Abstract
IL-15 is critical for natural killer (NK)–cell development and function and for memory CD8+ T-cell homeostasis. The IL-15 receptor consists of IL-15Rα, IL-2Rβ, and the common cytokine receptor γ chain (γc). IL-15Rα is known to “trans-present” IL-15 to an IL-2Rβ/γc heterodimeric receptor on responding cells to initiate signaling. To investigate the importance of the IL-15Rα cytoplasmic domain, we generated a chimeric receptor consisting of the extracellular domain of IL-15Rα and intracellular domain of IL-2Rα (IL-15Rαext/IL-2Rαint) and examined its function in 32D cells, in knock-in (KI) mice, and in adoptive-transfer experiments. The chimeric protein exhibited decreased cell-surface expression, and KI mice exhibited diminished NK, NKT, and CD8+ T-cell development and defects in T-cell functional responses. However, 32D cells expressing the chimeric receptor had less IL-15–induced proliferation than wild-type (WT) transfectants with similar levels of IL-15Rα expression, indicating a signaling role for the IL-15Rα cytoplasmic domain beyond its effect on expression, and demonstrating that the IL-2Rα and IL-15Rα cytoplasmic domains are functionally distinct. Interestingly, adoptive-transfer experiments indicated that the chimeric IL-15Rαext/IL-2Rαint receptor still supports trans-presentation. These experiments collectively indicate that IL-15Rα can act in cis in addition to acting in trans to present IL-15 to responding cells.
Introduction
IL-2 and IL-15 are evolutionarily related cytokines that have important actions on a number of lymphoid populations.1-3 IL-2 was discovered as a T-cell growth factor but in addition has other important roles, for example related to the development of regulatory T cells,4 in activation-induced cell death (AICD),5 and in the boosting of natural killer (NK)–cell cytolytic activity.6,7 The major actions of IL-15 are related to NK-cell and NKT-cell development and function8-10 and to memory CD8+ T-cell homeostasis.11-15 IL-2 signals via high-affinity receptors consisting of the IL-2 receptor α chain (IL-2Rα), IL-2Rβ, and the common cytokine receptor γ chain (γc), or via IL-2Rβ/γc intermediate affinity receptors.3,16 IL-15 receptors resemble IL-2 receptors in that there also are 3 important chains, IL-15Rα, IL-2Rβ, and γc.3,8,10,16 Thus, IL-2Rβ and γc are shared by IL-2 and IL-15. γc is also a component of the receptors for IL-4, IL-7, IL-9, and IL-21, and is mutated in patients with X-linked severe combined immunodeficiency.17,18 IL-2Rβ and γc are members of the type I cytokine receptor superfamily.17 IL-2Rα is specific for IL-2, whereas IL-15Rα is specific for IL-15. Both of these chains are distinctive cytokine receptor proteins that contain sushi domains but lack domains typical of type I cytokine receptor proteins.19,20 In contrast to IL-2Rα, which binds IL-2 with low affinity and is expressed mainly on activated T cells, IL-15Rα has a relatively high affinity for IL-15, and IL-15Rα mRNA is expressed more broadly.21,22
Consistent with the sharing of IL-2Rβ and γc, IL-15 has actions overlapping those of IL-2;23 however, these cytokines also have distinct functions. For example, Il2−/− and Il2ra−/− mice develop polyclonal T- and B-cell expansion that is associated with autoimmune disease,24,25 whereas Il15−/−26 and Il15ra−/− mice27 do not manifest lymphoid enlargement or autoimmune disease. Furthermore, IL-15 can inhibit IL-2–mediated AICD of CD4+ T cells.28 Thus, these cytokines have distinct as well as overlapping actions.
IL-2 and IL-15 share the same Jak1/Jak3 and Stat3/Stat5 signal transduction pathway mediated via the IL-2Rβ/γc complex.17,29 IL-2Rα binds IL-2 with low affinity but has a very short 13–amino acid cytoplasmic domain30 and cannot transduce a signal. Because of its rapid on-rate for IL-2, IL-2Rα is believed to “recruit” IL-2 to the cell surface and to present it to IL-2Rβ/γc in cis. In contrast, IL-15 signals by a process termed “trans-presentation”31 wherein IL-15Rα on one cell binds IL-15 and presents it to another cell that expresses IL-2Rβ and γc. Although this suggests that IL-15Rα may only be needed for binding ligand, it has a longer cytoplasmic domain (41 amino acids) than that of IL-2Rα (13 amino acids), and the cytoplasmic domain potentially could have a role for recruiting signaling molecules. Indeed, it was reported that Syk kinase32 and TRAF233 can interact with the IL-15Rα cytoplasmic domain, although the roles of these in vitro interactions are not well established for IL-15 function in vivo. To investigate the function of the IL-15Rα cytoplasmic domain, we transfected a chimeric receptor construct in which we replaced the cytoplasmic domain of IL-15Rα with that from IL-2Rα into 32D-IL-2Rβ cells and found significantly decreased proliferation in response to IL-15. To further investigate the role of the cytoplasmic domain in vivo, we also generated knock-in (KI) mice and examined the development and function of various cell populations in these KI mice, and we additionally performed adoptive transfer experiments with cells from wild-type (WT), IL-15Rα KI, and IL-15Rα knockout (KO) mice.
Methods
Cell culture, transient transfection, and proliferation assays
32D cells are IL-3–dependent myeloid progenitor cells. 32D cells transfected with IL-2Rβ chain (32D-IL-2Rβ cells) can proliferate in response to IL-2.34,35 Cells were cultured in RPMI 1640 medium containing 10% FBS, 50 μM 2-ME, 10% WEHI-3B cell–conditioned medium as a source of IL-3, 2 mM glutamine, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 0.5 mg/mL G418. pRV-GFP-WT plasmid was generated by subcloning the IL-15Rα coding region into pRV (a retroviral plasmid that directs expression of green fluorescent protein (GFP; provided by Dr Ken Murphy, Washington University, St Louis, MO). pRV-GFP-KI plasmid was generated by replacing the IL-15Rα intracellular domain with that from IL-2Rα (IL-15Rαext/IL-2Rαint). Plasmids were transfected into 32D-IL-2Rβ cells using a Cell Line Nucleofector Kit (Amaxa, Gaithersburg, MD). For measuring proliferation, after 24 hours, cells were washed, starved of growth factor for 4 hours, aliquoted at 3 × 104 cells per well in a 96-well plate, and treated in triplicate for 48 hours with medium alone or medium containing IL-15, IL-2, or IL-3. A total of 0.037 MBq (1 μCi) of [3H]-thymidine was added, cells were incubated for 16 hours, and incorporation was determined using a Betaplate 1205 counter (Wallac, Turku, Finland).
Mice
C57BL/6 and B6.SJL congenic mice were from Taconic (Germantown, NY). Il15ra−/− mice27 were from Dr Yutaka Tagaya, NCI, and analyzed at 8 to 16 weeks of age. Experimental protocols were approved by the National Heart, Lung and Blood Institute (NHLBI) Animal Use and Care Committees and followed the National Institutes of Health (NIH) guidelines titled “Using Animals in Intramural Research.”
