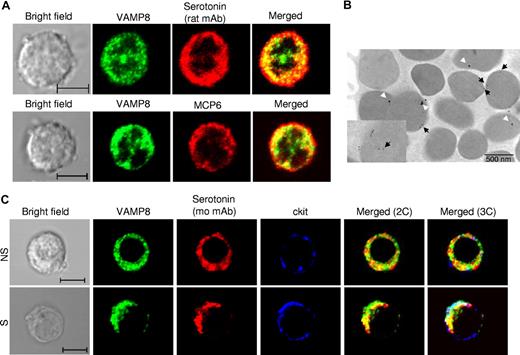
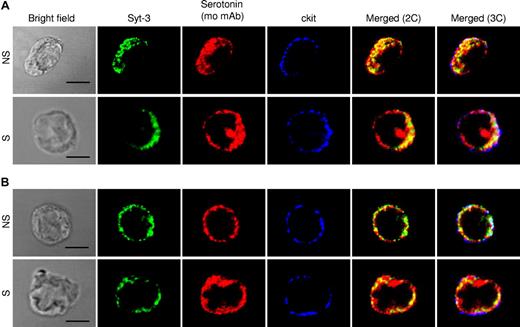
To gain further insight on the role of VAMP-8 in granule mobilization, we analyzed whether VAMP-8 localizes to SGs by assessing the colocalization with the SG marker serotonin in WT BMMCs. Similar to the data obtained in the RBL mast cells,18 a fraction of VAMP-8 protein overlapped with granule sero-tonin (mean ± SD: 34.1% ± 12.1%, rat antiserotonin and 30.3% ± 8.3%, mouse antiserotonin), however, some compartments appeared to have only VAMP-8 or serotonin (Figure 5A top panel). Partial overlap (34.3% ± 11.8%) was also seen with mMCP-6 as an SG marker (Figure 5A bottom panel). We also analyzed VAMP-8 localization at the ultrastructural level by immunoelectron microscopy using RPMCs that have a highly differentiated secretory granule compartment. Only weak staining for serotonin (20 nm GP) was seen with occasionally a detectable GP within secretory granules (Figure 5B). Staining for VAMP-8 (10 nm GP) was somewhat more prominent and was often observed at the surface of secretory granules. Interestingly, VAMP-8 was sometimes also detected inside. Closer inspection (Figure 5B inset) indicated that it might locate to vesicular-like structures resembling multivesicular bodies or remnants derived thereof.35 We next investigated the localization of VAMP-8 in stimulated cells versus unstimulated cells using confocal analysis. In agreement with a multigranular mode of exocytosis,36 our confocal analysis demonstrated that stimulation using calcium ionophore induced a coalescence of VAMP-8 and serotonin-containing vesicular structures with an increase in colocalization and relocation to the cell periphery as can be seen in comparison with the staining with the plasma membrane marker c-kit (Figure 5C and Figure S3A for quantitative analysis). A similar increase in colocalization between VAMP-8 and serotonin as well as VAMP-8 and mMCP-6 was also observed after IgE-dependent stimulation compared with PMA/ionomycin-induced stimulation (Figure S3B,C). The confocal images corresponding to IgE-stimulated cells are shown in Figure S4. As VAMP-8–deficient cells cannot be analyzed by VAMP-8 staining, we examined in parallel WT and VAMP-8–deficient BMMCs using syntaxin-3 as a SG marker. As shown previously,30 in unstimulated cells syntaxin-3 has a uniform granular distribution that colocalized with serotonin in both types of cells and thus may have a role in granule-granule fusion (Figure 6A). Syntaxin-3 showed a somewhat higher colocalization with serotonin in VAMP-8–deficient BMMCs. However, in agreement with a defective fusion mechanism, colocalization of syntaxin-3 and serotonin did not increase after stimulation with calcium ionophore in VAMP-8–deficient BMMCs, and syntaxin-3 appeared to relocate less extensively to the cell periphery compared with WT cells (Figure 6B; Figure S3D).
