Abstract
Macrophages are reservoirs of HIV-1 infection, proposed to transmit virus to CD4+ T cells, the primary target of the virus. Here we report that human monocyte-derived macrophages (MDMs) rapidly spread HIV-1 to autologous CD4+ T cells resulting in productive infection. Transmission takes place across transient adhesive contacts between T cells and MDMs, which have the features of a virological synapse including copolarization of CD4 on the T cell with HIV-1 Gag and Env on the macrophage. We propose that an infected MDM can infect at least one T cell every 6 hours. Since HIV-1–infected macrophages can survive for many weeks, these results highlight the central role played by macrophages in HIV-1 infection and pathogenesis.
Introduction
Macrophages are central players in HIV-1 pathogenesis: they are among the first cells infected by the virus, and have been proposed to spread infection to the brain and to form a long-lived virus reservoir.1,2 Two recent studies have shown that HIV-1 in macrophages assembles in a newly identified intracellular compartment that is a large and complex invaginated plasma membrane domain.3,4 The function of this compartment is unknown, but presumably HIV-1 may be stored here, protected from effector elements of the humoral immune system
In direct T-cell–T-cell spread of HIV-1, we and others have demonstrated that HIV-1 forms a virological synapse (VS).5-8 The VS is a multimolecular complex that forms at the interface between HIV-1–infected and uninfected receptor-expressing cells. Its assembly is driven by gp120-CD4–coreceptor interactions and depends upon stable cell-cell junctions maintained by adhesion molecules.5,9 Direct T-cell–T-cell spread is likely to be the dominant mode of viral spread between these cells in culture, and probably in vivo.7 HIV-1 also appears to spread from dendritic cells to T cells via a VS.10 HIV-1–infected monocyte-derived macrophages (MDMs) can also transmit virus to peripheral blood leukocytes (PBLs),2,11 but the underlying mechanism has not been elucidated.
Here, we investigated the transfer of HIV-1 from MDMs to autologous CD4+ T cells, and found that transfer of virus is rapid and takes place across transient VSs between T cells and MDMs.
Methods
Human MDMs were generated and infected with HIV-1BaL as described,4 and maintained in X-VIVO medium (Lonza, Walkersville, MD) containing 1% autologous heat-inactivated and filtered serum. Autologous CD4+ T cells were negatively selected from PBLs (Miltenyi-Biotec, Bergisch-Gladbach, Germany). Samples were fixed and stained as described,3,12 and were mounted in ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA), and analyzed at room temperature (RT) using a noninverted Zeiss LSM5 Pascal microscope (Carl Zeiss, Heidelberg, Germany), linked to Pascal software V4.2SP1 (Carl Zeiss). Images were acquired using a 63× oil-immersion objective (1.4 aperture) and processed using Adobe Photoshop v8.0 (Adobe Systems, San Jose, CA).
Staining/washing buffer contained 5% human and goat serum. Antibodies used were L12013 (Centre for AIDS Reagents [CFAR], Potters Bar, United Kingdom) with donkey anti–mouse FITC (Jackson ImmunoResearch, Suffolk, United Kingdom), mouse anti–Gag-p17 (4C9; NIBSC, Potters Bar, United Kingdom) with goat anti–mouse IgG2a alexa647 (Invitrogen), and biotinylated 2G1214 (endogenous biotin blocked with biotin-block; Invitrogen) with streptavidin-Tritc (Jackson ImmunoResearch). Samples for electron microscopy (EM) were prepared and analyzed as described4 using a JEM-2000EX microscope (JEOL, Tokyo, Japan) or a Tecnai F-30 microscope (FEI, Eindhoven, The Netherlands).
For flow cytometry (FC), T cells were collected, fixed, and stained for CD3 and CA-p24 as described.15 Inhibitors were 13B.8.216 (Coulter, Fullerton, CA), Q412013 (CFAR), T20 (National Institutes of Health [NIH], Bethesda, MD), 2D717 (BD Biosciences, San Jose, CA) (all 10 μg/mL), AZT (5 μM; NIH), and Tak-77918 (300 nM; NIBSC).
