Abstract
The antigen-specific interaction of a T cell with an antigen-presenting cell (APC) results in the formation of an immunologic synapse (IS) between the membranes of the 2 cells. β2 integrins on the T cell, namely, leukocyte function-associated antigen 1 (LFA-1) and its counter ligand, namely, immunoglobulin-like cell adhesion molecule 1 (ICAM-1) on the APC, critically stabilize this intercellular interaction. The small GTPase Rap1 controls T-cell adhesion through modulating the affinity and/or spatial organization of LFA-1; however, the upstream regulatory components triggered by the T-cell receptor (TCR) have not been resolved. In the present study, we identified a previously unknown function of a protein kinase C-θ (PKC-θ)/RapGEF2 complex in LFA-1 avidity regulation in T lymphocytes. After T-cell activation, the direct phosphorylation of RapGEF2 at Ser960 by PKC-θ regulates Rap1 activation as well as LFA-1 adhesiveness to ICAM-1. In OT-II TCR-transgenic CD4+ T cells, clustering of LFA-1 after antigen activation was impaired in the absence of PKC-θ. These data define that, among other pathways acting on LFA-1 regulation, PKC-θ and its effector RapGEF2 are critical factors in TCR signaling to Rap1. Taken together, PKC-θ sets the threshold for T-cell activation by positively regulating both the cytokine responses and the adhesive capacities of T lymphocytes.
Introduction
In lymphoid cells, β2-integrin receptors mediate adhesive events with other immune cells. β2 integrins on the T-cell surface, that is, leukocyte function-associated antigen 1 (LFA-1; CD11a/CD18), and their counterligands on the antigen-presenting cell (APC) surface, ie, immunoglobulin-like cell adhesion molecules (ICAM-1, -2, and -3) stabilize antigen-specific interactions between these cells. LFA-1/ICAM-1–mediated adhesion is critical for T-cell activation during the initiation of a productive immune response.1-3 LFA-1 is not constitutively adhesive, and dynamic modulation of the adhesive activity (avidity) of LFA-1 by external stimuli, such as cytokines, chemokines, or antigens, is a prerequisite for ICAM-1 binding.4,5
T-cell receptor (TCR) signals transiently enhance the functional activity of integrins via downstream signaling components in the so-called inside-out signaling pathway. Increased adhesion does not require changes in levels of LFA-1 expression on the cell surface of T lymphocytes. Activation-dependent inside-out signaling increases LFA-1 avidity via the control of integrin conformation6 and surface distribution.7 Cellular and genetic studies have shown that immune cell adhesion mediated by integrins is modulated by protein and lipid kinases, small GTPases of the Ras and Rho families, and their regulators and adaptor proteins. Signals from Ras/Rac/phosphatidylinositol-3-OH kinase (PI3-K)/cytohesin-1 and phospholipase C γ1 (PLC-γ1)/protein kinase C (PKC)/protein kinase D (PKD)/Rap1 are implicated in the functional activity regulation of the adhesion protein LFA-1.8-12 In particular, the small GTPase Rap1 is an important inside-out signaling molecule that controls cell adhesion through modulating the affinity and/or spatial organization of integrins.13,14 Stimulation with chemokines or antigen stimulation through the TCR activates Rap1, which is required for the transmigration of lymphocytes through endothelial cells and for immunologic synapse (IS) formation with APCs.10,15,16 Rap1 activation has been found to be defective in human leukocyte adhesion deficiency.17 Finally, Rap1A-deficient T cells show severely impaired LFA-1 clustering and adhesion after CD3 stimulation.18
The activation of resting T lymphocytes is known to require stimulation of the TCR/CD3 complex plus the CD28 auxiliary receptor or treatment with phorbol ester, a potent agonist for PKC, plus Ca2+ ionophore. Phorbol ester activates integrins, including LFA-1; consequently, PKC has been implicated in the inside-out signaling pathway.19 Notably, an enzymatic signaling function of PKC in LFA-1 activation in T cells was suggested by using the pan-PKC inhibitor GF109203X.20 Nevertheless, the physiologic and nonredundant PKC family member(s) as well as the biochemical basis by which the critical PKC isotype(s) mediate integrin activation in primary T cells remain undefined.
Methods
Reagents and plasmids
GF109203X was purchased from Alexis (Lausen, Switzerland). The pan-PKC low molecular weight inhibitor (LMWI)21 was provided by AltanaPharma (Konstanz, Germany). γ32-Adenosine triphosphate (γ32-ATP) was purchased from DuPont NEN (Vienna, Austria). Phorbol 12,13-dibutyrate (PDBu) and ionomycin were from Sigma-Aldrich (St Louis, MO). The antibodies used for stimulation of T cells were the anti-CD28 monoclonal antibody (mAb; clone 28.2) and the CD3-specific OKT3 (human) mAb and 2C11 (mouse) mAb. The RapGEF2 wild-type (wt) expression plasmid was obtained from J. L. Bos and F. Zwartkruis (Utrecht, The Netherlands). Human PKC-θ and βII, bovine PKC-α, and mouse PKC-ζ cDNAs were subcloned into the pEF-neo vector and CA mutants thereof have been described previously.22,23 Recombinant Escherichia coli-expressed glutathione S-transferase (GST)–RapGEF2 fusion proteins (containing a GST tag plus the critical RapGEF2 domain between amino acids 945 and 1108) were purified with glutathione-sepharose (GE Healthcare, Little Chalfont, United Kingdom). A (p)Ser960 antisera was raised in rabbits using the peptide RVRRS-S960(PO3H2)-FLNAK as immunogen. The antibody against PKC-θ used for immunoblotting was from BD Transduction Laboratory (Lexington, KY), and the antibody used for immunoprecipitates (IPs) of PKC-θ was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody against RapGEF2 was from Abnova (Taipei City, Taiwan). The phospho-Ser PKC substrate and (p)ERK1/2 antibodies were from Cell Signaling Technology (Danvers, MA).
Analysis of T-cell adhesion
Jurkat T cells were maintained in RPMI medium supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA). Naive CD3+ T cells were purified from pooled spleen and lymph nodes with mouse T-cell enrichment columns (R&D Systems, Minneapolis, MN). T-cell populations were typically 95% CD3+, as determined by staining and flow cytometry. Where indicated, CD4+ and CD8+ T cells were isolated with a negative CD4+ or CD8+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA). Cells were resuspended in RPMI medium and labeled with 12 μg/mL bisbenzimide Hoechst 33342 fluorochrome, trihydrochloride (Calbiochem, San Diego, CA) for 30 minutes at 37°C. After centrifugation and resuspension in Hank buffered saline solution (HBSS), the cells were delivered to 96-well plates (Maxisorp; Nalge Nunc International, Rochester, NY) at 1.5 × 105 cells/well as described.24 Before adhesion, plates were coated with antihuman IgG (Fcγ-specific) antibody at 0.9 μg/well overnight at 4°C, blocked 1 hour with 1% (wt/vol) bovine serum albumin (BSA) in phosphate-buffered saline (PBS), and incubated for 90 minutes at room temperature with recombinant mouse Fc-ICAM-1 fusion protein (R&D Systems) at 0.25 μg/well. Cells were allowed to adhere for 30 minutes at 37°C. For anti-CD3 stimulations, T cells were incubated with anti-CD3 antibody (10 μg/mL) during adhesion. Alternatively, PDBu (10 ng/mL; Sigma-Aldrich) plus Ca2+ ionophore (ionomycin, 125 ng/mL; Sigma-Aldrich) were used. Where indicated, soluble anti-CD28 (1 μg/mL; BD Biosciences, San Jose, CA) or MnCl2 (500 μM; Sigma-Aldrich) was added. Unbound cells were washed off carefully with 150 μL prewarmed HBSS. Bound cells were assayed in 100 μL HBSS using a fluorescence plate reader at 460 nm (Fluoroskan Ascent; Thermo Scientific, Waltham, MA). The adhesion signal of activated T cells (usually ∼ 40% of the absolute number of cells deposited in each well) was set as 100% adhesion for normalization purposes. The results represent an average of at least 3 independent experiments carried out in quadruplicate for each value. For inhibitor experiments, cells were pretreated with inhibitor (500 nM) at 37°C for 2 hours.