Gene targeting constructs
IL-15Rα is encoded by 7 exons, with part of exon 6 and all of exon 7 encoding the cytoplasmic domain.20,21 To replace the IL-15Rα cytoplasmic domain with that of IL-2Rα, a 5.4-kb genomic DNA fragment containing exon 6 and flanking sequences was polymerase chain reaction (PCR)–amplified and cloned 5′ to the PGK-neomycin (neo) cassette in a targeting vector, pNTK-LoxP.36 A 42-bp DNA sequence encoding the 13–amino acid-long IL-2Rα intracellular domain37 and a stop codon (5′-ACCTGGCAACACAGATGGAGGAAGAGCAGAAGAACCATCTAG-3′) (hatched box in Figure 2A) was inserted into exon 6 immediately 3′ to the sequence encoding the IL-15Rα transmembrane domain using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). A 2.5-kb genomic DNA fragment spanning exon 7 was then cloned 3′ to the PGK-neo cassette in the same plasmid. The sequence was verified by DNA sequencing.
Generation of chimeric IL-15Rαext/IL-2Rαint KI mice
The targeting vector was linearized with NotI, electroporated into strain 129 embryonic stem (ES) cells, and selected with G418.36 Genomic DNA from 192 ES clones was digested with HpaI and Southern-blotted with probe A (Figure 2A) to yield a 7.5-kb band for the WT allele versus a 9.3-kb band when the PGK-neo cassette was inserted by homologous recombination (Figure 2A,B). Homologous recombination was confirmed by digesting the ES cell DNA with XhoI and Southern blotting with probe B. ES cell clones with a chimeric IL-15Rαext/IL-2Rαint KI allele were injected into C57BL/6 blastocysts, resulting male KI mice were bred to C57BL/6 females, and progeny were analyzed for germ-line transmission of the KI allele by PCR and Southern blotting. Southern blotting with probe B after XhoI digestion yielded a band of 14.6 kb for the WT allele and 4.4 kb for the KI allele after homologous recombination (Figure 2C left panel). To avoid effects that might result from the neomycin cassette, mice carrying the KI allele were mated to EIIa-Cre transgenic mice,38 allowing excision of this LoxP-flanked cassette, yielding a 2.5-kb band as confirmed by Southern blotting (Figure 2C right panel). Mice heterozygous for the KI allele but lacking the neomycin cassette were intercrossed to generate IL-15Rαext/IL-2Rαint KI mice.
Flow cytometric analyses
Cells were stained with FITC-, PE-, and APC-conjugated mAbs (BD Pharmingen, San Diego, CA) and analyzed on a FACSort with CellQuest software (BD Biosciences, San Jose, CA). To measure IL-15Rα expression, biotinylated anti–mIL-15Rα and isotype-matched control antibodies (R&D Systems, Minneapolis, MN) were used, followed by staining with streptavidin-APC (BD Pharmingen).
Real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) from purified spleen naive T cells or T cells activated by 1 μg/mL plate-bound anti–mouse CD3ϵ and 1 μg/mL soluble anti–mouse CD28. First-strand cDNA was made using random hexamers and Omniscript Reverse Transcriptase (QIAGEN, Valencia, CA). Quantitation of IL-15Rα mRNA and 18S rRNA were performed by real-time PCR using the 7900H Sequence Detection System (Applied Biosystems, Foster City, CA).
Radiolabeled ligand binding assay
Carrier-free recombinant human IL-15 (Peprotech, Rocky Hill, NJ), was radiolabeled with [125I]-sodium iodide using a chloramine T method to obtain a specific activity of approximately 2000 cpm/fmol,39 and binding experiments were performed.40 Cells were incubated with increasing concentrations of 125I-labeled IL-15, and nonspecific binding was determined using a 100-fold excess of unlabeled IL-15 cytokine. Cell-bound and free 125I–IL-15 were separated by centrifugation through 90% dibutyl phthalate/10% paraffin oil. Nonlinear regression analyses of binding data were performed using a one-site equilibrium binding equation, and data were plotted in the Scatchard coordinate system (Prism; GraphPad Software, San Diego, CA).
Cytotoxicity assays
NK-cell cytolytic activity was determined by a 51Cr-release assay. Mice were injected with 100 μg of polyinosilic-polycytidylic acid (poly I:C). After 24 hours, effector NK cells were enriched from mouse spleens using DX5 MicroBeads (Miltenyi Biotec, Auburn, CA) and an AutoMACS separation system. Cells were counted and similar numbers of cells were incubated with 51Cr-labeled YAC-1 target cells at different effector-target (E/T) ratios at 37°C for 4 hours, and target cell lysis was determined.
Immunization of mice and measuring of tetramer and IFN-γ+CD8+ T cells
Replication-incompetent modified vaccinia Ankara virus strain (MVA) was from Drs Linda Wyatt and Bernard Moss, National Institutes of Allergy and Infectious Disease (NIAID; Bethesda, MD).41 WT and KI mice were injected intraperitoneally with 2 × 107 pfu of MVA. At 7 days or 1 month after immunization, splenocytes were isolated, stained with PerCP-labeled anti-CD8α (BD Pharmingen) and soluble tetrameric B8R20-27/H-2Kb complex conjugated to PE-labeled streptavidin (National Institutes of Health [NIH] Tetramer Core Facility, Bethesda, MD), and analyzed by flow cytometry. IFN-γ–producing cells were measured by enzyme-linked immunospot (ELISPOT) as previously described.42 C57BL/6 naive splenocytes were used as stimulator cells (0.2 × 106/well) and immunized with B8R20–27 peptide (TSYKFESV), which is a poxvirus cytotoxic T lymphocyte (CTL) epitope restricted by H-2Kb.43
Proliferation assays on purified cells
For [3H]-thymidine incorporation assays, CD8+ T cells were positively selected using paramagnetic microbeads conjugated to anti–mouse CD8α (Ly-2) mAb (Miltenyi Biotec). A total of 105 CD8+ T cells were activated by 1 μg/mL of plate-bound 2C11 anti–mouse CD3ϵ, 1 μg/mL anti–mouse CD28, 100 U/mL IL-2, or 5 or 50 ng/mL IL-15. After 48 hours, [3H]-thymidine (0.037 MBq [1 μCi]; 8-hour pulse) was added and incorporation determined with a Betaplate 1205 counter (Wallac).
For tracing cell division by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution, splenic CD8+ T cells were isolated with a CD8+ T cell isolation kit (Miltenyi Biotec) and then separated into CD44high and CD44low biotin-conjugated anti-CD44 mAb (BD Biosciences) and paramagnetic Microbeads conjugated to antibiotin mAb (Miltenyi Biotec). Isolated cells were labeled with 1 μM CFSE (Invitrogen-Molecular Probes) in PBS for 15 minutes at 37°C, cultured for 4 days in the medium with IL-21 (100 ng/mL) and IL-15 (5 or 50 ng/mL) as indicated, and then counted and analyzed on a FACSCalibur with CellQuest software (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Intracellular phospho-Stat5 staining
T cells were purified from mouse splenocytes by negative selection using a pan–T-cell isolation kit (Miltenyi Biotec), stimulated with 1 μg/mL each of plate-bound anti-CD3ϵ and soluble anti-CD28 for 3 days, washed, rested for 2 days, and then stimulated with IL-15 or IL-2. Cells were fixed in 2% paraformaldehyde/PBS and permeabilized in 90% methanol, followed by staining with anti–phospho-Stat5, anti-CD4, and anti-CD8α (BD Pharmingen).
Adoptive transfer
IL-15Rαext/IL-2Rαint KI mice were backcrossed to C57BL/6 mice for 8 to 9 generations. Splenic T cells were purified from donor mice by negative selection and labeled with CFSE, and 5 to 8 × 106 cells injected into recipient mice that had received 6 Gy (600 rad) total body radiation. On days 3, 5, or 6, single-cell suspensions from spleens were stained with fluorescent antibodies to CD4, CD8α, and CD45.2 (BD Pharmingen), and analyzed by flow cytometry using a FACSort with CellQuest software (BD Biosciences).