For quantitative real-time polymerase chain reaction (qPCR) samples, DNA was extracted as described.12 The pol-primers used were TGGGTTATGAACTCCATCCTGA (sense) and TGTCATTGACAGTCCAGCTGTCT (antisense); the reference gene used was human β-globin (AACTGGGCATGTGGAGACAGA (sense), CTAAGGGTGGGAAAATAGACCAATAG (antisense).
Results and discussion
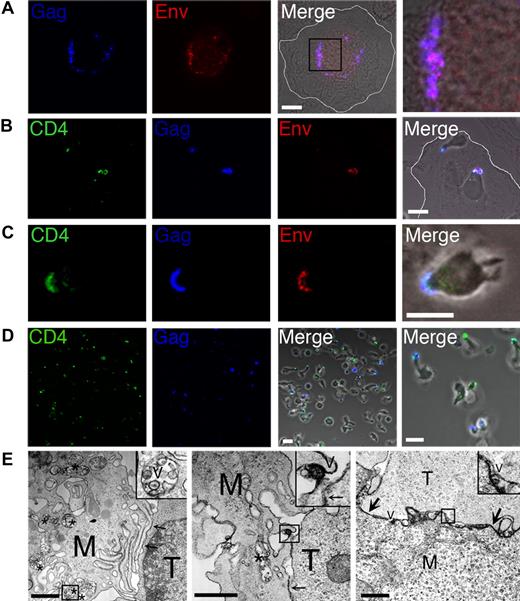
We cocultured HIV-1–infected MDMs (Figure 1A) with autologous CD4+ T cells for 6 hours, followed by fixation and staining for laser scanning confocal microscopy (LSCM) analysis. The T cells were prestained with CD4 nonblocking antibody,13 and after fixation and permeabilization, samples were stained for HIV-1 Gag and Env (Figure 1B,C). Since staining for HIV-1 Env in MDMs is complicated by human antibodies binding efficiently to human Fc-receptors,3 we blocked with human serum and used biotinylated 2G12 to reduce background. A high proportion of MDMs (> 80%) in the coculture had one or more T cells attached via the tip of a pseudopod-like extension of the T cell. In the majority of MDMs in which HIV-1 Gag and Env were detected (10%-30%, depending upon donor), these viral antigens colocalized with CD4 in the adherent T cells (Figure 1B,C). Colocalization of Gag, Env, and CD4 was observed within 1 hour, and the proportion of MDM–T-cell conjugates with colocalization of these antigens was maximal at 5 to 7 hours. Based on these data, we could not exclude the possibility that at least a proportion of the viral antigens was accumulating within the MDMs at the contact site with the T cell, but virus was not infecting the T cell. However, we noted that the T-cell contacts with the MDMs were transient, and many T cells detached from the MDMs after becoming Gag positive, confirming that Gag was transferring to the T cell. To investigate this further, we carefully aspirated detached T cells from the MDM–T-cell cocultures, and analyzed them by LSCM (Figure 1D). We observed that 5% to 10% of the T cells were Gag positive at 6 hours after coculture. This confirmed that the interactions between MDMs and T cells were short-lived, and combined with the data from Figure 1B,C suggested that HIV-1 is efficiently transmitted to CD4+ T cells across a transient VS-like structure.5,6 This was reinforced by EM analysis of the contact zone between HIV-1–infected MDMs and CD4+ T cells, which was characterized by tight adhesive junctions between the cells with regular gaps or pockets (Figure 1E). We found no evidence of cell-cell fusion, consistent with the lack of syncytium formation observed by LSCM. HIV-1 was detected within vesicular MDM compartments similar to those previously described3,4 (Figure 1E first panel), and virus was observed both proximal to, and in contact with, adherent T cells (Figure 1E second and third panels). Further research will be required to determine how this virus-containing compartment in MDMs relocates to the VS.
