Abstract
Plasmacytoid dendritic cells (pDCs) are antigen-presenting cells that develop into type-I interferon (IFN-I)–producing cells in response to pathogens. Their role in human immunodeficiency virus (HIV) pathogenesis needs to be understood. We analyzed their dynamics in relation to innate and adaptive immunity very early during the acute phase of simian immunodeficiency virus (SIV) infection in 18 macaques. pDC counts decreased in blood and increased in peripheral lymph nodes, consistent with early recruitment in secondary lymphoid tissues. These changes correlated with the kinetic and intensity of viremia and were associated with a peak of plasma IFN-I. IFN-I and viremia were positively correlated with functional activity of the immune suppression associated enzyme indoleamine-2,3-dioxygenase (IDO) and FoxP3+CD8+ T cells, which both negatively correlated with SIV-specific T-cell proliferation and CD4+ T-cell activation. These data suggest that pDCs and IFN-I play a key role in shaping innate and adaptive immunity toward suppressive pathways during the acute phase of SIV/HIV primary infection.
Introduction
Plasmacytoid dendritic cells (pDCs) are major type I interferon (IFN-I) producing cells in response to viral infections1 as a result of selective expression of Toll-like receptors 7 and 9 and constitutive expression of interferon response factor 7. They migrate to inflamed lymph nodes (LN) through high endothelium venules during viral and bacterial infections2,3 and provide an important link between innate and adaptive immunity, enhancing natural killer (NK) cell activity and adaptive immune responses.
Blood pDC counts and pDC-dependent IFN-I production levels in vitro decrease in patients infected with human immunodeficiency virus (HIV).4-8 These decreases are generally correlated with a fall in CD4+ T-cell counts, inversely correlated with plasma viral load, and are associated with opportunistic infections.5,6,9-12 The reduction in circulating pDC numbers during HIV infection may be related to their direct infection,6,9,13 or to redistribution to lymphoid organs, as suggested in the chronic asymptomatic stage of HIV infection.14
In nonhuman primate models of HIV infection, the IFN-I innate response is an early immunologic event.15-17 Rhesus macaque (Macaca mulatta) pDCs are activated and produce IFN-I in vitro in response to pathogenic simian immunodeficiency virus (SIV)18,19 like their human counterparts in response to HIV.20,21 Strong depletion of pDCs from blood and lymphoid tissues are reported in the end stage of SIV infection19,22 and attributed to infection and to higher levels of apoptosis. However, although the HIV/SIV interplay with the host immune response during primary infection is a key event, probably determining the later progression to disease, little is known about the role of pDCs in immune regulation at this early stage.
We predicted that HIV/SIV infection may have an impact on the dynamics of circulating and LN dendritic cells (DCs) and on their functions in the first few days of infection23,24 and that it may induce immune suppression through IFN-I production and indoleamine-2,3-dioxygenase (IDO) activity. IDO is the rate-limiting enzyme responsible for the extrahepatic catabolism of the essential amino acid tryptophan (Trp) and is triggered by type I and type II interferons.25-27 Abnormal production/activation of IDO is associated with inefficient immunologic responses to infections, including HIV/SIV and cancer26,28-30 and is induced by HIV in human pDCs in vitro.20,21 Growing evidence suggests that pDCs are involved in the induction of tolerance through IDO-dependent mechanisms.31 This suggests that pDCs may target immune suppression during the acute phase of HIV/SIV infection.
We focused on the dynamics of pDCs during primary infection by carrying out fine time-resolution sampling of blood, and a longitudinal analysis of peripheral lymph nodes, using absolute quantification, to investigate the possible homing of these cells into lymphoid tissues. In parallel, the kinetics of virus replication, IFN-I and II responses, and IDO activity were analyzed to improve our understanding of the contributions of virus and IFNs to IDO activation in vivo. We also analyzed pDCs and IDO dynamics as a function of the dynamics of FoxP3+CD25+CD8+ T cells and SIV-specific T-cell proliferative responses during primary infection.33 Experiments were performed in 18 cynomolgus macaques, and the role of virus infection was assessed by using 2 different infectious doses or by using postexposure antiretroviral treatment.
Methods
Animals
Young adult cynomolgus macaques (Macaca fascicularis) weighing 3 to 5 kg were imported from Mauritius and kept according to European guidelines for animal care (Journal Officiel des Communautés Européennes, L358, December 18, 1986). Blood sampling, biopsies, and treatment were carried out in macaques anesthetized by intramuscular injection of 10 mg/kg ketamine (Rhône-Mérieux, Lyon, France). Six macaques received 5000 animal infectious dose 50% (AID50) of SIVmac251 intravenously (5000 AID50 group), 6 received 50 AID50 intravenously (50 AID50 group), and 6 were infected intravenously with 50 AID50 and treated from 4 hours to 28 days after exposure. This AZT/3TC/indinavir antiretroviral treatment (ART) group was given a combination of 4.5 mg/kg 3′-azido-2′,3′-dideoxythymidine (AZT), 2.5 mg/kg 2′,3′-dideoxy-3′-thiacytidine (3TC), and 20 mg/kg indinavir twice daily, via the oral route, as described previously.32 Virus stock was kindly provided by Dr A. M. Aubertin (Université Louis Pasteur, Strasbourg, France).
Phenotypic characterization and intracellular staining
Blood samples were collected into cell preparation tubes for the isolation of peripheral blood mononuclear cells (PBMCs), according to the manufacturer's recommendations. Cells were incubated with FcR blocking reagent (Miltenyi Biotec, Auburn, CA) for 10 minutes at room temperature and labeled with monoclonal antibodies (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) specific for CD123, human leukocyte antigen-DR (HLA-DR), and either CD4, CD7, CD11b, CD36, CD40, CD45RA, CD80, CD86, CD184 (CXCR4), CD185 (CCR5), CD206, CD1c (BDCA-1), or with isotypic control antibodies for 20 minutes at +4°C. Cells were then washed in PBS, and fixed in Cell Fix (BD Biosciences, San Jose, CA). pDCs were gated on CD123bright, HLA-DR+ cells as described previously.24
For IFN-α intracellular labeling, PBMCs were stimulated by incubation for 6 hours with HSV-I at a multiplicity of infection of 0.5 or with 20 μg/mL total protein from aldrithiol-2 (AT-2)–inactivated SIVmac239 (SIV-AT2). Brefeldin A (Sigma-Aldrich, St Louis, MO) was added (10 μg/mL) for the last 4 hours. Mock stimulation with AT-2-treated SupT1 microvesicles was used as a negative control. AT-2-inactivated SIVmac239 (ARP1018.1) and its negative control (ARP1018.2) were obtained from Dr Jeff Lifson (National Cancer Institute, Frederick, MD), through the EU Program EVA Centralized Facility for AIDS Reagents (National Institute for Biological Standards and Control, Potters Bar, United Kingdom). Intracellular staining was performed using anti-human IFN-α2a monoclonal antibody (kindly provided by Dr Jacques Banchereau, Baylor Institute for Immunology Research, Dallas, TX), as described previously.24
Plasma viral load, T-cell counts, and T-cell proliferation assay
pDC quantification
Absolute numbers of lineage−HLA-DR+CD123+CD1c− pDCs were quantified in whole blood using TruCount tubes.24
Lymph node biopsies were weighed, dilacerated and passed through cell strainers (BD Biosciences). The percentage of lineage−HLA-DR+CD123+CD1c− pDCs was determined on freshly isolated cells.24 Absolute counts of pDCs were obtained by multiplying the total number of lymph node cells isolated per gram of tissue by the percentage of pDCs divided by 100.