Rap1 activation assay
Pull-down of Rap1-guanosine triphosphate (GTP) using a GST-RalGDS-Ras–binding domain (RBD) fusion protein was performed with the EZ-Detect Rap1 Activation Kit (Pierce, Rockford, IL) according to the manufacturer's protocol. Briefly, 2 × 107 cells lysed in ice-cold lysis buffer were incubated with GST-RalGDS-RBD fusion proteins coupled to glutathione agarose beads for 1 hour at 4°C. Beads were washed 3 times with lysis buffer, and immunoprecipitated protein was subjected to immunoblotting using a Rap1-specific antibody.
Two-hybrid screening and cDNA isolation
The Saccharomyces cerevisiae reporter strain EGY48 used for the LexA 2-hybrid screen was purchased from Clontech (Mountain View, CA). Strains were grown under standard conditions in rich or synthetic medium with appropriate supplements at 30°C. For the yeast 2-hybrid (Y2H) screening, EGY48 was cotransformed with (1) the bait construct encoding the catalytic domain of PKC-θ (PKC-θ-CD) fused to the LexA DNA-binding domain and (2) the human lymphocyte Matchmaker cDNA library (Clontech). Transformants were plated on synthetic dropout medium lacking leucine, tryptophan, and histidine but containing 5 mM 3-amino-1,2,4-triazole. His+ colonies were assayed for β-galactosidase activity using o-nitrophenyl β-D-galactopyranoside as substrate. At least 12 individual cotransformants were assayed. The results are presented as the mean plus or minus SD.
IP analysis
Jurkat TAg cells (107) were lysed in 1 mL lysis buffer (50 mM Tris-HCl, pH 7.3, 5 mM NaF, 5 mM Na3VO4, 2 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.5% Triton X-100, 50 μg/mL aprotinin, 50 μg/mL leupeptin). Lysates were precleared for 30 minutes at 4°C. Immunoprecipitation was performed at 4°C overnight followed by incubation with Protein G Sepharose (Amersham Pharmacia, Vienna, Austria) for 1 hour at 4°C. Beads were washed 5 times in lysis buffer, and immunoprecipitated protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on Bis/Tris-buffered gels (Novex, San Diego, CA). Proteins were transferred to a polyvinyldifluoride membrane (Millipore, Bedford, MA) by semidry blotting (90 mA/112 cm2, 90 minutes, 4°C). The primary antibodies were diluted in Tris-buffered saline containing 0.05% Tween-20 and 5% nonfat dry milk for incubation. Peroxidase-conjugated antibodies (Pierce) served as the secondary reagent (1:5000). Enhanced chemiluminescence (Super Signal; Pierce) was used for antigen detection.
Protein kinase assay
PKC-θ– and/or PKC-α–dependent phosphorylation was measured by incorporation of inorganic phosphate 32Pi from γ32P-ATP. Purified recombinant GST fusion proteins or myelin basic protein (MBP; 200 ng) was incubated in 50 μL kinase assay buffer (40 mM Tris, pH 7.5, 40 mM MgCl2, 0.2 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 0.2 mM dithiothreitol, 0.0002% Triton X-100, 0.3 μg/mL BSA) containing 1 μM ATP, 2 μCi γ32-ATP, 1 μM PDBu, and 160 μM phosphatidylserine. After 20 minutes at 30°C, the reaction was stopped by adding 5× SDS sample buffer (250 mM Tris, pH 11.5, 10% SDS, 50% glycerol, and 25% β-mercaptoethanol). Phosphorylation was analyzed by SDS-PAGE and autoradiography.
Cells and transfections
Jurkat SV40 large T antigen (TAg) cells were maintained in RPMI medium supplemented with 10% fetal calf serum (Invitrogen). Transient transfection of cells was performed by electroporation in a BTX-T820 Electro-SquarePorator (ITC Biotech, Heidelberg, Germany) apparatus using predetermined optimal conditions (2 × 107 cells at 450 V/cm, 5 pulses of 99 ms). Naive CD3+ T cells were negatively selected and purified from pooled spleen and lymph nodes with mouse T-cell enrichment columns (R&D Systems). T cells were resuspended in the solution from the Mouse CD3+ Cell Nucleofector Kit (Amaxa, Gaithersburg, MD), following the manufacturer's guidelines for cell line transfection. For siRNA-mediated silencing, we optimized the Nucleofector kit for use with mouse CD3+ cells. Briefly, up to 107 cells mixed with 1.5 μM ON-TARGETplus siRNA (Dharmacon, Lafayette, CO; smart pools for mouse Rap1A or mouse RapGEF2, respectively, or nontargeting siRNA controls) were transferred to the provided cuvette, nucleofected with an Amaxa Nucleofector apparatus, and immediately transferred into prewarmed 37°C culture medium. After transfection, cells were cultured for 72 hours before analysis.
Conjugate assay and fluorescence microscopy
OT-II CD4+ T cells from wt and PKC-θ−/− mice were purified by negative selection (Miltenyi Biotec). CD4+ T cells were then mixed to an equal number of LPS-activated APCs pulsed overnight with 2 μM OVA323-339 and incubated at 37°C for 10, 20, 30, and 40 minutes. The unstimulated condition corresponds to the addition of unpulsed LPS-activated B cells to OT-II CD4+ T cells. The conjugates were transferred on polylysine-coated slides and then fixed with 4% paraformaldehyde/4% sucrose in PBS. Briefly, cells were first blocked in 5% normal goat serum in PBS containing 0.2% BSA. Primary (monoclonal anti–LFA-1; BD PharMingen, San Diego, CA) and secondary (goat anti-mouse Alexa Fluor 594; Molecular Probes, Eugene, OR) were applied in PBS/BSA at room temperature. Preparations were analyzed on an AxioImager Z1 microscope (Carl Zeiss, Thornwood, NY) using 100×/1.46 NA and 25×/0.8 NA objectives. Images were recorded with a cooled CCD camera (SPOT; Diagnostic Instruments, Sterling Heights, MI) and MetaMorph image processing software (Universal Imaging, West Chester, PA). Pictures of 5 fields, each comprising a minimum of 50 conjugates, were obtained for each condition. To avoid any subjective bias, all analyses were performed double-blinded. The percentage of conjugates with polarized LFA-1 in each photographed field was determined. All animal experiments were approved by the Austrian authorities (bm:bwk-66.011/0052-BrGT/2006).
