Abstract
Thrombocytopenia is a critical problem that occurs in many hematologic diseases, as well as after cancer therapy and radiation exposure. Platelet transfusion is the most commonly used therapy but has limitations of alloimmunization, availability, and expense. Thus, the development of safe, small, molecules to enhance platelet production would be advantageous for the treatment of thrombocytopenia. Herein, we report that an important lipid mediator and a peroxisome proliferator–activated receptor gamma (PPARγ) ligand called 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2), increases Meg-01 maturation and platelet production. 15d-PGJ2 also promotes platelet formation from culture-derived mouse and human megakaryocytes and accelerates platelet recovery after in vivo radiation-induced bone marrow injury. Interestingly, the platelet-enhancing effects of 15d-PGJ2 in Meg-01 cells are independent of PPARγ, but dependent on reactive oxygen species (ROS) accumulation; treatment with antioxidants such as glutathione ethyl ester (GSH-EE); or N-acetylcysteine (NAC) attenuate 15d-PGJ2–induced platelet production. Collectively, these data support the concept that megakaryocyte redox status plays an important role in platelet generation and that small electrophilic molecules may have clinical efficacy for improving platelet numbers in thrombocytopenic patients.
Introduction
Platelets initiate clot formation and have important roles in both innate and adaptive immunity.1,2 Loss of platelets either by their destruction in the periphery or their reduced production can occur in diseases such as immune thrombocytopenic purpura, thrombotic thrombocytopenic purpura, human immunodeficiency virus (HIV) infection, aplastic anemia, and acute respiratory distress syndrome, and in approximately 1% to 5% of people receiving heparin therapy.3 In addition, cancer chemotherapy and radiation therapy are 2 of the most common causes of thrombocytopenia. Currently, platelet transfusions are the “gold standard” for treating the life-threatening complications of thrombocytopenia. However, platelet transfusions increase the risk of inflammation, disease transmission, and are costly and not always readily available.4,5 A catastrophic event such as mass radiation exposure would leave many victims without treatment. Currently, recombinant human interleukin-11 (IL-11), the only clinically approved drug for treating thrombocytopenia, is used as an alternative to platelet transfusions to modestly raise platelet counts.6 Therefore, there remains a need for more efficacious and readily available treatments to increase platelet number.
Platelets are derived from megakaryocytes, which reside in the bone marrow.7 During megakaryocyte maturation, the polyploid cell undergoes a complex process of cytoskeletal rearrangement, followed by proplatelet elongation, and the release of cytoplasmic fragments as circulating platelets.8,9 Proteomic studies have revealed that both megakaryocytes and platelets contain proteins of unknown function. Our laboratory reported that the ligand-activated transcription factor, peroxisome proliferator–activated receptor gamma (PPARγ), is present in both megakaryocytes and platelets.10 PPARγ functions as a heterodimer with the retinoid X receptor (RXR) to regulate adipogenesis, glucose metabolism, and inflammation.11,12 We have also shown that the PPARγ ligands rosiglitazone and 15d-PGJ2 dampen thrombin-induced human platelet activation and aggregation.13 Importantly, we recently determined that PPARγ is also found in platelet microparticles released during activation.14 These findings prompted us to study the effects of PPARγ ligands on megakaryocytes.
PGJ2 and 15d-PGJ2 are natural PPARγ ligands that are biologically active metabolites of PGD2.15,16 In addition to binding with high affinity to PPARγ, both PGJ2 and 15d-PGJ2 possess an electrophilic α,β-unsaturated carbonyl group in the cyclopentanone ring that reacts covalently with certain nucleophiles in some proteins.17,18 This action accounts for many of the PPARγ-independent activities of J-type prostaglandins, which include the potentiation of apoptosis, reorganization of cytoskeletal proteins, and generation of reactive oxygen species (ROS). In this study, we determined the effect of key synthetic and natural PPARγ ligands on megakaryocyte function using human and mouse models of thrombopoiesis. We found that the electrophilic prostaglandin, 15d-PGJ2, enhances platelet formation from megakaryocytes in vitro and in vivo.
Methods
Reagents and antibodies
15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) and rosiglitazone were purchased from Biomol (Plymouth Meeting, PA); 15-deoxy-Δ12,14-PG22 (15d-PGD2), PGJ2, 9,10 dihydro-15d-PGJ2 (CAY10410), PGE2, PGI2, PGF2α, and GW9662 were purchased from Cayman Chemical (Ann Arbor, MI); N-acetylcysteine (NAC), glutathione reduced ethyl ester (GSH-EE), and fibrinogen were all purchased from Sigma-Aldrich (St Louis, MO); 5-(and-6-)-carboxy-2′,7′-dicholorodihydrofluorescein diacetate (carboxy-H2DCFDA) and MitoSOX Red were purchased from Invitrogen (Carlsbad, CA); collagen from Chrono-log Corporation (Havertown, PA); CD61-FITC (GPIIIa), CD41-FITC (GPIIb), CD61-PE, and annexin V-FITC were purchased from BD Biosciences (San Jose, CA); GPIbβ and GPV were purchased from Emfret Analytics (Wurtzburg, Germany); recombinant human thrombopoietin (rhTPO) was purchased from R&D Systems (Minneapolis, MN); BIT9500 was purchased from StemCell Technologies (Vancouver, BC); and total actin (CP-01) was from Oncogene (Cambridge, MA).
Cell line culture and treatment conditions
Meg-01 and M07e cells were purchased from ATCC (Rockville, MD), and Dami cells were a generous gift from Dr Patricia J. Simpson-Haidaris (University of Rochester). All cells were cultured in RPMI-1640 tissue culture medium (Invitrogen) supplemented with 5% fetal bovine serum (FBS; Invitrogen), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma-Aldrich), 2 mM l-glutamine (Invitrogen), 4.5 g/L glucose (Invitrogen), and 50 μg/mL gentamicin (Invitrogen). M07e cells were also supplemented with 100 ng/mL of granulocyte monocyte–colony stimulating factor (GM-CSF, R&D Systems). All treatments were performed in normal growth media. Dimethyl sulfoxide (DMSO) was used as a vehicle (control) in the following experiments. Animal protocols used in this study were approved by the University of Rochester Committee on Animal Resources.
Megakaryocyte differentiation from mouse bone marrow
Bone marrow was isolated from the femora of male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) and plated at a concentration of 106 cells per well in 12-well plates in Iscove modified Dulbecco media (IMDM; Invitrogen) with 20% BIT 9500 (bovine serum albumin [BSA], insulin, transferrin) and supplemented with 100 ng/mL of rhTPO. Cells were cultured for 5 days, after which 15d-PGJ2 (10 μM) was added to the culture medium for 24 hours. On the sixth day, cells were examined by phase-contrast microscopy using an inverted Olympus IX81 microscope (Olympus, Center Valley, PA), and images were captured using SPOT RT software (Diagnostic Instruments, Sterling Heights, MI). Megakaryocytes were identified by preincubating with anti–mouse CD16/32 (FcγR-blocking antibody) and then incubating with phycoerythrin (PE)-conjugated hamster anti–mouse CD41 antibody.
Megakaryocyte differentiation from human cord blood-derived CD34+ cells
Human CD34+ cord blood cells were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). Cells were plated at 2.5 × 105 cells per well in a 12-well plate and cultured in serum-free medium as previously described19 and supplemented with 100 ng/mL rhTPO. After 14 days in culture, megakaryocytes were identified by staining with a CD61-FITC antibody and analyzed on a BD Biosciences FACSCalibur flow cytometer. Data were analyzed using FlowJo software (TreeStar, Ashland, OR). Cell images were captured using an Olympus IX81 inverted microscope.
Platelet isolation and platelet production analysis
Platelets were isolated from megakaryocytes by centrifugation at 150g for 10 minutes. Supernatants underwent sequential centrifugation (500g for 10 minutes and 1000g for 10 minutes). The remaining platelet pellet was fixed then permeabilized with a CD61 or CD41 antibody. 7-AAD (BD Pharmingen) was added for 10 minutes before analyzing the cells by flow cytometry. Platelets were identified using 3 well-described parameters7,20-22 : size (using normal human platelets as a control), presence of CD61 or CD41, and lack of DNA as determined by the absence of 7-AAD staining. Ten thousand platelet events were collected, and platelets were quantified by rate (platelet events/second).23
Human blood platelet isolation
Whole blood was obtained under University of Rochester Institutional Review Board approval from male and female donors by venipuncture into a citrate phosphate dextrose adenine solution containing collection bag (Baxter Fenwal, Round Lake, IL) or vacutainer tubes containing buffered citrate sodium (BD Biosciences, Franklin Lakes, NJ). Platelets were then isolated as described.13,14 On average, 5.5 × 1010 platelets/unit of blood were obtained, and the platelet purity was greater than 99%. Informed consent was obtained from all donors in accordance with the Declaration of Helsinki.
Platelet staining and collagen-induced functional assays
Platelets were isolated from the Meg-01 cells and human blood. Both normal human platelets and Meg-01–derived platelets were stained with annexin V-FITC then treated with 10 μg/mL of collagen for 15 minutes. Cells were analyzed for collagen-induced annexin V binding and change in size and shape by flow cytometry. Platelets were isolated from primary human megakaryocytes and primary mouse megakaryocytes. Primary human platelets were stained with CD41-FITC, and primary mouse platelets were stained with CD61-PE then treated with 10 μg/mL of collagen. Cells were analyzed for collagen-induced CD61/CD41 up-regulation and change in size and shape using flow cytometry.
