Abstract
Ceramide, a tumor-suppressor lipid, is generated by sphingomyelin hydrolysis or by de novo synthesis when cells are activated by various stress stimuli as well as when cancer cells are subjected to genotoxic chemotherapy. Ceramide may modulate apoptotic signaling pathways; however, its transcription-dependent effects remain unclear. Our data showed that actinomycin D partially inhibited ceramide-induced apoptosis. Using microarray analysis, we found that ceramide up-regulated a tumor suppressor gene called thioredoxin-interacting protein (Txnip). Similarly, the chemotherapeutic agent etoposide induced Txnip expression en route to apoptosis, which was blocked by inhibitors of ceramide production. Txnip colocalized with thioredoxin and reduced its activity, which caused dissociation of thioredoxin from apoptosis signal-regulating kinase 1 (ASK1). Cells expressing ASK1 siRNA were more resistant to ceramide-induced apoptosis. Ceramide caused ASK1-regulated p38 mitogen-activated protein kinase (MAPK) and JNK activation, as well as activation of the endoplasmic reticulum (ER) stress cascade, and pharmacologic or siRNA-mediated inhibition of p38 MAPK or JNK partially reduced ceramide-induced mitochondria-mediated apoptosis. Furthermore, ceramide-induced ASK1, p38, and JNK phosphorylation and cell apoptosis were inhibited by Txnip siRNA transfection. Taken together, we show that ceramide exhibits a mechanism of transcriptional regulation involving up-regulation of Txnip expression, also induced by etoposide, which results in ASK1 activation, ER stress, and p38 and JNK phosphorylation, all leading to apoptosis.
Introduction
Ceramide is generated from sphingolipid metabolism by sphingomyelinase or through de novo synthesis when cells encounter various stimuli of apoptotic signaling, such as death receptors (CD95 and TNF receptor), radiation (UV and γ-irradiation), and anticancer agents.1-6 Previous reports showed ceramide modulates cell apoptosis through diverse signaling pathways, which involve the inhibition of prosurvival kinases, such as Akt/PKB and PKCα, and the activation of stress-activated kinases, such as JNK, PKCζ, kinase suppressors of Ras (KSR), and protein phosphatases, such as protein phosphatase 2A (PP2A) and PP1.6-10 Mitochondria represent another potential regulating target for ceramide. Ceramide causes the change of mitochondrial transmembrane potential (MTP) through forming channels or by targeting MTP-controlling proteins, such as Bcl-2 family members, resulting in translocation of cytochrome c and AIF proteins from mitochondria to cytoplasm, followed by caspase-3 activation.11-17 In addition, transcriptional and translational regulatory mechanisms may also be involved in ceramide-induced apoptosis.18,19
The mitogen-activated protein kinases (MAPKs), including ERK, p38 MAPK, and JNK, play the central role in stress-induced cell death as well as in apoptotic signaling of ceramide. Ceramide causes ERK dephosphorylation, and induces p38 MAPK and JNK phosphorylation.17,20-23 Previous studies showed that p38 MAPK causes Bax and Bak activation, while JNK causes Bim activation, followed by their translocation to mitochondria.23-26 Also, p38 MAPK phosphorylates Bcl-2 and Bcl-xL to prevent their accumulation in mitochondria.27 Likewise, ceramide-activated p38 MAPK and JNK contribute to cell apoptosis through mitochondrial damage and caspase activation.17,21,23 However, the upstream mechanisms regarding p38 MAPK and JNK activation by ceramide remain unresolved.
Thioredoxin-interacting protein (Txnip), also called vitamin D3 up-regulated protein 1, is an endogenous inhibitor of thioredoxin.28-30 In addition to 1,25-dihydroxyvitamin D3, various stress stimuli, including serum deprivation, H2O2, glucose, irradiation, and anticancer agents have been reported to stimulate Txnip expression.31-35 Thioredoxin blocks apoptosis signal-regulating kinase 1 (ASK1) activity and the subsequent ASK1-dependent apoptosis, and this thioredoxin-mediated inhibitory effect is abolished by Txnip.31,36 Therefore, Txnip functions as a stress-responsive protein and possesses a proapoptotic role. Txnip-deficient mice have been shown to develop hepatocellular carcinoma.37 In addition, overexpression of Txnip has been reported to induce cell apoptosis through an increase in the Bax/Bcl-2 ratio, and caspase-3 expression.34 In our present study, using microarray analysis, we showed that ceramide induced up-regulation of Txnip expression, which plays, at least, a partial role in ceramide-induced apoptosis. Furthermore, Txnip is involved in ASK1-, p38 MAPK- and JNK-mediated pathways, as well as endoplasmic reticulum (ER) stress apoptotic signaling pathways.
Methods
Cell cultures and reagents
The mouse T hybridoma cell line 10I was kindly provided by Dr M. Z. Lai (Institute of Molecular Biology, Academia Sinica, Taiwan). The human Jurkat T-cell line was obtained from Dr B. C. Yang (Department of Microbiology and Immunology, National Cheng Kung University, Taiwan). 10I and Jurkat T cells were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, 50 U/mL penicillin, and 0.05 mg/mL streptomycin and maintained at 37°C in 5% CO2. Cells were washed with serum-free medium and resuspended in hybridoma medium (Gibco BRL; for 10I cells), and RPMI serum-free medium (for Jurkat T cells) before experiments. Ceramide analog C2-ceramide (BioMol, Plymouth Meeting, PA), etoposide (Bio-Vision, Mountain View, CA), actinomycin D, fumonisin B1, myriocin, chlorpromazine, desprimine (Sigma-Aldrich, St Louis, MO), p38 MAPK inhibitor SB203580, and JNK inhibitor SP600125 (TOCRIS, Ellisville, MO) were dissolved in dimethyl sulfoxide (DMSO).
Analysis of cell apoptosis and mitochondrial functional assay
For apoptosis detection, cells were fixed with 70% ethanol in phosphate-buffered saline (PBS) for propidium iodide (PI; Sigma-Aldrich) staining and then were analyzed using flow cytometry (FACScan; BD Biosciences, San Jose, CA). Apoptotic cell membrane disruption characterized by the presence of phosphatidylserine was performed using the annexin V–phycoerythrin detection kit (BioVision). The mitochondrial dehydrogenase activities of viable cells were determined using a WST-8 assay kit (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. The mitochondrial transmembrane potential value was determined using rhodamine 123 (Sigma-Aldrich), followed by flow cytometric analysis.15,16 For the detection of cellular caspase activation, the ApoAlert caspase colorimetric assay kit (Clontech, Palo Alto, CA) for caspase-3 and an ApoAlert caspase fluorescent assay kit for caspase-9 according to the manufacturer's instructions were performed. Optical density (OD) measurements were performed using a microplate reader, and the substrate activities shown as p-nitroanilide (nmol) were calculated.
cDNA microarray
The isolated RNA was reverse-transcribed to generate Cy3- and Cy5-labeled cDNA probes. Labeled probes were then hybridized to a commercial cDNA microarray, Mouse Chip 15K-1 (eGenomix Technology, Taipei, Taiwan), which contains 15 488 genes. The hybridized slide was scanned using the GenePix 4000B scanner (Axon Instruments, Molecular Devices, Sunnyvale, CA), the image was processed using the GenePix Pro 3.0 (Axon Instruments), and microarray data were analyzed by the eGenomix V1.0 (eGenomix Technology Corp). The ratio of Cy5 to Cy3 of microarray spots demonstrated the mRNA abundance of this gene expression in the 2 study groups. After determining the ratio of Cy5 to Cy3, significant changes in gene expression were determined as greater than 2 (up-regulated) or less than 0.5 (down-regulated).