Statistical analysis
Statistical analysis was performed with SPSS and the statistical environment R (www.cran.r-project.org), as described previously.25,26
Results
Up-regulation of LFA-1-dependent adhesion by PKC-θ
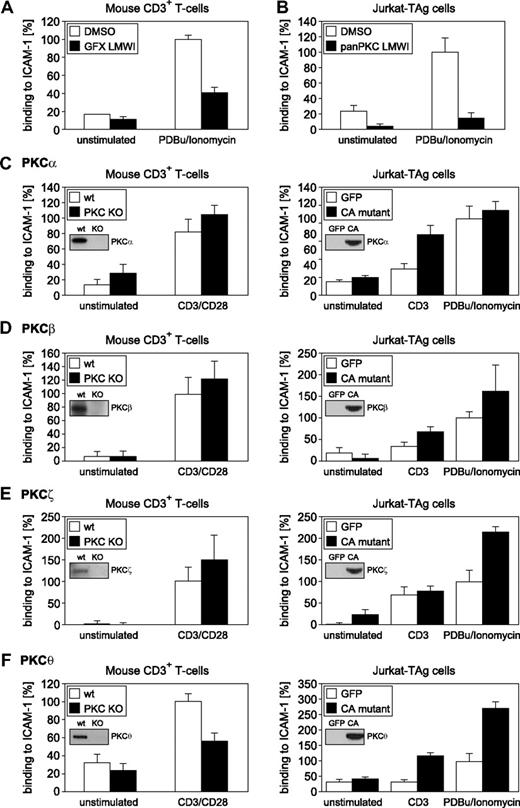
To evaluate the functions of individual PKC isotypes with regard to adhesiveness regulation of LFA-1 on CD3+ T lymphocytes ex vivo, we used an established assay system for activation-dependent adhesion through LFA-1/ICAM-1.14,18,24 As a first step, we used pan-PKC LMWIs.21,27 Consistent with published reports,20 selective pan-PKC LMWIs were able to significantly reduce phorbol ester activation-induced adhesion in both Jurkat and purified mouse CD3+ T cells (Figure 1A,B). Similarly, pan-PKC LMWIs impaired phorbol ester-induced β1-integrin adhesion. This decrease in CD3/CD28-induced adhesiveness in pan-PKC LMWI-treated cells was accompanied by a strong reduction in CD3/CD28-induced cytokine responses, confirming the efficacy of these inhibitors of TCR-mediated signaling21 (data not shown).
PKC pathways required for CD3+ T-cell adhesion. Adhesion responses of primary CD3+ (A) and Jurkat (B) T cells in the presence of GF109203X or pan-PKC LMWI.21 The PKC inhibitors significantly reduced binding of T cells to ICAM-1 compared with dimethylsulfoxide (DMSO) controls (DMSO, n = 3; LMWI at 500 nM, n = 3; split-plot ANOVA, P = .013). (C-F) (Left panels) Activation-dependent adhesion of LFA-1 to ICAM-1 in CD3+ T cells derived from wt and PKC isotype-deficient mice. (Right panels) Adhesion of LFA-1 to ICAM-1 in Jurkat T cells expressing CA PKC isotype mutants.22,63 After a 30-minute incubation with medium or stimuli (CD3, CD3/CD28, or PDBu plus ionomycin, as indicated), T cells were analyzed. (Insets) Immunoblots of KO, wt, or transfected T cell lysates, as indicated. Binding to ICAM-1 was reduced significantly in PKC-θ KO CD3+ T cells compared with wt controls (PKC-θ+/+, n = 6; PKC-θ−/−, n = 6; split-plot ANOVA, P = .035). Under CD3-stimulated conditions, the binding of Jurkat T cells transfected with the PKC-θ CA mutant to ICAM-1 was higher than that of the GFP-transfected control but was just above the 0.5% significance level (GFP, n = 5; CA-PKC-θ, n = 5; split-plot ANOVA, P = .052). Results shown are the mean plus or minus SE of at least 3 independent experiments.
PKC pathways required for CD3+ T-cell adhesion. Adhesion responses of primary CD3+ (A) and Jurkat (B) T cells in the presence of GF109203X or pan-PKC LMWI.21 The PKC inhibitors significantly reduced binding of T cells to ICAM-1 compared with dimethylsulfoxide (DMSO) controls (DMSO, n = 3; LMWI at 500 nM, n = 3; split-plot ANOVA, P = .013). (C-F) (Left panels) Activation-dependent adhesion of LFA-1 to ICAM-1 in CD3+ T cells derived from wt and PKC isotype-deficient mice. (Right panels) Adhesion of LFA-1 to ICAM-1 in Jurkat T cells expressing CA PKC isotype mutants.22,63 After a 30-minute incubation with medium or stimuli (CD3, CD3/CD28, or PDBu plus ionomycin, as indicated), T cells were analyzed. (Insets) Immunoblots of KO, wt, or transfected T cell lysates, as indicated. Binding to ICAM-1 was reduced significantly in PKC-θ KO CD3+ T cells compared with wt controls (PKC-θ+/+, n = 6; PKC-θ−/−, n = 6; split-plot ANOVA, P = .035). Under CD3-stimulated conditions, the binding of Jurkat T cells transfected with the PKC-θ CA mutant to ICAM-1 was higher than that of the GFP-transfected control but was just above the 0.5% significance level (GFP, n = 5; CA-PKC-θ, n = 5; split-plot ANOVA, P = .052). Results shown are the mean plus or minus SE of at least 3 independent experiments.
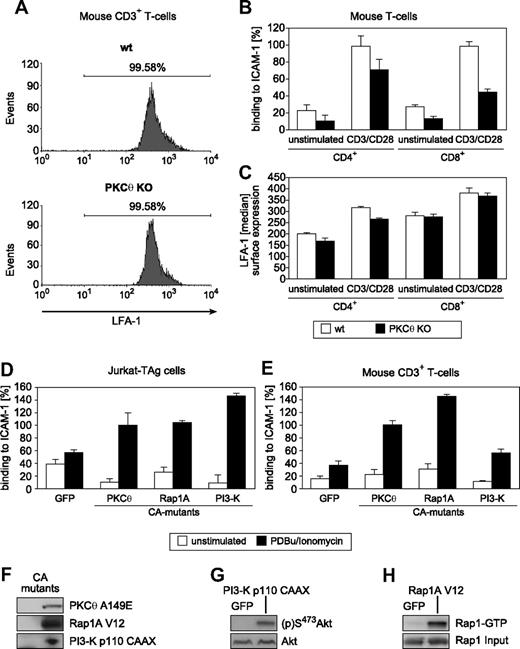
Because T lymphocytes express multiple isotypes of PKC,28-30 pharmacologic pan-PKC inhibition studies cannot resolve PKC isotype-specific roles. Because of their established roles in T-cell functions, we focused on the phorbol ester-responsive PKC-α, -β, and -θ isotypes, as well as the atypical PKC-ζ isotype,31-34 the latter of which is known to function downstream of PKC-θ–mediated signaling.35 Constitutively active (CA) forms of these PKC isotypes were expressed in Jurkat T cells, and the resulting adhesion responses were investigated. In parallel and physiologically relevant, the adhesion responses of primary CD3+ T cells derived from our established panel of PKC knockout (KO) mice were compared with those of wt sibling controls (Figure 1C-F). Among the 4 critical PKC isotypes tested, only PKC-θ demonstrated a nonredundant role in LFA-1–dependent adhesion. Expression of the recombinant CA-PKC-θ A148E mutant significantly enhanced both TCR- and phorbol ester (PDBu)–induced T-cell adhesion to ICAM-1. Furthermore, PKC-θ gene ablation (in our established KO mouse line32 ) significantly reduced LFA-1–mediated T-cell adhesiveness (Figure 1F). Cell surface expression levels of both the TCR/CD3 complex32 (data not shown) and LFA-1 (Figure 2A) were found to be normal in PKC-θ–deficient T cells, excluding adhesion defects caused by altered expression levels of these receptor molecules. Similarly, the loss of PKC-θ impaired CD3/CD28-induced ICAM-1 adhesiveness but not LFA-1 surface expression in purified CD4+ and CD8+ T cells (Figure 2B,C).
T-cell adhesion depends on PKC-θ function. (A) Flow cytometric analysis of LFA-1 expression on wt and PKC-θ KO T cells. (B) Altered activation-dependent adhesion to ICAM-1 through LFA-1 in the PKC-θ–deficient CD4+ and CD8+ subsets. (C) Intact surface expression levels of LFA-1 in the PKC-θ–deficient CD4+ and CD8+ subsets. (D,E) Adhesion-stimulatory activities of the transiently transfected with GFP control or CA mutants PKC-θ A149E, Rap1A V12, and p110-CAAX in Jurkat (D) and primary CD3+ (E) T cells with or without PDBu/ionomycin stimulation. Results shown are the mean plus or minus SD of at least 3 independent experiments. (F,G) As experimental controls, both immunoblot for the transfected CA mutant proteins p110-CAAX and Rap1A V12, and the (p)S473 status of the PI3-K effector Akt/PKB or Rap1-GTP loading of the recombinant Rap1A V12 mutant, respectively, in lysates from unstimulated Jurkat cells are shown.