Macrophages transmit HIV-1 to CD4+ T cells across a virological synapse-like structure. Human MDMs were differentiated on glass coverslips for 7 days prior to infection with 6 ng CA-p24 of the HIV-1BaL isolate for a further 7 days. MDMs were subsequently cocultured with 5 × 105 PHA/IL-2–activated autologous CD4+ T cells that were prestained for CD4. Unattached T cells were carefully removed by aspiration at various times after coculture and incubated on poly-l-lysine–treated coverslips for 1 hour followed by fixation in 4% PFA. MDMs were gently washed with warm RPMI, and were fixed together with remaining attached T cells. Samples were subsequently permeabilized and stained for Gag and Env, and analyzed by LSCM. (A) HIV-1–infected MDMs in the absence of T cells. We used clone C49 to stain Gag-p17, which recognizes p17 cleaved from p55, representing mature virions. The right-hand panel is a magnified image of the boxed region from the merged image. A white line is drawn to indicate the MDM cell membrane in panels A and B. (B,C) MDM–T-cell cocultures stained for CD4, Gag, and Env. (D) Detached CD4+ T cells stained for HIV-1 Gag and CD4. White scale bars in panels A-D represent 10 μm. (E) EM analysis of the contact zone between HIV-1–infected MDMs and CD4+ T cells. Cells were cocultured for 5 hours in the presence of trace amounts of PHA (0.06 μg/mL) to stabilize clustered cells for sample preparation. M represents MDMs; T, CD4+ T cell; *, virus-containing vacuolar compartment; and V, HIV-1 particles; arrows point to closely apposed plasma membranes of MDMs and T cells. Note that many of the cell and viral membranes appear dense and strongly contrasted by the presence of ruthenium red label. Black scale bars represent 1 μm.
Macrophages transmit HIV-1 to CD4+ T cells across a virological synapse-like structure. Human MDMs were differentiated on glass coverslips for 7 days prior to infection with 6 ng CA-p24 of the HIV-1BaL isolate for a further 7 days. MDMs were subsequently cocultured with 5 × 105 PHA/IL-2–activated autologous CD4+ T cells that were prestained for CD4. Unattached T cells were carefully removed by aspiration at various times after coculture and incubated on poly-l-lysine–treated coverslips for 1 hour followed by fixation in 4% PFA. MDMs were gently washed with warm RPMI, and were fixed together with remaining attached T cells. Samples were subsequently permeabilized and stained for Gag and Env, and analyzed by LSCM. (A) HIV-1–infected MDMs in the absence of T cells. We used clone C49 to stain Gag-p17, which recognizes p17 cleaved from p55, representing mature virions. The right-hand panel is a magnified image of the boxed region from the merged image. A white line is drawn to indicate the MDM cell membrane in panels A and B. (B,C) MDM–T-cell cocultures stained for CD4, Gag, and Env. (D) Detached CD4+ T cells stained for HIV-1 Gag and CD4. White scale bars in panels A-D represent 10 μm. (E) EM analysis of the contact zone between HIV-1–infected MDMs and CD4+ T cells. Cells were cocultured for 5 hours in the presence of trace amounts of PHA (0.06 μg/mL) to stabilize clustered cells for sample preparation. M represents MDMs; T, CD4+ T cell; *, virus-containing vacuolar compartment; and V, HIV-1 particles; arrows point to closely apposed plasma membranes of MDMs and T cells. Note that many of the cell and viral membranes appear dense and strongly contrasted by the presence of ruthenium red label. Black scale bars represent 1 μm.
