Abstract
The fludarabine-melphalan-alemtuzumab combination is a well tolerated conditioning regimen for HCT. Unfortunately recurrence rates are high, particularly for those with active disease entering HCT. Clofarabine (Clo), a second generation nucleoside analog with excellent activity in acute leukemia, might enhance disease control. Here we report the results of a phase I–II study of clofarabine-melphalan-alemtuzumab conditioning for allogeneic peripheral blood HCT. GVHD prophylaxis used tacrolimus. For the phase I cohort, one pt was enrolled per dose level, until the first DLT or until 2 had gr 3 toxicity. Dose level 1 consisted of: clofarabine 10 mg/m2/day on d-7 to -3 and melphalan 100 mg/m2 on day-2. Clo was increased by 10 mg/m2/day per cohort until 40 mg/m2/day. Then, melphalan was increased by 20 mg/m2 until 140 mg/m2.
Twelve pts were accrued in the phase I portion. There were four deaths from transplant mortality (TRM); two patients who had failed prior transplant and were pancytopenic prior to conditioning, died of early sepsis unrelated to regimen related toxicity. One early death from multi-organ failure in a patient with t-AML, residual breast cancer and a history of heart disease was attributed to possible regimen related toxicity. One additional pt with multiple comorbidities died at day 159 from sepsis. The phase II dose was established at Clo 40 mg/m2/day × 5 days + melphalan 140 mg/m2. Sixteen pts have been accrued in the ongoing phase II study. For the entire phase I–II cohort, median age was 52 (20–69); 13 had AML, 13 NHL, 1 CML, 1CLL; 23 had high or intermediate and 5 had low risk disease. 5 had relapsed after alloHCT, 4 after autoHCT. HCT-CI was >= 3 in 9 and ECOG PS >=1 in 11. Sixteen had HLA-identical sibling donors and 12 had 8/8 Matched unrelated donors.
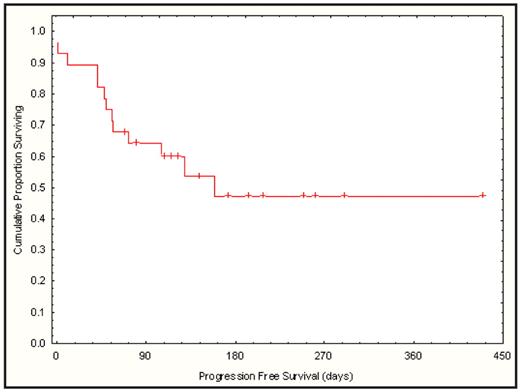
All evaluable patients recovered ANC >0.5 by d+11 (9–20) and plt count > 20 by d+12 (9–27). Median d 30 donor chimerism was 95% (range 14–100) with no late graft failures. In addition to the four transplant related deaths during the phase I portion, two additional transplant related deaths occurred during the phase II portion. One patient with multiple comorbidities died during conditioning from possible regimen related toxicity and one pt who had failed a prior transplant died from GVHD. Other toxicities included: gr 2–3 reversible ALT elevation in 14 pts, between day-2 and day +5; gr 2 reversible bilirubin elevation in 1 pt; gr 2–3 hand-foot syndrome in 9 pts; gr 3 mucositis and pulmonary hemorrhage in 1 pt; gr 2 renal failure in 3 pts. With a median follow-up 140 days (51–412), 3 of 12 pts in the phase I portion and 12 of 16 pts in the phase II portion of the study remain in remission. Estimated day 180 progression-free survival is 47% (95% CI: 26–69) (figure)
Conclusions: Clofarabine-melphalan-alemtuzumab conditioning induces durable engraftment in related and unrelated donor HCT. The most common toxicities are reversible elevation of transaminases and hand foot syndrome. Grade II renal failure can occur. There were six transplant related deaths, two attributed to possible regimen related toxicity. All transplant related deaths occurred in patients with high risk disease, prior transplant and/or multiple comorbidities. Response rates and duration of response are encouraging.
Disclosures: Van Besien:Genzyme: Research Funding. Godley:MGI Pharma, Inc.: Consultancy; Pharmion: Research Funding. Rich:Genzyme: Research Funding. Stock:Genzyme: Membership on an entity’s Board of Directors or advisory committees, Research Funding.
Author notes
Corresponding author