In situ immunofluorescence
Small blocks of lymph node tissue were snap-frozen in liquid nitrogen and stored at −80°C. Sections (10-μm) were cut with a CM1500 cryotome (Thermo Fisher Scientific, Courtaboeuf, France), dried for 30 minutes, fixed for 10 minutes in acetone, and used directly for labeling or stored at −20°C. Sections were washed in PBS, and nonspecific sites were blocked by incubation in 0.3% bovine serum albumin, 0.5% human AB serum, 2% normal goat serum in PBS for 30 minutes. Endogenous biotins were blocked using an avidin biotin blocking kit (Vector Laboratories, Peterborough, United Kingdom). Sections were then incubated for 90 minutes with the following primary antibodies: 25 μg/mL anti-CD123 (clone 7G3; BD Biosciences), 0.2 μg/mL anti-CD20 (clone L26; Dako Denmark A/S, Glostrup, Denmark), 12 μg/mL anti-CD3 (rabbit polyclonal; Dako) and appropriate isotypic controls. Sections were washed twice in 0.5% Tween 20 (Sigma-Aldrich) in PBS. They were then incubated for 1 hour with secondary antibodies (0.5 μg/mL biotinylated goat-antimouse IgG1or IgG2a [Invitrogen, Carlsbad, CA] and either 5 μg/mL Alexa Fluor 546-conjugated goat-antimouse IgG1 or IgG2a [Invitrogen] or 10 μg/mL TRITC-conjugated goat-antirabbit IgG [Invitrogen]). Sections were washed twice, and biotinylated antibodies were detected by incubation for 15-minute incubation with 1 μg/mL streptavidin-Alexa Fluor 488 (Invitrogen). The sections were washed again, stained for nuclei for 10 minutes in 1/20 000 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), then incubated with 0.5 M glycine in PBS for 30 minutes. Slides were mounted with the Fluoromount Kit (Dako). All sections were analyzed under an Axiovert 100M microscope (Zeiss, Le Pecq, France) equipped with an Orca-ER CCD camera 1344 × 1024 pixels (Hamamatsu Photonics, Hamamatsu City, Japan), and images were colorized using Image J 1.38 software (National Institutes of Health [NIH], http://rsbweb.nih.gov/ij/).
Determination of IDO activity
Kynurenine (Kyn) and Trp concentrations were measured in macaque plasma from ethylenediaminetetraacetic acid-treated blood, using high-performance liquid chromatography (HPLC) with coulometric detection.34 The Kyn/Trp ratio was considered as an index of IDO activity, independent of baseline Trp concentrations.35
Quantification of type I IFN, IFN-γ, and IL-18
IFN-I levels were measured by assessing reduction of the cytopathic effect of vesicular stomatitis virus-infected Madin-Darby bovine kidney cells.36 A laboratory reference sample of human IFN-α, standardized against the NIH Ga 023-902-530 reference, was included for each titration. In uninfected cynomolgus macaques, plasma IFN-I concentration was less than 2 IU/mL. Enzyme-linked immunosorbent assay kits for monkey IFN-γ (BioSource International, Camarillo, CA) and human interleukin-18 (IL-18; MBL, Nagoya, Japan), known to cross-react with macaque cytokine, were used. IFN-γ was not detectable in most animals before infection, but in some, the IFN-γ concentration exceeded 100 pg/mL (2 in the 50 AID50 group and 1 in the 5000 AID50 group). These macaques with high background levels were excluded from subsequent analysis.
In vitro stimulation of pDCs
AT-2-inactivated SIVmac239 (2 μg/mL total protein, equivalent to 560 ng/mL p27) or herpes simplex virus (HSV) 1 (6 × 105 plaque-forming units) was added to 1 mL whole blood for 18 hours at 37°C. Plasma samples were collected and stored at −80°C until use for IFN-I titration. Negative controls of stimulation included mock-stimulation and stimulation with AT-2-treated SupT1 microvesicles.
Statistical analysis
The nonparametric Spearman rank correlation test was used to investigate the relationships between parameters. The nonparametric Mann-Whitney test was used to compare different groups of macaques, and the nonparametric Wilcoxon rank test was used to compare data from the same macaques at different time points before and after SIV infection. In some cases, we calculated the area under the curve (AUC) for analysis of the amplitude of variation of a given parameter during primary infection. Statistical analyses were carried out with Statview software (SAS Institute, Cary, NC). In 2-tailed tests, P values of .05 or lower were considered significant.
Results
Changes in pDC numbers during primary SIV infection
Twelve macaques were exposed intravenously to 50 AID50 (n = 6) or to 5000 AID50 (n = 6) of SIVmac251.
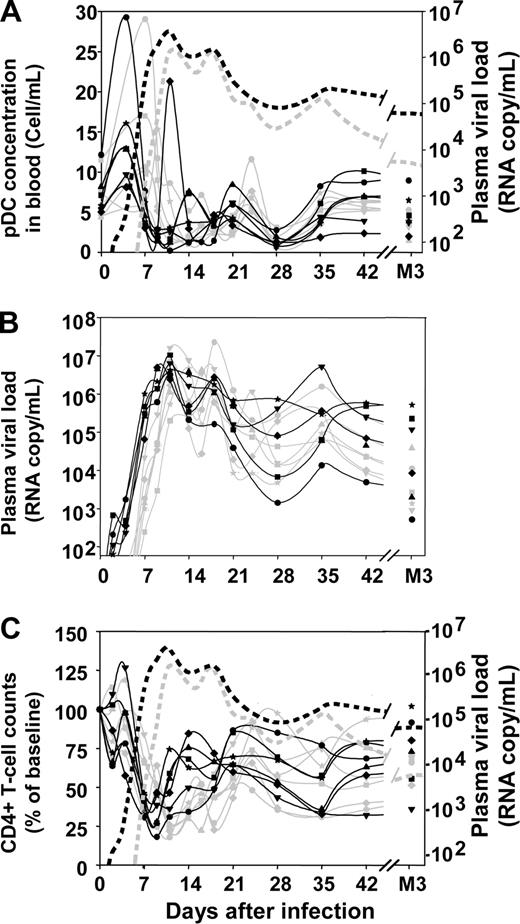
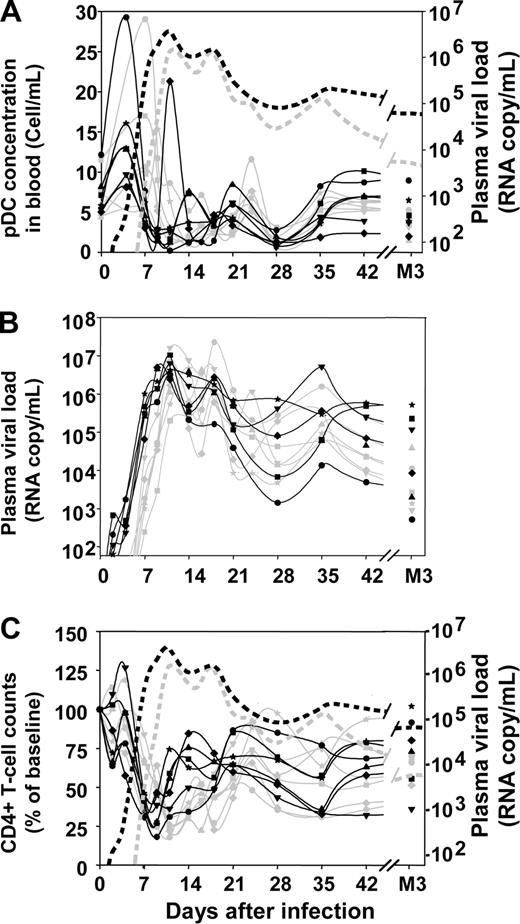
Profound changes in pDC numbers were observed in the blood after infection (Figure 1A). The earliest change was a significant transient increase during the first week of infection (mean 2.28-fold increase; range, 1.23-4.22). This increase was rapidly followed by an acute decrease between days 9 and 14 (mean, 16% of baseline; range, 2%-31% of baseline). Plasmacytoid DC counts decreased earlier in the 5000 AID50 group than in the 50 AID50 group, but the nadir was not significantly different, mirroring kinetics and amplitudes of the viral load peak (Figure 1B). pDC numbers then gradually increased to subnormal values at 3 months after infection, staying below baseline from plasma viral load setpoint (month 3) to month 9 after infection (data not shown, P < .0001).