Phalloidin staining
Cells were fixed and permeabilized with 0.1% Triton-100 for 1 hour at room temperature (RT). Subsequently, cells were blocked with 1% BSA in phosphate buffered saline (PBS)–Tween 20 (0.1%) for 1 hour at RT, then stained with phalloidin-Alexa Fluor 488 (Invitrogen, 1:200) at RT for 2 hours. Cells were mounted and visualized using an Olympus BX51 light microscope (Olympus, Melville, NY).
Transmission electron microscopy
Cells were fixed for 4 hours in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. After fixation, cells were placed in 1% osmium tetroxide in phosphate buffer, dehydrated in ethanol, embedded in Epon/Araldite epoxy resin, and analyzed on a Hitachi H-7100 electron microscope.
Platelet spreading
Coverslips were coated with 100 μg/mL fibrinogen for 1 hour and blocked with 0.5 mg/mL BSA for 1 hour. Platelets were added to the fibrinogen-coated coverslips in Tyrode buffer and fixed with glutaraldehyde, postfixed with 1% osmium tetroxide, and dehydrated. Platelets were then coated with a gold/palladium film. Images were acquired using the Zeiss-Leo 982 FE-SEM (scanning electron microscope).
PPARγ gene reporter analysis
Meg-01 cells were plated at a density of 1.6 × 107 cells per well in a 6-well plate and transfected, using lipofectamine LTX (Invitrogen), with a PPRE (peroxisome proliferator response element)–luciferase reporter plasmid containing 3 copies of the ACO-PPRE (a gift from Dr B. Seed, Massachusetts General Hospital, Boston, MA). Cells were then treated with 10 μM 15d-PGJ2, 9,10 dihydro-15d-PGJ2, or rosiglitazone 24 hours after transfection. Transfection efficiency was determined by transfecting the cells with green fluorescent protein (GFP) plasmid and determining the percentage of cells that were GFP+ by flow cytometry. All cell treatment groups exhibited similar transfection efficiencies. Luciferase activity was assessed 24 hours after ligand treatment using the promega luciferase assay system, and relative light units (RLUs) were determined using a lumicount microplate luminometer (Packard Instrument, Meriden, CT).
Construction of lentiviral vector expressing PPARγ siRNA
A 19-bp target sequence was selected to knock down PPARγ. PPARγ was cloned downstream of the RNA polymerase III U6 promoter and subcloned into a FG12 lentiviral vector, which expresses GFP under the ubiquitin C promoter. HEK 293 cells expressing this siRNA PPARγ had reduced PPARγ protein levels (> 90%; data not shown). To make the lentivirus, HEK 293 cells were cotransfected with 3 plasmids: the envelope vector (CMV-VSVG), the transfer vector (FG12-shRNAPPAR), and the packaging construct (pCMVΔ89.2 [gag/pol proteins]). Viral supernatants were collected every 8 hours for 2 days and concentrated by ultracentrifugation at 50 000g for 2 hours at 4°C using a Beckman SW 28 rotor. Viral titers were determined by infection of HeLa cells with serial dilutions of the viral stocks. Meg-01 cells were infected at a multiplicity of infection (MOI) of 20. After 48 hours, approximately 56% of the PPARγ siRNA cells were transduced as determined by flow cytometry. The GFP+ cells were then sorted by FACS and grown in RPMI with 5% FBS.
Western blotting for PPARγ
Western blot for PPARγ was performed as previously described.13,14 Briefly, a rabbit antihuman polyclonal primary antibody against PPARγ (Biomol) was added at a 1:1000 dilution in 5% nonfat milk for 1 hour at room temperature. The secondary Ab (Jackson ImmunoResearch Laboratories) was added at a 1:10 000 dilution for 1 hour in 2.5% nonfat milk. Membranes were visualized with enhanced chemiluminescence (ECL; Pierce, Rockford, IL).
Reactive oxygen species production
Ten micromoles carboxy-H2DCFDA was added to cells for 20 minutes at RT. The cells were washed and immediately analyzed by flow cytometry. Five micromoles MitoSOX Red dye in Hanks balanced salt solution (HBSS) containing Mg 2+ and Ca 2+ was added to the cells for 15 minutes at 37°C. The cells were washed in HBSS and analyzed by flow cytometry.
DNA content analysis
Cells were fixed in 95% ethanol, treated with RNAse (Sigma-Aldrich) followed by 20 μg/mL propidium iodide (Sigma-Aldrich) to stain DNA, and analyzed by flow cytometry.
Megakaryocyte progenitor (Meg-CFCs) assay
Primary mouse bone marrow cells were suspended in IMDM at a concentration of 8 × 106 cells/mL. Two hundred thousand cells were plated in IMDM supplemented with 20% BIT9500, 0.2% 2-mercaptoethanol, 2% glutamax, and 20% cellgro-H2O (Mediatech, Herndon, VA), and cultured with 50 ng/mL rhTPO, 10 ng/mL IL-3, 20 ng/mL IL-6, 50 ng/mL IL-11 (all Preprotech, Rocky Hill, NJ), and collagen at 37° for 7 days.24 Collagen gels were dehydrated, fixed, and labeled with GPIbβ and GPV, modified by ABC (Vector Labs, Burlingame, CA), and developed in Vector Red (Vector Labs). Meg-CFCs were defined by their ability to generate colonies containing at least 3 megakaryocytes.
Irradiation-induced thrombocytopenia
Seven- or eight-week-old male C57BL/6 mice (Jackson Laboratories) were either exposed to 5 Gy cesium (Cs137) total body irradiation (TBI) using a Model 8114 6000 Curie Shepherd Cs137 irradiator (approximately 3200 Curie sealed Cs137 source) with a dose rate of 177.9 Gy/min or left unexposed. Mice (n = 4) received intravenous injections of vehicle (8% DMSO) or 15d-PGJ2 (1 mg/kg) for 4 consecutive days from day 1 (days 1, 2, 3, and 4 after irradiaton). Blood was obtained from the aorta or the orbital sinus. Platelets were counted before radiation exposure and on days 10, 22, and 31 after radiation exposure using the Heska CBC-Diff veterinary analyzer (Fort Collins, CO). Data are expressed as average platelet counts from 2 separate experiments. For the unirradiated mice, platelets were counted on days 4, 10, and 15.
Statistical analysis
Results are expressed as the mean plus or minus standard deviation (SD). Statistical analysis was performed using a paired, 2-tailed Student t test with P values less than .05 deemed statistically significant. All experiments were repeated at least 3 times unless otherwise stated.
Results
Small electrophilic molecules induce platelet formation
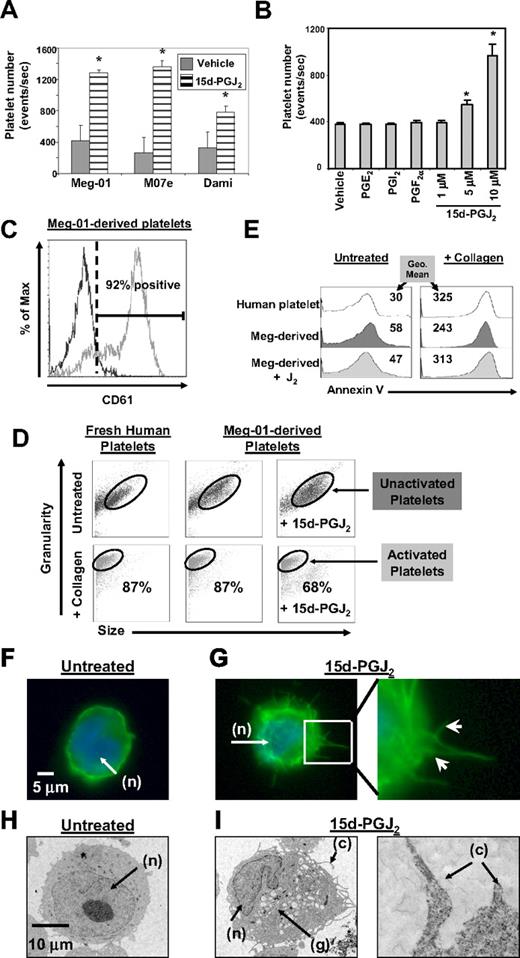
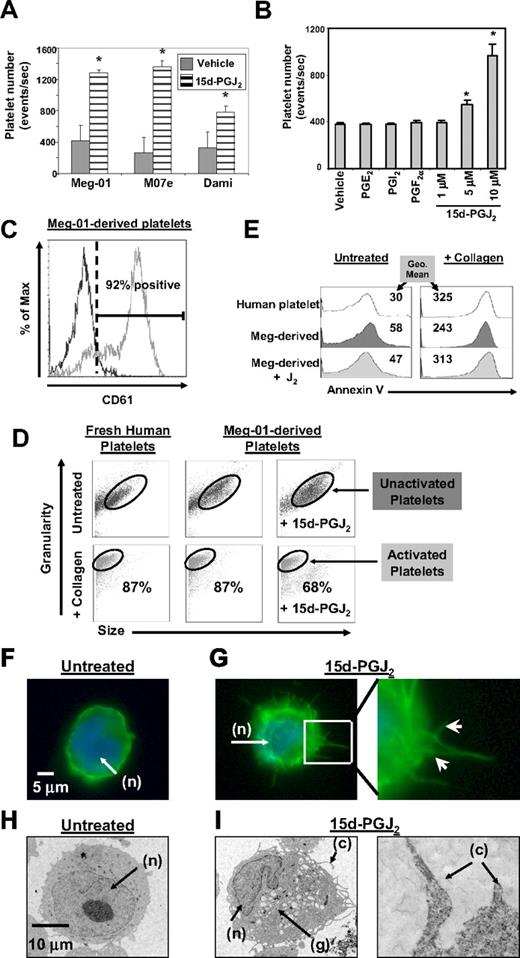
Our initial studies demonstrated that 3 well-described megakaryocytic cell lines (Meg-01, M07e, Dami) all increased platelet production after exposure for 24 hours to 15d-PGJ2 (Figure 1A). Further studies were completed on Meg-01 cells, as this cell produces platelets similar in structure and function to freshly isolated human platelets.25 Treatment of Meg-01 cells with increasing concentrations of 15d-PGJ2 caused a dose-dependent increase in platelet production (Figure 1B). However, not every type of prostaglandin enhanced platelet production. For example, PGE2, PGI2, and PGF2α, which are not PPARγ ligands, failed to enhance platelet production (Figure 1B). Platelets derived from Meg-01 cells express the platelet marker CD61 (glycoprotein IIIa, Figure 1C).
