Abstract
The 6 nucleotide insertion-deletion polymorphism (-6526N del) in the CASP8 promoter has been demonstrated to destroy an Sp1 binding site, resulting in reduced caspase-8 activity and expression and a reduced susceptibility of 652 6N del/del T-lymphocytes to undergo apoptosis in response to anti-FAS and to tumour infiltrating lymphocytes. As the susceptibility of T-lymphocytes to undergo apoptosis could affect outcome following allogeneic HSCT we have investigated the impact of the CASP8-652 6N del polymorphism in both donors and recipients on the outcomes of patients undergoing unrelated donor HSCT.
Genotyping was performed on 186 donor/recipient pairs who underwent an HSCT from a donor from the Anthony Nolan Trust in one of 25 transplant centres in the United Kingdom between 1997 and 2006. All patients were transplanted for a haematological malignancy and had long term clinical data collated. Diagnoses were CML: 40.3%; MDS: 4.3%, ALL: 16.6%; AML: 23.6 %, lymphoproliferative disorders: 10.4 %, multiple myeloma: 4.8%. The frequency of the 3 genotypes in the Caspase 8 promoter region was as follows: RECIPIENTS: WT/WT=0.26; WT/del=0.49; del/del=0.24 and DONORS: WT/WT=0.33; WT/del=0.40; del/del=0.25. Frequencies in both the donor and recipient populations were in Hardy-Weinberg equilibrium.
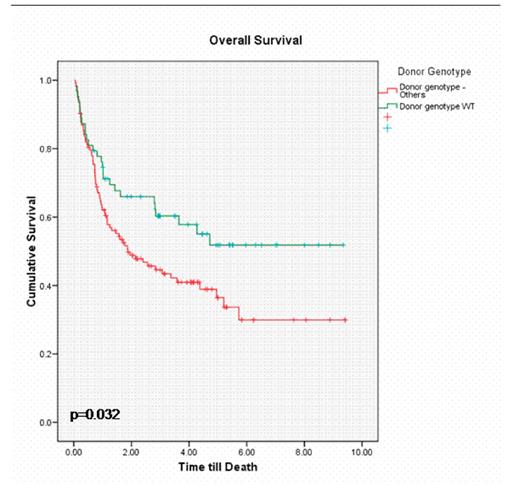
Our findings show that the overall survival (OS) in recipients who received a transplant from a donor with a WT/WT genotype was significantly better as compared to donors with WT/del or del/del genotype (52% v 34% at 5 years; p=0.03). The disease free survival (DFS) at 3 years was also significantly better (39% v 18%; p=0.03). In addition, we found a significant reduction in grade II-IV acute GVHD (17% v 30%; p=0.04) and a trend towards lower incidence of chronic GVHD (42% v 63%; p=0.06) and transplant related mortality (TRM) (19% v 35%; p=0.06) in these patients. No significant difference in disease relapse was seen.
The results were particularly striking in the subgroup of recipients where one or two deletions were present (WT/del or del/del genotype). The OS was 62% in those who received a graft from a donor with WT/WT genotype compared to 36% with other donors (p=0.01). This remained significant in multivariate analysis (HR 0.53; CI 0.29, 0.98; p=0.04). This subgroup also had a lower risk of relapse (p=0.02).
In this cohort, the majority (156/186) of recipients had in-vivo T-cell depletion using Alemtuzumab. As Alemtuzumab depletes T-cells in part by apoptosis induced via a caspase 8 dependent pathway, we hypothesize that recipients who receive a transplant from a donor with WT/WT genotype are likely to get more effective T-cell depletion and hence less acute GVHD and reduced TRM resulting in improved survival. The mechanism of interaction between donor and recipient genotypes remains to be explored.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author