Changes in blood pDC counts, plasma viral load, and blood CD4+ T-cell counts over time after inoculation with SIVmac251. Absolute counts of pDCs in the blood (A), plasma viral loads (B), proportion of CD4+ T cells with respect to baseline (C). Solid gray lines in panels A, B, and C: macaques infected with 50 AID50; solid black lines in panels A, B, and C: macaques infected with 5000 AID50; dotted gray lines in panels A and C: median plasma viral load of the 50 AID50 group; dotted black lines in panels A and C: median plasma viral load of the 5000 AID50 group.
Changes in blood pDC counts, plasma viral load, and blood CD4+ T-cell counts over time after inoculation with SIVmac251. Absolute counts of pDCs in the blood (A), plasma viral loads (B), proportion of CD4+ T cells with respect to baseline (C). Solid gray lines in panels A, B, and C: macaques infected with 50 AID50; solid black lines in panels A, B, and C: macaques infected with 5000 AID50; dotted gray lines in panels A and C: median plasma viral load of the 50 AID50 group; dotted black lines in panels A and C: median plasma viral load of the 5000 AID50 group.
The decrease in pDC counts paralleled the decrease in CD4+ T-cell counts (Figure 1C) and pDC counts were correlated with CD4+ T-lymphocyte counts at setpoint (P = .05) and 9 months after infection (P = .03). pDC counts were not correlated with plasma viral loads, although dynamics of pDCs in the blood were strongly related to the kinetics of viremia, higher infectious doses resulting in earlier peak of viremia and earlier changes in pDC blood counts than lower doses.
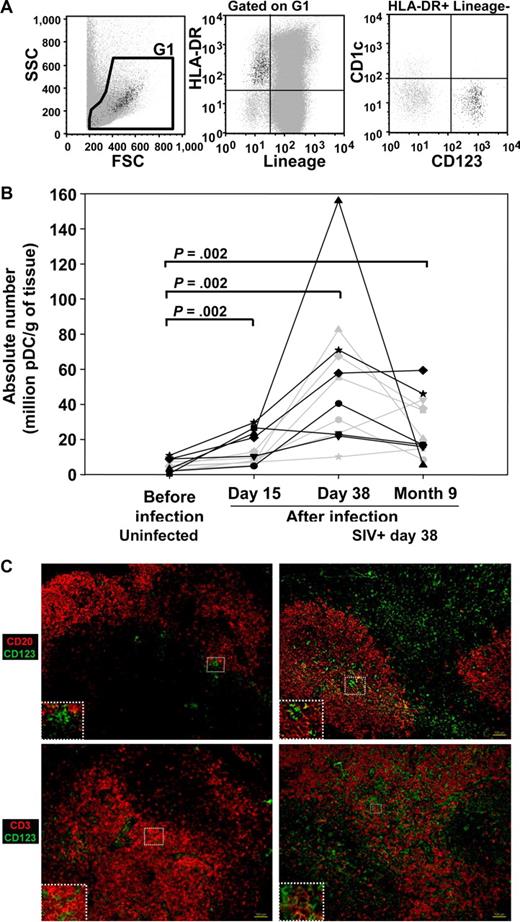
As blood pDC counts were not correlated with viral load and the acute decline of pDC levels in the blood was partly corrected during chronic infection in cynomolgus macaques, we investigated potential homing of pDC to peripheral LN. pDCs were counted sequentially by flow cytometry in peripheral LN cells (Figure 2A,B). Before infection, pDCs represented 0.6 × 106 to 11 × 106 pDCs/g of tissue (Figure 2B), which corresponds to 0.039 (± 0.024%) of total LN cells. On day 15, they had increased significantly (Wilcoxon, P = .002), and a further increase was observed in 11 of the 12 macaques between days 15 and 38, reaching 10 × 106 to 156 × 106 pDCs/g (increase from baseline levels by a factor of 2 to 49 on day 38). Nine months after infection, the number of pDCs in lymph nodes was lower than during primary infection, but remained greater than preinfection levels (P = .002). The percentages of pDCs in total lymph node cells paralleled that of absolute numbers (data not shown).
pDC dynamics in the peripheral lymph nodes of cynomolgus macaques after infection with SIV. (A) Representative dot plot showing CD123+ CD1c− pDCs in a lineage (CD3, CD8, CD14, and CD20)− HLA-DR+ gate, in lymph node cells. (B) Changes in the numbers of pDCs in sequential peripheral lymph node biopsies over time. Solid gray lines: macaques infected with 50 AID50; solid black lines: macaques infected with 5000 AID50. P values are given for the Wilcoxon rank test. (C) Distribution of CD123+ cells in lymph nodes of SIV-uninfected (left panel) and SIV-infected macaques (day 38 after exposure, right panel). Photographs are representative of 2 SIV-negative and 3 SIV-positive macaques and of the entire section observed. Serial lymph node sections (10 μm) were either double-labeled for CD123 and CD20 (top panel), or CD123 and CD3 (bottom panel), or stained with isotypic control antibodies or DAPI (Figure S1). Magnification: ×100. Inset magnification: ×200 (top left, top right, bottom left) and ×400 (bottom right).
pDC dynamics in the peripheral lymph nodes of cynomolgus macaques after infection with SIV. (A) Representative dot plot showing CD123+ CD1c− pDCs in a lineage (CD3, CD8, CD14, and CD20)− HLA-DR+ gate, in lymph node cells. (B) Changes in the numbers of pDCs in sequential peripheral lymph node biopsies over time. Solid gray lines: macaques infected with 50 AID50; solid black lines: macaques infected with 5000 AID50. P values are given for the Wilcoxon rank test. (C) Distribution of CD123+ cells in lymph nodes of SIV-uninfected (left panel) and SIV-infected macaques (day 38 after exposure, right panel). Photographs are representative of 2 SIV-negative and 3 SIV-positive macaques and of the entire section observed. Serial lymph node sections (10 μm) were either double-labeled for CD123 and CD20 (top panel), or CD123 and CD3 (bottom panel), or stained with isotypic control antibodies or DAPI (Figure S1). Magnification: ×100. Inset magnification: ×200 (top left, top right, bottom left) and ×400 (bottom right).