15d-PGJ2 enhances platelet production from megakaryoblast cell lines. (A) Meg-01, M07e, or Dami cells (106) were treated with 15d-PGJ2 (10 μM) for 24 hours. 15d-PGJ2 increases platelet production in Meg-01, M07e, and Dami cells after 24 hours. (B) Meg-01 (106) cells were treated with prostaglandins for 24 hours. 15d-PGJ2 dose-dependently increased platelet production in Meg-01 cells after 24 hours, unlike PGE2 (10 μM), PGI2 (10 μM), and PGF2α (10 μM). (C) 92% of the Meg-01–derived platelets expressed the platelet surface marker CD61. Isotype control is shown by the left histogram. Results are presented as mean plus or minus SD (P < .05). (D) Forward and side scatter plots showing that platelets produced from untreated and 15d-PGJ2–treated (10 μM for 24 hours). Meg-01 cells mimic freshly isolated human platelets in their ability to undergo shape change with 15 minutes of collagen treatment (10 μg/mL). Eighty-seven percent of human platelets, 87% of Meg-01–derived platelets, and 68% of the platelets produced from 15d-PGJ2–treated Meg-01 cells decreased their size (forward scatter) and increased their granularity (side scatter) in response to collagen. (E) Histogram plots showing that Meg-01–derived platelets are similar to normal human platelets in their ability to undergo annexin V binding in both the presence and absence of collagen. Values are geometric mean fluorescence intensity. (F) Meg-01 cells were untreated or treated with DMSO (vehicle) or 15d-PGJ2 (10 μM) for 2 hours. Phalloidin staining of f-actin fibers shown by fluorescence microscopy in an untreated cell. Note DAPI-stained nucleus (n) and smooth rounded cell surface. (G) Phalloidin staining of f-actin fibers shown by fluorescence microscopy. Left picture shows heavy phalloidin staining of organized f-actin bundles in a cell treated with 10 μM of 15d-PGJ2 for 2 hours. Right picture shows enlarged (×40) section highlighting membrane demarcations. Arrows show heavy phalloidin staining of f-actin bundles. (H) Meg-01 cells were untreated or treated with 10 μM of 15d-PGJ2 for 24 hours. Transmission electron microscopy (TEM) shows an untreated cell; note smooth round nucleus (n) and the absence of granules. (I) Left TEM picture shows a cell treated with 10 μM of 15d-PGJ2 for 24 hours. Note horseshoe-shaped nucleus (n), granule content (g), and cytoplasmic extensions (c). Right picture shows enlarged section highlighting cytoplasmic extensions.
15d-PGJ2 enhances platelet production from megakaryoblast cell lines. (A) Meg-01, M07e, or Dami cells (106) were treated with 15d-PGJ2 (10 μM) for 24 hours. 15d-PGJ2 increases platelet production in Meg-01, M07e, and Dami cells after 24 hours. (B) Meg-01 (106) cells were treated with prostaglandins for 24 hours. 15d-PGJ2 dose-dependently increased platelet production in Meg-01 cells after 24 hours, unlike PGE2 (10 μM), PGI2 (10 μM), and PGF2α (10 μM). (C) 92% of the Meg-01–derived platelets expressed the platelet surface marker CD61. Isotype control is shown by the left histogram. Results are presented as mean plus or minus SD (P < .05). (D) Forward and side scatter plots showing that platelets produced from untreated and 15d-PGJ2–treated (10 μM for 24 hours). Meg-01 cells mimic freshly isolated human platelets in their ability to undergo shape change with 15 minutes of collagen treatment (10 μg/mL). Eighty-seven percent of human platelets, 87% of Meg-01–derived platelets, and 68% of the platelets produced from 15d-PGJ2–treated Meg-01 cells decreased their size (forward scatter) and increased their granularity (side scatter) in response to collagen. (E) Histogram plots showing that Meg-01–derived platelets are similar to normal human platelets in their ability to undergo annexin V binding in both the presence and absence of collagen. Values are geometric mean fluorescence intensity. (F) Meg-01 cells were untreated or treated with DMSO (vehicle) or 15d-PGJ2 (10 μM) for 2 hours. Phalloidin staining of f-actin fibers shown by fluorescence microscopy in an untreated cell. Note DAPI-stained nucleus (n) and smooth rounded cell surface. (G) Phalloidin staining of f-actin fibers shown by fluorescence microscopy. Left picture shows heavy phalloidin staining of organized f-actin bundles in a cell treated with 10 μM of 15d-PGJ2 for 2 hours. Right picture shows enlarged (×40) section highlighting membrane demarcations. Arrows show heavy phalloidin staining of f-actin bundles. (H) Meg-01 cells were untreated or treated with 10 μM of 15d-PGJ2 for 24 hours. Transmission electron microscopy (TEM) shows an untreated cell; note smooth round nucleus (n) and the absence of granules. (I) Left TEM picture shows a cell treated with 10 μM of 15d-PGJ2 for 24 hours. Note horseshoe-shaped nucleus (n), granule content (g), and cytoplasmic extensions (c). Right picture shows enlarged section highlighting cytoplasmic extensions.
We next determined whether the platelets produced by 15d-PGJ2 treatment were responsive to known activators. In normal human platelets, collagen promotes shape-change and phosphatidylserine becomes highly expressed on the surface of platelets.26-28 Therefore, annexin V staining was used to distinguish platelets from apoptotic bodies and as a quantitative measure of platelet activation. Meg-01 cells were untreated or treated with 15d-PGJ2 and the Meg-01–derived platelets were isolated and treated with collagen. After collagen activation, Meg-01–derived platelets changed size and shape, as indicated by a decrease in forward scatter (size) and an increase in side scatter (granularity; Figure 1D). In addition, collagen increased the binding of annexin V in both Meg-01–derived platelets and normal human platelets (Figure 1E). These data indicate that Meg-01–derived platelets and normal human platelets have similar responses to collagen stimulation.
Morphologic changes, characteristic of megakaryopoiesis, in Meg-01 cells were evident within 1 to 2 hours of 15d-PGJ2 treatment, as revealed by staining for filamentous (f)-actin (Figure 1F-G). Arrows in Figure 1G show actin bundles protruding from the cell membrane. The formation of these protrusions are associated with megakaryopoiesis, as actin is found in proplatelet extensions and is important for the bending and bifurcation of the branches.8,29 These membrane protrusions were absent in untreated Meg-01 cells (Figure 1F). Meg-01 cells were also analyzed by transmission electron microscopy (TEM). Within 24 hours of 15d-PGJ2 treatment, Meg-01 cells exhibited structural characteristics consistent with differentiating cells, such as granule formation (g), a horseshoe-shaped nucleus (n), and the elongation of cytoplasmic extensions (c) (Figure 1I). Figure 1I confirms the presence of the cytoplasmic extensions seen with phalloidin staining. These phenotypic changes are absent in untreated cells (Figure 1H).
15d-PGJ2 promotes platelet production from mouse megakaryocytes
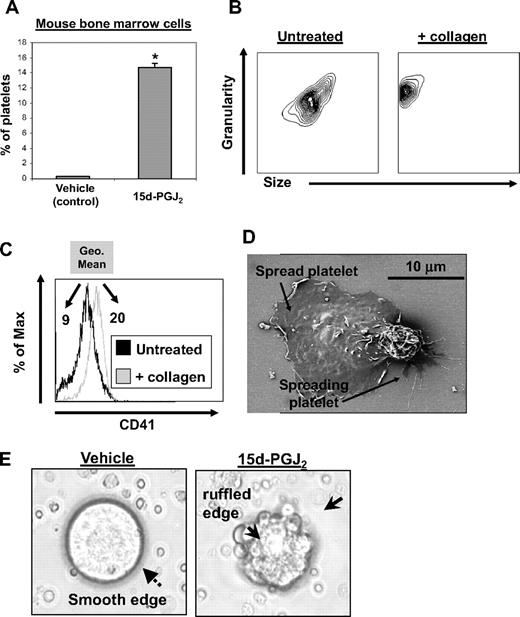
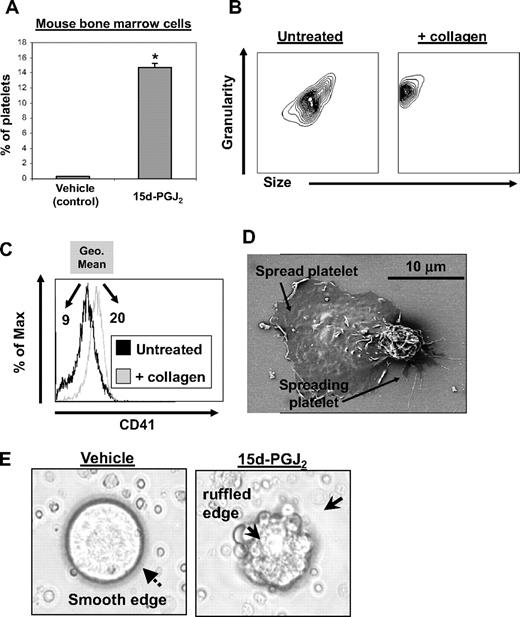
To evaluate whether 15d-PGJ2 affects platelet production from normal mouse megakaryocytes, primary mouse bone marrow cells were cultured for 5 days with rhTPO to promote megakaryocyte enrichment and maturation. After culture, bone marrow cells were treated with 15d-PGJ2 for 24 hours. 15d-PGJ2 increased the percentage of CD61 + platelets in culture by approximately 14% (Figure 2A). These mouse bone marrow–derived platelets exhibited normal responses to collagen, as they changed shape (decreased in size and increased in granularity) and increased their surface expression of CD41 (GPIIb, Figure 2B,C). Geometric mean fluorescence intensity increased from 9 to 20 after collagen treatment. Mouse bone marrow culture–derived platelets show functionality, as they spread in response to fibrinogen (Figure 2D). 15d-PGJ2 also induced the appearance of ruffled edges on the megakaryocytes, commonly observed during membrane blebbing of platelets, whereas vehicle-treated megakaryocytes exhibited a smooth surface (Figure 2E).