Results
Replacement of the cytoplasmic domain of IL-15Rα with that from IL-2Rα decreases IL-15–induced proliferation in transfected 32D-IL-2Rβ cells
To investigate whether the cytoplasmic domain of IL-15Rα has a key signaling role, we generated a chimeric receptor plasmid (denoted pRV-GFP-KI) by replacing the intracellular domain of IL-15Rα with that from IL-2Rα (IL-15Rαext/IL-2Rαint), and transiently transfected 32D-IL-2Rβ cells with pRV-GFP-WT and pRV-GFP-KI plasmids. The expression level of the chimeric receptor was lower than WT IL-15Rα when similar amounts of WT and KI plasmids were transfected (data not shown), perhaps resulting from a role of the IL-15Rα cytoplasmic domain in recycling IL-15/IL-15Rα complexes to the cell surface.31 We therefore transfected 32D-IL-2Rβ cells with different amounts of each plasmid and selected transfectants with similar cell surface IL-15Rα expression; this was reproducibly achieved when we used approximately 1 μg pRV-GFP-WT and 2 μg pRV-GFP-KI plasmids (Figure 1A right panel). The transfection efficiency, indicated by the percentage of GFP+ cells, was similar in pRV-GFP-WT– and pRV-GFP-KI–transfected 32D-IL-2Rβ cells (Figure 1A left panel). At low doses of IL-15 (0.01, 0.1, and 1 ng/mL, corresponding to 0.77, 7.7, and 77 pM, respectively) that can activate cells through binding to high-affinity IL-15 receptors, less proliferation was seen in 32D-IL-2Rβ/KI cells than in 32D-IL-2Rβ/WT cells, despite similar levels of IL-15Rα expression, and 32D-IL-2Rβ cells completely lacking IL-15Rα had essentially no proliferation (Figure 1B). Higher doses of IL-15 can activate cells through binding to moderate affinity IL-2Rβ/γc complexes, and as expected, at 10 ng/mL (770 pM) of IL-15, similar proliferation was seen, including in the 32D-IL-2Rβ cells that do not express IL-15Rα and in transfectants that express either WT IL-15Rα or the KI IL-15Rα mutant (Figure 1B). As expected, all of the cells proliferated normally to either IL-3 or IL-2 (Figure 1B). The defective proliferation of 32D-IL-2Rβ/KI cells in response to low doses of IL-15 stimulation indicated a key role for the IL-15Rα intracellular domain.
Proliferation of 32D-IL-2Rβ cells reconstituted with similar expression levels of WT and KI IL-15Rα. The coding region of WT IL-15Rα or an IL-15Rαext/IL-2Rαint chimeric construct was subcloned into pRV-GFP vector. Different doses of the plasmids (pRV-GFP-WT or pRV-GFP-KI) were transfected into 32D-IL-2Rβ cells. One day after the transfection, cells were rested in cytokine-free medium for 4 hours, then incubated in medium alone, or with IL-15 (0.01, 0.1, 1.0, or 10 ng/mL), IL-3 (50 ng/mL), or IL-2 (100 U/mL). At the end of the incubation, IL-15Rα expression on transfected cells was measured by flow cytometry. We chose 2 groups of transfected cells with similar transfection efficiency (percentage of GFP+ cells, indicated on left panels) and expression levels of WT and KI IL-15Rα (mean fluorescence intensity, indicated on right panels; A) and examined their proliferation (B) by measuring [3H]-thymidine uptake in triplicate as described in “Methods.” Means plus or minus SEM are shown. The results shown are representative of 3 separate experiments.
Proliferation of 32D-IL-2Rβ cells reconstituted with similar expression levels of WT and KI IL-15Rα. The coding region of WT IL-15Rα or an IL-15Rαext/IL-2Rαint chimeric construct was subcloned into pRV-GFP vector. Different doses of the plasmids (pRV-GFP-WT or pRV-GFP-KI) were transfected into 32D-IL-2Rβ cells. One day after the transfection, cells were rested in cytokine-free medium for 4 hours, then incubated in medium alone, or with IL-15 (0.01, 0.1, 1.0, or 10 ng/mL), IL-3 (50 ng/mL), or IL-2 (100 U/mL). At the end of the incubation, IL-15Rα expression on transfected cells was measured by flow cytometry. We chose 2 groups of transfected cells with similar transfection efficiency (percentage of GFP+ cells, indicated on left panels) and expression levels of WT and KI IL-15Rα (mean fluorescence intensity, indicated on right panels; A) and examined their proliferation (B) by measuring [3H]-thymidine uptake in triplicate as described in “Methods.” Means plus or minus SEM are shown. The results shown are representative of 3 separate experiments.
Generation of IL-15Rαext/IL-2Rαint KI mice
To further examine the role of the IL-15Rα intracellular domain in vivo, we generated mice in which the cytoplasmic domain of IL-15Rα was replaced with that from IL-2Rα by homologous recombination (the chimeric KI allele herein is also denoted IL-15Rαext/IL-2Rαint; Figure 2A). ES cells expressing the chimeric KI gene were identified by Southern blotting (Figure 2B), with the targeted KI allele yielding a 9.3-kb band, 1.8 kb larger than the 7.5 kb WT allele due to the presence of the PGK-neo cassette. The ES cells were injected into C57BL/6 embryos and germ-line transmission was confirmed by Southern blotting (Figure 2C left panel). Mice heterozygous for the targeted allele were crossed to EIIa-Cre mice to delete the neo cassette, as confirmed by Southern blotting (Figure 2C right panel), to yield a KI allele containing the IL-2Rα cytoplasmic domain and a single LoxP site 500 bp 3′ of exon 6. The correctness of this insertion was verified by DNA sequencing (data not shown). The KI allele encodes a transcript 42 nucleotides longer than WT IL-15Rα mRNA due to this insert in exon 6 (Figure 2D); RT-PCR of the KI allele confirmed its larger size than a WT IL-15 cDNA (Figure 2E).
Generation of the IL-15Rαext/IL-2Rαint KI mice. (A) Schematics showing the gene structure of IL-15Rα, targeting construct, targeted allele after homologous recombination, and the KI allele after deletion of the neo cassette by Cre recombinase. ■ represents exons, and the hatched box in exon 6 indicates the insertion of the coding sequence of IL-2Rα intracellular domain. (B) ES cells containing the KI allele. Genomic DNA from the ES cells was digested with HpaI and Southern-blotted with probe A indicated in panel A. (C) After homologous recombination, an XhoI site was inserted 5′ to the first LoxP site. Southern blotting with probe B after XhoI digestion exhibited a band of 14.6 kb in the WT allele, a 4.4-kb band in the KI allele after homologous recombination, and a 2.5-kb band in the KI allele after excision of the neo cassette by Cre recombinase. (D) Schematic of partial structure of IL-15Rα cDNA. The numbers represent the corresponding exon numbers in the genome. 6a and 6b are from exon 6 but separated by the insertion, which is indicated by a hatched box. The location of forward (Fwd) and reverse (Rev) primers used to amplify the IL-15Rα cDNA is shown. (E). Total RNA was isolated from total splenocytes, reverse-transcribed, and amplified with primers as in panel D.