The ratios of pDCs to other cell populations counted by flow cytometry (CD20+ B cells, CD4+ and CD8+ T cells, CD3−CD8+/NK cells) were all increased after infection, the highest increases being observed during primary infection (data not shown), suggesting that the enrichment in pDCs is selective.
The increase of pDCs in lymph nodes was confirmed by in situ immunofluorescence labeling of CD123 on peripheral lymph node sections (Figure 2C, Figure S1). In uninfected animals, CD123+ cells were mostly in the T-cell area, as shown by CD3/CD123 double staining, and were sometimes close to the B-cell area. Both the T-cell area and the B-cell follicles were infiltrated with large numbers of CD123+ cells after 38 days of infection.
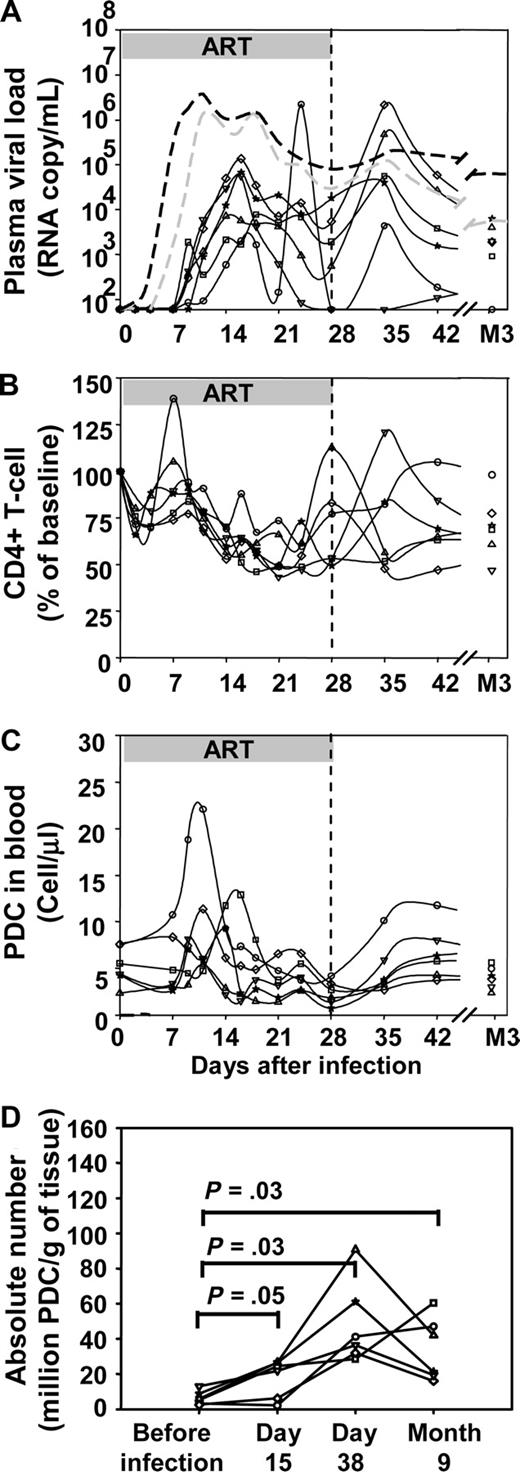
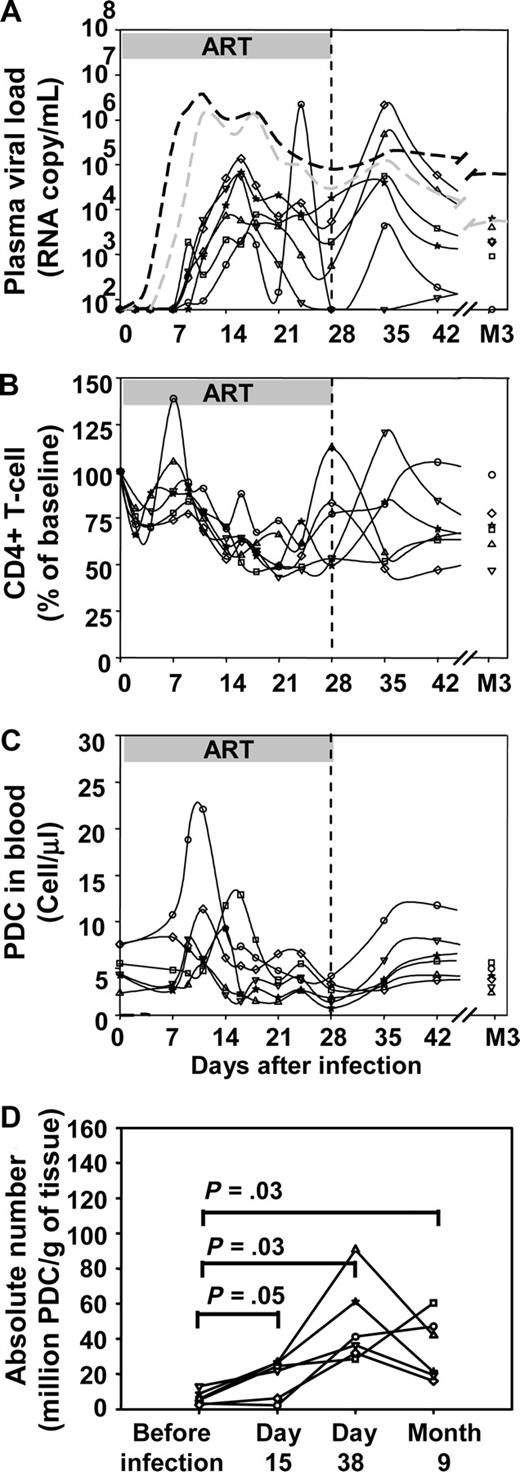
To further highlight the impact of virus replication on pDC dynamics, a third group of 6 macaques was exposed to 50 AID50 of SIVmac251 and then treated with ART 4 hours to 28 days after infection. As shown in Figure 3A, macaques in this group showed a delay of the viral load peak of 2 days on average (mean 17 days ± 3 days vs 15 ± 3 days for the 50 AID50 untreated group, Mann Whitney test P = .005). Viral load peaks were also lower in the treated group (median peak viral load 3.06 × 104 copy/mL versus 1.39 × 106 copy/mL, P = .025). In treated macaques, the transient decrease in CD4+ T-cell counts was delayed and attenuated (mean nadir 45% ± 9% of baseline CD4+ T-cell count vs 21% ± 7%, P = .004; Figure 3B). Likewise, the transient decrease in blood pDC absolute counts was smaller in the treated group than in the untreated group (Figures 1C,3C). Indeed, in ART-treated macaques, pDCs decreased to a mean nadir of 41% (± 16%) of baseline counts whereas in untreated macaques the nadir of pDCs was lower 17% (± 8%) of baseline counts (P = .02). The absolute numbers of pDCs increased in peripheral lymph nodes after infection (Figure 3D), and this increase did not differ significantly from those in untreated macaques.