Total RNA extraction and RT-PCR
Cells were resuspended in TRIzol reagent (Gibco BRL) according to the manufacturer's procedures. After phenol/chloroform extraction and isopropanol precipitation, total RNAs were washed in 75% ethanol and quantified using a spectrophotometer (Gene Quant II; GE Healthcare, Little Chalfont, United Kingdom) at 260 nm. For cDNA synthesis, 5 μg of total RNA was incubated with 25 U of Moloney murine leukemia virus reverse transcriptase (RT; Promega, Madison, WI) and 2 μg/mL of oligo primers (Promega) at 37°C for 2 hours, followed by 95°C for 5 minutes. Primers for polymerase chain reactions (PCRs) were based on GenBank-published sequences and are as follows: mouse Txnip, 5′-CATTATCTCAGGGACTTGCG-3′ (sense) and 5′-CTCACTGCACGTTGCTGT-3′ (antisense); mouse β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (sense) and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (antisense). Reactions were performed using the following PCR protocol: 95°C for 30 seconds, 56°C for 1 minute, and 72°C for 1 minute for 35 cycles. PCR products were resolved on 1% agarose gels and stained with ethidium bromide. β-actin was used as internal control.
Thioredoxin activity assay
Thioredoxin activity was detected using insulin disulfide reduction assay as described38 with modifications. Cellular proteins were first extracted in a lysis buffer containing 20 mM HEPES (pH 7.9), 100 mM KCl, 300 mM NaCl, 10 mM EDTA, 0.1% Nonidet P-40, and protease inhibitor cocktail (Sigma-Aldrich). Quantified proteins (100 μg) were preincubated with 2 μL dithiothreitol activation buffer (50 mM HEPES [pH 7.6], 1 mM EDTA, 1 mg/mL bovine serum albumin, and 2 mM dithiothreitol) in a total volume of 35 μL at 37°C for 15 minutes to reduce thioredoxin. Then, 20 μL of reaction buffer (200 μL of 1 M HEPES [pH 7.6], 40 μL of 40 mg/mL NADPH, 40 μL of 0.2 M EDTA, and 500 μL of 10 mg/mL insulin) was added to the mixture. Reactions were started by adding 5 μL bovine thioredoxin reductase (Sigma-Aldrich) and incubated at 37°C for 40 minutes and then stopped by adding 250 μL stop mixture (0.25 mL of 6 M guanidine hydrochloride, 0.4 mg/mL 5,5′-dithiobis-nitrobenzoic acid in 0.2 M Tris-HCl [pH 8.0]). For the control group, equal amount of water (5 μL) was used. After reaction, the absorbance at 412 nm was determined.
Immunoprecipitation and Western blot analysis
To detect indicated proteins, total cell lysates were extracted using a Triton X-100–based lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris [pH 7.5], 5 mM EDTA, 5 mM NaN3, 10 mM NaF, and 10 mM sodium prophosphate) with a protease inhibitor mix and a phosphatase inhibitor cocktail I (Sigma-Aldrich). For immunoprecipitation assay, 500 μg total-cell lysates were incubated with protein G–Sepharose (Upstate Biotechnology, Lake Placid, NY) and antithioredoxin antibody (American Diagnostica, Stamford, CT) for 16 hours at 4°C. After washing, thioredoxin-bound beads were denatured at 100°C in Laemmli sample buffer. Proteins were separated using SDS-PAGE and then transferred to a PVDF membrane (Millipore, Billerica, MA). After blocking, blots were developed with a series of antibodies as indicated. Antibodies specific for ASK1, phospho-ASK1 (serine 83), phospho-ASK1 (threonine 845), p38 MAPK, phospho-p38 MAPK (threonine180/tyrosine182), JNK, phospho-JNK (threonine183/tyrosine185), and Bip were purchased from Cell Signaling Technology (Beverly, MA). Antithioredoxin, anti-CHOP, anti–phospho-PERK (threonine 981), anti-PERK (Santa Cruz Biotechnology, Santa Cruz, CA), anti–cleaved caspase-4 (Sigma-Aldrich) antibodies, and Txnip monoclonal antibody (Medical Biological Laboratories, Nagoya, Japan) were also used. Mouse antibodies specific for α-tubulin, β-actin, and EGFP (Santa Cruz Biotechnology) were used for internal controls. Finally, blots were hybridized with horseradish peroxidase–conjugated goat anti–rabbit IgG or anti–mouse IgG (Cell Signaling Technology) and developed using an ECL Western blot detection kit (Millipore) according to the manufacturer's instructions.
siRNA preparation and transient expression
Plasmids expressing short hairpin RNA were constructed using standard techniques. The pSUPER/enhanced GFP (EGFP) contained the GFP gene from pEGFP-N1 (Clontech) inserted into pSUPER vector39 (kindly provided by Dr R. Agami, The Netherlands Cancer Institute, Amsterdam, The Netherlands). To generate pSUPER-hTxnip/EGFP, pSUPER/EGFP was digested with BglII and HindIII, and the annealed targeting oligonucleotides ACAGACUUCGGAGUACCUG for hTxnip40 were ligated into the vector. The pSUPER-hASK1/EGFP, pSUPER-hp38 MAPKα/EGFP, and pSUPER-hJNK/EGFP were reconstructed from pHSU6-hASK1 (provided by Dr M. D. Lai, Department of Biochemistry and Molecular Biology, National Cheng Kung University, Taiwan), and pKD-p38α/SAPK2a-v3 and pKD-JNK2a2/SAPK1a-v5 (Upstate Biotechnology), respectively. For transfection, cells were resuspended at 1 × 107 cells/0.5 mL in OPTI-MEM (Gibco BRL), and mixed with 40 μg plasmid DNA. The mixture was then added to an electroporation cuvette (0.4-cm electrode gap) and subjected to 270 V and 950 μF (Gene Pulser II Electroporation System; Bio-Rad). After electroporation, the cells were cultured for 16 to 48 hours before analysis. A FACSAria cell sorter (BD Biosciences) was used to sort EGFP-positive cells for vector control and short interfering RNA (siRNA)–expressing cells.
Immunostaining
For intracellular immunostaining, cells were fixed and permeabilized with 1% formaldehyde/methanol in PBS for 10 minutes at room temperature. After the cells were washed, a series of antibodies was used as indicated, followed by FITC- or TRITC-conjugated goat anti–mouse and anti–rabbit IgG (Calbiochem, San Diego, CA) staining. For confocal microscopy, mouse anti-Txnip, rabbit antithioredoxin, anti–phospho-p38, and anti–phospho-JNK were used. Monoclonal antibody against ceramide (Sigma-Aldrich) and rabbit anti–phospho-p38 and anti–phospho-JNK were also used for flow cytometric analysis. 4′,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR), Hoechst 33342, and PI (Sigma-Aldrich) were used for nuclear staining.
Statistical analysis
All in vitro experiments were performed at least 3 times, and the data are expressed as means plus or minus SD. Data were analyzed by the Student t test, and P values less than .05 was considered statistically significant.