Localization of VAMP-8 and SG markers in resting and stimulated WT BMMCs. (A) Colocalization of VAMP-8 with the SG markers serotonin and mMCP-6 was analyzed in BMMCs using rat antiserotonin (top panel) and rat antimouse mMCP-6 (bottom panel) using confocal microscopy. Representative single optical sections and overlay (merge) images are shown. (B) Ultrastructural colocalization analysis of RPMCs using VAMP-8 (10 nm GP, black arrows) and mouse antiserotonin (20 nm GP, white arrows). The inset shows VAMP-8 granule matrix labeling on vesicular-like structures. (C) Colocalization of VAMP-8 with SG marker serotonin was analyzed in unstimulated (NS) and PMA/ionomycin (3 minutes)–stimulated (S) BMMCs. Cells were stained with rabbit anti–VAMP-8 and mouse antiserotonin. For comparison, relative localization to plasma membrane marker c-kit (rat mAb) is shown. Cells were analyzed by confocal microscopy. Representative single optical sections as well as 2-color (2C) and 3-color (3C) overlay images are shown. Compartments showing overlap for 2 colors and 3 colors appear as yellow and white, respectively. Bars represent 5 μm.
Localization of VAMP-8 and SG markers in resting and stimulated WT BMMCs. (A) Colocalization of VAMP-8 with the SG markers serotonin and mMCP-6 was analyzed in BMMCs using rat antiserotonin (top panel) and rat antimouse mMCP-6 (bottom panel) using confocal microscopy. Representative single optical sections and overlay (merge) images are shown. (B) Ultrastructural colocalization analysis of RPMCs using VAMP-8 (10 nm GP, black arrows) and mouse antiserotonin (20 nm GP, white arrows). The inset shows VAMP-8 granule matrix labeling on vesicular-like structures. (C) Colocalization of VAMP-8 with SG marker serotonin was analyzed in unstimulated (NS) and PMA/ionomycin (3 minutes)–stimulated (S) BMMCs. Cells were stained with rabbit anti–VAMP-8 and mouse antiserotonin. For comparison, relative localization to plasma membrane marker c-kit (rat mAb) is shown. Cells were analyzed by confocal microscopy. Representative single optical sections as well as 2-color (2C) and 3-color (3C) overlay images are shown. Compartments showing overlap for 2 colors and 3 colors appear as yellow and white, respectively. Bars represent 5 μm.
Localization of syntaxin-3, serotonin, and c-kit in resting and stimulated WT and VAMP-8–deficient BMMCs. Colocalization of SG marker syntaxin-3 and serotonin was analyzed in nonstimulated (NS) and PMA/ionomycin (3 minutes)–stimulated (S) WT (A) and VAMP-8–deficient (B) BMMCs. Cells were stained with rabbit anti–syntaxin-3 and mouse antiserotonin. For comparison, relative localization to the plasma membrane marker c-kit (rat mAb) is shown. Cells were analyzed by confocal microscopy. Representative single optical sections as well as 2-color (2C) and 3-color (3C) overlay images are shown. Compartments showing overlap for 2 colors and 3 colors appear as yellow and white, respectively. Bars represent 5 μm.
Localization of syntaxin-3, serotonin, and c-kit in resting and stimulated WT and VAMP-8–deficient BMMCs. Colocalization of SG marker syntaxin-3 and serotonin was analyzed in nonstimulated (NS) and PMA/ionomycin (3 minutes)–stimulated (S) WT (A) and VAMP-8–deficient (B) BMMCs. Cells were stained with rabbit anti–syntaxin-3 and mouse antiserotonin. For comparison, relative localization to the plasma membrane marker c-kit (rat mAb) is shown. Cells were analyzed by confocal microscopy. Representative single optical sections as well as 2-color (2C) and 3-color (3C) overlay images are shown. Compartments showing overlap for 2 colors and 3 colors appear as yellow and white, respectively. Bars represent 5 μm.