Generation of the IL-15Rαext/IL-2Rαint KI mice. (A) Schematics showing the gene structure of IL-15Rα, targeting construct, targeted allele after homologous recombination, and the KI allele after deletion of the neo cassette by Cre recombinase. ■ represents exons, and the hatched box in exon 6 indicates the insertion of the coding sequence of IL-2Rα intracellular domain. (B) ES cells containing the KI allele. Genomic DNA from the ES cells was digested with HpaI and Southern-blotted with probe A indicated in panel A. (C) After homologous recombination, an XhoI site was inserted 5′ to the first LoxP site. Southern blotting with probe B after XhoI digestion exhibited a band of 14.6 kb in the WT allele, a 4.4-kb band in the KI allele after homologous recombination, and a 2.5-kb band in the KI allele after excision of the neo cassette by Cre recombinase. (D) Schematic of partial structure of IL-15Rα cDNA. The numbers represent the corresponding exon numbers in the genome. 6a and 6b are from exon 6 but separated by the insertion, which is indicated by a hatched box. The location of forward (Fwd) and reverse (Rev) primers used to amplify the IL-15Rα cDNA is shown. (E). Total RNA was isolated from total splenocytes, reverse-transcribed, and amplified with primers as in panel D.
IL-15Rαext/IL-2Rαint KI mice have reduced numbers of thymic NKT cells and of splenic NK, NKT, and memory-phenotype CD8+ T cells
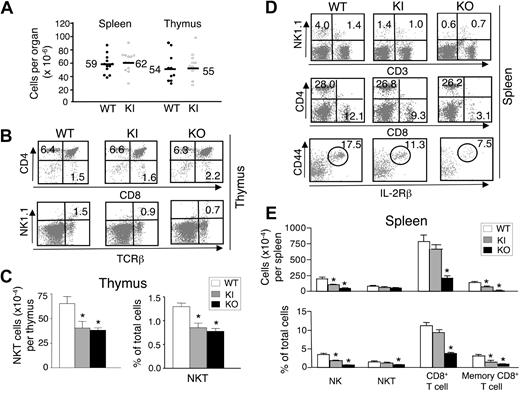
We next evaluated the size and cellularity of spleen and thymus in mice homozygous for the IL-15Rαext/IL-2Rαint KI allele and found no significant difference from WT littermates (Figure 3A). IL-15 is critical for the maintenance of NK, NKT, and memory CD8+ T cells, and Il15−/− and Il15ra−/− mice are deficient in these populations.26,27 Although flow cytometric analyses revealed no differences in total numbers of thymic CD4+ and CD8+ T-cell subsets in WT and KI mice, thymic NKT cells (NK1.1+TCRβ+; Figure 3B,C) as well as splenic NK cells (NK1.1+CD3−), NKT cells (NK1.1+CD3+), CD8+ T cells, and memory CD44highIL-2Rβ+ CD8+ T cells, were modestly decreased in the KI mice, and more reduced in the KO mice (Figure 3D,E).
Lymphoid organ cellularities and lymphocyte staining in WT, KI, and KO mice. (A) Total cellularity was counted in spleen and thymus from multiple experiments using age- and sex-matched littermates. The values shown are from both male and female mice between 8 and 16 weeks of age. Horizontal bars and numbers shown represent the mean value of each group. (B) Thymocytes from individual WT, KI, and KO mice were analyzed for expression of T cell (CD4+/CD8+) and NKT cell (NK1.1+ TCRβ+) markers by flow cytometry (n = 3/group; 8-12 weeks of age). The numbers shown represent the percentages of cells in each quadrant. The results shown are representative of 3 experiments. (C) Absolute numbers and percentage of thymic NKT cells from WT, KI, and KO mice. Means plus or minus SEM are shown. Statistical analysis was performed by one-way analysis of variance (ANOVA) using Prism. *P < .05 when compared with WT. (D) Splenocytes from WT, KI, and KO mice were analyzed for expression of NK cells (NK1.1+CD3−), NKT cells (NK1.1+CD3+), T cells (CD4+/CD8+) and memory CD8+ T cells (cells were gated on CD8+ CD3+ then gated on CD44highIL-2Rβ+; n = 3/group; 8-12 weeks of age). Numbers shown represent the percentage of cells in each quadrant or gate. The results shown are representative of 3 experiments. (E) Absolute numbers and percentage of splenic NK, NKT, CD8+ cells, and memory CD8+ T cells from WT, KI, and KO mice. Means plus or minus SEM are shown. Statistical analysis was performed by one-way ANOVA using Prism. *P < .05 when compared with WT.
Lymphoid organ cellularities and lymphocyte staining in WT, KI, and KO mice. (A) Total cellularity was counted in spleen and thymus from multiple experiments using age- and sex-matched littermates. The values shown are from both male and female mice between 8 and 16 weeks of age. Horizontal bars and numbers shown represent the mean value of each group. (B) Thymocytes from individual WT, KI, and KO mice were analyzed for expression of T cell (CD4+/CD8+) and NKT cell (NK1.1+ TCRβ+) markers by flow cytometry (n = 3/group; 8-12 weeks of age). The numbers shown represent the percentages of cells in each quadrant. The results shown are representative of 3 experiments. (C) Absolute numbers and percentage of thymic NKT cells from WT, KI, and KO mice. Means plus or minus SEM are shown. Statistical analysis was performed by one-way analysis of variance (ANOVA) using Prism. *P < .05 when compared with WT. (D) Splenocytes from WT, KI, and KO mice were analyzed for expression of NK cells (NK1.1+CD3−), NKT cells (NK1.1+CD3+), T cells (CD4+/CD8+) and memory CD8+ T cells (cells were gated on CD8+ CD3+ then gated on CD44highIL-2Rβ+; n = 3/group; 8-12 weeks of age). Numbers shown represent the percentage of cells in each quadrant or gate. The results shown are representative of 3 experiments. (E) Absolute numbers and percentage of splenic NK, NKT, CD8+ cells, and memory CD8+ T cells from WT, KI, and KO mice. Means plus or minus SEM are shown. Statistical analysis was performed by one-way ANOVA using Prism. *P < .05 when compared with WT.
Decreased IL-15Rαext/IL-2Rαint cell-surface expression but normal IL-15 binding affinity in KI T cells
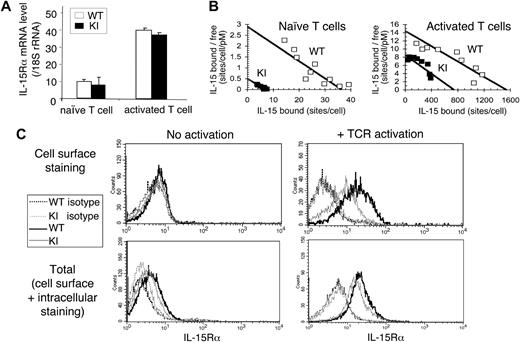
We next investigated whether replacement of the IL-15Rα intracellular domain with that from IL-2Rα affected the expression level, as we had observed in the 32D-IL-2Rβ cells. As shown in Figure 4A, freshly isolated T cells from WT and KI mice activated with anti-CD3ϵ+ anti-CD28 showed similar IL-15Rα mRNA expression. However, although unstimulated T cells from both WT or KI mice bound 125I-labeled IL-15 with similar high affinity, indicating an interaction of the cytokine with the IL-15Rα chain and/or the IL-15Rα/IL-2Rβ/γc complex (Kd = 10-100 pM),40,44 the WT cells had approximately 5-fold more binding sites/cell than the KI T cells (see Scatchard plots in Figure 4B). Following activation with anti-CD3ϵ plus anti-CD28 for 3 days, receptor expression increased in both WT and KI T cells, with the WT cells having more receptors than the KI T cells (Figure 4B).