Results
Actinomycin D reduces ceramide-induced cell apoptosis
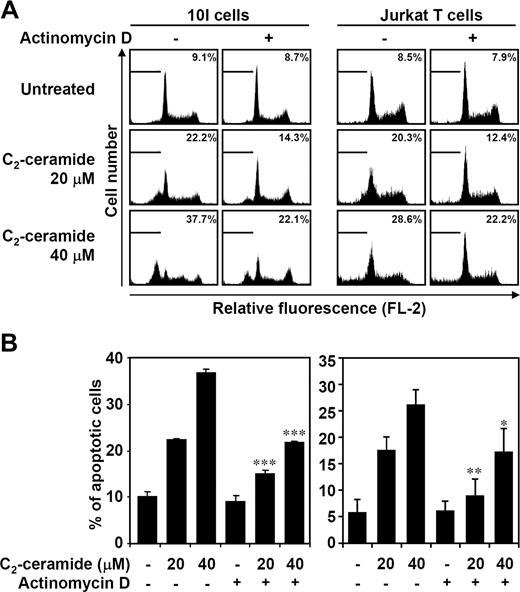
Although the roles of ceramide involved in apoptosis have been extensively studied, the molecular mechanisms of ceramide-mediated apoptotic signaling are not fully resolved. In the present study, ceramide-induced transcription-dependent apoptotic regulation was investigated. Since our previous studies15,16 demonstrated that ceramide induced mouse 10I T-hybridoma and human Jurkat T cell apoptosis, we determined whether actinomycin D treatment reduced ceramide-induced apoptosis. Using PI staining followed by flow cytometric analysis, ceramide-induced subG0/G1 phase accumulation (apoptotic cells) was partially inhibited by pretreatment with actinomycin D (Figure 1). Ceramide-induced caspase-3 activation was also partially reversed by actinomycin D (data not shown). These results suggested that ceramide-induced apoptotic signaling may act, at least in part, through a transcription-dependent mechanism.
Ceramide induces transcription-regulated cell apoptosis. In the absence or presence of actinomycin D (0.15 μg/mL), mouse T-hybridoma 10I cells and human Jurkat T cells were treated with 20 or 40 μM C2-ceramide for 12 and 24 hours, respectively. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. Representative histograms (A) and the percentages of apoptotic cells (B) are shown (means ± SD of triplicate cultures; *P < .05; **P < .01; ***P < .001 as compared with the groups without actinomycin D treatment).
Ceramide induces transcription-regulated cell apoptosis. In the absence or presence of actinomycin D (0.15 μg/mL), mouse T-hybridoma 10I cells and human Jurkat T cells were treated with 20 or 40 μM C2-ceramide for 12 and 24 hours, respectively. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. Representative histograms (A) and the percentages of apoptotic cells (B) are shown (means ± SD of triplicate cultures; *P < .05; **P < .01; ***P < .001 as compared with the groups without actinomycin D treatment).
Microarray analysis reveals Txnip up-regulation after ceramide stimulation
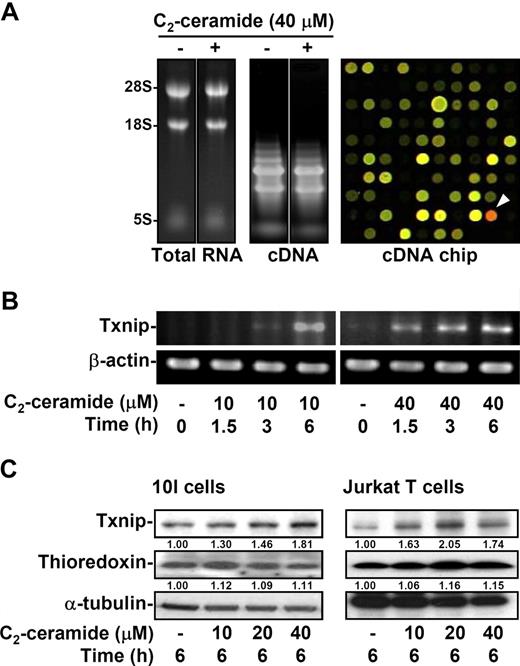
We next verified the gene expression profile after ceramide stimulation. We previously showed that ceramide caused 10I cells to undergo apoptosis within 6 hours.15,16,41 For detection of gene expression, 10I cells were treated with ceramide for 2 hours, followed by microarray analysis. The quality of extracted total RNA and generated cDNA, and the image of the hybridized cDNA array chip are shown (Figure 2A). According to the data from microarray analysis, there were 27 up-regulated genes and 11 down-regulated genes. The highly up-regulated and down-regulated expression genes are listed in Table 1. Based on the array chip results, the expression of Txnip (Figure 2A right panel, arrowhead) was most significantly increased by ceramide treatment. The Txnip mRNA expression after ceramide stimulation was confirmed by RT-PCR, showing a time- and dose-dependent manner (Figure 2B). Furthermore, the Txnip protein levels were up-regulated dose-dependently by ceramide in both 10I and Jurkat T cells as shown by Western blotting (Figure 2C). In contrast, the protein expression of thioredoxin after ceramide treatment remained similar levels (Figure 2C).
Ceramide induces Txnip mRNA and protein expression. (A) 10I cells were treated with 40 μM C2-ceramide for 2 hours. Cells were collected and total RNA was purified followed by cDNA synthesis. Total RNA (left) and cDNA (middle) were confirmed by agarose gel electrophoresis. After microarray analysis, the results of hybridization in the cDNA chip (right) revealed the up-regulated (red; arrowhead) and down-regulated (green) genes. (B) The effects of C2-ceramide on the mRNA expression of Txnip via a time- and dose-dependent manner were determined by RT-PCR. The expression of β-actin was used as an internal control. (C) The protein expression of Txnip and thioredoxin after various doses of C2-ceramide treatment was detected in 10I and Jurkat T cells using Western blot analysis. The α-tubulin expression was used as an internal control. The relative ratios of Txnip–α-tubulin and thioredoxin–α-tubulin are shown.
Ceramide induces Txnip mRNA and protein expression. (A) 10I cells were treated with 40 μM C2-ceramide for 2 hours. Cells were collected and total RNA was purified followed by cDNA synthesis. Total RNA (left) and cDNA (middle) were confirmed by agarose gel electrophoresis. After microarray analysis, the results of hybridization in the cDNA chip (right) revealed the up-regulated (red; arrowhead) and down-regulated (green) genes. (B) The effects of C2-ceramide on the mRNA expression of Txnip via a time- and dose-dependent manner were determined by RT-PCR. The expression of β-actin was used as an internal control. (C) The protein expression of Txnip and thioredoxin after various doses of C2-ceramide treatment was detected in 10I and Jurkat T cells using Western blot analysis. The α-tubulin expression was used as an internal control. The relative ratios of Txnip–α-tubulin and thioredoxin–α-tubulin are shown.
Ceramide-up-regulated Txnip colocalizes with thioredoxin and decreases its activity
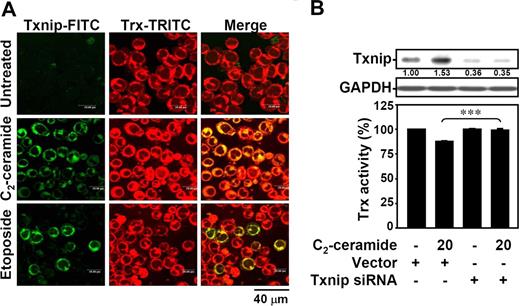
Txnip acts as a negative regulator of thioredoxin.29-32 We therefore investigated whether ceramide-induced expression of Txnip may decrease thioredoxin activity. Using immunostaining followed by confocal microscopic observation, the up-regulated expression of Txnip after ceramide treatment was demonstrated in Jurkat T cells (Figure 3A green). The expression of thioredoxin remained unchanged (Figure 3A red). Ceramide-up-regulated Txnip mostly colocalized with thioredoxin (Figure 3A yellow). A similar result was observed in 10I cells (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Using an insulin reduction assay, ceramide was found to reduce the thioredoxin activity (Figure S1B). To further verify the reduced thioredoxin activity caused by ceramide-up-regulated Txnip, we used human Txnip siRNA to knock down ceramide-induced Txnip expression in Jurkat T cells (Figure 3B). Results showed that the decreased thioredoxin activity elicited by ceramide was rescued after Txnip siRNA treatment (Figure 3B).