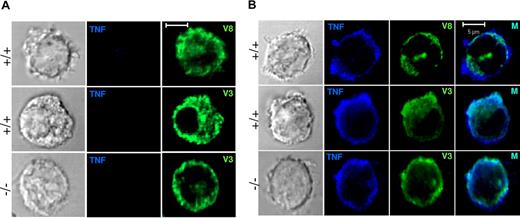
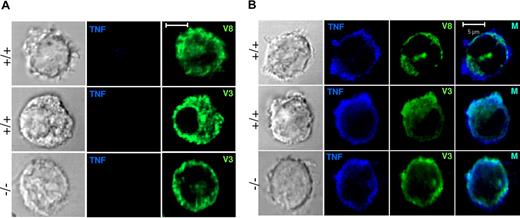
Since no differences in cytokine secretion were found between WT and VAMP-8–deficient cells, we also analyzed trafficking of TNF. In agreement with the absence of release after short-term stimulation, no TNF staining was detectable in unstimulated cells (Figure 7A). To enable detection after stimulation, cells were treated with TNF-alpha–converting enzyme (TACE) inhibitor TAPI-1, which prevents cleavage of the membrane-bound precursor of TNF into the mature secreted form once it has reached the surface. Upon such treatment, significant amounts of TNF accumulated at the cell periphery in cells stimulated for 3 hours with IL-1β (Figure 7B). The latter has been shown to be a potent inducer of cytokine/chemokine release in mast cells in the absence of degranulation.37 By contrast, staining of TNF after stimulation with PMA/ionomycin was very weak (not shown). Analysis of VAMP-8–containing compartments showed that after the 3-hour stimulation period VAMP-8 was largely intracellular and does not substantially redistribute to the periphery in conformity with the absence of degranulation under these stimulation conditions. VAMP-8 also did not considerably colocalize with the TNF found at the cell periphery (mean ± SD: 16.1 ± 10.7). By contrast, staining of cells with VAMP-3 revealed large patches of vesicular-like structures at the periphery, sometimes extending into rufflelike projections and a strong overlap with TNF both in WT and in VAMP-8–deficient BMMCs (mean ± SD: 52.3 ± 10.1 and 51.3 ± 14.7, respectively). This is in agreement with the lack of effect of VAMP-8 on cytokine secretion. These results suggest that the terminal trafficking step of TNF release and processing in mast cells similar to macrophages6 may include VAMP-3–containing vesicular compartments.
Localization of unprocessed TNF in IL-1β–stimulated WT and VAMP-8–deficient BMMCs. (A) VAMP-8–deficient and WT BMMCs were exposed to vehicle (3 hours). Cells were then stained with anti-TNF and anti–VAMP-8 or anti–VAMP-3 as indicated. (B) VAMP-8–deficient and WT BMMCs were stimulated with 25 ng/mL IL-1β (3 hours) in the presence of 50 μM TAPI, which inhibits cleavage of the membrane precursor form of TNF. Cells were stained with anti-TNF and anti–VAMP-8 or anti–VAMP-3 as indicated and were then analyzed by confocal microscopy. Representative single optical sections are shown. In the 2-color overlay image (M), compartments containing both markers appear as light blue.
Localization of unprocessed TNF in IL-1β–stimulated WT and VAMP-8–deficient BMMCs. (A) VAMP-8–deficient and WT BMMCs were exposed to vehicle (3 hours). Cells were then stained with anti-TNF and anti–VAMP-8 or anti–VAMP-3 as indicated. (B) VAMP-8–deficient and WT BMMCs were stimulated with 25 ng/mL IL-1β (3 hours) in the presence of 50 μM TAPI, which inhibits cleavage of the membrane precursor form of TNF. Cells were stained with anti-TNF and anti–VAMP-8 or anti–VAMP-3 as indicated and were then analyzed by confocal microscopy. Representative single optical sections are shown. In the 2-color overlay image (M), compartments containing both markers appear as light blue.
Discussion
In the present study, we demonstrate in mast cells that the v-SNARE VAMP-8 functions in the exocytosis of granular stored inflammatory mediators, but does not play a role in the exocytosis of newly synthesized cytokines/chemokines. In vitro experiments demonstrated an approximately 50% inhibition in IgE-stimulated release of β-hexosaminidase and histamine in VAMP-8–deficient mast cells. These results were confirmed in passive systemic anaphylaxis experiments where a similar reduction in blood plasma histamine levels known to depend on the activation of mast cells32,33 was noted. By contrast, the secretion of TNF, IL-6, and MIP-1α was not affected by the absence of VAMP-8.