15d-PGJ2 enhances platelet production from mouse megakaryocytes in vitro. Bone marrow isolated from C57BL/6 mice was cultured for 5 days in the presence of rhTPO. On day 5 of culture, cells were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours. Cells were photographed in culture and platelet production was analyzed by flow cytometry. (A) Shown are the percentage of total cells from bone marrow cultures that are CD61+ platelets. Bone marrow cultures that were treated with either vehicle or 15d-PGJ2. Results are presented as mean plus or minus SD (P < .01). (B) Platelets were isolated from other cells in culture by gradient centrifugation from mouse bone marrow cultures treated with 15d-PGJ2. Left plot shows the forward and side scatter of untreated platelets. Right plot shows the forward and side scatter of platelets treated with collagen (10 μg/mL for 15 minutes). Note the decrease in size and increase in granularity. (C) Histogram showing the up-regulation of surface CD41 with collagen treatment. Values are a measure of geometric mean fluorescence intensity. (D) Scanning electron microscopy showing 2 culture-derived mouse platelets spread on a fibrinogen-coated slide. (E) Left picture shows microscopy of a mouse megakaryocyte cultured in the absence of 15d-PGJ2. Note the smooth surface. Right picture shows microscopy of a mouse megakaryocyte cultured in the presence of 15d-PGJ2. Note the ruffled surface, characteristic of morphologic changes that promote proplatelet formation.
15d-PGJ2 enhances platelet production from mouse megakaryocytes in vitro. Bone marrow isolated from C57BL/6 mice was cultured for 5 days in the presence of rhTPO. On day 5 of culture, cells were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours. Cells were photographed in culture and platelet production was analyzed by flow cytometry. (A) Shown are the percentage of total cells from bone marrow cultures that are CD61+ platelets. Bone marrow cultures that were treated with either vehicle or 15d-PGJ2. Results are presented as mean plus or minus SD (P < .01). (B) Platelets were isolated from other cells in culture by gradient centrifugation from mouse bone marrow cultures treated with 15d-PGJ2. Left plot shows the forward and side scatter of untreated platelets. Right plot shows the forward and side scatter of platelets treated with collagen (10 μg/mL for 15 minutes). Note the decrease in size and increase in granularity. (C) Histogram showing the up-regulation of surface CD41 with collagen treatment. Values are a measure of geometric mean fluorescence intensity. (D) Scanning electron microscopy showing 2 culture-derived mouse platelets spread on a fibrinogen-coated slide. (E) Left picture shows microscopy of a mouse megakaryocyte cultured in the absence of 15d-PGJ2. Note the smooth surface. Right picture shows microscopy of a mouse megakaryocyte cultured in the presence of 15d-PGJ2. Note the ruffled surface, characteristic of morphologic changes that promote proplatelet formation.
15d-PGJ2 promotes platelet production from human megakaryocytes
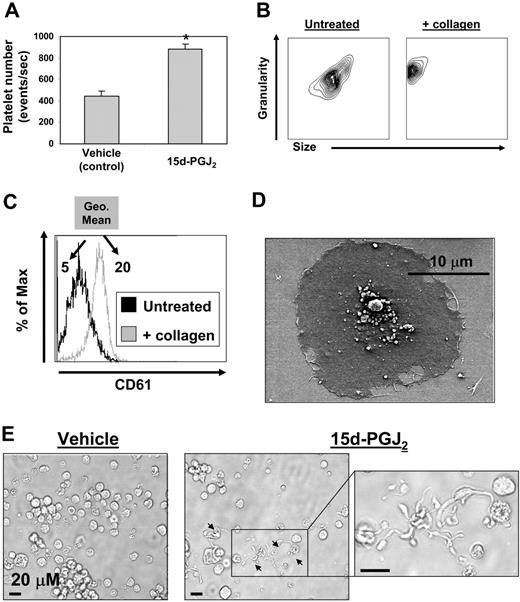
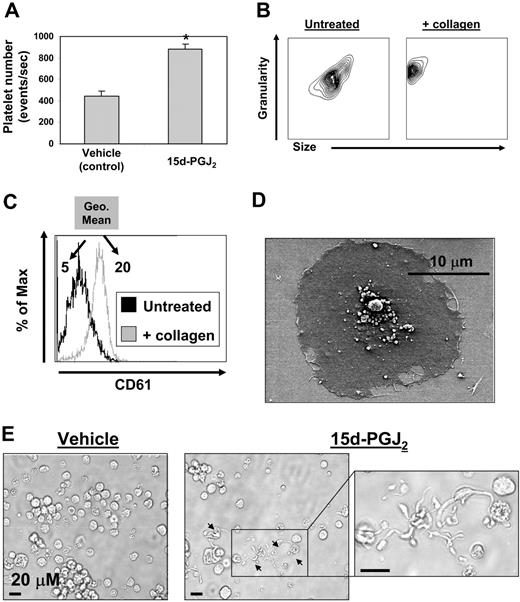
Cord blood–derived CD34+ cells (99% pure) were cultured for 14 days with rhTPO, at which time more than 90% of the cells expressed CD61 (data not shown). The cultures consisted of a mixed population of both immature and mature megakaryocytes as determined by ploidy observed by Diff-Quik staining (data not shown). These CD61+ cells were then treated with 15d-PGJ2 (10 μM) for 24 hours and platelet production was assessed by flow cytometry. Treatment with 15d-PGJ2 doubled the number of platelets generated from these human megakaryocytes as determined by presence of the platelet marker CD61 and the absence of DNA (Figure 3A). Flow cytometric data showed that human culture–derived platelets exhibited normal responses to collagen, as they changed shape (decreased in size and increased in granularity) and increased their surface expression of CD61 (Figure 3B,C). Geometric mean fluorescence intensity increased from 5 to 20 after collagen treatment. In addition, these culture-derived platelets showed functionality, as they spread in response to fibrinogen (Figure 3D). 15d-PGJ2 also induced proplatelet formation (Figure 3E).
Megakaryocytes generated from human CD34+ cells in vitro exhibit increased platelet production with 15d-PGJ2 treatment. Generating megakaryocytes and platelets from human cord blood–derived CD34+ cells in vitro. CD34+ cells cultured in the presence of rhTPO for 14 days are 92% positive for CD61 (data not shown). CD61 expressing cells generated from cord blood were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours, and cells were photographed in culture and platelet production was analyzed by flow cytometry. (A) Bar graph showing that 15d-PGJ2 increases the number of platelets derived from primary human megakaryocytes. Results are presented as mean plus or minus SD (P < .01). (B) Platelets were isolated by gradient centrifugation from CD61+ cell cultures treated with 15d-PGJ2. Left plot shows the forward and side scatter of untreated platelets. Right plot shows the forward and side scatter of platelets treated with collagen (10 μg/mL for 15 minutes). Note the decrease in size and increase in granularity. (C) Histogram showing the up-regulation of surface CD61 with collagen treatment. Values are a measure of geometric mean fluorescence intensity. (D) SEM showing a culture-derived human platelet spread on a fibrinogen-coated slide. (E) Left picture shows microscopy of a human megakaryocyte cultured in the absence of 15d-PGJ2. Middle picture shows microscopy of a human megakaryocyte cultured in the presence of 15d-PGJ2. Arrows indicate proplatelets. Far right picture shows magnification of proplatelet extensions.
Megakaryocytes generated from human CD34+ cells in vitro exhibit increased platelet production with 15d-PGJ2 treatment. Generating megakaryocytes and platelets from human cord blood–derived CD34+ cells in vitro. CD34+ cells cultured in the presence of rhTPO for 14 days are 92% positive for CD61 (data not shown). CD61 expressing cells generated from cord blood were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours, and cells were photographed in culture and platelet production was analyzed by flow cytometry. (A) Bar graph showing that 15d-PGJ2 increases the number of platelets derived from primary human megakaryocytes. Results are presented as mean plus or minus SD (P < .01). (B) Platelets were isolated by gradient centrifugation from CD61+ cell cultures treated with 15d-PGJ2. Left plot shows the forward and side scatter of untreated platelets. Right plot shows the forward and side scatter of platelets treated with collagen (10 μg/mL for 15 minutes). Note the decrease in size and increase in granularity. (C) Histogram showing the up-regulation of surface CD61 with collagen treatment. Values are a measure of geometric mean fluorescence intensity. (D) SEM showing a culture-derived human platelet spread on a fibrinogen-coated slide. (E) Left picture shows microscopy of a human megakaryocyte cultured in the absence of 15d-PGJ2. Middle picture shows microscopy of a human megakaryocyte cultured in the presence of 15d-PGJ2. Arrows indicate proplatelets. Far right picture shows magnification of proplatelet extensions.