Impact of early antiretroviral treatment on plasma viral load, CD4+ T-cell counts, and pDC dynamics in blood and lymph nodes. Changes of each parameter in 6 individual macaques infected with 50 AID50 of SIV (solid black lines) that received ART from 4 hours to 28 days after virus inoculation (50 AID50 + ART group). (A) Plasma viral loads. (B) Blood CD4+ T-cell counts. (C) Absolute pDCs counts in the blood. (D) Absolute numbers of pDCs in peripheral lymph nodes. Dotted lines in A: median plasma viral load of the 50 AID50 group (gray) and of the 5000 AID50 group (black).
Impact of early antiretroviral treatment on plasma viral load, CD4+ T-cell counts, and pDC dynamics in blood and lymph nodes. Changes of each parameter in 6 individual macaques infected with 50 AID50 of SIV (solid black lines) that received ART from 4 hours to 28 days after virus inoculation (50 AID50 + ART group). (A) Plasma viral loads. (B) Blood CD4+ T-cell counts. (C) Absolute pDCs counts in the blood. (D) Absolute numbers of pDCs in peripheral lymph nodes. Dotted lines in A: median plasma viral load of the 50 AID50 group (gray) and of the 5000 AID50 group (black).
Acute peaks of plasma IFN-I correlate with IDO activity and IL-18 production all being closely synchronized to the increase of viremia during the acute phase
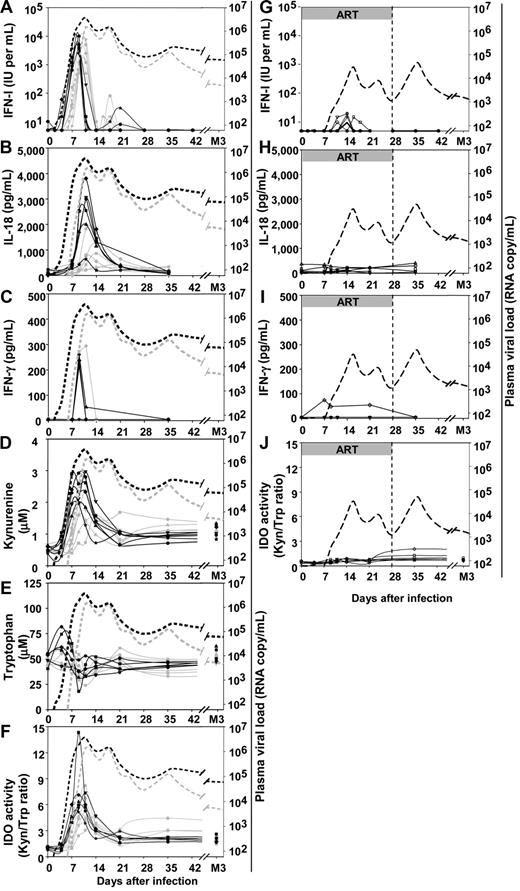
As previously reported for rhesus macaques,15,17 IFN-I levels peaked during the increase in viremia (Figure 4A) 2 to 3 days earlier in the 5000 AID50 group than in the 50 AID50 group (between days 7 and 9, mean 8.8 ± 0.4 days, vs between days 9 and 11, mean 10.2 ± 1.0, P = .02). IFN-I concentration in plasma rapidly returned to undetectable levels in all animals on day 14. A small rebound was observed between days 14 and 28, then IFN-I was no longer detectable between days 28 and 91. The increases in plasma IFN-I concentration and plasma viral load occurred at the same time. The area under the curve (days 0 to 21) of plasma IFN-I concentration was positively correlated with that of plasma viral load (ρ = 0.67, P = .03).
Changes in plasma concentration of different soluble factors over time after inoculation with SIVmac251 in untreated and ART-treated macaques. Untreated macaques: (A) IFN-I, (B) IL-18, (C) IFN-γ, (D) kynurenine, (E) tryptophan, (F) kynurenine:tryptophan ratio. Solid gray lines: macaques infected with 50 AID50; solid black lines: macaques infected with 5000 AID50. Dotted lines in panels A to F: median plasma viral load of the 50 AID50 group (gray) and 5000 AID50 group (black). Macaques that received post-exposure ART: (G) IFN-I, (H) IL-18, (I) IFN-γ, (J) tryptophan and kynurenine ratio. Dotted lines in panels G to J: median plasma viral load of the 50 AID50+ART group. Duration of ART treatment is indicated by a gray horizontal bar.
Changes in plasma concentration of different soluble factors over time after inoculation with SIVmac251 in untreated and ART-treated macaques. Untreated macaques: (A) IFN-I, (B) IL-18, (C) IFN-γ, (D) kynurenine, (E) tryptophan, (F) kynurenine:tryptophan ratio. Solid gray lines: macaques infected with 50 AID50; solid black lines: macaques infected with 5000 AID50. Dotted lines in panels A to F: median plasma viral load of the 50 AID50 group (gray) and 5000 AID50 group (black). Macaques that received post-exposure ART: (G) IFN-I, (H) IL-18, (I) IFN-γ, (J) tryptophan and kynurenine ratio. Dotted lines in panels G to J: median plasma viral load of the 50 AID50+ART group. Duration of ART treatment is indicated by a gray horizontal bar.
We also determined plasma concentrations of IL-18, because this proinflammatory cytokine is induced by IFN-I37 and induces IFN-γ in NK cells.17,38 IL-18 is also a potent chemoattractant for human pDCs.39 A peak of IL-18 was evidenced in the plasma (Figure 4B). The peak of IL-18 occurred 2 to 3 days after the peak of IFN-I.
A peak of plasma IFN-γ concentrations was observed in a limited number of macaques: one of the 5 animals of the 50 AID50 group and 3 of 4 animals of the 5000 AID50 group studied (Figure 4C). When detectable, the IFN-γ peak was temporally associated with the peak of IL-18, and the macaques with a detectable IFN-γ peak were among those with the largest increase in IL-18 levels.
Because IFN-I and HIV are both inducers of the immune suppression-associated enzyme IDO in human pDCs in vitro,21 we analyzed functional IDO activity in vivo. IDO catalyzes extrahepatic Trp catabolism in the Kyn pathway, and measurement of their respective concentrations in serum is used as a marker of IDO activity.40 A peak was observed for Kyn (mean peak value, 2.24 ± 0.56 μM compared with 0.53 ± 0.08 μM at baseline), coinciding with the increase in plasma viremia (Figure 4D). This transient acute Kyn peak was associated with a transient decrease in Trp concentration, which returned to normal values by 3 months after infection (Figure 4E). As a consequence, the Kyn/Trp ratio increased as viral load increased toward its peak (Figure 4F), with significantly more rapid kinetics (P = .03) in the 5000 AID50 group (peaking from days 9 to 11; mean, 9.3 ± 0.8) than in the 50 AID50 group (days 9 to 14; mean, 11.2 ± 1.6). IDO activity decreased after the peak but remained higher at set point than at baseline (P = .002) by a factor of 1.23 to 2.29 (mean 1.83 ± 0.27). Thus, the dynamics of IFN-I, IDO, and IL-18 were highly coordinated with the exponential increase in viral load during primary infection. IFN-I and Kyn were synchronous with the exponential increase of viremia, whereas IL-18 and IFN-γ increased later. Virus and IFN-I seemed to be the earliest inducers of IDO detected in the acute phase.