Expression of IL-15Rα in T cells from WT and KI mice. (A) IL-15Rα mRNA levels in T cells. mRNA were prepared from naive and anti-CD3ϵ plus anti-CD28 activated splenic T cells from WT and KI mice. IL-15Rα mRNA levels were quantitated by real-time PCR and normalized to the level of 18S ribosomal RNA. Means plus or minus SEM are shown (n = 3/group). (B) Binding of 125I–IL-15 to WT and KI T cells purified from WT or KI mouse spleens (6-mouse pool). Both naive and anti-CD3ϵ plus anti-CD28 activated T cells were analyzed (see “Methods”). The data are presented as Scatchard plots (bound vs bound/free: radiolabeled IL-15–specific binding expressed in sites per cell on the abscissa, and the ratio of specifically bound fraction in sites per cell to the concentration of free iodinated IL-15 expressed in picomoles is on the ordinate). (C) Expression levels of total and cell-surface IL-15Rα in WT and KI T cells. T cells were isolated from WT and KI splenocytes and activated by anti-CD3ϵ plus anti-CD28 for 3 days. Cell-surface IL-15Rα expression were detected by staining both naive and activated T cells with biotinylated anti–mIL-15Rα followed by streptavidin-APC. In parallel, some of the cells were fixed and permeabilized, then stained with biotinylated anti–mIL-15Rα, thus staining both cell-surface and intracellular IL-15Rα. Biotinylated mouse IgG was used as an isotype control.
Expression of IL-15Rα in T cells from WT and KI mice. (A) IL-15Rα mRNA levels in T cells. mRNA were prepared from naive and anti-CD3ϵ plus anti-CD28 activated splenic T cells from WT and KI mice. IL-15Rα mRNA levels were quantitated by real-time PCR and normalized to the level of 18S ribosomal RNA. Means plus or minus SEM are shown (n = 3/group). (B) Binding of 125I–IL-15 to WT and KI T cells purified from WT or KI mouse spleens (6-mouse pool). Both naive and anti-CD3ϵ plus anti-CD28 activated T cells were analyzed (see “Methods”). The data are presented as Scatchard plots (bound vs bound/free: radiolabeled IL-15–specific binding expressed in sites per cell on the abscissa, and the ratio of specifically bound fraction in sites per cell to the concentration of free iodinated IL-15 expressed in picomoles is on the ordinate). (C) Expression levels of total and cell-surface IL-15Rα in WT and KI T cells. T cells were isolated from WT and KI splenocytes and activated by anti-CD3ϵ plus anti-CD28 for 3 days. Cell-surface IL-15Rα expression were detected by staining both naive and activated T cells with biotinylated anti–mIL-15Rα followed by streptavidin-APC. In parallel, some of the cells were fixed and permeabilized, then stained with biotinylated anti–mIL-15Rα, thus staining both cell-surface and intracellular IL-15Rα. Biotinylated mouse IgG was used as an isotype control.
Given the normal mRNA level, the decreased cell-surface IL-15Rα expression on the KI T cells may have resulted from reduced translation, increased “trapping,” or increased shedding, and we investigated these possibilities. Using a biotinylated antibody and flow cytometry, as expected given the low receptor number by binding assays (Figure 4B), we found little if any cell-surface IL-15Rα expression on unstimulated T cells by flow cytometry (Figure 4C top left panel), whereas expression was increased on T-cell receptor (TCR)–activated T cells, with higher expression on WT than KI T cells (Figure 4C top right panel). When cells were fixed, permeabilized, and stained with a biotin-conjugated mAb to IL-15Rα to detect total (both cell surface and intracellular) IL-15Rα, there was less of a difference between WT and KI mice (Figure 4C bottom panels), suggesting that the reduced cell surface IL-15Rα expression in KI T cells did not result from diminished translation. One possibility is that the decreased receptor expression may result from the “trapping” of the chimeric IL-15Rαext/IL-2Rαint receptor in an intracellular compartment. Thus, even though IL-2Rα,45 like IL-15Rα,31 is known to be recycled to the cell surface, at least in the context of the chimeric protein, the cytoplasmic domain of IL-2Rα could be less efficient than that of IL-15Rα for this function. A second possibility that may also contribute to reduced IL-15Rα cell-surface expression on KI T cells is an increase in the release from the cells of the chimeric IL-15Rαext/IL-2Rαint receptor. Recent data have revealed that a secreted form of IL-15Rα exists in humans and mice and that this soluble IL-15Rα (sIL-15Rα) is the result of alternative splicing or is due to a proteolytic cleavage event at the cell surface. These sIL-15Rα molecules associate with IL-15 to either promote or inhibit IL-15 activity.46-48 Although the decreased cell-surface IL-15Rα expression in KI mice could have resulted from increased receptor release, when we evaluated the serum level of sIL-15Rα in WT and KI mice using the DuoSet ELISA kit from R&D Systems according to the manufacturer's recommendations, the sIL-15Rα levels in the mouse sera tested (10 WT sera and 11 KI sera) were below the detection limit of the kit (data not shown).
Cells with the chimeric IL-15Rαext/IL-2Rαint receptor have defective antigen-specific responses, CD8+ T-cell proliferation, and Stat5 activation
We next investigated whether cells expressing the chimeric receptor exhibited any functional defects by examining the function of NK cells from WT, KI, and KO mice using YAC-1 target cells. As previously reported,27 cells from KO mice had essentially no cytolytic activity, but the KI cells did not reproducibly display less cytolytic activity against YAC-1 target cells than did WT cells (data not shown), with variations in cytolytic activity potentially being attributable to the decreased number of NK cells in these animals as compared with WT mice (Figure 3D,E). We next immunized mice with MVA virus, and evaluated their immune response against an immunodominant CTL epitope (see “Methods”). The relative percentage of epitope-specific memory CD8+ T cells in WT and KI mice was similar (Figure 5A). Although IFN-γ–producing T-cell numbers were not significantly altered at day 7 (data not shown), by 1 month, they were reproducibly diminished (Figure 5B).
Functional analyses of T cells in WT, KI, and KO mice. (A) Relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells in the spleen. Cells were isolated 1 month after MVA immunization and prepared for flow cytometry. Data represent the relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells out of total CD8+ T cells. Data are representative of 2 independent experiments. (B) Functional activity of IL-15Rαext/IL-2Rαint KI versus WT mice by ELISPOT for IFN-γ production (see “Methods”). Means plus or minus SEM are shown (n = 5/group). Data are representative of 2 independent experiments. (C) Proliferation of CD8+ T cells. Splenic CD8+ T cells were purified from WT, KI, and KO mice and cultured in medium alone, 1 μg/mL of plate-bound anti-CD3ϵ or 1 μg/mL of anti-CD3ϵ plus anti-CD28 supplemented with IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL). Proliferation was assessed by measuring [3H]-thymidine uptake as described in “Methods.” Means plus or minus SEM are shown (n = 3/group). Data are representative of 4 separate experiments. (D) Proliferation of memory and naive CD8+ T cells. Splenic effector/memory (CD44high) and naive (CD44low) CD8+ T cells from WT and KI mice were labeled with CFSE and then cultured for 4 days in medium alone, 100 ng/mL IL-21 alone, or IL-21 plus IL-15 (5 ng/mL or 50 ng/mL) before flow cytometric analysis. Percentage of CFSE dilution is indicated. The experiment was performed twice with similar cooperative effects of IL-15 and IL-21 on CFSE dilution. (E) Intracellular staining of Stat5 phosphorylation. T cells isolated from WT, KI, and KO mice spleen were preactivated in anti-CD3ϵ plus anti-CD28 for 3 days, washed, and rested for 2 days. IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL) was added to the medium. After 15 minutes of stimulation, cells were harvested and stained for CD4+/CD8+ subsets and intracellular phosphorylated Stat5. The Stat5 phosphorylation level was analyzed from CD8+ T cells. Means plus or minus SEM are shown (n = 6/group).