Platelet production induced by 15d-PGJ2 is independent of PPARγ
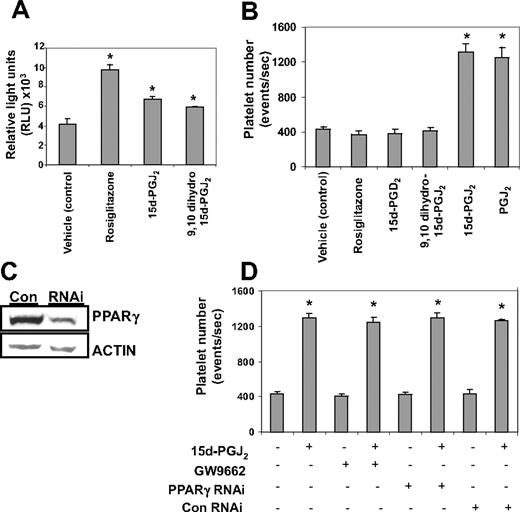
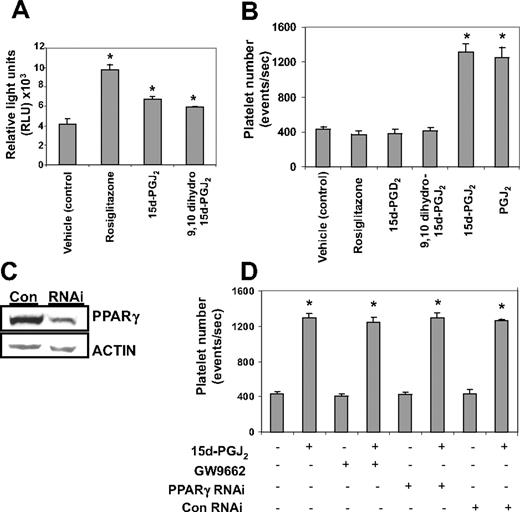
We next investigated whether 15d-PGJ2 enhanced platelet production was PPARγ-dependent or -independent. All PPARγ ligands tested (15d-PGJ2, 9,10 dihydro-15d-PGJ2, and rosiglitazone) activated PPARγ (Figure 4A). Despite this, only the electrophilic prostaglandins (PGJ2 and 15d-PGJ2) enhanced Meg-01 platelet production (Figure 4B). Nonelectrophilic thiazolidinedione-type drugs such as rosiglitazone and the nonelectrophilic prostaglandins,9,10 dihydro-15d-PGJ2 and 15d-PGD2, failed to enhance platelet production. (Figure 4B). This suggests that the effects of these agents are independent of PPARγ. The involvement of PPARγ in the platelet-generating effects of 15d-PGJ2 was further analyzed by infecting Meg-01 cells with a PPARγ siRNA lentivirus (Figure 4C). As shown in Figure 4D, knocking down PPARγ protein failed to attenuate the platelet-enhancing effects of 15d-PGJ2. In addition, the ability of 15d-PGJ2 to enhance platelet production was not blocked by the small molecule, irreversible PPARγ antagonist, GW9662.30 This further supports that the effects of 15d-PGJ2 are PPARγ-independent.
Platelet production from Meg-01 cells by 15d-PGJ2 is independent of PPARγ. (A) Cells were transiently transfected with a PPRE-luciferase construct and treated with either 10 μM 15d-PGJ2, 9,10 dihydro-15d-PGJ2, or rosiglitazone. Twenty-four hours after ligand treatment, a luciferase assay was performed. Cells treated with PPARγ ligands had increased luciferase activity compared with the untreated cells. (B) Meg-01 cells were treated with DMSO (vehicle control) or with 10 μM rosiglitazone, 15d-PGD2, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, or PGJ2 and platelet number was assessed by flow cytometry. Results are presented as mean plus or minus SD (P < .01). (C) Western blot showing that cells infected with PPARγ-siRNA have 66% less PPARγ protein compared with cells infected with the control (con) virus. (D) Cells either infected with lentivirus PPARγ-siRNA or pretreated for 2 hours with 100 nM GW9662, an irreversible PPARγ antagonist, were treated with 15d-PGJ2 for 24 hours. Platelet production was assessed by flow cytometry. Results are presented as mean plus or minus SD (P < .01, n = 3).
Platelet production from Meg-01 cells by 15d-PGJ2 is independent of PPARγ. (A) Cells were transiently transfected with a PPRE-luciferase construct and treated with either 10 μM 15d-PGJ2, 9,10 dihydro-15d-PGJ2, or rosiglitazone. Twenty-four hours after ligand treatment, a luciferase assay was performed. Cells treated with PPARγ ligands had increased luciferase activity compared with the untreated cells. (B) Meg-01 cells were treated with DMSO (vehicle control) or with 10 μM rosiglitazone, 15d-PGD2, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, or PGJ2 and platelet number was assessed by flow cytometry. Results are presented as mean plus or minus SD (P < .01). (C) Western blot showing that cells infected with PPARγ-siRNA have 66% less PPARγ protein compared with cells infected with the control (con) virus. (D) Cells either infected with lentivirus PPARγ-siRNA or pretreated for 2 hours with 100 nM GW9662, an irreversible PPARγ antagonist, were treated with 15d-PGJ2 for 24 hours. Platelet production was assessed by flow cytometry. Results are presented as mean plus or minus SD (P < .01, n = 3).
15d-PGJ2 induces reactive oxygen species formation
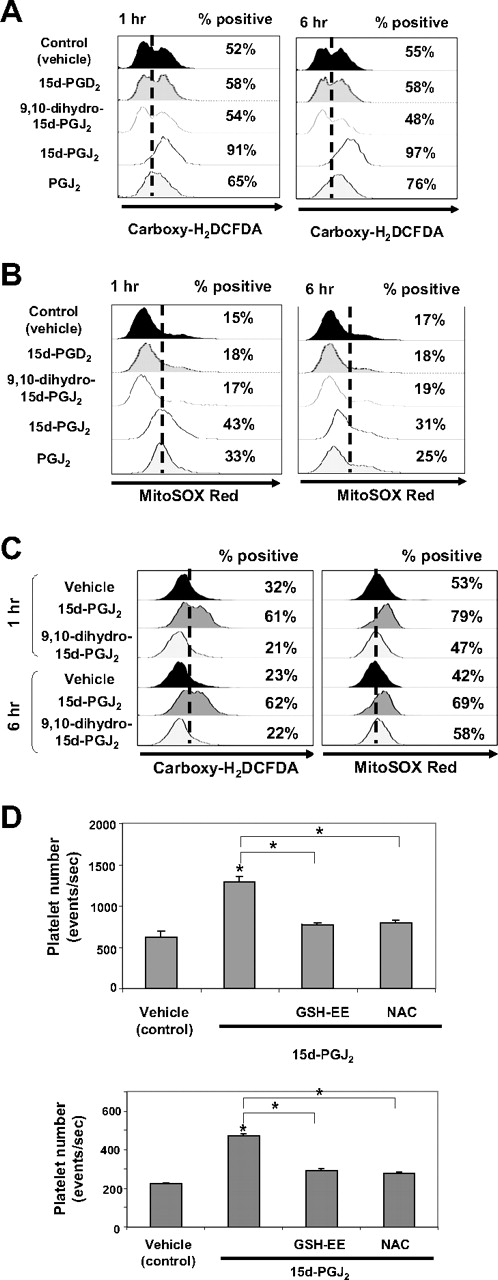
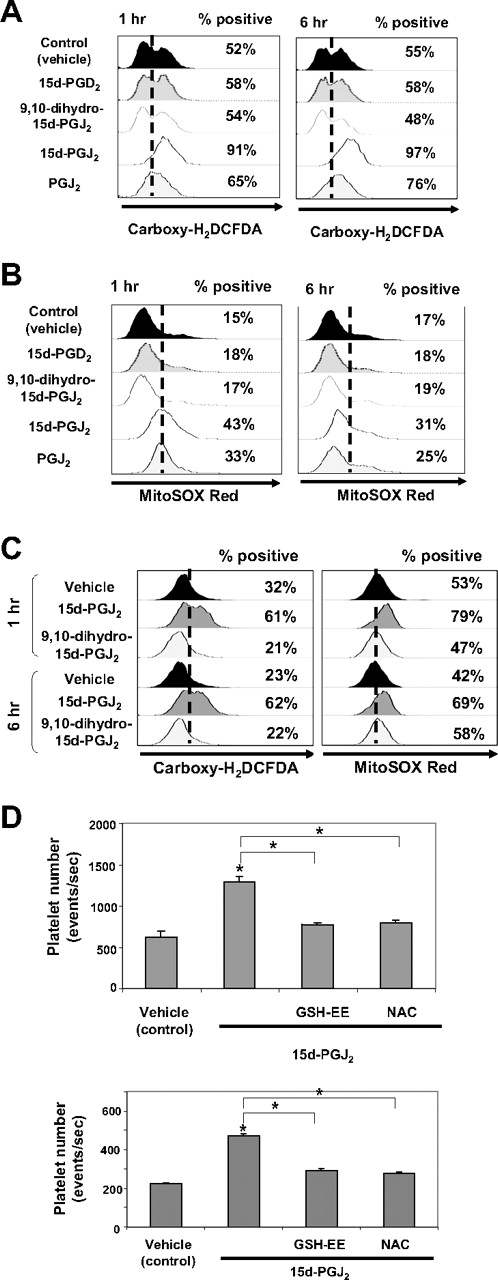
15d-PGJ2 can act independently of PPARγ by modulating cellular redox status.17,31,32 Subsequent experiments were performed to address whether 15d-PGJ2 and other prostaglandins alter intracellular ROS levels in megakaryocytes. We used carboxy-H2DCFDA to detect a broad range of oxidants, including superoxide, peroxynitrate, hydrogen peroxide, and nitric oxide (NO). Flow cytometric analysis (Figure 5A) demonstrates an increase in the percentage of Meg-01 cells that express ROS from 52% to 91% after 1 hour and 97% after 6 hours of 15d-PGJ2 treatment. The nonelectrophilic compounds, 9,10 dihydro-15d-PGJ2 and 15d-PGD2, failed to increase the percentage of cells generating ROS. However, another electrophilic prostaglandin, PGJ2, also increased the percentage of cells expressing ROS (65% at 1 hour and 76% at 6 hours).