Innate immunity was strongly reduced in ART-treated macaques, which showed reduced and delayed peaks of plasma IFN-I (Figure 4G). No increase in IL-18 concentration was observed (Figure 4H), and only 1 of 4 macaques analyzed in this group showed a transient, atypical increase in plasma IFN-γ concentration (Figure 4I). In these animals, IDO activity did not increase significantly during treatment, a slight increase being observed after treatment interruption (Figure 4J), suggesting that postexposure treatment might attenuate IDO-dependent immune suppression. Thus, the intensity of innate immunity is closely associated with the viral load during primary infection in vivo, strongly suggesting that viremia needs to reach a certain threshold for an acute burst of innate immunity to occur.
AUCs were calculated for each parameter, between days 0 and 21, taking into account the entire acute phase of infection in the 18 macaques (treated or untreated; Figure S2). IDO activity (Kyn/Trp ratio) and Kyn concentration were positively correlated with IFN-I concentration (ρ = 0.79, P = .001 and ρ = 0.85, P = .0004, respectively) but not with IFN-γ concentration. Kyn and IFN-I concentrations were also positively correlated with plasma viral loads (ρ = 0.75, P = .002 and ρ = 0.86, P = .0004, respectively). Therefore, acute IDO activity was strongly correlated with both IFN-I concentration and viremia, but not with IFN-gamma concentration, during primary infection.
As viral load peaked after Kyn concentration, whereas IFN-I concentration peaked earlier, these data suggest that IFN-I plays a key role in the early phase of acute IDO activation, although a direct virus-induced IDO activation in this study in vivo is not excluded as reported in human pDCs in vitro.21
Primary infection is associated with a transient acute reduction in IFN-I production by pDCs in response to viral stimulation in vitro
Human pDCs rapidly become refractory to secondary stimulation in vitro,41 a phenomenon proposed to account for decreased in vitro production of IFN-I in patients infected with HIV.42 As IFN-I became undetectable soon after its concentration peaked in the plasma, despite the persistence of high viral loads in our study, we compared the capacity of pDCs to respond to SIV and HSV-1 stimulation before and after this peak, to evaluate a possible refractory phase in vivo.
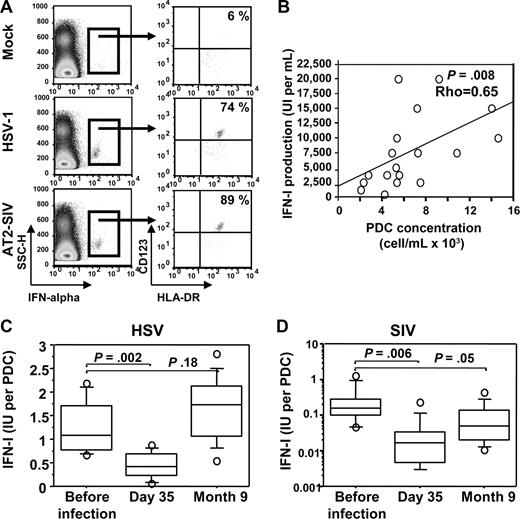
In vitro, cynomolgus macaque pDCs underwent rapid IFN-α synthesis in response to SIV stimulation (Figure 5A), as previously shown in response to HSV stimulation.24 These cells were also characterized extensively by flow cytometry (Figure S3). Cynomolgus pDCs share common characteristics with human and rhesus macaque pDCs in response to HIV and SIV, respectively.43,44 In PBMC stimulation assays, most IFN-positive cells clustered in the pDC gate, and no other IFN-positive cell population was evidenced outside the pDC gate (Figure 5A). In addition, in whole blood stimulation assays, IFN-I production was strongly correlated with the amount of pDCs in the blood sample (Figure 5B). Then, we tested blood samples after infection to follow IFN-I production by pDCs in response to in vitro stimulation.
pDC-dependent IFN-I production in response to de novo stimulation with HSV and SIV in vitro. (A) Dot plot set showing intracellular staining of IFN-α2a. Left, SSC/IFN-α dot plot. Right, CD123/HLA-DR dot plot of gated IFN-α+ cells. PBMCs were stimulated 6 hours with either HSV-1 or with inactivated SIV (AT-2) or were mock stimulated. Representative data for 2 uninfected macaques are shown. (B) Spearman rank correlation analysis of IFN-I production (whole blood assay) in response to HSV and pDC concentration in blood samples for 18 uninfected macaques. (C) Longitudinal changes in IFN-I production in response to HSV. (D) Longitudinal changes in IFN-I production in response to SIV. IFN-I production per pDC is expressed as the ratio between plasma IFN-I concentration after 18 hours of stimulation and the absolute number of pDCs in whole blood samples.
pDC-dependent IFN-I production in response to de novo stimulation with HSV and SIV in vitro. (A) Dot plot set showing intracellular staining of IFN-α2a. Left, SSC/IFN-α dot plot. Right, CD123/HLA-DR dot plot of gated IFN-α+ cells. PBMCs were stimulated 6 hours with either HSV-1 or with inactivated SIV (AT-2) or were mock stimulated. Representative data for 2 uninfected macaques are shown. (B) Spearman rank correlation analysis of IFN-I production (whole blood assay) in response to HSV and pDC concentration in blood samples for 18 uninfected macaques. (C) Longitudinal changes in IFN-I production in response to HSV. (D) Longitudinal changes in IFN-I production in response to SIV. IFN-I production per pDC is expressed as the ratio between plasma IFN-I concentration after 18 hours of stimulation and the absolute number of pDCs in whole blood samples.
Before infection, IFN-I production per pDC was close to 1 IU per pDC (Figure 5C), as reported for human pDCs.1,45 On day 35 after infection, IFN-I production per pDC in response to HSV was significantly lower, by a factor of approximately 2 on average (Wilcoxon P = .002), and then it gradually increased, with preinfection values recovered 9 months after infection. IFN-I production per pDC in response to SIV was also strongly decreased (by a log on average) on day 35 after infection (Figure 5D). Then it was partly restored but by contrast to what was observed for HSV stimulation, responses to SIV remained low 9 months after infection (P = .05 compared with uninfected macaques).
These data demonstrate that in addition to profound dynamic changes, pDCs rapidly become refractory to IFN-I production in response to de novo stimulation during acute infection. This decrease probably results from intense stimulation in vivo during the acute phase of SIV infection, accounting for the rapid, but transient, acute plasma IFN-I peak.