Functional analyses of T cells in WT, KI, and KO mice. (A) Relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells in the spleen. Cells were isolated 1 month after MVA immunization and prepared for flow cytometry. Data represent the relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells out of total CD8+ T cells. Data are representative of 2 independent experiments. (B) Functional activity of IL-15Rαext/IL-2Rαint KI versus WT mice by ELISPOT for IFN-γ production (see “Methods”). Means plus or minus SEM are shown (n = 5/group). Data are representative of 2 independent experiments. (C) Proliferation of CD8+ T cells. Splenic CD8+ T cells were purified from WT, KI, and KO mice and cultured in medium alone, 1 μg/mL of plate-bound anti-CD3ϵ or 1 μg/mL of anti-CD3ϵ plus anti-CD28 supplemented with IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL). Proliferation was assessed by measuring [3H]-thymidine uptake as described in “Methods.” Means plus or minus SEM are shown (n = 3/group). Data are representative of 4 separate experiments. (D) Proliferation of memory and naive CD8+ T cells. Splenic effector/memory (CD44high) and naive (CD44low) CD8+ T cells from WT and KI mice were labeled with CFSE and then cultured for 4 days in medium alone, 100 ng/mL IL-21 alone, or IL-21 plus IL-15 (5 ng/mL or 50 ng/mL) before flow cytometric analysis. Percentage of CFSE dilution is indicated. The experiment was performed twice with similar cooperative effects of IL-15 and IL-21 on CFSE dilution. (E) Intracellular staining of Stat5 phosphorylation. T cells isolated from WT, KI, and KO mice spleen were preactivated in anti-CD3ϵ plus anti-CD28 for 3 days, washed, and rested for 2 days. IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL) was added to the medium. After 15 minutes of stimulation, cells were harvested and stained for CD4+/CD8+ subsets and intracellular phosphorylated Stat5. The Stat5 phosphorylation level was analyzed from CD8+ T cells. Means plus or minus SEM are shown (n = 6/group).
Because IL-15 is important for the homeostatic proliferation and maintenance of memory CD8+ T cells,11,13,49 we also examined the response to IL-15 of naive splenic CD8+ T cells from WT, KI, and KO mice. No significant difference in CD8+ T-cell proliferation was observed in vitro in WT, KI, and KO cells stimulated with either anti-CD3ϵ or anti-CD3ϵ plus anti-CD28, and as expected, the addition of IL-2 augmented proliferation of these cells (Figure 5C). However, when TCR-activated CD8+ T cells were costimulated with 5 ng/mL (385 pM) of IL-15, a dose that titrates mainly the high-affinity IL-15Rα and induces little if any proliferation in the KO cells, the proliferation of KI T cells was somewhat diminished. In contrast, a higher dose of IL-15 (50 ng/mL) induced similar proliferation in cells from WT and KI mice, whereas CD8+ T cells from KO mice still exhibited decreased proliferation (Figure 5C). When we used a very high dose of IL-15 (500 ng/mL) sufficient to fully titrate the lower affinity IL-2Rβ/γc complex, as anticipated, the KO CD8+ T cells now exhibited similar proliferation to WT cells (data not shown). Because the expression levels of IL-2Rβ and γc were similar on activated CD8+ T cells from WT, KI, and KO mice as evaluated by flow cytometry (data not shown), the decreased proliferation of CD8+ T cells from KO mice in response to 5 or 50 ng/mL of IL-15 indicates the need for IL-15Rα on the T cell for an optimal response to IL-15. To further investigate the effect of IL-15Rα on the proliferation of effector/memory and naive CD8+ T cells, splenic CD8+ T cells from WT and KI mice were further separated based on CD44 expression and then labeled with CFSE. Cells were cultured for 4 days in medium with IL-15 plus IL-21 but in the absence of anti-CD3ϵ and anti-CD28 costimulation to avoid further TCR-mediated activation of these cell subpopulations. Consistent with our previous report,50 the rate of cell division of both memory (CD44high) and naive (CD44low) phenotype CD8+ T cells from WT mice was accelerated by the combination of IL-21 with either low-dose (5 ng/mL) or high dose (50 ng/mL) IL-15, as compared with cells cultured with medium or IL-21 alone (Figure 5Dc,d vs 5Da,b; Figure 5Dg,h vs 5De,f). Both memory- and naive-phenotype CD8+ T cells from KI mice exhibited lower cell division than cells from WT mice when cultured with IL-21 plus high-dose IL-15 (50 ng/mL; Figure 5Dh vs 5De and Figure 5Dp vs 5Dl). However, cells from KI mice showed strongly reduced cell division when cultured with IL-21 plus low-dose IL-15 (5 ng/mL; Figure 5Dg vs 5Dc and Figure 5Do vs 5Dk), indicating that the chimeric IL-15Rαext/IL-2Rαint receptor mediates defective proliferation of both memory- and naive-phenotype CD8+ T cells. Interestingly, Lodolce et al51 suggested that differences in proliferation of Il15ra−/− versus Il15ra+/− CD8+ T cells could be explained by the observation that Il15ra−/− mice have lower numbers of memory phenotype CD44highIL-2Rβ+CD8+ T cells than do controls. Our data generally indicated that the defect seen in Figure 5C was not due to a difference in the expression of IL-2Rβ, although we cannot exclude a partial contribution.
We next investigated whether the KI cells could mediate normal Stat5 activation, as might be expected given that the essential signaling molecules associate with IL-2Rβ (Jak1, Stat5a, and Stat5b) and γc (Jak3) rather than IL-15Rα. Analogous to the proliferation results in Figure 5C, 5 ng/mL of IL-15 induced less Stat5 phosphorylation in KI than in WT CD8+ T cells, and almost no Stat5 phosphorylation was induced in the KO cells (Figure 5E). Interestingly, at 50 ng/mL, Stat5 phosphorylation in KI cells was reproducibly more defective (Figure 5E) than was proliferation (Figure 5C), whereas 500 ng/mL of IL-15 (which fully saturates IL-2Rβ/γc receptors) induced maximal Stat5 activation even in the KO cells (data not shown). Retroviral transduction of WT IL-15Rα into the IL-15Rα KO T cells restored partial Stat5 phosphorylation in response to the lower concentration of IL-15 (data not shown), and IL-2 induced similar Stat5 phosphorylation in each cell type (Figure 5E), excluding a general intrinsic defect in the ability of the KI and KO cells to activate the Jak-STAT pathway.