Small electrophilic molecules induce the generation of ROS. (A) Meg-01 cells (106) were untreated or treated with DMSO, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, PGJ2, or 15d-PGD2, at a concentrations up to 10 μM, for 1 hour and 6 hours. Cells were harvested, carboxy-H2DCFDA was added for 30 minutes, and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (B) Meg-01 cells were exposed to DMSO or to 10 μM 15d-PGD2, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, or PGJ2 for 1 or 6 hours. Cells were harvested, and MitoSOX red was added for 15 minutes and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (C) Primary human megakaryocytes (106) were untreated or treated with DMSO, 9,10 dihydro-15d-PGJ2, 15d-PGJ2 at concentrations up to 10 μM, for 1 hour and 6 hours. Cells were harvested and carboxy-H2DCFDA was added for 30 minutes or MitoSOX red was added for 15 minutes and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (D) Cells were pretreated with either 1 mM NAC or 5 mM GSH-EE for 2 hours followed by treatment with 15d-PGJ2 (10 μM) or a cotreatment of NAC (1 mM) and 15d-PGJ2 (10 μM) for 24 hours. Top bar graph shows the effects of antioxidants on platelet production from Meg-01 cells. Bottom bar graph shows the effects of antioxidants on platelet production from primary human megakaryocytes.
Small electrophilic molecules induce the generation of ROS. (A) Meg-01 cells (106) were untreated or treated with DMSO, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, PGJ2, or 15d-PGD2, at a concentrations up to 10 μM, for 1 hour and 6 hours. Cells were harvested, carboxy-H2DCFDA was added for 30 minutes, and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (B) Meg-01 cells were exposed to DMSO or to 10 μM 15d-PGD2, 9,10 dihydro-15d-PGJ2, 15d-PGJ2, or PGJ2 for 1 or 6 hours. Cells were harvested, and MitoSOX red was added for 15 minutes and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (C) Primary human megakaryocytes (106) were untreated or treated with DMSO, 9,10 dihydro-15d-PGJ2, 15d-PGJ2 at concentrations up to 10 μM, for 1 hour and 6 hours. Cells were harvested and carboxy-H2DCFDA was added for 30 minutes or MitoSOX red was added for 15 minutes and the cells were analyzed by flow cytometry. The percentage of ROS-positive cells is shown. (D) Cells were pretreated with either 1 mM NAC or 5 mM GSH-EE for 2 hours followed by treatment with 15d-PGJ2 (10 μM) or a cotreatment of NAC (1 mM) and 15d-PGJ2 (10 μM) for 24 hours. Top bar graph shows the effects of antioxidants on platelet production from Meg-01 cells. Bottom bar graph shows the effects of antioxidants on platelet production from primary human megakaryocytes.
In addition to total intracellular ROS, we next investigated the ability of different prostaglandins to increase mitochondrial superoxide levels using MitoSOX Red. MitoSOX Red is a live-cell permeant indicator that is rapidly targeted to the mitochondria, where it reacts with superoxides and binds to nucleic acids, resulting in fluorescence.33 Flow cytometric analysis (Figure 5B) demonstrates an increase in the percentage of Meg-01 cells that produce mitochondrial superoxide at 1 hour (43%) and 6 hours (31%) after 15d-PGJ2 treatment (10 μM). Similar to carboxy-H2DCFDA analysis, the nonelectrophilic 9,10 dihydro-15d-PGJ2 and 15d-PGD2 failed to increase the percentage of cells generating mitochondrial superoxide. However, the electrophilic PGJ2 also increased the percentage of cells expressing superoxide at 1 hour (33%) and 6 hours (25%). Thus, electrophilic prostaglandins induce ROS in Meg-01 cells.
We extended our Meg-01 studies by examining intracellular ROS production in primary human megakaryocytes in response to 15d-PGJ2 and 9,10 dihydro-15d-PGJ2. Flow cytometric analysis (Figure 5C) demonstrates an increase in the per-centage of ROS-positive cells in the 15d-PGJ2 cultures after carboxy-H2DCFDA staining and Mitosox Red staining at 1 hour and 6 hours.
Antioxidants attenuate platelet production
The effects of 15d-PGJ2 on platelet production are partially reversed with pretreatment using thiol antioxidants such as GSH-EE and NAC. Meg-01 cells and primary human megakaryocytes were pretreated with 5 mM of GSH-EE or 1 mM of NAC for 2 hours and extensively washed before 15d-PGJ2 addition. After 24 hours, cells were stained for CD61 expression and platelet number was evaluated. Flow cytometric data (Figure 5D) reveal that both GSH-EE and NAC attenuate 15d-PGJ2–induced platelet production by reducing the number of platelets produced to approximately control levels. Collectively, these data suggest that the production of ROS is important for platelet production from megakaryocytes.
15d-PGJ2 increases megakaryocyte ploidy and proplatelet formation
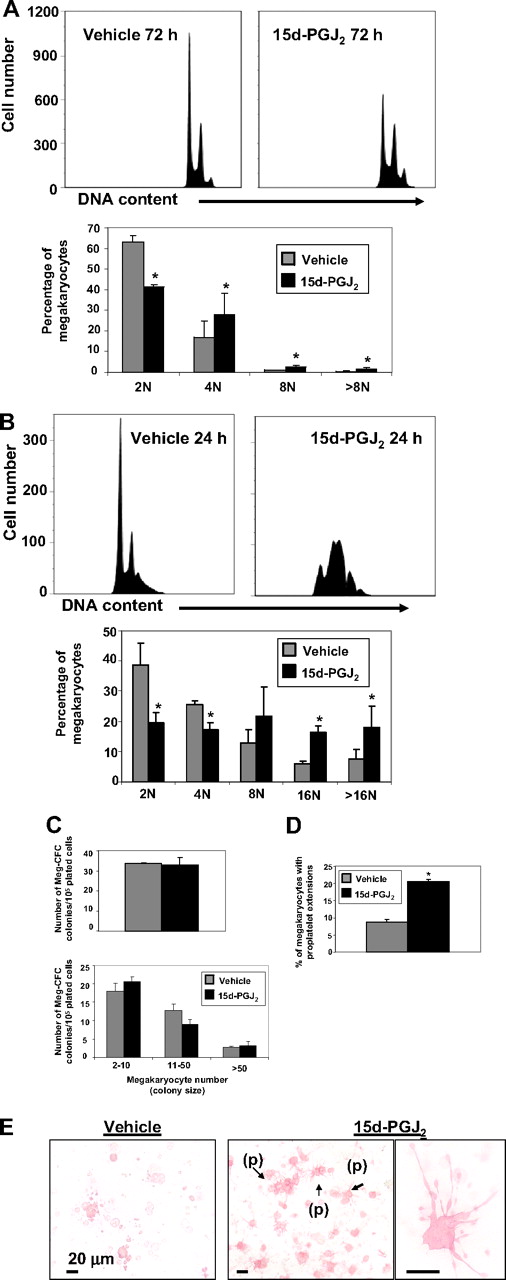
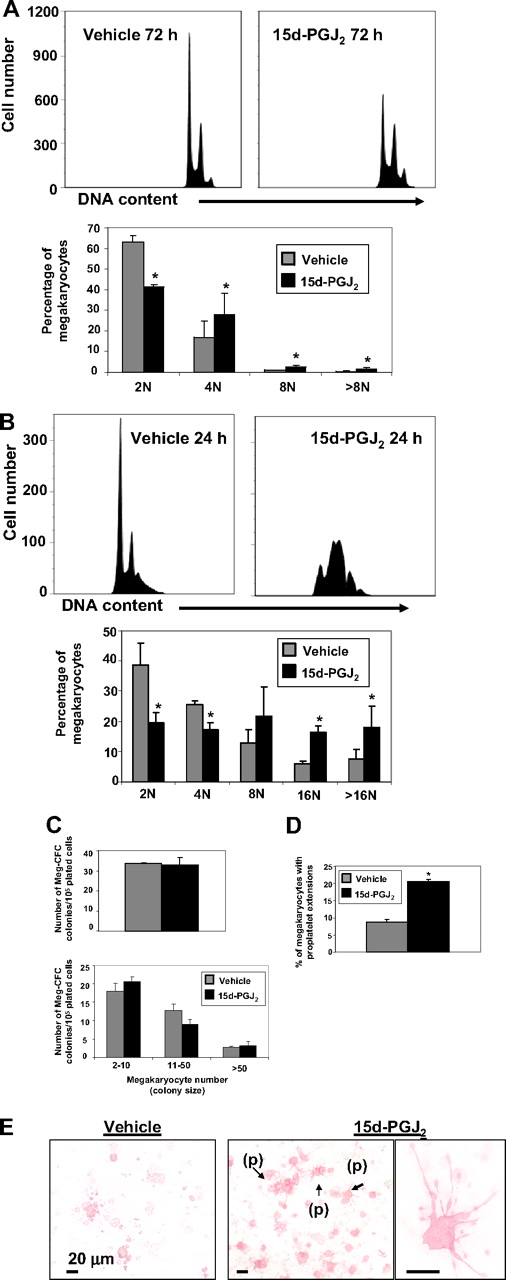
To determine the mechanism of increased platelet production, we examined the effect of 15d-PGJ2 on megakaryocyte colony formation and maturation. Meg-01 cells were treated with 15d-PGJ2 for 24, 48, or 72 hours. Although DNA content was unchanged at 24 and 48 hours (data not shown), histograms show that 15d-PGJ2 addition increased DNA content in Meg-01 cells by 72 hours (Figure 6A). In addition, DNA histograms show that 15d-PGJ2 increased DNA content in primary mouse megakaryocytes by 24 hours (Figure 6B). To determine the effect of 15d-PGJ2 on early-stage thrombopoiesis, primary mouse bone marrow cells were harvested from mice that were injected with 1 mg/kg of 15d-PGJ2 or 1 mg/kg of vehicle for 2 consecutive days. After 7 days of culture, Meg-CFCs were identified by labeling with GPV and GPIbβ. 15d-PGJ2 did not influence megakaryocyte colony number or colony size (Figure 6C), however, 15d-PGJ2 significantly augmented the percentage of megakaryocytes producing proplatelets (Figure 6D,E).