T-cell adhesion depends on PKC-θ function. (A) Flow cytometric analysis of LFA-1 expression on wt and PKC-θ KO T cells. (B) Altered activation-dependent adhesion to ICAM-1 through LFA-1 in the PKC-θ–deficient CD4+ and CD8+ subsets. (C) Intact surface expression levels of LFA-1 in the PKC-θ–deficient CD4+ and CD8+ subsets. (D,E) Adhesion-stimulatory activities of the transiently transfected with GFP control or CA mutants PKC-θ A149E, Rap1A V12, and p110-CAAX in Jurkat (D) and primary CD3+ (E) T cells with or without PDBu/ionomycin stimulation. Results shown are the mean plus or minus SD of at least 3 independent experiments. (F,G) As experimental controls, both immunoblot for the transfected CA mutant proteins p110-CAAX and Rap1A V12, and the (p)S473 status of the PI3-K effector Akt/PKB or Rap1-GTP loading of the recombinant Rap1A V12 mutant, respectively, in lysates from unstimulated Jurkat cells are shown.
Both phosphatidylinositol-3-OH kinase (PI3-kinase)36-38 and Rap1A15,17,18,39 are known to regulate the avidity of LFA-1. Expression of the CA-mutant forms of Rap1A and PI3-K enhanced LFA-1 binding to ICAM-1 in both Jurkat and primary CD3+ T cells (Figure 2D-H). Furthermore, expression of the CA form of PKC-θ also regulated the avidity of LFA-1 (Figure 2D-H). Notably, only the CA-mutant-transfected T cells exhibited reproducibly enhanced stimulation-dependent adhesion levels compared with the GFP controls. Consistent with the pharmacologic inhibition studies (Figure 1A,B), overexpressed dominant negative (DN)-mutant PKC-θ K409R did not enhance activation-induced CD3+ T-cell adhesiveness (not shown). Thus, a simple scaffold function of PKC-θ in this pathway can be excluded. Notably, whereas in PKC-θ–deficient T cells a significant defect in TCR-induced T-cell adhesiveness has been observed, phorbol ester-induced LFA-1/ICAM-1 interaction was mostly intact in PKC-θ−/− T cells (data not shown). This indicates redundancy of PKC-θ with other PKC isotypes and/or phorbol ester receptors in inside-out signaling. Together, these results indicate that the catalytic activity of PKC-θ (but not of the other representative PKC isotypes tested) is involved in the physiologic process of T-cell adhesiveness, affecting particularly the avidity but not the cell surface expression of LFA-1.
Association of RapGEF2 with PKC-θ
Little is known about PKC-θ isotype-selective downstream effectors and substrates. To explore the molecular mechanisms by which PKC-θ regulates LFA-1 functions, we used a Y2H screen for PKC-θ–interacting proteins, using the catalytic domain of PKC-θ (PKC-θ CD) fused to the LexA DNA-binding domain as “bait.” This analysis identified a group of clones that interacted strongly with PKC-θ CD. Neither the PKC-α CD nor the regulatory domain (RD) of PKC-α or PKC-θ interacted with these clones, indicating some degree of domain and PKC isotype specificity. DNA sequencing revealed the interacting “prey” protein as the NH2-terminal domain of human RapGEF2, also known as PDZ-GEF1,40 an activator of the Rap1GTPase (Figure 3A). Immunoprecipitation analysis in Jurkat T cells transfected with an epitope-tagged RapGEF2 expression vector confirmed the interaction between RapGEF2 and PKC-θ in T cells (Figure 3B). This PKC-θ/RapGEF2 interaction was observed to be constitutive, and CD3 stimulation did not further enhance the association. In contrast, no association of RapGEF2 with the PKC-α or PKC-β isotype was detectable (not shown). These results identify RapGEF2 as a potential interacting molecule with the PKC-θ isotype in the inside-out signaling pathway.
PKC-θ physically interacts with and phosphorylates RapGEF2. (A) PKC-θ/RapGEF2 complex formation was first observed in the LexA Y2H library screen with the COOH-terminal CD of PKC-θ as bait. In retransformation analysis, the PKC-θ CD, but not the PKC-θ RD or the PKC-α CD or RD domain, interacted with RapGEF2 in parallel assays. Values are mean plus or minus SE of at least 3 independent experiments. (Inset) Immunoblot of the distinct subfragments of the cloned bait and prey proteins, the latter minimally representing the NH2-terminal region (amino acids [aa] 1-173) of RapGEF2, obtained from the Y2H system. (B) Coimmunoprecipitation analysis of Jurkat T cells transiently cotransfected with hemagglutinin (HA)–tagged RapGEF2 and PKC-θ or GFP expression control. Twenty-four hours after transfection, RapGEF2 wt and SS959/960AA mutant IPs were immunostained with the PKC-θ–specific antibody. (Bottom panel) The RapGEF2 input control. No PKC-θ coimmunoprecipitated with control serum (MOCK), indicating the specificity of the interaction. Similar results were obtained in 3 independent experiments. (C) Jurkat T cells were stimulated with anti-CD3 antibodies or with PDBu/ionomycin, as indicated. Then, RapGEF2 was immunoprecipitated and immunostained with a broadly reactive (p)Ser (top panel) or RapGEF2 (bottom panel) antibody. *A nonspecific upper protein band, recognized by the pan RapGEF2 antiserum. (D) Similarly, a phosphopeptide antibody to Ser960 reacted with immunoprecipitates of RapGEF2 in an epitope-specific manner on CD3 stimulation of transfected Jurkat cells. (E) RapGEF2 is selectively phosphorylated by PKC-θ but not PKC-α in vitro. Recombinant RapGEF2 wt or neutral exchange SS959/960AA mutant protein was incubated with γ32P-ATP as indicated. The reaction was stopped, and 32Pi incorporation was analyzed by SDS-PAGE and autoradiography (top panel). Equal loading of RapGEF2 GST fusion proteins was validated by an anti-GST immunoblot (middle panel). Comparable enzymatic activities of these baculo-derived PKC-α and PKC-θ preparations were verified with an MBP substrate phosphorylation reaction (bottom panel). Experiments were repeated at least 3 times, with similar results.
PKC-θ physically interacts with and phosphorylates RapGEF2. (A) PKC-θ/RapGEF2 complex formation was first observed in the LexA Y2H library screen with the COOH-terminal CD of PKC-θ as bait. In retransformation analysis, the PKC-θ CD, but not the PKC-θ RD or the PKC-α CD or RD domain, interacted with RapGEF2 in parallel assays. Values are mean plus or minus SE of at least 3 independent experiments. (Inset) Immunoblot of the distinct subfragments of the cloned bait and prey proteins, the latter minimally representing the NH2-terminal region (amino acids [aa] 1-173) of RapGEF2, obtained from the Y2H system. (B) Coimmunoprecipitation analysis of Jurkat T cells transiently cotransfected with hemagglutinin (HA)–tagged RapGEF2 and PKC-θ or GFP expression control. Twenty-four hours after transfection, RapGEF2 wt and SS959/960AA mutant IPs were immunostained with the PKC-θ–specific antibody. (Bottom panel) The RapGEF2 input control. No PKC-θ coimmunoprecipitated with control serum (MOCK), indicating the specificity of the interaction. Similar results were obtained in 3 independent experiments. (C) Jurkat T cells were stimulated with anti-CD3 antibodies or with PDBu/ionomycin, as indicated. Then, RapGEF2 was immunoprecipitated and immunostained with a broadly reactive (p)Ser (top panel) or RapGEF2 (bottom panel) antibody. *A nonspecific upper protein band, recognized by the pan RapGEF2 antiserum. (D) Similarly, a phosphopeptide antibody to Ser960 reacted with immunoprecipitates of RapGEF2 in an epitope-specific manner on CD3 stimulation of transfected Jurkat cells. (E) RapGEF2 is selectively phosphorylated by PKC-θ but not PKC-α in vitro. Recombinant RapGEF2 wt or neutral exchange SS959/960AA mutant protein was incubated with γ32P-ATP as indicated. The reaction was stopped, and 32Pi incorporation was analyzed by SDS-PAGE and autoradiography (top panel). Equal loading of RapGEF2 GST fusion proteins was validated by an anti-GST immunoblot (middle panel). Comparable enzymatic activities of these baculo-derived PKC-α and PKC-θ preparations were verified with an MBP substrate phosphorylation reaction (bottom panel). Experiments were repeated at least 3 times, with similar results.