These results place VAMP-8 as a fusion protein that segregates preformed mediator release from cytokine/chemokine molecular trafficking pathways in mast cells. Previous studies have shown that mast cell SGs are secretory lysosomes with a close connection between the secretory and endocytic pathway.8-10,35 Three types of SGs have been identified35 : (1) type I granules, which contain multivesicular bodies, are accessible to endocytotic tracers, and are devoid of serotonin, (2) type II SGs, composed of both multivesicular bodies and electron dense material, are accessible to endocytic tracers and positive for serotonin, and (3) type III SGs, containing only electron-dense material, are positive for serotonin but are inaccessible to endocytotic tracers. VAMP-8 was originally characterized as a v-SNARE connected to endocytotic and endosomal-lysosomal molecular trafficking.24-26 More recently, it was also shown to function in granule exocytosis in exocrine cells and platelets.27,28 In agreement with the connection between endocytic and exocytic pathways, we found an overlap of VAMP-8 and serotonin and MCP-6–containing secretory granules in BMMCs using confocal analysis similar to the results with tumor mast cells.18 The overlap was partial for both markers, which can be explained by the above-described heterogeneity of the granular compartment and previous data showing that VAMP-8 also localizes to endosomes.25 In immunoelectronmicroscopy analysis besides on the granule membrane it was also sometimes found in intracellular structures that could be endosomal-derived multivesicular bodies or remnants derived thereof.35 It is also in agreement with our data showing that release was not inhibited completely, either in vitro or in vivo. Thus, besides VAMP-8 other v-SNAREs may also be operative. Possible candidates are VAMP-2 and VAMP-7 as both proteins partially colocalize with SGs in mast cells18 and exogenously expressed fluorescent-tagged forms of these SNAREs relocated to the cell periphery upon stimulation.20,38
Biochemical evidence also supports the direct implication of VAMP-8 in fusion during degranulation. Initial experiments showed that absence of VAMP-8 had no effect on expression levels of other trafficking proteins differing from the report in platelets where some compensatory effects were observed.28 Furthermore, in addition to IgE-stimulated release, ionomycin- or thapsigargin-initiated release was also inhibited, placing VAMP-8 at a late step likely situated at the level of fusion. Indeed, we demonstrated that VAMP-8 within 1 to 3 minutes forms complexes with both SNAP-23 and syntaxin-4 in WT but not VAMP-8–deficient BMMCs. As SNAP-23 and syntaxin-4 were previously implicated in SG fusion in mast cells,17,18 our data strongly support the role of VAMP-8 in fusion. We also detected complexes between SNAP-23 and VAMP-2. In VAMP-8–deficient BMMCs, the complexes showed a tendency to increase upon stimulation, while this was less the case in WT BMMCs, supporting our earlier statement that VAMP-2 may also play a role or compensate for the loss of VAMP-8 in SG fusion events.
The direct implication of VAMP-8 in the membrane fusion during degranulation was further supported by our data showing that it relocated to the periphery in stimulated cells similar to syntaxin-3, another marker for SGs in mast cells. Interestingly, colocalization between VAMP-8 and serotonin increased in short-term stimulated cells (where serotonin was still detectable), suggesting that degranulation may involve additional fusion events, such as recently proposed for secretory lysosomal exocytosis in cytotoxic T cells. In these cells, cytotoxic vesicles become primed for exocytosis after interaction with a Munc13-4/Rab27a–positive “exocytic vesicle.”11 Interestingly, both Munc13-4 and Rab27b, a Rab27a homologue, have recently been described to play a role in mast cell degranulation,39,40 and similar mechanisms may therefore also be operative in mast cells. In VAMP-8–deficient BMMCs, somewhat more overlap between syntaxin-3 and serotonin was observed. This relates to our electron microscopy (EM) data showing that VAMP-8–deficient BMMCs contain more mature granules (Table 1). However, in agreement with the inhibition of degranulation observed in these cells, no increase in colocalization became apparent in stimulated cells and relocation of syntaxin-3 to the plasma membrane (PM) was less prominent compared with WT BMMCs.