Ceramide-up-regulated Txnip interacts with thioredoxin and reduces its activity. (A) Jurkat T cells were treated with 20 μM C2-ceramide or 25 μM etoposide for 6 hours, and the interaction of Txnip with thioredoxin was determined by confocal microscopic observation. After fixation with 1% formaldehyde in PBS, cells were incubated with Txnip and thioredoxin antibodies, followed by FITC-labeled (Txnip-FITC; green) and TRITC-labeled (Trx-TRITC; red) secondary antibodies, respectively. The colocalization of Txnip and thioredoxin was visualized (Merge; yellow). (B) In vector control– and hTxnip siRNA-transfected Jurkat T cells, Txnip protein expression was detected using Western blot analysis. The GAPDH expression was used as an internal control. The relative ratio of Txnip-GAPDH is shown. The thioredoxin activity was detected using an insulin reduction assay after 20 μM C2-ceramide treatment for 12 hours. Results are shown as means plus or minus SD of triplicate cultures. The vector control group without ceramide treatment was normalized as 100%. (***P < .001).
Ceramide-up-regulated Txnip interacts with thioredoxin and reduces its activity. (A) Jurkat T cells were treated with 20 μM C2-ceramide or 25 μM etoposide for 6 hours, and the interaction of Txnip with thioredoxin was determined by confocal microscopic observation. After fixation with 1% formaldehyde in PBS, cells were incubated with Txnip and thioredoxin antibodies, followed by FITC-labeled (Txnip-FITC; green) and TRITC-labeled (Trx-TRITC; red) secondary antibodies, respectively. The colocalization of Txnip and thioredoxin was visualized (Merge; yellow). (B) In vector control– and hTxnip siRNA-transfected Jurkat T cells, Txnip protein expression was detected using Western blot analysis. The GAPDH expression was used as an internal control. The relative ratio of Txnip-GAPDH is shown. The thioredoxin activity was detected using an insulin reduction assay after 20 μM C2-ceramide treatment for 12 hours. Results are shown as means plus or minus SD of triplicate cultures. The vector control group without ceramide treatment was normalized as 100%. (***P < .001).
Etoposide causes ceramide generation and Txnip expression
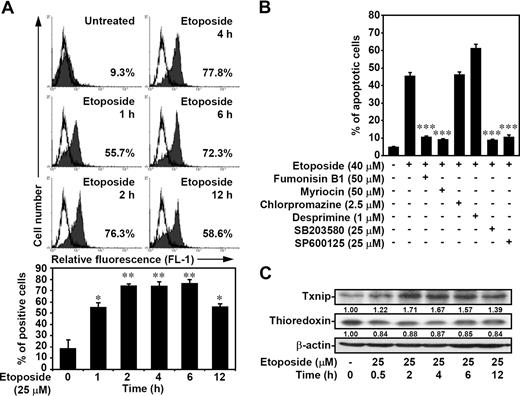
Our previous studies showed that etoposide may induce an apoptotic signaling pathway similar to that induced by ceramide.15,16 We thus determined the generation of ceramide in etoposide-treated Jurkat T cells. Results showed that etoposide induced ceramide generation within 1 hour (Figure 4A). Treatment with fumonisin B1, an inhibitor of ceramide synthase, and myriocin, an inhibitor of serine palmitoyl transferase, but not chlorpromazine and desprimine, inhibitors of acid sphingomyelinase, inhibited etoposide-induced cell apoptosis (Figure 4B), thus indicating a requirement for de novo ceramide generation. Furthermore, we demonstrated the increased expression of Txnip by etoposide (Figure 4C) and the colocalization of up-regulated Txnip with thioredoxin after etoposide treatment (Figure 3A).
Etoposide induces ceramide generation, ceramide- and p38 MAPK/JNK-dependent cell apoptosis, and Txnip up-regulation. (A) Jurkat T cells were treated with 25 μM etoposide for different time periods as indicated, and the generation of ceramide was determined by flow cytometric analysis. After fixation with 1% formaldehyde in PBS, cells were incubated with ceramide antibodies, followed by FITC-labeled secondary antibodies. Representative histograms (top) and the percentages of positive cells (bottom) are shown (means ± SD of triplicate cultures; *P < .05; **P < .01). (B) Jurkat T cells were pretreated with fumonisin B1, myriocin, chlorpromazine, desprimine, SB203580, or SP600125 for 1 hour, followed by etoposide treatment for 24 hours. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. The percentages of apoptotic cells are shown (means ± SD of triplicate cultures; ***P < .001). (C) The Txnip and thioredoxin protein expression after 25 μM etoposide treatment for different time periods was detected using Western blot analysis. The expression of β-actin was used as an internal control. The relative ratio of Txnip–β-actin and thioredoxin–β-actin are shown.
Etoposide induces ceramide generation, ceramide- and p38 MAPK/JNK-dependent cell apoptosis, and Txnip up-regulation. (A) Jurkat T cells were treated with 25 μM etoposide for different time periods as indicated, and the generation of ceramide was determined by flow cytometric analysis. After fixation with 1% formaldehyde in PBS, cells were incubated with ceramide antibodies, followed by FITC-labeled secondary antibodies. Representative histograms (top) and the percentages of positive cells (bottom) are shown (means ± SD of triplicate cultures; *P < .05; **P < .01). (B) Jurkat T cells were pretreated with fumonisin B1, myriocin, chlorpromazine, desprimine, SB203580, or SP600125 for 1 hour, followed by etoposide treatment for 24 hours. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. The percentages of apoptotic cells are shown (means ± SD of triplicate cultures; ***P < .001). (C) The Txnip and thioredoxin protein expression after 25 μM etoposide treatment for different time periods was detected using Western blot analysis. The expression of β-actin was used as an internal control. The relative ratio of Txnip–β-actin and thioredoxin–β-actin are shown.