The chimeric IL-15Rαext/IL-2Rαint KI construct has normal trans-presentation
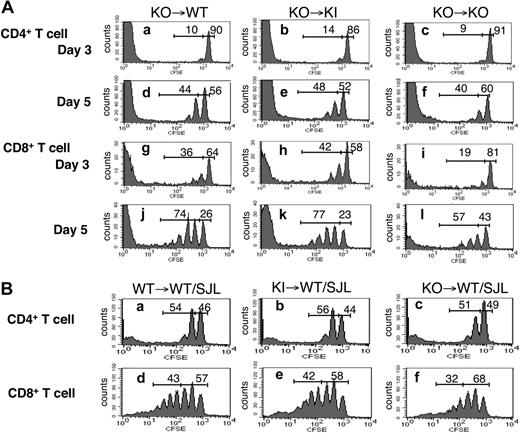
As noted, IL-15Rα binds IL-15 with high affinity and presents it in trans to cells that express the lower affinity IL-2Rβ/γc complex.31 To determine the importance of the IL-15Rα cytoplasmic domain for trans-presentation, we transferred CFSE-labeled KO T cells into irradiated WT, KI, or KO mice and used CFSE dilution as a measure of cell-cycle progression. In both the spleen (Figure 6A) and lymph nodes (data not shown), CD4+ and CD8+ KO T cells exhibited similar CFSE dilution in WT and KI recipients at days 3 and 5 (Figure 6Ab vs 6Aa, Figure 6Ae vs 6Ad, Figure 6Ah vs 6Ag, and Figure 6Ak vs 6Aj), but cell-cycle progression of CD4+ T cells in KO recipients was slightly decreased at day 5 (Figure 6Af vs 6Ad), and cell-cycle progression of CD8+ T cells was markedly decreased at both days 3 and 5 (Figure 6Ai vs 6Ag and Figure 6Al vs 6Aj) in KO recipients. These results are consistent with the importance of IL-15Rα for trans-presentation.52 The similar results in WT and KI recipient mice indicate that the sequence of the IL-15Rα cytoplasmic domain is not essential for trans-presentation.
Adoptive transfer of T cells to WT, KI, or KO host mice. (A) CFSE-labeled T cells from KO mice were injected into irradiated WT, KI, or KO mice (5 × 106 cells each). On days 3 and 5, splenocytes from host mice were stained for CD4+/CD8+ subsets. Proliferation of transferred CD4+/CD8+ T cells was visualized by CFSE dilution. Data are representative of 4 mice/group. (B) CFSE-labeled T cells from WT, KI, or KO mice were injected into irradiated WT B6.SJL congenic mice (8 × 106 cells each). On day 6, splenocytes were isolated from host mice and stained for CD45.2, CD4, and CD8. Cells were gated on CD45.2+ to exclude endogenous T cells. Proliferation of transferred CD4+/CD8+ T cells was visualized by CFSE dilution. Data are representative from 5 mice/group.
Adoptive transfer of T cells to WT, KI, or KO host mice. (A) CFSE-labeled T cells from KO mice were injected into irradiated WT, KI, or KO mice (5 × 106 cells each). On days 3 and 5, splenocytes from host mice were stained for CD4+/CD8+ subsets. Proliferation of transferred CD4+/CD8+ T cells was visualized by CFSE dilution. Data are representative of 4 mice/group. (B) CFSE-labeled T cells from WT, KI, or KO mice were injected into irradiated WT B6.SJL congenic mice (8 × 106 cells each). On day 6, splenocytes were isolated from host mice and stained for CD45.2, CD4, and CD8. Cells were gated on CD45.2+ to exclude endogenous T cells. Proliferation of transferred CD4+/CD8+ T cells was visualized by CFSE dilution. Data are representative from 5 mice/group.
A role for IL-15Rα in cis signaling
In addition to its role in trans-presentation, we investigated whether IL-15Rα could also cooperate in cis with IL-2Rβ/γc complexes on the same cell, allowing IL-15 signaling to also occur in a more “traditional” fashion via an IL-15Rα/IL-2Rβ/γc heterotrimer on a single cell, analogous to the IL-2 high-affinity IL-2Rα/IL-2Rβ/γc receptor. To examine this, we transferred CFSE-labeled T cells from WT, KI, and KO mice into irradiated WT B6.SJL congenic mice so that IL-15 could be trans-presented by IL-15Rα–expressing host cells (Figure 6B). In this experiment, any difference in the responsiveness of the donor T cells necessarily resulted from differences in the IL-15Rα status of these cells. Whereas KI and WT donor cells exhibited similar cell-cycle progression (Figure 6Bb vs 6Ba and Figure 6Be vs 6Bd), there was a reproducible partial decrease in cell-cycle progression of both CD4+ (Figure 6Bc vs 6Ba) and CD8+ (Figure 6Bf vs 6Bd) KO donor T cells. The fact that there still was significant cell-cycle progression of the donor KO T cells indicates a role for trans-presentation in supporting CD8+ T-cell homeostasis; however, the greater proliferation by the donor WT and KI cells than by the KO cells also indicates a role for IL-15Rα on the responding donor cells, and thus a role for IL-15Rα function in cis signaling as well as trans signaling. In addition, we cannot exclude the possibility that the lower proliferation of the KO cells could potentially be partially explained by these cells having a less activated phenotype than the WT and KI cells.
Discussion
IL-15 is important for the development and normal function of NK cells, NKT cells, and memory CD8+ T cells. In this study, we investigated the importance of the cytoplasmic domain of IL-15Rα by replacing it with that from IL-2Rα. Although the chimeric construct had similar mRNA expression to that observed for WT IL-15Rα, its cell-surface expression was diminished, ostensibly due to increased intracellular “trapping” of the chimeric receptor. Like IL-15Rα, IL-2Rα also recycles to the cell surface; thus, these results indicate that the IL-15Rα cytoplasmic domain is more efficient for this process than is the IL-2Rα cytoplasmic domain. In addition to the importance of the IL-15Rα cytoplasmic domain for cell-surface expression, it is also needed for normal signaling, given defective proliferation to submaximal concentrations of IL-15 in 32D–IL-2Rβ transfectants in which similar levels of WT and the KI constructs were expressed.
IL-15Rαext/IL-2Rαint KI mice had reduced numbers of thymic NKT cells, as well as splenic NK, NKT, and memory-phenotype CD8+ T cells, albeit not as low as found in IL-15Rα KO mice. Moreover, when the functions of CD8+ T cells in the KI mice were examined, we observed decreased proliferation of CD8+ T cells in response to stimulation with TCR plus picomolar concentrations of IL-15 and reduced cell division of both memory- and naive-phenotype CD8+ T cells following stimulation by IL-21 plus picomolar concentrations of IL-15. The development of IFN-γ–producing CD8+ T cells was also decreased in KI mice immunized with a peptide antigen. Moreover, IL-15–induced Stat5 phosphorylation in CD8+ T cells was also decreased in the KI mice, although not as severely as in IL-15Rα KO mice. These abnormalities potentially result from a combination of diminished expression of the chimeric protein as well as from defective signaling.