With the use of a broadly reactive phospho-Ser PKC substrate antibody that detects phosphorylated serine residues with Arg or Lys at the −2 and +2 positions and a hydrophobic residue at the +1 position,41 we determined that RapGEF2 is inducibly phosphorylated at serine(s) in Jurkat T cells. Stimulation of RapGEF2-transfected Jurkat T cells led to strong reactivity of the anti-(p)Ser PKC substrate antibody with the RapGEF2 immunoprecipitates (Figure 3C). A pan-PKC inhibitor blocked the activation-induced serine phosphorylation of RapGEF2 in intact Jurkat T cells (data not shown), similar to the effect of the pan-PKC LMWIs on LFA-1 activation (Figure 1A,B). Next, to define the particular residue(s) recognized by the anti-(p)Ser antibody, the sequence of RapGEF2 was analyzed using a phosphorylation site prediction program.42 The sequence analysis strongly identified Ser959 and Ser960, whose surrounding sequences were predicted to be the least unfavorable for phosphorylation by PKC, as potential phosphorylation sites. Phospho-status analysis of RapGEF2 using our (p)Ser960 site-specific antisera reacted with immunoprecipitates of RapGEF2 wt protein on stimulation (Figure 3D). This reaction appeared site-specific as no immunoreactivity with this (p)Ser960 antisera is seen when the RapGEF2 S960A mutant was expressed (Figure 3D). Consistently, we examined whether RapGEF2 is indeed a direct substrate of PKC-θ. Identical amounts of GST fusion proteins of the wt and SS959/960AA double mutants of RapGEF2 were used in an in vitro kinase assay, together with purified PKC-θ and PKC-α enzymes (Figure 3E). Both protein kinases were similarly active toward the ectopic substrate MBP, indicating comparable enzymatic activities of these baculo-derived PKC-α and PKC-θ preparations. PKC-θ, but not PKC-α, readily phosphorylated the recombinant RapGEF2 in vitro. In contrast, PKC-θ exhibited reduced activity in mutant constructs lacking phosphorylation sites at Ser959 and Ser960. Of note, RapGEF2 was phosphorylated by PKC-θ quite similarly to the well-suited PKC substrate MBP, indicating that PKC-θ has a high activity for RapGEF2 phosphorylation in vitro. These data define RapGEF2 as a novel kinase substrate and indicate that PKC-θ is likely to be involved in activation-induced RapGEF2 serine phosphorylation at Ser960.
Activation of Rap1 by RapGEF2
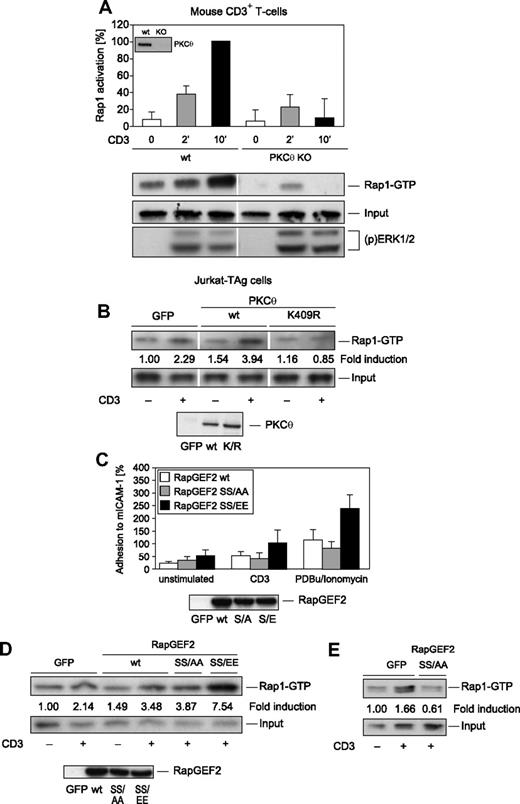
Guanine nucleotide exchange factors (GEFs) such as RapGEF2 activate Rap1 by inducing the active GTP-bound state and are the key link between cell surface receptors and Rap1 activation. To examine whether PKC-θ regulates Rap1 activation downstream of the TCR/CD3 receptor, a GST fusion protein containing the RBD of RalGDS was used to selectively precipitate activated Rap1.43 The recovery of activated Rap1 was monitored by immunoblotting with anti-Rap1 antibodies. Initiating TCR/CD3 signaling after CD3 ligation increased the amount of activated Rap1 in T cells (Figure 4A). A highly reproducible increase in the amount of activated Rap1 was seen 10 minutes after CD3 stimulation. Consistent with the proposed ability of PKC-θ to phosphorylate RapGEF2 and thereby enhance the Rap1 activation pathway, PKC-θ–deficient CD3+ T cells did not show significant CD3-induced Rap1 activation compared with wt control CD3+ T cells (Figure 4A). CD3-induced Rap1 activation also was reduced significantly in kinase-dead K409R mutant PKC-θ–overexpressing Jurkat T cells (Figure 4B). No defects on CD3-induced activation of the Ras/mitogen-activated protein kinase (MAPK) pathways, however, were seen in either kinase-dead K409R mutant PKC-θ–overexpressing Jurkat T cells or PKC-θ−/− mouse CD3+ T cells (Figure 4A; and data not shown). Thus, PKC-θ is essential in regulating the signaling pathway leading to Rap1 but not to Ras/MAPK activation in T lymphocytes.
Rap1 activation is a novel PKC-θ effector function. (A) To analyze the role of PKC-θ in CD3-induced Rap1 activation, wt and PKC-θ–KO CD3+ T cells were subjected to a Rap1 GTP pull-down assay. As a result, PKC-θ–deficient CD3+ T cells did demonstrate a severe Rap1 activation defect. As reported,32 CD3-induced MAPK activation, via detection of dually phosphorylated ERK1/2, was intact, validating our activation protocol of PKC-θ-KO T cells. (B) Transient transfection of Jurkat T cells with wt PKC-θ or kinase-dead PKC-θ K409R mutant cDNA. Vertical lines have been inserted to indicate a repositioned gel lane. Equal expression levels of the transfected wt and K409R mutant PKC-θ were confirmed by immunoblotting (inset). To analyze the modulation of LFA-1 avidity (C) and Rap1 activation (D) by RapGEF2 phosphorylation at Ser960, Jurkat T cells were transiently transfected with 10 μg RapGEF2 SS959/960AA or SS959/960EE mutant cDNA expression vector or with GFP control, as indicated. Transfected Jurkat T cells were left unstimulated or were stimulated with anti-CD3 antibodies or PDBu/ionomycin. Equal expression levels of the transfected wt and mutant RapGEF2 were confirmed by immunoblotting (insets). On stimulation with PDBu/ionomycin, the RapGEF2 SS/EE mutant caused significantly stronger adhesion to ICAM-1 than did the RapGEF2 wt or SS/AA mutant (wt RapGEF2, n = 3; RapGEF2 SS/AA, n = 3; RapGEF2 SS/EE, n = 3; P = .003). (E) To induce stronger overexpression, 30 μg RapGEF2 SS959/960AA mutant cDNA expression vector or GFP control was used to transfect Jurkat T cells. Results shown in panels B, D, and E are representative results of 3 experiments with similar outcomes; data in panels A and C are shown as the mean plus or minus SD of 3 independent experiments.