15d-PGJ2 augments DNA content and enhances proplatelet formation. (A) Meg-01 cells were treated with vehicle or 15d-PGJ2 (10 μM) for 24, 48, or 72 hours. Left histogram shows vehicle-treated Meg-01 cells (72 hours). Right histogram shows 15d-PGJ2–treated Meg-01 cells (72 hours). Bar graph demonstrates that by 72 hours Meg-01 cells in the presence of 15d-PGJ2 exhibit higher DNA contents compared with Meg-01 cells in the presence of vehicle. Results are presented as mean plus or minus SD (P < .05). (B) Primary mouse megakaryocytes were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours. Left histogram shows vehicle-treated cells (24 hours). Right histogram shows 15d-PGJ2–treated cells (24 hours). Bar graph demonstrates that by 24 hours, cells in the presence of 15d-PGJ2 exhibit higher DNA content compared with cells in the presence of vehicle. Results are presented as mean plus or minus SD (P < .05). (C) Left bar graph shows the number of Meg-CFC colonies. Right bar graph shows the size of Meg-CFC colonies. Results are presented as mean plus or minus SD (P < .05). (D) Bar graph showing the percentage of megakaryocytes exhibiting proplatelet extensions. Results are presented as mean plus or minus SD (P < .05). (E) Immunohistochemical GP1bβ and GPV staining of megakaryocyte progenitor-derived colonies. Left picture shows colonies grown from bone marrow of vehicle-treated mice. Middle picture shows colonies grown from bone marrow of 15d-PGJ2–treated mice. Note proplatelet extensions (p). Far right picture shows a magnification of the proplatelet extensions.
15d-PGJ2 augments DNA content and enhances proplatelet formation. (A) Meg-01 cells were treated with vehicle or 15d-PGJ2 (10 μM) for 24, 48, or 72 hours. Left histogram shows vehicle-treated Meg-01 cells (72 hours). Right histogram shows 15d-PGJ2–treated Meg-01 cells (72 hours). Bar graph demonstrates that by 72 hours Meg-01 cells in the presence of 15d-PGJ2 exhibit higher DNA contents compared with Meg-01 cells in the presence of vehicle. Results are presented as mean plus or minus SD (P < .05). (B) Primary mouse megakaryocytes were treated with vehicle or 15d-PGJ2 (10 μM) for 24 hours. Left histogram shows vehicle-treated cells (24 hours). Right histogram shows 15d-PGJ2–treated cells (24 hours). Bar graph demonstrates that by 24 hours, cells in the presence of 15d-PGJ2 exhibit higher DNA content compared with cells in the presence of vehicle. Results are presented as mean plus or minus SD (P < .05). (C) Left bar graph shows the number of Meg-CFC colonies. Right bar graph shows the size of Meg-CFC colonies. Results are presented as mean plus or minus SD (P < .05). (D) Bar graph showing the percentage of megakaryocytes exhibiting proplatelet extensions. Results are presented as mean plus or minus SD (P < .05). (E) Immunohistochemical GP1bβ and GPV staining of megakaryocyte progenitor-derived colonies. Left picture shows colonies grown from bone marrow of vehicle-treated mice. Middle picture shows colonies grown from bone marrow of 15d-PGJ2–treated mice. Note proplatelet extensions (p). Far right picture shows a magnification of the proplatelet extensions.
15d-PGJ2 enhances platelet recovery after ionizing radiation
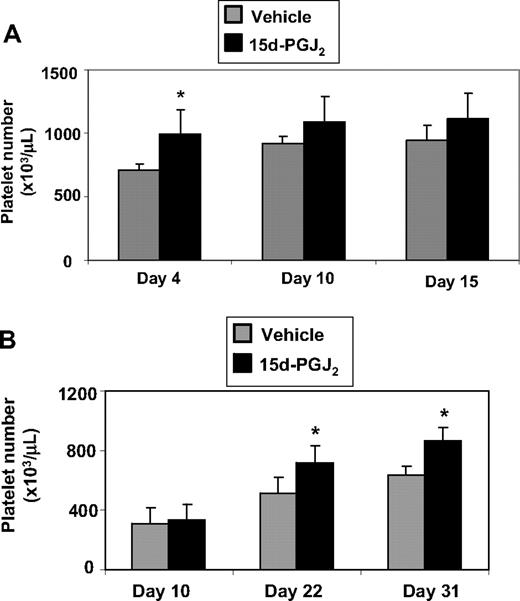
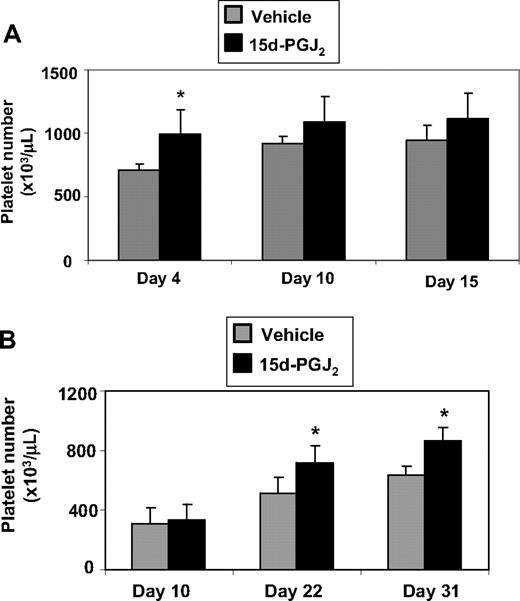
Mice that received intravenous injections of 15d-PGJ2 for 4 consecutive days had higher platelet numbers compared with mice that received vehicle (Figure 7A). To evaluate the effect of 15d-PGJ2 on platelet counts in a model of thrombocytopenia, we exposed mice to Cs137 (5 Gy). Mice exhibited a platelet nadir at approximately 10 days after irradiation (approximately 250 000/μL). The mice that received intravenous injections of 15d-PGJ2 for 4 consecutive days exhibited accelerated platelet recovery on days 22 and 31 compared with the vehicle mice (Figure 7B). Platelet numbers completely recovered (approximately 800 000 μL) by day 31 in the 15d-PGJ2–treated mice. There were no differences in weight between vehicle-treated and 15d-PGJ2–treated mice (data not shown).
15d-PGJ2 enhances platelet number in vivo and accelerates recovery of platelets after ionizing radiation. (A) C57BL/6 mice were injected intravenously with 1 mg/kg 15d-PGJ2 for 4 consecutive days. Platelet number was measured on days 4, 10, and 15. There is a significant increase in the levels of circulating platelets on day 4 in mice treated with 15d-PGJ2. Results are presented as mean plus or minus SD (P < .01; n = 4). (B) C57BL/6 mice were exposed to 5 Gy of total body ionizing irradiation on day 0 and on the following 4 consecutive days, were injected intravenously with 1 mg/kg 15d-PGJ2. Platelet number was measured on days 10, 22, and 31. There is a significant increase in the levels of circulating platelets on days 22 and 31 in mice treated with 15d-PGJ2. Results are presented as means plus or minus SD (P < .01; n = 8).
15d-PGJ2 enhances platelet number in vivo and accelerates recovery of platelets after ionizing radiation. (A) C57BL/6 mice were injected intravenously with 1 mg/kg 15d-PGJ2 for 4 consecutive days. Platelet number was measured on days 4, 10, and 15. There is a significant increase in the levels of circulating platelets on day 4 in mice treated with 15d-PGJ2. Results are presented as mean plus or minus SD (P < .01; n = 4). (B) C57BL/6 mice were exposed to 5 Gy of total body ionizing irradiation on day 0 and on the following 4 consecutive days, were injected intravenously with 1 mg/kg 15d-PGJ2. Platelet number was measured on days 10, 22, and 31. There is a significant increase in the levels of circulating platelets on days 22 and 31 in mice treated with 15d-PGJ2. Results are presented as means plus or minus SD (P < .01; n = 8).
Discussion
Thrombocytopenia causes significant morbidity and mortality, and few therapies are useful except for transfusion. Understanding the molecular mechanisms that underlie both megakaryocyte maturation and platelet release will provide insight into new ways to enhance platelet production from their precursor cells, the megakaryocytes. The results presented herein demonstrate that 15d-PGJ2 enhances platelet production from both mouse and human megakaryocytes. We show that ROS generation is critical for platelet production and that small electrophilic prostaglandins, such as 15d-PGJ2, which enhance ROS, may provide therapeutic benefit in the treatment of thrombocytopenia.