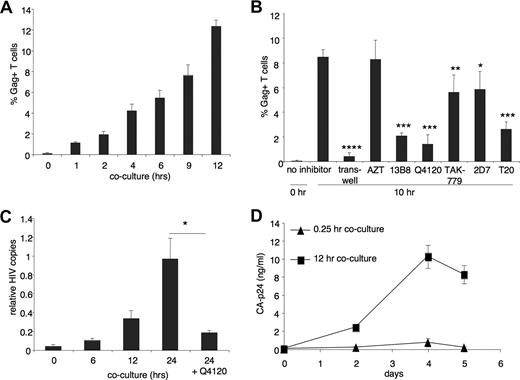
To quantify viral transmission, we harvested all T cells from cocultures with MDMs at various times, and analyzed the cells by FC. We stained for CD3 and intracellular HIV-1 Gag p24 to determine the percentage of HIV-1–positive T cells, and found that significant numbers (P < .002 [ANOVA], compared with t = 0) of Gag-positive T cells could be detected after 1 hour (Figure 2A). After 12 hours, more than 12% of 5 × 105 T cells were Gag positive. With an estimated average of 3 × 104 infected MDMs per well, based on LSCM analysis and counting, we propose that each infected MDM is able to infect at least one T cell every 6 hours. To investigate the requirement for cell-cell spread compared with cell-free virus spread, we performed an experiment using a transwell system in which infected MDMs were separated from T cells by a virus-permeable membrane. Figure 2B shows that after 10-hour coculture, 8.5% of T cells that were in contact with HIV-1–infected MDMs were infected. By contrast, cell-free virus infection resulted in 0.5% of HIV-1–infected cells, indicating that cell-cell viral spread is at least 10-fold more efficient than cell-free spread. The presence of RT inhibitor AZT did not reduce the percentage of Gag-positive T cells, demonstrating that the Gag measured by FC fully represents transmitted virus, and not newly produced Gag in the T cells. To investigate the role of HIV-1 receptor interactions, we tested antibody inhibitors of gp120-CD4 (13B.8.2,16 Q412013 ) and gp120-CCR5 (2D717 ), a small molecule inhibitor of gp120-CCR5 interaction (TAK-77918 ), and a fusion inhibitor (T2019 ). Viral transmission was substantially inhibited by CD4-blocking antibodies (Figure 2B) without any obvious reduction in the amount of MDM–T-cell clustering, as confirmed by LSCM analysis (not shown). Blocking CCR5 with either 2D7 or TAK-779 gave more subtle but nevertheless significant inhibition, whereas T20 reduced transmission by more than 50%, indicating that gp41-mediated fusion plays a role in transmission by MDMs, just as in dendritic cell15 – and T cell5 –mediated transmission. The inability of CCR5 antagonists to potently inhibit the presence of a HIV-1 Gag signal in the T cells might be interpreted as representing a proportion of CCR5-indepenent entry, in accord with another recent study.8 However, another possibility is that HIV-1 particles released from infected MDMs attach to the surface of T cells rendering them Gag positive, but entry is inhibited due to CCR5 antagonists or T20. Part of the Gag signal in this experiment therefore may represent extracellular virus. We therefore set out to investigate whether MDM-mediated HIV-1 transfer established a productive infection in T cells. To do this, we carefully aspirated detached T cells from MDM–T-cell cocultures at 0, 6, 12, and24 hours, extracted DNA, and performed qPCR for HIV-1 pol (Figure 2C). Our results demonstrated significant (P < .02) de novo viral DNA synthesis within 6 hours of coculture, rising to more than 20-fold above baseline at 24 hours, indicating that HIV-1 transmission results in rapid initiation of viral entry and reverse transcription in CD4+ T cells. Finally, productive infection of the T cells was confirmed in a replication assay, where T cells harvested from MDM–T-cell cocultures were depleted of any residual MDMs, cultured for an additional 5 days, and supernatant p24 measured (Figure 2D).