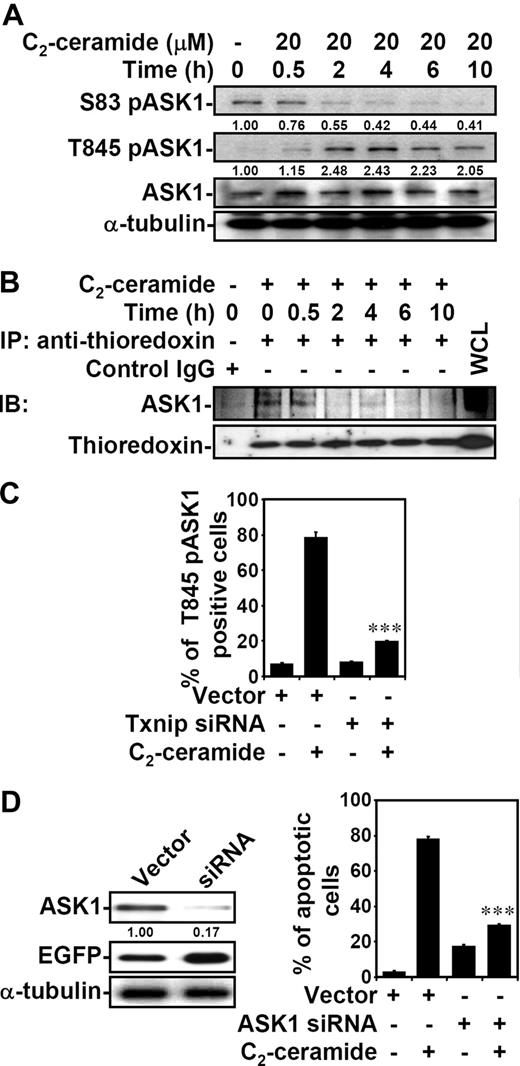
Ceramide causes ASK1 activation
Thioredoxin functions as a direct inhibitor of ASK1, which is required for stress-induced apoptosis.36,43-46 Because ceramide up-regulated Txnip expression and reduced thioredoxin activity, we therefore investigated the effect of ceramide on ASK1 activity. The inhibitory residue serine 83 (Ser83) of ASK1 was dephosphorylated after ceramide treatment in both Jurkat T cells (Figure 5A) and 10I cells (Figure S2). This is consistent with the previous findings that Akt/PKB phosphorylates ASK1 Ser83,47 whereas ceramide inhibits Akt/PKB.4,6,48,49 We also confirmed the inactivation of Akt/PKB by ceramide (data not shown). Moreover, the critical residue threonine 845 (Thr845) within the activation loop of ASK1 was phosphorylated by ceramide (Figures 5A,S2). The increased phosphorylation of ASK1 Thr845 correlates with ASK1 activation.45,50 Therefore, ceramide caused ASK1 activation by inducing dephosphorylation at Ser83 and phosphorylation at Thr845. Also, the association of thioredoxin with ASK1 was disrupted after ceramide treatment in Jurkat T cells (Figure 5B). To verify the relationship between Txnip and ASK1 activation, we transfected Txnip siRNA into Jurkat T cells and found that the ceramide-induced ASK1 phosphorylation at Thr845 was inhibited by Txnip siRNA (Figures 5C,S3A). We further used ASK1 siRNA to verify the involvement of ASK1 in ceramide-induced cell death. A reduced ASK1 protein level in ASK1 siRNA-transfected Jurkat T cells was confirmed as compared with vector control-transfected cells (Figure 5D left panel). After ceramide treatment, ASK1 siRNA-transfected EGFP+ cells showed lower percentages of apoptotic cells than the vector control group (Figure 5D right panel; Figure S3B).
ASK1 activation is involved in ceramide-induced apoptosis. (A) Jurkat T cells were treated with 20 μM C2-ceramide for different time periods as indicated. The expression of phospho-ASK1 at serine 83 (S83 pASK1) and at threonine 845 (T845 pASK1), and ASK1, were determined using Western blot analysis. The relative ratio of pASK1 to total ASK1 is shown. The expression of α-tubulin was an internal control. (B) The cell lysate from untreated or 20 μM C2-ceramide–treated Jurkat T cells for different time periods was immunoprecipitated using antithioredoxin antibody, followed by immunoblotting using anti-ASK1 and antithioredoxin antibodies. Cell lysate without ceramide treatment and immunoprecipitation (WCL indicates whole-cell lysate) was used as a positive control; mouse IgG (first lane) was the negative control. (C) Vector control– and hTxnip siRNA-transfected Jurkat T cells were treated with 20 μM C2-ceramide for 6 hours, and the expression of T845 pASK1 was measured by flow cytometric analysis. The percentages of positive cells are shown as means plus or minus SD of triplicate cultures (***P < .001 as compared with the vector control group). (D) ASK1 protein expression in vector control– and ASK1 siRNA-transfected Jurkat T cells were detected by Western blotting (left). The relative ratio of ASK1 to EGFP is shown. The expression of α-tubulin was used as an internal control. Vector control– and ASK1 siRNA-transfected cells were treated with 20 μM C2-ceramide for 24 hours, and cell apoptosis was measured by annexin V staining. The percentages of apoptotic cells are shown (means ± SD of triplicate cultures; ***P < .001 as compared with the vector control group).
ASK1 activation is involved in ceramide-induced apoptosis. (A) Jurkat T cells were treated with 20 μM C2-ceramide for different time periods as indicated. The expression of phospho-ASK1 at serine 83 (S83 pASK1) and at threonine 845 (T845 pASK1), and ASK1, were determined using Western blot analysis. The relative ratio of pASK1 to total ASK1 is shown. The expression of α-tubulin was an internal control. (B) The cell lysate from untreated or 20 μM C2-ceramide–treated Jurkat T cells for different time periods was immunoprecipitated using antithioredoxin antibody, followed by immunoblotting using anti-ASK1 and antithioredoxin antibodies. Cell lysate without ceramide treatment and immunoprecipitation (WCL indicates whole-cell lysate) was used as a positive control; mouse IgG (first lane) was the negative control. (C) Vector control– and hTxnip siRNA-transfected Jurkat T cells were treated with 20 μM C2-ceramide for 6 hours, and the expression of T845 pASK1 was measured by flow cytometric analysis. The percentages of positive cells are shown as means plus or minus SD of triplicate cultures (***P < .001 as compared with the vector control group). (D) ASK1 protein expression in vector control– and ASK1 siRNA-transfected Jurkat T cells were detected by Western blotting (left). The relative ratio of ASK1 to EGFP is shown. The expression of α-tubulin was used as an internal control. Vector control– and ASK1 siRNA-transfected cells were treated with 20 μM C2-ceramide for 24 hours, and cell apoptosis was measured by annexin V staining. The percentages of apoptotic cells are shown (means ± SD of triplicate cultures; ***P < .001 as compared with the vector control group).