Rap1 activation is a novel PKC-θ effector function. (A) To analyze the role of PKC-θ in CD3-induced Rap1 activation, wt and PKC-θ–KO CD3+ T cells were subjected to a Rap1 GTP pull-down assay. As a result, PKC-θ–deficient CD3+ T cells did demonstrate a severe Rap1 activation defect. As reported,32 CD3-induced MAPK activation, via detection of dually phosphorylated ERK1/2, was intact, validating our activation protocol of PKC-θ-KO T cells. (B) Transient transfection of Jurkat T cells with wt PKC-θ or kinase-dead PKC-θ K409R mutant cDNA. Vertical lines have been inserted to indicate a repositioned gel lane. Equal expression levels of the transfected wt and K409R mutant PKC-θ were confirmed by immunoblotting (inset). To analyze the modulation of LFA-1 avidity (C) and Rap1 activation (D) by RapGEF2 phosphorylation at Ser960, Jurkat T cells were transiently transfected with 10 μg RapGEF2 SS959/960AA or SS959/960EE mutant cDNA expression vector or with GFP control, as indicated. Transfected Jurkat T cells were left unstimulated or were stimulated with anti-CD3 antibodies or PDBu/ionomycin. Equal expression levels of the transfected wt and mutant RapGEF2 were confirmed by immunoblotting (insets). On stimulation with PDBu/ionomycin, the RapGEF2 SS/EE mutant caused significantly stronger adhesion to ICAM-1 than did the RapGEF2 wt or SS/AA mutant (wt RapGEF2, n = 3; RapGEF2 SS/AA, n = 3; RapGEF2 SS/EE, n = 3; P = .003). (E) To induce stronger overexpression, 30 μg RapGEF2 SS959/960AA mutant cDNA expression vector or GFP control was used to transfect Jurkat T cells. Results shown in panels B, D, and E are representative results of 3 experiments with similar outcomes; data in panels A and C are shown as the mean plus or minus SD of 3 independent experiments.
To study the potential functional role of SS559/960 phosphorylation of RapGEF2, the influence of RapGEF2 SS559/960 mutation on activation-dependent signaling pathways leading to LFA-1 adhesiveness was determined. Transient expression of the wt, neutral exchange SS959/960AA mutant, or acidic exchange SS959/960EE (phosphomimic) mutant form of RapGEF2 in Jurkat T cells revealed that the SS959/960EE mutant substantially enhanced the adhesiveness of LFA-1 to ICAM-1 in stimulated cells (Figure 4C). This result indicates that RapGEF2 phosphorylation enhances the inside-out signaling pathway leading to LFA-1 activation in T cells. Consistently, CD3-induced Rap1 activation was enhanced in the SS959/960EE mutant RapGEF2-expressing cells (Figure 4D). Furthermore, the SS959/960AA mutant RapGEF2 significantly inhibited Rap-1 activation on pronounced overexpression of the mutant protein (Figure 4E). Consistent with the established role of RapGEF2 as activator of Rap1 and not Ras,40 CD3 ligation-induced MAPK activation was not affected by RapGEF2 wt or mutant protein expression (not shown).
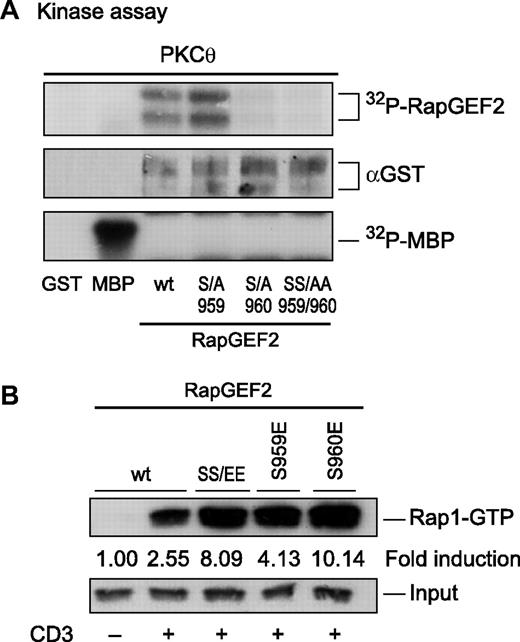
To formally confirm the exact Ser residue that is phosphorylated by PKC-θ, we used GST fusion proteins of the S959A and S960A single mutants of RapGEF2 in an in vitro kinase assay (Figure 5A). PKC-θ efficiently phosphorylated RapGEF2 wt and S959A mutant proteins but failed to phosphorylate the neutral exchange mutants SS959/960AA and S960A. These data indicate that Ser960 on RapGEF2 is directly phosphorylated by PKC-θ. Consistently, CD3-induced Rap1 GTP loading was enhanced in both the SS959/960EE double and S960E single mutant-expressing cells (Figure 5B). These results support our hypothesis that, downstream of the TCR/CD3 receptor, (p)Ser960 on RapGEF2 is a critical PKC-θ phosphorylation site in the signaling pathway to Rap1 in T lymphocytes.
Ser960 on RapGEF2 is selectively phosphorylated by PKC-θ. (A) Recombinant RapGEF2 wt and neutral exchange SS959/960AA, S959A, and S960A mutant proteins were incubated with γ32P-ATP as indicated. The reaction was stopped, and 32Pi incorporation was analyzed by SDS-PAGE and autoradiography (top panel). Equal loading of RapGEF2 GST fusion proteins was validated by immunoblotting (middle panel). The enzymatic activity of PKC-θ was controlled in an MBP substrate phosphorylation reaction (bottom panel). Experiments were repeated at least 3 times, with similar results. (B) To analyze the effects of RapGEF2 phosphorylation at Ser960 on Rap1 GTP loading, Jurkat T cells were transiently transfected with RapGEF2 wt or SS959/960EE, S959E, or S960E mutant cDNA expression vectors or with GFP control, as indicated. CD3-induced Rap1 activation, as determined by GTP loading, was enhanced in the SS959/960EE as well as in the S960E mutant RapGEF2-expressing cells. Equal expression levels of the transfected wt and mutant RapGEF2 were confirmed by immunoblotting (not shown). Results shown are representative results of 3 experiments with similar outcomes.
Ser960 on RapGEF2 is selectively phosphorylated by PKC-θ. (A) Recombinant RapGEF2 wt and neutral exchange SS959/960AA, S959A, and S960A mutant proteins were incubated with γ32P-ATP as indicated. The reaction was stopped, and 32Pi incorporation was analyzed by SDS-PAGE and autoradiography (top panel). Equal loading of RapGEF2 GST fusion proteins was validated by immunoblotting (middle panel). The enzymatic activity of PKC-θ was controlled in an MBP substrate phosphorylation reaction (bottom panel). Experiments were repeated at least 3 times, with similar results. (B) To analyze the effects of RapGEF2 phosphorylation at Ser960 on Rap1 GTP loading, Jurkat T cells were transiently transfected with RapGEF2 wt or SS959/960EE, S959E, or S960E mutant cDNA expression vectors or with GFP control, as indicated. CD3-induced Rap1 activation, as determined by GTP loading, was enhanced in the SS959/960EE as well as in the S960E mutant RapGEF2-expressing cells. Equal expression levels of the transfected wt and mutant RapGEF2 were confirmed by immunoblotting (not shown). Results shown are representative results of 3 experiments with similar outcomes.