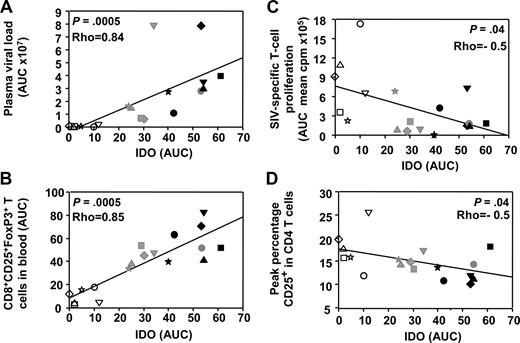
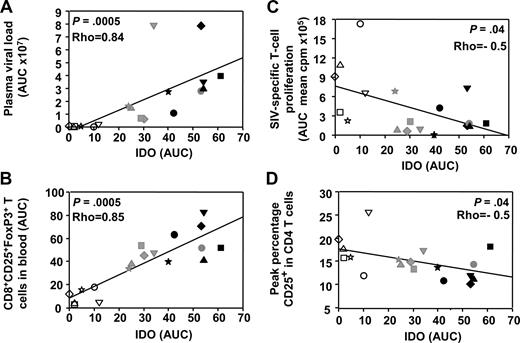
IDO activity correlates positively with virus load and FoxP3+CD25+CD8+ T cells and negatively with both antigen-specific T-cell proliferation and CD4+ T-cell activation
We then analyzed IFN-I and IDO dynamics in relation with the adaptive immune response. We recently described the dynamics of adaptive immunity in these macaques33,46 and showed a transient expansion of CD25brightCD8+ T cells, which express FoxP-3 and CTLA-4—2 molecules expressed by regulatory T cells (Tregs)—9 to 35 days after infection.33 Because IDO was recently shown to be induced by HIV in pDCs in vitro21 and to correlate with viral load in HIV infection,29 and because pDCs expressing IDO activate Tregs in a mouse tumor model,47 we searched for the correlation between IDO activity and FoxP3+CD8+ T-cell subset expansion in vivo. IDO activity correlated not only with plasma viral load (Spearman rank correlation P = .0005, ρ = 0.84) but also with transient expansion of the FoxP3+CD25+CD8+ T-cell subset (P = .0005, ρ = 0.85; Figure 6A,B). Transient expansion of the FoxP3+CD25+CD8+ T-cell subset also correlated with IFN-I concentrations (P = .0015, ρ = 0.77; data not shown). In contrast, acute IDO activity negatively correlated with both SIV-specific T-cell proliferation and CD4+ T-cell activation (Figure 6C,D).
Acute IFN-I and IDO responses correlate with the transient induction of FoxP3+CD25+CD8+ T cells. Spearman rank correlations between IDO activity and plasma viral load (A), IDO activity and CD8+CD25+FoxP3+ T cells (B), IDO activity and SIV-specific T-cell proliferation (C), and IDO activity and CD4+ T-cell activation (D). AUCs were used for plasma viral loads, CD8+CD25+FoxP3+ T-cell numbers (days 9-42), and SIV-specific T-cell proliferation (days 1-100), to take into account the optimal periods of time covering acute dynamic changes in each of these parameters during primary infection. ART-treated macaques are represented by open symbols, whereas untreated macaques are represented by gray (50 AID50 group) or black (5000 AID50 group).
Acute IFN-I and IDO responses correlate with the transient induction of FoxP3+CD25+CD8+ T cells. Spearman rank correlations between IDO activity and plasma viral load (A), IDO activity and CD8+CD25+FoxP3+ T cells (B), IDO activity and SIV-specific T-cell proliferation (C), and IDO activity and CD4+ T-cell activation (D). AUCs were used for plasma viral loads, CD8+CD25+FoxP3+ T-cell numbers (days 9-42), and SIV-specific T-cell proliferation (days 1-100), to take into account the optimal periods of time covering acute dynamic changes in each of these parameters during primary infection. ART-treated macaques are represented by open symbols, whereas untreated macaques are represented by gray (50 AID50 group) or black (5000 AID50 group).
These data, confronted to the dynamic of pDCs, are consistent with acute pDC-dependent IFN-I production during acute infection creating a favorable environment for immune suppression by inducing IDO functional activity and FoxP3+CD25+CD8+ T cells, which may participate in dampening the CD4+ T-cell SIV-specific response.
Discussion
Our data, based on longitudinal analysis of a large number of macaques with close time-course measurement in the blood and the lymph nodes, describe for the first time a rise in circulating pDCs within a week after SIVmac251 infection in cynomolgus macaques, suggesting early involvement of these cells in virus/host interaction. This was followed by a striking coincidence between the accumulation of pDCs in the lymph nodes, and pDCs decrease in the blood during the acute phase of primary SIV infection; nadir was attained 2 weeks after infection. The dynamics of pDCs were closely correlated with the dynamics of CD4+ T-cell counts and plasma viral load. The transient increase of circulating pDCs during the first week may be due to mobilization from lymphoid organs to the blood or to differentiation from blood precursors.48,49 Later during acute HIV or SIV infection, previous reports have shown that DCs accumulate in the lymph node paracortex but without specific characterization of pDCs.50,51 The increase in pDCs counts in the lymph nodes of SIV-infected macaques, demonstrated here for the first time during the acute phase of primary infection, is consistent with the increase in CD123+ cell numbers reported in the lymph nodes of patients infected with HIV later during chronic asymptomatic infection.14 These data contrast with other reports, both in a group of patients infected with HIV-1 who had low CD4+ T-cell counts52 and in macaques infected with SIV that had simian AIDS.19,22 One possible interpretation that would reconcile these divergent results is that pDCs increase in lymphoid tissues in early stage of infection and are depleted in later stages as a consequence of infection19 and/or increased cell death.22 Several factors may account directly or indirectly to early relocation of pDCs: the high levels of viral replication observed in lymphoid organs53,54 ; the acute IL-18 burst we observed, because this cytokine was recently shown to act as a chemoattractant for human pDCs in vitro39 ; or chemokines and their receptors, such as CCL19/MIP3-β/CCR7 and CCL-20/MIP-3α/CCR6, because their mRNA levels increase in the spleen and lymph nodes during acute SIV infection55 and because HIV induces CCR7 expression on human pDCs in vitro.56
In ART-treated macaques, although transient decrease of pDCs in the blood was lower than in untreated macaques, increase of pDCs in peripheral lymph nodes was similar. Possible interpretations could be that pDCs relocation to other lymphoid organs such as spleen or mucosal tissues could be affected by ART or that cell death and/or infection of pDCs are reduced in these macaques. Our data strongly suggest that pDCs contribute to the rapid and acute increase in plasma IFN-I concentration in vivo, even though other cells, such as macrophages, might also contribute to the peak in plasma IFN-I concentration in vivo.
First, we observed that the relocation of pDCs to lymph nodes and the decrease in blood pDC counts were concomitant with the increase in viral load and plasma IFN-I concentration, suggesting production at the site of virus replication in the lymphoid organs. Second, we showed that macaque pDCs underwent activation, maturation, and rapid IFN-I production in response to SIV stimulation in vitro, as described previously in human pDCs.18-20 Third, pDCs became unresponsive to secondary stimulation once the acute IFN-I response was over, suggesting that they were desensitized for IFN-I production in vivo as observed previously for human pDCs in vitro after primary stimulation with Toll-like receptor-9 ligand41 and consistent with the reduced ability of pDCs to produce IFN-I in response to in vitro stimulation in patients infected with HIV.42 Fourth, we did not evidence IFN-I–positive cell clusters outside the pDCs gate in our in vitro stimulation studies.