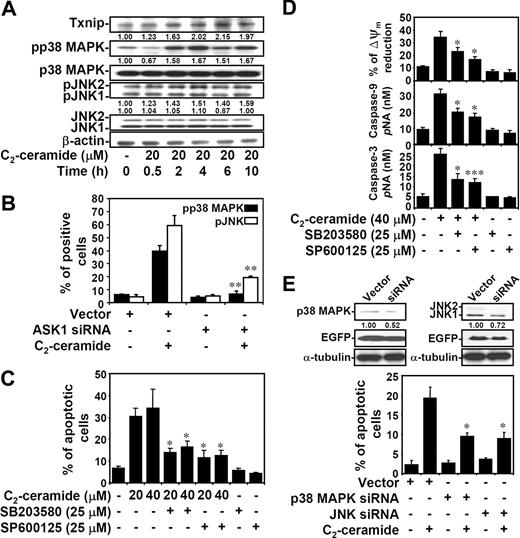
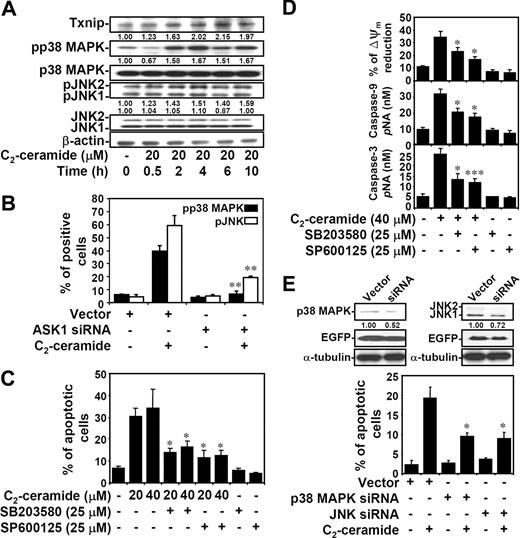
p38 MAPK and JNK activation in ceramide-induced apoptotic pathway is transcription dependent
ASK1 is involved in the stress-activated SEK1-JNK and MKK3/MKK6-p38 MAPK cascades, and ASK1 is part of a complex in ER stress which accesses these stress kinases.45,51,52 We therefore determined whether ceramide-induced activation of p38 MAPK and JNK was through a transcription-dependent mechanism. Results showed a time-dependent response of increased Txnip expression and p38 MAPK and JNK phosphorylation by ceramide in both Jurkat T cells (Figure 6A) and 10I cells (Figure S4A). Thioredoxin protein expression remained similar after ceramide treatment (Figure S4A). To further examine whether p38 MAPK and JNK were activated through the transcriptional regulation, 10I cells were treated with ceramide in the presence or absence of actinomycin D. Actinomycin D inhibited ceramide-induced p38 MAPK and JNK phosphorylation as demonstrated by immunostaining followed by confocal microscopic observation (Figure S4B), flow cytometric analysis (Figure S4C), and Western blotting (Figure S4D). The earlier time point of Txnip expression and transcription-dependent regulation implied that the ceramide-elevated Txnip may act upstream of p38 MAPK and JNK phosphorylation. Since Txnip is involved in ceramide-induced ASK1 activation, the regulation of ASK1 on ceramide-activated p38 MAPK and JNK was subsequently determined. In ASK1 siRNA-transfected EGFP+ Jurkat T cells, ceramide-induced phosphorylation of p38 MAPK and JNK was inhibited (Figures 6B,S4E,F). These results demonstrated that ceramide induces p38 MAPK and JNK activation through a Txnip-ASK1-regulated pathway. Further experiments showed that inhibitors of p38 MAPK and JNK reduced ceramide-induced cell apoptosis, but the magnitudes of their apoptosis-sparing effect was modest (Figures 6C,S4G). Studies using inhibitors of p38 MAPK and JNK also showed the requirement of p38 MAPK and JNK in etoposide-induced cell apoptosis (Figure 4B). Furthermore, inhibitors of p38 MAPK and JNK reduced ceramide-induced apoptosis, in part, via a mitochondrial pathway, as demonstrated by the 30% to 40% reduction of mitochondrial transmembrane potential (ΔΨm) and caspase-9 and caspase-3 activation (Figure 6D). In p38 MAPK or JNK siRNA-transfected EGFP+ Jurkat T cells, ceramide-induced cell apoptosis was inhibited (Figures 6E, S4H,I). Therefore, ceramide regulates ASK1-p38 MAPK and JNK activation and their contribution to apoptosis. Because ASK1 is also involved in the ER stress pathway in the activating complex Ire1-ASK1-TRAF2,52 we demonstrated activation of the ER stress pathway occurred with similar kinetics (Figure S5).
Ceramide causes ASK1-regulated p38 MAPK and JNK activation, and p38 MAPK- and JNK-mediated apoptosis. (A) Jurkat T cells were treated with C2-ceramide for different time periods as indicated. The expression of phospho-p38 MAPK (pp38 MAPK) at threonine180/tyrosine182, p38 MAPK, phospho-JNK (pJNK) at threoine183/tyrosine185, JNK, and Txnip were determined using Western blot analysis. The relative ratios of pp38 MAPK to p38 MAPK and pJNK to JNK are shown. The expression of β-actin was an internal control. (B) Vector control– and ASK1 siRNA-transfected Jurkat T cells were treated with 20 μM C2-ceramide for 6 hours, and the expression of pp38 MAPK and pJNK were determined by flow cytometric analysis. The percentages of positive cells are shown as means plus or minus SD of triplicate cultures (**P < .01 as compared with the vector control group). (C) Cells were pretreated with SB203580 (25 μM) or SP600125 (25 μM) for 1 hour, followed by C2-ceramide treatment for 24 hours. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. The percentages of apoptotic cells are shown as means plus or minus SD of triplicate cultures (*P < .05 as compared with the groups without inhibitor treatment). (D) Meanwhile, activation of caspase-9 and caspase-3 at 24 hours as determined by caspase activity assay kit are shown (means ± SD of triplicate cultures). The mitochondrial transmembrane potential (ΔΨm) reduction in cells with C2-ceramide stimulation for 12 hours was detected by rhodamine 123 followed by flow cytometric analysis, and are shown as means plus or minus SD of triplicate cultures (*P < .05; ***P < .001 as compared with the groups without inhibitor treatment). (E) The p38 MAPK and JNK protein expression in vector control– and p38 MAPK or JNK siRNA-transfected Jurkat T cells were detected by Western blotting (top). The relative ratios of p38 MAPK to EGFP and JNK to EGFP are shown. The expression of α-tubulin was used as an internal control. Cells were treated with 20 μM C2-ceramide for 24 hours, and cell apoptosis was measured by annexin V staining. The percentages of apoptotic cells are shown as means plus or minus SD of triplicate cultures (*P < .05 as compared with the vector control group).
Ceramide causes ASK1-regulated p38 MAPK and JNK activation, and p38 MAPK- and JNK-mediated apoptosis. (A) Jurkat T cells were treated with C2-ceramide for different time periods as indicated. The expression of phospho-p38 MAPK (pp38 MAPK) at threonine180/tyrosine182, p38 MAPK, phospho-JNK (pJNK) at threoine183/tyrosine185, JNK, and Txnip were determined using Western blot analysis. The relative ratios of pp38 MAPK to p38 MAPK and pJNK to JNK are shown. The expression of β-actin was an internal control. (B) Vector control– and ASK1 siRNA-transfected Jurkat T cells were treated with 20 μM C2-ceramide for 6 hours, and the expression of pp38 MAPK and pJNK were determined by flow cytometric analysis. The percentages of positive cells are shown as means plus or minus SD of triplicate cultures (**P < .01 as compared with the vector control group). (C) Cells were pretreated with SB203580 (25 μM) or SP600125 (25 μM) for 1 hour, followed by C2-ceramide treatment for 24 hours. Apoptotic cells were detected using PI staining followed by flow cytometric analysis. The percentages of apoptotic cells are shown as means plus or minus SD of triplicate cultures (*P < .05 as compared with the groups without inhibitor treatment). (D) Meanwhile, activation of caspase-9 and caspase-3 at 24 hours as determined by caspase activity assay kit are shown (means ± SD of triplicate cultures). The mitochondrial transmembrane potential (ΔΨm) reduction in cells with C2-ceramide stimulation for 12 hours was detected by rhodamine 123 followed by flow cytometric analysis, and are shown as means plus or minus SD of triplicate cultures (*P < .05; ***P < .001 as compared with the groups without inhibitor treatment). (E) The p38 MAPK and JNK protein expression in vector control– and p38 MAPK or JNK siRNA-transfected Jurkat T cells were detected by Western blotting (top). The relative ratios of p38 MAPK to EGFP and JNK to EGFP are shown. The expression of α-tubulin was used as an internal control. Cells were treated with 20 μM C2-ceramide for 24 hours, and cell apoptosis was measured by annexin V staining. The percentages of apoptotic cells are shown as means plus or minus SD of triplicate cultures (*P < .05 as compared with the vector control group).