Meg-01 cells undergo differentiation reflective of megakaryocyte maturation and platelet release.25 Our data indicate that 15d-PGJ2 has a strong platelet production–enhancing effect in this cell line. When Meg-01 cells were treated with 15d-PGJ2, they lost their smooth rounded appearance, exhibited significant cytoplasmic protrusions, and increased their granule content (Figure 1I). Meg-01 cells also up-regulated active-caspase protein in the presence of 15d-PGJ2 (data not shown). All these features are consistent with a megakaryocyte that is actively making platelets.34-36 These platelets appeared to be functional, as they expressed platelet surface markers and changed shape and elevated surface levels of phosphatidylserine in response to collagen (Figure 1D,E). Phosphatidylserine is up-regulated on the surface of platelets with collagen activation and is important for blood coagulation.26,27 We expanded our findings based on Meg-01 cells by also evaluating differentiating megakaryocytes from mouse bone marrow and from human cord blood–derived CD34+ cells. In both mouse and human cultures, more platelets were generated from megakaryocytes after 15d-PGJ2 treatment. Culture-derived platelets showed similar morphologic and functional features to normal human platelets. They expressed platelet-specific receptors and were activated in response to either collagen or fibrinogen. Culture-derived platelets up-regulated CD61 and CD41, changed shape in response to collagen stimulation, and spread on fibrinogen-coated slides. Platelet spreading is an irreversible process necessary for platelet-surface contact during hemostasis and is, therefore, a good measure of platelet function.37
15d-PGJ2 is a potent PPARγ ligand.15 Although PPARγ is important in adipogenesis and inflammation,11 recent studies suggest that PPARγ ligands influence the hematopoietic system.38 Nagasawa et al published that certain PPARγ ligands impaired erythrocyte maturation,39 and Kasono et al demonstrated that some PPARγ ligands elevated platelet numbers in a mouse model of thrombocytopenia by reducing the phagocytic activity of macrophages.40 Our laboratory previously reported that megakaryocytes and platelets express PPARγ.13 Therefore, herein, we examined the effects of PPARγ ligands on megakaryocytes. Our findings show that prostaglandins such as PGE2, PGI2, and PGF2α, which are poor activators of PPARγ, failed to enhance platelet production in Meg-01 cells. In contrast, the natural PPARγ ligand, 15d-PGJ2, potently enhanced platelet production, a novel finding. Interestingly, 15d-PGJ2 induced platelet production in Meg-01 cells that was independent of PPARγ. We demonstrated this by first showing that 2 highly selective synthetic PPARγ ligands, rosiglitazone and 9,10 dihydro-15d-PGJ2, do not have the same platelet enhancing effects as 15d-PGJ2. Next, we demonstrated PPARγ independence by knocking down PPARγ protein or by inhibiting PPARγ activation with the irreversible PPARγ antagonist. Under these conditions, no changes were observed in the ability of 15d-PGJ2 to induce platelet production. The PPARγ-independent effects of 15d-PGJ2 may be due to its electrophilic carbon in the cyclopentanone ring.10,16,41 9,10 dihydro-15d-PGJ2, a structural analog of 15d-PGJ2, which binds to PPARγ with the same affinity as 15d-PGJ2 but lacks the electrophilic carbon in the cyclopentanone ring, failed to enhance platelet production. In contrast, the electrophilic PGJ2 also enhanced platelet production similarly to 15d-PGJ2. We conclude that the electrophilic properties of 15d-PGJ2 are important for its platelet-enhancing effects.
The electrophilic nature of 15d-PGJ2 promotes mechanisms that accompany apoptotic related events such as cytoskeletal rearrangement and ROS generation.10,18,42 Our data are consistent with these findings because both cytoskeletal changes (Figure 1G,I, Figure 2E, Figure 3E) and ROS generation (Figure 5A-C) occurred after 15d-PGJ2 exposure, supporting the observation that platelet production is, in part, a specialized form of apoptosis.35,43 One recent study showed that the overexpression of scinderin, an f-actin–severing protein, in Meg-01 cells led to megakaryoblast differentiation and platelet production.44 In addition, 15d-PGJ2 can interact with the cytoskeleton and oxidize susceptible cysteine residues leading to f-actin depolymerization.18,42,45 Actin is a main scavenger of electrophilic lipids because of its high abundance and nucleophilic cysteine residues.46 Thus, 15d-PGJ2 may promote platelet production by a mechanism involving interaction with cytoskeletal proteins and f-actin depolymerization.
Several studies have implicated the cytoskeleton as an important regulator of the redox state of the cell, and conversely, the redox state of the cell may also influence the cytoskeleton.47,48 Our data show that 15d-PGJ2 promotes the generation of ROS within 1 hour. While it is well-known that redox status regulates cell proliferation, differentiation, and survival, the role of ROS in platelet production has been unclear until now. We demonstrate that ROS, and more specifically, mitochondrial superoxide, play a role in the dynamic process of megakaryocyte maturation and platelet release. Only the electrophilic molecules that generated ROS enhanced platelet production (Figure 5A-C). Pretreating Meg-01 cells or primary human megakaryocytes with either GSH-EE or NAC before 15d-PGJ2 treatment attenuated both ROS induction (data not shown) and platelet formation (Figure 5D). Interestingly, many disorders associated with oxidative stress have platelet abnormalities, such as those seen in type 2 diabetes and atherosclerosis.49 Further studies will be useful to determine whether oxidative stress in the bone marrow and, more specifically, in the megakaryocytes affects megakaryocyte maturation and platelet function.
15d-PGJ2 not only stimulated platelet production, but also stimulated megakaryocyte maturation. While 15d-PGJ2 failed to increase megakaryocyte number (data not shown), megakaryocyte polyploidization was increased. 15d-PGJ2 did not significantly enhance Meg-01 ploidy until 72 hours after treatment, suggesting that this is a longer-term effect and did not directly promote the platelet release that was demonstrated by 24 hours. In contrast, 15d-PGJ2 enhanced primary mouse megakaryocyte ploidy by 24 hours, suggesting that megakaryocyte maturation may play a direct role in promoting the platelet release. In addition, while 15d-PGJ2 failed to promote the proliferation of megakaryocyte progenitors, the number of megakaryocytes with proplatelet extensions was significantly higher. Collectively, these results suggest that 15d-PGJ2 increases platelet numbers by stimulating megakaryocyte maturation and/or subsequent proplatelet formation.
The platelet-enhancing activity of 15d-PGJ2 in vitro raised the possibility that it may exhibit a similar effect in vivo. As shown, 15d-PGJ2 had a significant effect on enhancing platelet production (Figure 7A) and accelerating platelet recovery in our model of radiation-induced thrombocytopenia (Figure 7B). We observed a significant increase in platelet number at days 22 and 31 after radiation exposure. These data suggest that 15d-PGJ2 may be having an indirect effect on platelet number by up-regulating a cytokine, initiating a protein cascade, or influencing another cell type that regulates megakaryocyte maturation and platelet release; these effects may take additional time to manifest a phenotype. The elevation in platelet number has potential clinical significance through promoting platelet formation in immune-mediated thrombocytopenias where megakaryocyte numbers in the marrow are normal or increased and through reducing the risk for hemorrhage during platelet recovery after myelosuppression. The latter platelet-enhancing effect may be particularly important after chemotherapy or radiation exposure, where platelet depletion is accompanied by endothelial cell damage. Another complication associated with radiation exposure is scarring of vital organs such as lung and bone marrow. Importantly, 15d-PGJ2 has demonstrated significant anti-scarring activities in models of lung scarring.50
Our findings suggest that 15d-PGJ2 is a promising therapeutic target for treating thrombocytopenia and may have advantages over other thrombopoietic agents that are being developed. 15d-PGJ2 is small, cheap to make, and can readily penetrate tissues. In addition, 15d-PGJ2 is highly conserved between species and is endogenously produced, raising the possibility that high endogenous levels of 15d-PGJ2 in the bone marrow could potentially be associated with conditions characterized by high platelet counts.
Bone marrow suppression is the most common severe adverse effect after cytotoxic chemotherapy or radiation exposure and results in anemia, leucopenia, and thrombocytopenia. These can be ameliorated by transfusion support, but this has unwanted side effects including allergy, disease transmission, alloimmunization, limited availability, and expense. Pharmacologic treatment with myeloid growth factors and with erythropoietin has improved our ability to accelerate myeloid and erythroid recovery, respectively. However, no comparable cytokine therapy is currently available to accelerate thrombopoiesis. Our discovery that the electrophilic prostaglandin 15d-PGJ2 exerts a potent thrombopoietic effect provides insight into the molecular mechanisms regulating both megakaryopoiesis and thrombopoiesis. This may lead to identification of new therapeutic agents to accelerate platelet recovery after marrow injury.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the technical support of the Electron Microscope Research Core, specifically Karen L. de Mesy Bentley, Ian M. Spinelli, and Gayle Schneider. The authors also thank Dr Craig T. Jordan and Randall M. Rossi for use of the Heska Veterinary Analyzer.
This work was supported by National Institutes of Health (Bethesda, MD) grants T32ES07026, DE011390, ES01247, HL078603, HL086367, EY017123, and DK09361, and by the PhRMA Foundation (Washington, DC).
National Institutes of Health
Authorship
Contribution: J.J.O'B. designed and performed research, analyzed data, and wrote the paper. S.L.S. contributed vital new reagents, analyzed data, and edited the manuscript. J.T. and K.E.S. performed research. N.B., C.W.F., and M.B.T. analyzed data and edited the manuscript. J.M.G. contributed vital new reagents. J.P. edited the manuscript. R.P.P. designed research, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard P. Phipps, Department of Environmental Medicine, Box 850, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Rochester, NY 14642; e-mail: richard_phipps@urmc.rochester.edu.