For the first time, our study monitored the acute transient burst of IDO activity in parallel with acute changes in pDCs dynamics and IFN-I concentration in the early phase of viremia. The kinetic of IDO activity in cynomolgus macaques in our study and the positive correlation observed between IDO activity and viral load are consistent with observations made in Indian rhesus macaques.57 The positive correlation observed between IDO activity and viral load is also consistent with published data suggesting that the virus itself induces IDO directly, through its own proteins, and indirectly, by inducing pDC-dependent IFN-I production.21,58
Although IFN-γ is a major inducer of IDO in vitro,25,59 and correlates positively with IDO activity during chronic HIV infection,40,60 in our study, during acute infection, transient increase in plasma IFN-γ concentration only occurred in a small number of macaques, over a limited time period, consistent with findings in HIV-1 acute infection.61 IFN-γ concentrations were correlated with plasma viral loads, but increased later and were not correlated with Kyn concentrations. Therefore, in contrast to IFN-I and the virus, IFN-γ does not appear as a major inducer in the initial acute induction of IDO activity. This does not exclude the possible later involvement of IFN-γ in the amplification of IDO activation.
We also investigated the relationship between innate and adaptive immune responses. We recently reported the dynamics of FoxP3+CD4+ and CD8+ T cells during infection in the same animal cohort.33 In the present work, we analyzed the relationship between these and innate immunity. IFN-I and IDO were shown to provide important signals for the induction of CD4+ Tregs in vitro.62,63 We found no correlation of FoxP3+CD4+ Tregs, which declined in the blood and were not increased in peripheral lymph nodes,33 with IFN-I or IDO activity (data not shown), although we cannot exclude a different picture from other lymphoid organs that were not available in this study.
In contrast, we observed a transient expansion of CD25brightCD8+ T cells in the blood, at approximately day 21 after infection. These cells bear regulatory T-cell markers such as FoxP3 and CTLA-4, were not correlated to the activation of CD8+ T cells,33 and were positively correlated with plasma viral load and negatively correlated with antigen-specific proliferative responses to SIV. We suggested they might participate in the control of helper T-cell responses during primary infection.33 In the present study, we show that this transient expansion strongly correlates with IDO activity and IFN-I concentration. These factors may therefore be important to create favorable environmental conditions in vivo for the expansion of CD25brightFoxP3+CD8+ T cells, as previously reported in vitro for CD4+ Tregs,62,63 probably through the activation of pDCs.64 Although direct evidence of regulatory function and increase of these cells in lymphoid tissues are yet lacking, because acute IDO activity and FoxP3+CD8+ T-cell expansion both negatively correlated with antigen-specific T-cell proliferation and CD4+ T-cell activation, we speculate that FoxP3+CD8+ T cells may therefore synergize to impair SIV-specific CD4+ T-cell responses during primary infection
Indeed, these cells share phenotype features with some of the human CD8+ regulatory T cells described in the literature.65-68 Alternatively, we cannot exclude the possibility that FoxP3 expression on CD8+ T cells during primary infection could result from activation with no regulatory function as reported in vitro for human CD8+ T cells69 although remarkably the dynamic of CD25+CD8+ T cells (total CD25+CD8+ T cells, CD25 being used as an activation marker) did not correlate with FoxP3+CD8+ T cells.33
Likewise, FoxP3+CD4+ Tregs were suggested to contribute to the suppression of SIV specific CD8+ T-cell responses.30 Indeed, cycling CD4+ and CD8+ T lymphocytes are barely detectable before the end of the IFN-I peak at day 14 after SIV infection in cynomolgus macaques32 (data not shown). This observation supports the hypothesis that the antiproliferative activity of IFN-I70 and/or IDO-dependent suppression71 may contribute to delay the expansion of antigen-specific T cells important for the control of viremia. This environment, during increasing viremia, may provide favorable conditions for the establishment of viral reservoirs and chronic infection by dampening cellular immunity.
Postexposure antiretroviral treatment has major repercussions on both viremia and innate immunity, weakening the IFN-I response, abolishing the IL-18 peak, limiting pDC dynamic changes during acute infection, and suppressing acute increase in IDO activity. This may favor the early development of more efficient adaptive immune responses and better long-term viremia control as shown in this and our previous study.32 Low levels of IFN-I production were also observed in macaques infected with attenuated Δnef SIVmac,15 which also display lower viral load and develop an efficient adaptive immune response.72,73 Therefore, low viremia during primary infection is associated with lower levels of IFN-I production, weaker immune suppression, better adaptive immunity, and improved control of viremia in the long term. This suggests that acute production of IFN-I early during primary HIV/SIV infections may limit the capacity of the host to fight infection as recently shown in the mouse/mouse cytomegalovirus infection model.74
In conclusion, our in vivo longitudinal study strongly suggests that greater viral expansion during primary infection leads to a stronger interferon response and transient induction of immunosuppressive agents, dampening antigen-specific CD4+ T-cell responses. IFN-I plays a major role in antiviral immunity,75 but our results show a strong correlation between IFN-I concentration, virus replication levels, and markers of immune suppression during primary infection, suggesting opposite effects on cellular immunity. Because plasmacytoid DCs home to lymph nodes during the acute phase of infection and are most likely involved in the acute production of IFN-I, they appear as key elements in this duality.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Lennanders Foundation, the Royal Swedish Academy of Sciences, the Faculty of Medicine of Lund University, Sweden, and Becton Dickinson France. We thank Dr Jacques Banchereau (Baylor Institute for Immunology Research, Baylor Research Institute, Dallas, TX) for providing the anti-human IFN-α2a monoclonal antibody, Dr Anne-Marie Aubertin (University Louis Pasteur, Strasbourg, France) for the virus stock, and GlaxoSmithKline (Brentford, United Kingdom) for providing Lamivudine and Zidovudine. We also thank Drs Nathalie Bosquet, Anne Couëdel-Courteille, Magali Rancez, Cécile Butor, Michaela Muller-Trutwin, and all members of the Dendritic Cells work group from the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS; AC19/AC31) for helpful discussions. Finally, we thank Jean-Claude Mascaro, Christophe Jouy, Hélène Juin, Patrick Flament, and Christophe Joubert for animal care.
This work was supported by the French national AIDS agency, ANRS, and by EUROPRISE (LSHP-CT-2006-037611). B. Malleret and I.K. were supported, respectively, by doctoral and postdoctoral fellowships from the ANRS, M.N. by a postdoctoral fellowship from Sidaction, and L.P. by a doctoral fellowship (BDI) from the CNRS. B. Manéglier was supported by the Unité de Formation et de Recherche Médicale Paris Ile de France Ouest Bonus Qualité Recherche (BQR).
Authorship
Contribution: B. Malleret helped to write the manuscript, performed research, and analyzed data; B. Manéglier, I.K., P.L., M.N., and L.P. performed research and analyzed data; P.B., B.D., J.C., and T.A. performed research; O.S.-V. analyzed data. A.H. and R.L.G. analyzed data and helped to write the manuscript; B.V; designed experiments, performed research, analyzed data, and helped to write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Bruno Vaslin, CEA, Institut des Maladies Emergentes et Thérapies Innovantes (IMETI), Service d'Immuno-Virologie (SIV), 18, route du panorama, BP 6, 92265 Fontenay-aux-Roses, Cedex, France; e-mail: bruno.vaslin@cea.fr.
References
Author notes
*B. Manéglier and I.K. contributed equally to this work.