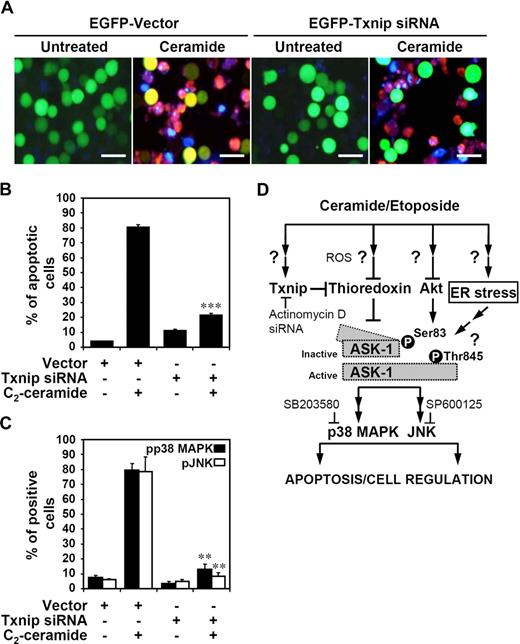
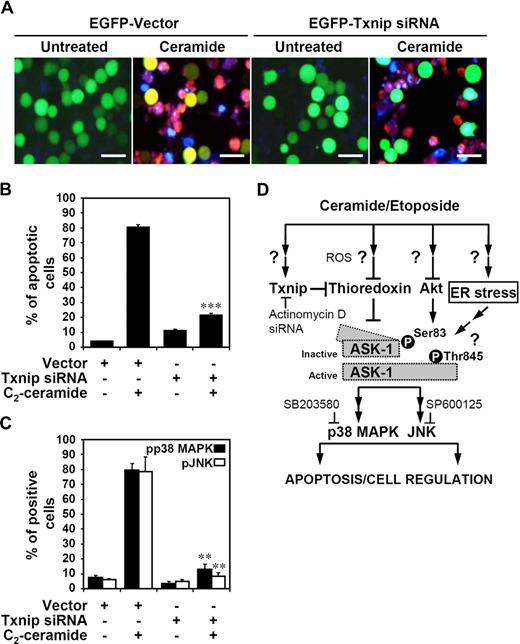
Txnip is involved in ceramide-induced p38 MAPK and JNK phosphorylation and cell death
To further confirm an essential role of Txnip in ceramide-induced p38 MAPK and JNK activation and cell death, we used specific siRNA to knock down Txnip expression in Jurkat T cells. Compared with the vector control group, there was a lower level of ceramide-induced Txnip expression in Txnip siRNA-transfected Jurkat T cells (Figure 3B). Using immunostaining followed by fluorescence microscopic observation, ceramide-induced Jurkat T-cell apoptosis was markedly inhibited by Txnip siRNA treatment (Figure 7A). Ceramide-induced apoptosis (Figure 7A annexin V+ cells, red) was observed in both EGFP+ and EGFP− cells. In EGFP-positive cells, however, ceramide-induced apoptosis could only be detected in cells transfected with vector alone (Figure 7A yellow) but not in cells transfected with Txnip siRNA (Figure 7A green). These results were quantified using flow cytometric analysis to determine the percentages of EGFP vector or EGFP-Txnip siRNA and annexin V–PE double-positive cells (Figures 7B,S6A). Meanwhile, flow cytometric analysis (Figures 7C,S6B,C) showed that ceramide–up-regulated p38 MAPK and JNK phosphorylation were inhibited by Txnip siRNA, but not by the vector control. Taken together, ceramide-induced ASK1 and p38 MAPK and JNK activations and cell death are, at least in part, regulated by Txnip. The origins and relative roles of Akt inactivation in this process are currently not fully understood.
Down-regulation of Txnip reduces ceramide-induced p38 MAPK and JNK phosphorylation and cell apoptosis. (A) After treatment with 20 μM C2-ceramide for 24 hours, vector control– and hTxnip siRNA-transfected Jurkat T cells were measured using PE-conjugated annexin V staining (red), followed by fluorescence microscopic observation. Hoechst 33342 (blue) was used for nuclear staining. The scale bar equals 40 μm. (B) Using flow cytometric analysis, the percentages of apoptotic cells (annexin V+) are shown (***P < .001 as compared with the vector control group). (C) After C2-ceramide treatment for 6 hours, the status of pp38 MAPK and pJNK in vector- and hTxnip siRNA-transfected Jurkat T cells were measured by flow cytometric analysis using pp38 MAPK and pJNK antibodies followed by rhodamine (red)–conjugated secondary antibody staining. The percentages of positive cells are shown (means ± SD of triplicate cultures; **P < .01 as compared with the vector control group). (D) A model for ceramide and etoposide-induced transcriptional apoptotic signaling which involves Txnip-regulated ASK1, p38 MAPK, and JNK activation. By microarray analysis, we show a novel role of ceramide on Txnip expression. Ceramide–up-regulated Txnip binds to thioredoxin and decreases the thioredoxin activity, which causes dissociation of thioredoxin from ASK1. ASK1 is activated via Akt-mediated dephosphorylation at serine 83 and phosphorylation at threonine 845 with unknown mechanism. The requirement of Txnip in ceramide-activated p38 MAPK and JNK was demonstrated using Txnip siRNA. Furthermore, Txnip-regulated p38 MAPK and JNK activation are, at least in part, involved in ceramide-induced cell apoptosis.
Down-regulation of Txnip reduces ceramide-induced p38 MAPK and JNK phosphorylation and cell apoptosis. (A) After treatment with 20 μM C2-ceramide for 24 hours, vector control– and hTxnip siRNA-transfected Jurkat T cells were measured using PE-conjugated annexin V staining (red), followed by fluorescence microscopic observation. Hoechst 33342 (blue) was used for nuclear staining. The scale bar equals 40 μm. (B) Using flow cytometric analysis, the percentages of apoptotic cells (annexin V+) are shown (***P < .001 as compared with the vector control group). (C) After C2-ceramide treatment for 6 hours, the status of pp38 MAPK and pJNK in vector- and hTxnip siRNA-transfected Jurkat T cells were measured by flow cytometric analysis using pp38 MAPK and pJNK antibodies followed by rhodamine (red)–conjugated secondary antibody staining. The percentages of positive cells are shown (means ± SD of triplicate cultures; **P < .01 as compared with the vector control group). (D) A model for ceramide and etoposide-induced transcriptional apoptotic signaling which involves Txnip-regulated ASK1, p38 MAPK, and JNK activation. By microarray analysis, we show a novel role of ceramide on Txnip expression. Ceramide–up-regulated Txnip binds to thioredoxin and decreases the thioredoxin activity, which causes dissociation of thioredoxin from ASK1. ASK1 is activated via Akt-mediated dephosphorylation at serine 83 and phosphorylation at threonine 845 with unknown mechanism. The requirement of Txnip in ceramide-activated p38 MAPK and JNK was demonstrated using Txnip siRNA. Furthermore, Txnip-regulated p38 MAPK and JNK activation are, at least in part, involved in ceramide-induced cell apoptosis.