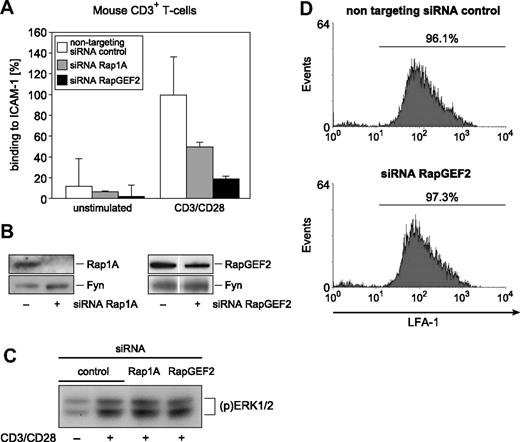
Next, RapGEF2 gene ablation was used to determine whether RapGEF2 is involved physiologically in TCR-dependent signaling pathways leading to the activation of LFA-1 adhesiveness. Small interfering RNA (siRNA)–mediated gene silencing of either RapGEF2 or Rap1A abrogated the endogenous LFA-1 transactivation pathway, as defined by ICAM-1 adhesiveness of siRNA-transfected CD3+ T cells (Figure 6A,B). MAPK activation and LFA-1 surface expression, however, were not affected in both control siRNA and gene-targeting siRNA-transfected cells, validating the apparently intact signaling machinery (Figure 6C,D). Thus, similar to Rap1A, RapGEF2 appears to be functionally required for CD3/CD28-mediated activation of LFA-1 adhesion responses. Of note, siRNA-mediated RapGEF2 inhibition repeatedly appeared more profound than Rap1A and PKC-θ inhibition. The relatively milder phenotypes of Rap1A and PKC-θ gene ablation are likely a consequence of functional complementation by the Rap1B gene and other protein kinases than PKC-θ functioning upstream of RapGEF2, respectively. Nevertheless, these results together support the biologic significance of a RapGEF2 function in mouse CD3+ T cells.
RapGEF2 siRNA-mediated gene ablation reduces adhesion responses. (A) Effects of Rap1A and RapGEF2 knockdown (via synthetic siRNA transfections) on the CD3/CD28-induced LFA-1 activation of primary CD3+ T cells. Results shown are the mean plus or minus SE. (B) The modulation of LFA-1 avidity by Rap1A knockdown was correlated with the siRNA-mediated down-regulation of Rap1A and RapGEF2 proteins, respectively. Vertical lines have been inserted to indicate a repositioned gel lane. (C) As controls, CD3 ligation-induced MAPK activation and (D) LFA-1 surface expression on siRNA-transfected CD3+ T cells remained intact. Experiments were repeated at least 3 times, with similar results.
RapGEF2 siRNA-mediated gene ablation reduces adhesion responses. (A) Effects of Rap1A and RapGEF2 knockdown (via synthetic siRNA transfections) on the CD3/CD28-induced LFA-1 activation of primary CD3+ T cells. Results shown are the mean plus or minus SE. (B) The modulation of LFA-1 avidity by Rap1A knockdown was correlated with the siRNA-mediated down-regulation of Rap1A and RapGEF2 proteins, respectively. Vertical lines have been inserted to indicate a repositioned gel lane. (C) As controls, CD3 ligation-induced MAPK activation and (D) LFA-1 surface expression on siRNA-transfected CD3+ T cells remained intact. Experiments were repeated at least 3 times, with similar results.
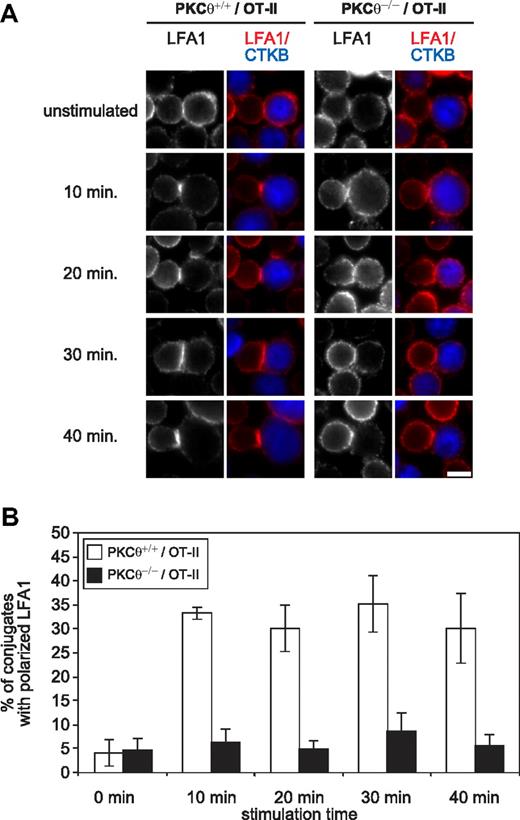
The β2-integrin LFA-1 is involved in the formation of the immunologic synapse in T cells and in conjugate formation between APC and T cells at the onset of an immune response. To assess whether loss of PKC-θ impairs LFA-1/ICAM-1 interaction, we loaded freshly isolated splenic B cells with the model peptide OVA323-339 and then incubated the peptide-loaded B cells with T cells of OT-II TCR-transgenic mice. Subsequently, conjugate formation as well as LFA-1 clustering between T and B cells was evaluated by fluorescence microscopy in a double-blinded fashion. Consistent with our proposed model, a partially impaired clustering of LFA-1 in PKC-θ–deficient OT-II CD4+ T cells was reproducibly observed (Figure 7). Our study is the first to test PKC-θ function in a model for LFA-1 clustering in OT-II TCR-transgenic mice. LFA-1 clustering in mouse OT-II wt CD4+ T cells was also altered by pharmacologic PKC inhibition (via PKC LMWI treatment; not shown), and this independently confirmed an essential and enzymatic role of the PKC-θ in the RapGEF2/Rap1 pathway during OVA323-339–mediated OT-II T-cell adhesion regulation.
PKC-θ promotes T cell LFA-1/ICAM-1 binding during antigen recognition. (A) Fluorescence microscopy images of OT-II/PKC-θ+/+ and OT-II/PKC-θ−/− CD4+ T cell/B cell conjugates.66,67 OT-II CD4+ T cells were added to LPS-activated B cells (blue) pulsed overnight with or without (unstimulated control) 2 μM of OVA323-339 and incubated at 37°C for 10, 20, 30, and 40 minutes. After fixation, the conjugates were stained for LFA-1 (red). Bar scale represents 5 μm. (B) Graph represents the percentages of conjugates with polarized LFA-1 staining. These results correspond to the data obtained with 2 independent experiments where at least 40 conjugates in each of 5 micrographic fields were counted. Interaction of genotype and stimulation: F(4,10) = 4324; P = .028; factorial ANOVA (SPSS).
PKC-θ promotes T cell LFA-1/ICAM-1 binding during antigen recognition. (A) Fluorescence microscopy images of OT-II/PKC-θ+/+ and OT-II/PKC-θ−/− CD4+ T cell/B cell conjugates.66,67 OT-II CD4+ T cells were added to LPS-activated B cells (blue) pulsed overnight with or without (unstimulated control) 2 μM of OVA323-339 and incubated at 37°C for 10, 20, 30, and 40 minutes. After fixation, the conjugates were stained for LFA-1 (red). Bar scale represents 5 μm. (B) Graph represents the percentages of conjugates with polarized LFA-1 staining. These results correspond to the data obtained with 2 independent experiments where at least 40 conjugates in each of 5 micrographic fields were counted. Interaction of genotype and stimulation: F(4,10) = 4324; P = .028; factorial ANOVA (SPSS).