A distinctive function for IL-15Rα is “trans-presentation.”31,53 To investigate the role of the IL-15Rα intracellular domain in this process, we adoptively transferred T cells from IL-15Rα KO mice into WT, KI, and KO recipient mice. Interestingly, we did not observe any difference in the reconstitution of T cells in WT and KI recipients, whereas the reconstitution of CD8+ T cells was delayed/defective in the KO recipients, indicating that the CD8+ T-cell homeostatic repopulation process is dependent on trans-presentation from IL-15Rα, but the similar results for WT and KI recipients indicated that the sequence of the IL-15Rα intracellular domain is not essential for this function. Given the data from 32D cells suggesting a signaling function for IL-15Rα, we also evaluated if IL-15Rα on T cells could act in cis with the IL-2Rβ/γc complex on the same cell to transduce an IL-15 signal. We explored this possibility by adoptively transferring T cells from WT, KI, and KO mice into WT congenic mice so that any differences in cell-cycle progression would be due to the IL-15Rα expression on the responding transferred T cells. The fact that a partial defect in cell-cycle progression was detected in the KO T cells indicates that IL-15Rα expression on the responding T cells also contributes to cell-cycle progression. A few groups also reported the possible cis function of IL-15Rα expressed on responding cells. One in vitro study showed that IL-15Rα+ CD8+ T cells had a survival advantage over IL-15Rα–deficient CD8+ T cells at low concentrations of IL-15, which suggests that IL-15Rα may facilitate higher affinity binding to the functional receptor complex or induce a survival signal in absence of the βγ subunits.12 Another study also showed that purified high- but not low-avidity CD8+ cytotoxic T lymphocytes reproducibly produced low levels of IFN-γ when stimulated only with IL-15 without presenting cells. This makes it plausible that IL-15Rα on T cells could act in cis to increase sensitivity to low levels of IL-15, conferring to these cells expressing high IL-15Rα a survival advantage for greater homeostatic proliferation in response to limited IL-15 in the immune quiescent host.54 Our data provide in vivo evidence in support of cis signaling function for IL-15Rα.
In summary, the generation of IL-15Rαext/IL-2Rαint KI transfectants and mice have helped to clarify that the IL-15Rα cytoplasmic domain does more than merely anchor IL-15Rα in the cell membrane. Our studies reveal that although IL-2Rα and IL-15Rα are both sushi domain–containing proteins that cooperate with IL-2Rβ and γc, their cytoplasmic domains are functionally distinct. Moreover, our study indicates that the IL-15Rα cytoplasmic domain sequence is not critical for trans-presentation, but that it is required for mediating normal IL-15 function.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Cheng-Yu Liu for generating the IL-15Rαext/IL-2Rαint KI mice; Constance Robinson for maintaining the mice strains; and Drs. Yutaka Tagaya, Christian Hinrichs (both from the National Cancer Institute [NCI]), Keji Zhao, Rosanne Spolski, and Jian-Xin Lin (all from NHLBI) for valuable discussions.
This work was supported by the Intramural Research Programs of NHLBI and NCI, NIH.
National Institutes of Health
Authorship
Contribution: Z.W., H.-H.X., J.B., R.Z., D.I., I.M.B., and S.K.O. designed and performed research, collected and analyzed data, and wrote the paper; J.B.-R. performed research; and J.A.B. and W.J.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren J. Leonard, Laboratory of Molecular Immunology, NHLBI, NIH, Bldg 10, Rm 7B05, Bethesda, MD 20892-1674; e-mail: wjl@helix.nih.gov.

![Figure 1. Proliferation of 32D-IL-2Rβ cells reconstituted with similar expression levels of WT and KI IL-15Rα. The coding region of WT IL-15Rα or an IL-15Rαext/IL-2Rαint chimeric construct was subcloned into pRV-GFP vector. Different doses of the plasmids (pRV-GFP-WT or pRV-GFP-KI) were transfected into 32D-IL-2Rβ cells. One day after the transfection, cells were rested in cytokine-free medium for 4 hours, then incubated in medium alone, or with IL-15 (0.01, 0.1, 1.0, or 10 ng/mL), IL-3 (50 ng/mL), or IL-2 (100 U/mL). At the end of the incubation, IL-15Rα expression on transfected cells was measured by flow cytometry. We chose 2 groups of transfected cells with similar transfection efficiency (percentage of GFP+ cells, indicated on left panels) and expression levels of WT and KI IL-15Rα (mean fluorescence intensity, indicated on right panels; A) and examined their proliferation (B) by measuring [3H]-thymidine uptake in triplicate as described in “Methods.” Means plus or minus SEM are shown. The results shown are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2007-03-080697/7/m_zh80230827350001.jpeg?Expires=1767902000&Signature=iD1K78fvP51Pw7SxzDHkxpngGIpA2RrUkEzaqiEWBXZSRFBfGkshWuZNAeM5VsERJ3uKpEJnXMcfjajTTNjkHa1aNY61sq550ONsyEoDOXhNmJvSZ7OYyop-siHZujzjDJOXBUXl2SdNscrQRKyeMXjH5EdMVAD-4odvxfbVV6DUTSMbS4w67AXsn5PNKOPUowmAxmOf6Nc3G4NgP4pnu0bGs-0kXbVwaLRCCay05v97upz27-ixNJK9V-Y6QKl4VEomwrMkvGyc3vqfatY44FvG~v96babUQju-9rm4r2GAKkxVUfp8c67DdWQ0UfOB2sROjPjjOSE3rpzxIusOZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Functional analyses of T cells in WT, KI, and KO mice. (A) Relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells in the spleen. Cells were isolated 1 month after MVA immunization and prepared for flow cytometry. Data represent the relative percentage of B8R20–27/H-2Kb tetramer+ CD8+ T cells out of total CD8+ T cells. Data are representative of 2 independent experiments. (B) Functional activity of IL-15Rαext/IL-2Rαint KI versus WT mice by ELISPOT for IFN-γ production (see “Methods”). Means plus or minus SEM are shown (n = 5/group). Data are representative of 2 independent experiments. (C) Proliferation of CD8+ T cells. Splenic CD8+ T cells were purified from WT, KI, and KO mice and cultured in medium alone, 1 μg/mL of plate-bound anti-CD3ϵ or 1 μg/mL of anti-CD3ϵ plus anti-CD28 supplemented with IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL). Proliferation was assessed by measuring [3H]-thymidine uptake as described in “Methods.” Means plus or minus SEM are shown (n = 3/group). Data are representative of 4 separate experiments. (D) Proliferation of memory and naive CD8+ T cells. Splenic effector/memory (CD44high) and naive (CD44low) CD8+ T cells from WT and KI mice were labeled with CFSE and then cultured for 4 days in medium alone, 100 ng/mL IL-21 alone, or IL-21 plus IL-15 (5 ng/mL or 50 ng/mL) before flow cytometric analysis. Percentage of CFSE dilution is indicated. The experiment was performed twice with similar cooperative effects of IL-15 and IL-21 on CFSE dilution. (E) Intracellular staining of Stat5 phosphorylation. T cells isolated from WT, KI, and KO mice spleen were preactivated in anti-CD3ϵ plus anti-CD28 for 3 days, washed, and rested for 2 days. IL-15 (5 ng/mL or 50 ng/mL) or IL-2 (100 U/mL) was added to the medium. After 15 minutes of stimulation, cells were harvested and stained for CD4+/CD8+ subsets and intracellular phosphorylated Stat5. The Stat5 phosphorylation level was analyzed from CD8+ T cells. Means plus or minus SEM are shown (n = 6/group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2007-03-080697/7/m_zh80230827350005.jpeg?Expires=1767902000&Signature=Oq4ZEQ8vkqC~EljUfMngXdKq3dmCQJ4WAXzMw9cGDplKGzCiGF5BujO5u4IJ09H3PQLHQ0tgqdorAdFxI6F4kzoAWkFBbk1waeDy2PSJKa4UiNL3oLvk2WhapAzrOf6Hk0jH2uiEsCKroa7AJvtbH~udyJaOG4850BVE-STv1dqjtrgP2yXYcQXZFLxLyil-B5ZCfXZlw9phc0Xrg-RpJY5jZ36anYSaNxOPX9EHWcxghInYGfRNPbsSq7J1uMyJPBiKxVoi-liAELEq1JumtEwTU3tjV8nUMUHyfV2B0etvMkkEdmrH1tNS41qMty1iFn2uGiSZD~dpDE9yF3XyXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)