With the notable exception of TNF, which can be found at least in part in cytoplasmic granules in some types of mast cells,41-43 secretion of cytokines/chemokines depends on protein synthesis and subsequent secretion by a still ill-defined intracellular trafficking pathway. Cytokines/chemokine in mast cells are released by various stimuli in the absence of degranulation,37,44,45 suggesting that their trafficking pathways are different. Our data demonstrate that absence of VAMP-8 does not affect TNF, IL-6, IL-4, and MIP-1α secretion from BMMCs, supporting that cytokine secretion does not use the secretory lysosomal pathway defined by VAMP-8. Cytokine trafficking has been difficult to study, likely due to the transient nature of the trafficking events. In agreement, only addition of a TNF-converting enzyme inhibitor that blocks proteolytic release of plasma membrane–exposed cytokine allowed visualization of some protein after stimulation with ionomycin or thapsigargin. This was further enhanced when cells were stimulated with IL-1β, which does not induce degranulation. It is possible that proteases released by degranulation may elicit nonspecific cleavage of unprocessed surface-exposed TNF,46 while this may be less the case after degranulation-independent activation with IL-1β. Comparative analysis of unprocessed TNF with VAMP-8–containing compartments showed that after 3 hours of stimulation, VAMP-8 was largely intracellular and did not substantially redistribute to the cell periphery in conformity with the absence of degranulation under these stimulation conditions. VAMP-8 also did not considerably colocalize with surface-exposed TNF. We therefore looked at VAMP-3, previously reported to traffic from the Golgi to recycling endosomes, where it mediates release into the phagocytic cup.6 Interestingly, although mast cell secretory events and not phagocytosis were examined, we found that surface-exposed TNF concentrated at the periphery together with VAMP-3–positive compartments that sometimes extended into rufflelike projections. This indicates that TNF trafficking in mast cells similar to macrophages may involve VAMP-3–positive compartments. As VAMP-3 has previously been associated with the endocytotic recycling compartment, this could indicate interception with a constitutive intracellular trafficking pathway.6 However, further functional studies are necessary to elucidate whether VAMP-3 may be involved in regulated fusion events.
Taken together, the present findings demonstrate an important role for VAMP-8, a v-SNARE originally localized to endosomes and lysosomes, in mast cell degranulation. A role for VAMP-8 in exocytosis has also be demonstrated in the exocrine system.27,28 Together with recent data, which show a role for VAMP-8 in lysosomal granules exocytosis in platelets,28 the evolving view is that regulated exocytosis of secretory lysosomes in hematopoietic cells may generally depend on VAMP-8 as one of the major v-SNAREs involved in fusion. These findings extend the role of VAMP-8 as a specific regulator of secretory lysosomal exocytosis. It further demonstrates the intimate connection between endosomal and secretory pathways in these cells. Our results show that VAMP-8 does not affect cytokine release, indicating that cytokine trafficking uses distinct trafficking pathways independent of VAMP-8. For TNF, as observed in macrophages,6,7 this may involve a VAMP-3–containing recycling compartment. This distinction of VAMP-3–containing cytokine-occupied vesicular-like structures versus VAMP-8–containing SGs affords the possibility that interference with intracellular trafficking pathways regulated by VAMP-8 and possibly other fusion proteins might represent a specific therapeutic strategy to selectively interfere with degranulation, but not cytokine release, in mast cells. However, side effects on other exocrine pathways that involve VAMP-8 such as secretion from pancreatic acinar cells have to be considered.27 The utility of this approach would be to ameliorate immediate hypersensitivity while keeping intact some of the innate immune function of the mast cell.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cécile Pouzet and Samira Benadda (IFR 02, Paris-Nord) for the help with confocal image acquisition. We also thank Hwee Chien Liew and Jie Li for technical assistance. We also thank Dr Lisa Scandiuzzi, Dr Meetu Tiwari, and Dr Renato C. Monteiro for critical and thoughtful discussions.
N.T. and U.B. were supported by the Fondation de Recherche Médicale (program Défis de la Recherche en Allergologie). The research project of U.B. and C.B. has been supported by a Marie Curie Early Stage Research Training Fellowship of the European Community's Sixth Framework Program under contract number 504926. The research of J.R. is supported by the intramural research program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: N.T. performed most of the experiments, analyzed the data, and wrote the paper; C.-C.W. and C.B. performed experiments and analyzed the data; G.K. performed in vivo experiments, F.V. and M.R.S. performed EM studies and analysis; Z.Q. performed histologic analysis; J.R. provided critical reagents, helped with data analysis, and participated in paper writing; G.Z. designed experiments and analyzed data; W.H. supervised experiments and participated in study design; U.B. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrich Blank, Inserm U699, Immunopathologie rénale, récepteurs et inflammation, Faculté de Medicine Paris 7, Site Xavier Bichat, 16-rue Henri Huchard, BP416 75870 Paris, France; e-mail: ublank@bichat.inserm.fr.