HIV-1 transmission to CD4+ T cells is receptor and fusion dependent, and results in productive infection. (A) HIV-1–infected MDMs were cocultured with autologous CD4+ T cells for 1 to 12 hours, followed by harvesting of the T cells with cold 5 mM EDTA/PBS. Cells were subsequently stained for CD3 and intracellular CA-p24 and analyzed by FC to determine the percentage of HIV-1–positive T cells. Data shown represent means of 3 independent experiments, and error bars represent SEM. (B) HIV-1–infected MDMs were cocultured with CD4+ T cells that had been preincubated with several inhibitors (1 hour, 37°C), followed by harvesting of the T cells after 10 hours, and staining for FC as in panel A. Alternatively, T cells were not added to MDMs directly, but were separated by a transwell (0.3-μm pore size; Costar, Cambridge, MA) to prevent cell-cell contact. Data represent the mean of triplicates in a single experiment and error bars represent + 1 SD. *P < .02; **P < .03; ***P < .001; ****P < .001, ANOVA. (C) Detection of HIV-1 reverse transcription using qPCR. HIV-1–infected MDMs were cocultured with autologous CD4+ T cells for 0, 6, 12, and 24 hours, followed by gentle aspiration of the T cells with warm RPMI, lysis, and DNA isolation and purification. qPCR using HIV-1 pol primers was performed to measure de novo viral DNA synthesis. Data were normalized to human β-globin. Data represent the mean of triplicates in a single experiment and error bars represent + 1 SD and *P < .02, ANOVA. (D) Replication of HIV-1 after transmission. CD4+ T cells were collected from 12-hour cocultures with HIV-1–infected MDMs, depleted of all MDMs with CD14 beads (Miltenyi-Biotec), and cultured for an additional week (105/well/250 μL). Viral replication was detected by p24 released into the supernatant by ELISA. Data represent the mean of quadruplicates in a single experiment and error bars represent + 1 SD.
HIV-1 transmission to CD4+ T cells is receptor and fusion dependent, and results in productive infection. (A) HIV-1–infected MDMs were cocultured with autologous CD4+ T cells for 1 to 12 hours, followed by harvesting of the T cells with cold 5 mM EDTA/PBS. Cells were subsequently stained for CD3 and intracellular CA-p24 and analyzed by FC to determine the percentage of HIV-1–positive T cells. Data shown represent means of 3 independent experiments, and error bars represent SEM. (B) HIV-1–infected MDMs were cocultured with CD4+ T cells that had been preincubated with several inhibitors (1 hour, 37°C), followed by harvesting of the T cells after 10 hours, and staining for FC as in panel A. Alternatively, T cells were not added to MDMs directly, but were separated by a transwell (0.3-μm pore size; Costar, Cambridge, MA) to prevent cell-cell contact. Data represent the mean of triplicates in a single experiment and error bars represent + 1 SD. *P < .02; **P < .03; ***P < .001; ****P < .001, ANOVA. (C) Detection of HIV-1 reverse transcription using qPCR. HIV-1–infected MDMs were cocultured with autologous CD4+ T cells for 0, 6, 12, and 24 hours, followed by gentle aspiration of the T cells with warm RPMI, lysis, and DNA isolation and purification. qPCR using HIV-1 pol primers was performed to measure de novo viral DNA synthesis. Data were normalized to human β-globin. Data represent the mean of triplicates in a single experiment and error bars represent + 1 SD and *P < .02, ANOVA. (D) Replication of HIV-1 after transmission. CD4+ T cells were collected from 12-hour cocultures with HIV-1–infected MDMs, depleted of all MDMs with CD14 beads (Miltenyi-Biotec), and cultured for an additional week (105/well/250 μL). Viral replication was detected by p24 released into the supernatant by ELISA. Data represent the mean of quadruplicates in a single experiment and error bars represent + 1 SD.
Our data are consistent with a central role of MDMs as an in vivo viral reservoir that efficiently seeds large numbers of T cells with HIV-1 via cell-cell spread across a VS. This finding has implications for viral pathogenesis in all immune tissues that contain cells of the macrophage lineage, with particular relevance to dense secondary lymphoid tissue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nicky Martin and Clare Jolly for helpful discussions, the Centre for AIDS Reagents, NIBSC, and the NIH AIDS Research and Reference Reagent Program for reagents, and Fondation Dormeur for the generous gift of equipment.
This work was supported by fellowship number 106731 from The American Foundation for AIDS Research (amfAR), grant G0400453 from the MRC, UK, and an award from the Neutralizing Antibody Consortium (NAC) of the International AIDS Vaccine Initiative (IAVI, New York, NY).
Authorship
Contribution: F.G. designed and performed the research, and wrote the paper; S.W. performed research; Q.J.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fedde Groot, The Sir William Dunn School of Pathology, University of Oxford, South Parks Road, Oxford OX1 3RE, United Kingdom; e-mail: fedde.groot@path.ox.ac.uk.