Discussion
A number of studies regarding ceramide-mediated apoptotic signaling have been reported; however, the mechanisms of ceramide-induced transcriptional regulation are less known. This study is the first to show that ceramide induces Txnip expression and the subsequent activation of ASK1, which is involved in ceramide-induced cell apoptosis. Txnip expression is inducible by various stress stimuli, including H2O2, irradiation, UV, serum deprivation, and growth-inhibitory signals such as TGF-β1.31-33,53 The anticancer agents, including 5-fluorouracil and suberoylanilide hydroxamic acid, can also induce Txnip expression in cancer cells.35,54 In the present study, we have also demonstrated the induction of Txnip expression by etoposide. Actually, the involvement of ceramide signaling in chemotherapy-induced apoptosis has been reported via both de novo synthesis55,56 and sphingomyelin hydrolysis.57 Similar to the findings in the human T-cell line Molt-4,55,56 we found that etoposide-induced Jurkat T-cell apoptosis requires de novo ceramide synthesis. Inhibitors of acid sphingomyelinase did not cause an effect on etoposide-induced Jurkat T-cell apoptosis. Whether neutral sphingomyelinase is involved as previously described in glioma cells57 remains to be determined. Since Txnip expression is down-regulated in various tumor tissues and overexpression of Txnip sensitizes cells undergoing apoptosis, Txnip itself is considered as a tumor suppressor.32,37,53 We showed that ceramide and etoposide dramatically induced Txnip up-regulation. As a matter of fact, ceramide functions as a tumor-suppressor lipid because of its proapoptotic role in cellular stress responses.4,58 The observation of increased expression of Txnip by ceramide, therefore, suggests an involvement of ceramide and Txnip in tumor suppression.
Ceramide-up-regulated Txnip colocalized with thioredoxin, and thereby modulated thioredoxin activity. Previous studies showed the potential mechanism of thioredoxin inhibition by reactive oxygen species (ROS).59,60 Although ceramide may induce oxidative damage by increasing ROS generation,61-63 we could not observe significant changes of ROS production within 6 hours in ceramide-treated cells (data not shown), at which time the reduced activity of thioredoxin was already detected. Thus, we speculate that the reduction of thioredoxin activity is mostly caused by an increased expression of Txnip, but the possibility of ROS-mediated inhibition of thioredoxin cannot be completely excluded (Figure 7D).
Thioredoxin associates with ASK1 and inhibits ASK1 kinase activity36 or promotes ASK1 ubiquitination and degradation44 to inhibit ASK1-mediated apoptosis. In this study, we found that ceramide elevated Txnip expression and down-regulated thioredoxin activity, resulting in ASK1 activation. For ASK1 activation, ASK1 oligomerization and phosphorylation on critical threonine residues within its activation loop cause the dissociation from thioredoxin.45,46,50 In accordance with Txnip induction by ceramide, we observed an increased phosphorylation of ASK1 at the activation residue Thr845 and dephosphorylation at the inhibitory residue Ser83. The disruption of ASK1 and thioredoxin interaction was also confirmed. ASK1 plays a pivotal role in TNF-α–, stress-, and anticancer drug–induced apoptosis, and inhibition of ASK1 kinase activity protects cells from apoptosis.36,46,51,64,65 Meanwhile, various stress stimuli induce Txnip expression.31-33,53 Whether stress stimulation leads to the generation of ceramide, which then causes Txnip expression and ASK1 activation, as shown in this study, remains to be investigated.
ASK1 is a member of the MAPK kinase kinase (MAPKKK) family, which acts upstream of p38 MAPK and JNK through MKK3/6 and MKK4/7, respectively.45,51,66 Ceramide induces p38 MAPK and JNK phosphorylation, followed by mitochondrial damage and cytochrome c release, leading to apoptosis.17,20-23 However, the upstream mechanisms of ceramide-activated p38 MAPK and JNK are not clear. We show an inhibition by actinomycin D of ceramide-induced apoptosis. Consistent with our findings, previous reports have indicated the transcriptional and translational mechanisms in ceramide-induced apoptosis.18,19 Interestingly, actinomycin D also blocked ceramide-induced p38 MAPK and JNK phosphorylation. Therefore, the activation of p38 MAPK and JNK appear to be transcription dependent. Specific silencing using Txnip siRNA demonstrated that ceramide-activated ASK1, p38 MAPK, and JNK are regulated by Txnip expression. We conclude that Txnip, which inhibits thioredoxin and subsequently activates ASK1 acts upstream of ceramide-induced p38 MAPK and JNK activation and apoptosis (Figure 7D).
The Txnip gene promoter has various transcription factor–binding regions in human embryonic kidney cell line 293–derived Bosc cells, including TATA box, CCAAT box, AP-1, CREB, NFAT, and heat-shock factor binding elements,33 and in mouse myeloma cell line Sp2/0 cells, including TATA box, CCAAT box, GATA-1, Sp1, NF-κB, and MZF1.67 The binding of serum deprivation–induced heat-shock factor to the Txnip gene promoter resulted in Txnip gene expression, which was observed in human Bosc cells,33 but not in mouse Sp2/0 cells.67 The detail mechanisms of ceramide-activated Txnip gene expression are still unclear. Which transcription factors are induced by ceramide leading to Txnip expression remain to be investigated.
Our previous results showed that ceramide-induced 10I, Jurkat T, or A549 cell apoptosis was markedly blocked by PP2A, GSK-3, caspase inhibition, and mitochondrial stabilization.15,16,41,49 In addition to ceramide-induced transcription-independent cell apoptosis, we herein further show that ceramide may induce Txnip expression and subsequently activate ASK1-p38 MAPK/JNK–mediated mitochondrial apoptosis. Whether there is cross-talk between ceramide-induced transcription-dependent and -independent pathways is under intensive investigation. Our preliminary results show that the PP2A inhibitor okadaic acid, and GSK-3 inhibitors lithium chloride and SB415286, reduce ceramide-induced Txnip expression (data not shown).
Recent studies have suggested that ceramide acts as a key regulator in the response of cancer cells to the taxane group of anticancer drugs.68,69 A ceramide transport protein, CERT, was identified, and its down-regulation sensitized cancer cells to cytotoxic agents via elevating ER ceramide and potentiating ER stress and apoptosis. An earlier study showed that ceramide bound to the ER and induced calcium influx from the ER to mitochondria.70 Our studies also showed that ceramide and etoposide may cause ER stress responses, including Bip and CHOP up-regulation, caspase-4 activation, and PERK phosphorylation at Thr981. The roles of ER stress–regulated Txnip expression followed by ASK1-p38 MAPK/JNK activation need to be further investigated. In addition to a proapoptotic role, Txnip is also involved in vascular inflammation and hyperglycemia regulation.40,71 Ceramide causes increased ASK1 expression and activity during keratinocyte differentiation.72 Whether the ceramide-induced Txnip/thioredoxin/ASK1 signaling pathway is also involved in regulating other cellular functions is also unclear.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Robert Anderson and Chi-Wu Chiang for critical reading of this manuscript. We also thank Keng-Hsun Hu and Chin-Wu Yeh for providing the ASK1 siRNA, p38 MAPK siRNA, and JNK siRNA plasmids, and Ming-Chen Yang for performing cell sorting.
This work was supported by grant no. 91-B-FA09-1-4 from the Ministry of Education (MOE) Program for Promoting Academic Excellence of University (Taiwan).
Authorship
Contribution: C.L.C. designed and performed the research and analyzed data; C.F.L. helped to design the research; W.T.C. and C.F.T prepared the Txnip siRNA plasmids; W.C.H performed some of the experiments; and C.L.C. and Y.S.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yee-Shin Lin, Department of Microbiology and Immunology, National Cheng Kung University Medical College, Tainan 701, Taiwan; e-mail: yslin1@mail.ncku.edu.tw.