Discussion
The central role of PKC-θ in signaling the immune response combined with the restricted expression of PKC-θ in T cells have focused extensive efforts toward developing immunosuppressive therapeutics targeting PKC-θ.44,45 Mechanistically, the role of PKC-θ in immune responses is clearly established in mouse models of autoimmunity, asthma, and arthritis46-50 ; nevertheless, the exact biochemical mechanisms of PKC-θ function(s) are enigmatic. Although several transcription factors essential for interleukin-2 (IL-2) induction (ie, nuclear factor κB [NF-κB], activator protein 1 [AP-1], and nuclear factor of activated T cells [NF-AT]) are regulated in part by PKC-θ,44 additional physiologic and nonredundant functions of PKC-θ in T cells must be considered. Indeed, the present study reports an additional key role of PKC-θ in TCR-dependent inside-out signaling leading to LFA-1 activity in CD3+ T cells. This study provides a more complete understanding of the essential and most probably additive roles of PKC-θ during T-cell effector responses in vivo.
LFA-1–mediated adhesiveness has long been known to be regulated positively by phorbol ester, a pleiotropic activator of several PKC isotypes. However, neither the PKC family member(s) involved nor the biochemical basis by which phorbol ester mediates integrin activation had been defined. Pharmacologic PKC inhibition (by inhibitors of kinase catalytic activity) abrogated the positive effects of phorbol ester on adhesion, excluding an essential involvement of non-PKC phorbol ester receptors,51 such as chimaerins, Unc-13/Munc-13, and CalDAG-GEF (also known as RasGRP) in this LFA-1 activation pathway. Furthermore, these PKC inhibitor studies excluded a scaffold function of PKC and established that PKC exerts its cellular function via an enzymatic role, ie, direct phosphorylation of an unknown protein substrate critical to the adhesion process.
However, T lymphocytes express multiple isotypes of PKC,29,44,47-50 which are thought to exert substrate preferences through distinct subcellular localizations established via selective kinase/lipid and kinase/protein interactions. Recent studies have shown that at least 4 PKC isotypes are critical for T-cell activation. PKC-θ has been shown to play an important role in IL-2 pro-moter transactivation as well as in T-cell immune responses in vivo.32,45,48-50,52 PKC-α is critical for interferon-γ production and Th1 responses in vivo,31 whereas PKC-β appears to function in LFA-1-mediated outside-in signaling leading to T-cell locomotion.33 Furthermore, a significant, albeit partial, Th2 defect in PKC-ζ KO mice indicated a biologic role of this atypical PKC isotype in T cell–dependent immune responses in vivo.34 Using both the T lymphoid Jurkat tumor cell line as well as primary mouse CD3+ T cells, we defined PKC-θ, but not PKC-α, -β, or -ζ, as a nonredundant and essential PKC isotype in this inside-out pathway. Consistent with this novel molecular-functional link between PKC-θ and LFA-1 avidity regulation, PKC-θ is the only isotype that is rapidly recruited to the IS on T-cell engagement.53
On investigating the biochemical basis of PKC-θ–mediated LFA-1 activation, we revealed that PKC-θ is essential in TCR/CD3-mediated Rap1 activation in Jurkat as well as in primary CD3+ T cells. Rap1 was demonstrated previously to be dependent on PKC function54,55 ; however, the physiologic roles of distinct PKC isotypes or of RapGEF family members in TCR-mediated Rap1 activation were unknown. The present study defined a PKC-θ/RapGEF2 complex as a novel regulatory component upstream of Rap1 in CD3+ T cells. The nonconserved NH2-terminal region of RapGEF2 (minimally amino acids 1-173 in RapGEF2) selectively interacts with the PKC-θ catalytic domain, indicating a probable kinase/substrate interaction.
RapGEF2 has an established function in the regulation of cell growth in nonhematopoietic cells. Ohtsuka et al56 determined that RapGEF2 contains an incomplete cAMP-binding region (RCBD) followed by a PDZ domain, RBD, a Ras guanosine diphosphate (GDP)/GTP exchange protein domain, and a COOH-terminal consensus PDZ-binding motif. Consistently, cAMP and cGMP had no effect on the GEF activity of RapGEF2. de Rooij et al57 identified a Ras exchange motif between the RCBD and PDZ domain in the NH2-terminal half of RapGEF2. These authors showed that the transfection of COS-7 cells with RapGEF2 increased the binding of GTP to Rap1A and Rap1B but not to other small GTPases. By assaying the GEF activity of the truncated protein M5, the RCBD on RapGEF2 was determined to be a GEF inhibitory domain.57 RapGEF2 bound Rap1A through its RBD in a GTP-dependent manner, and RapGEF2 stimulated GDP/GTP exchange in Rap1A through its Ras exchange motif and GEF domains in vitro and in vivo.58,59
Consistent with the ability of PKC-θ, but not PKC-α, to interact with RapGEF2, PKC-θ, but not PKC-α, directly phosphorylated Ser960 on RapGEF2 in vitro. T-cell stimulation by phorbol ester (a potent PKC agonist) as well as by CD3 ligation induced the phosphorylation of RapGEF2 in the Jurkat T-cell line. This inducible serine phosphorylation of RapGEF2 was abrogated by cotreatment with a pan-PKC inhibitor (not shown), indicating that RapGEF2 is a PKC substrate in intact T cells. Importantly, our results showed that RapGEF2 plays an essential role in up-regulation of LFA-1 adhesiveness through the TCR/CD3 receptor. Gene ablation of RapGEF2 by siRNA inhibited CD3/CD28-mediated LFA-1 activation in primary CD3+ T cells, confirming the requirement of RapGEF2 in the inside-out signaling pathway. Furthermore, the phospho-status of Ser960 in RapGEF2 appears to regulate Rap1 activation as well as LFA-1 adhesiveness to ICAM-1, as shown by the acidic exchange phospho-mimic S960E RapGEF2 mutant protein. As specificity controls stimulation-induced MAPK activation remained intact under these experimental conditions. Correspondingly, gene ablation of PKC-θ in our PKC-θ KO mouse line32 as well as the expression of kinase-dead K409R mutant PKC-θ abrogated CD3-mediated Rap1A activation in T cells.
Finally and consistent with our proposed model, we now establish PKC-θ as critical intermediary in antigen-induced LFA-1 clustering in OT-II TCR-transgenic CD4+ T cells (Figure 7). The strongly altered LFA-1 clustering observed in the absence of PKC-θ confirms that, in T cells, the LFA-1/ICAM-1 interaction is regulated in a PKC-θ–dependent manner and PKC-θ is critical for T-cell LFA-1/ICAM-1 binding dynamics during antigen recognition. This finding is also consistent with the observation60 that PKC-θ is required for dynamic regulation of the IS during early T-cell priming.
Taken together, the present results provide evidence that RapGEF2 is a direct target of PKC-θ in a pathway involved in Rap1-dependent LFA-1 activation in CD3+ T cells. The findings that support this conclusion are the following: (1) PKC-θ selectively regulates TCR/CD3-mediated Rap1 activation and LFA-1 adhesion responses; (2) PKC-θ physically interacts with RapGEF2, and in vitro–purified PKC-θ phosphorylates recombinant RapGEF2 at Ser960; (3) expression of the RapGEF2 S960E phospho-mimic mutant enhances Rap1 as well as LFA-1 activation; and (4) RapGEF2 is essential for the CD3/CD28-induced positive adhesion regulation of LFA-1. Thus, a nonredundant functional role of PKC-θ/RapGEF2 in Rap1 activation and LFA-1 avidity modulation in CD3+ T cells has been elucidated. On TCR/CD3 stimulation, RapGEF2 appears as a direct substrate and molecular effector of PKC-θ in the PKC-θ/RapGEF2/Rap1A inside-out signaling cascade. This model represents a previously unknown pathway downstream of PKC-θ that leads to enhanced T-cell adhesion, which is one crucial step in promoting sustained T-cell activation responses. The strongly altered LFA-1 clustering observed in the absence of PKC-θ physiologically confirm that in T cells, the LFA-1/ICAM-1 interaction is regulated in a PKC-θ–dependent manner and PKC-θ is critical for T cell LFA-1/ICAM-1 binding dynamics during antigen recognition. Notably, PKC-θ function is critical for the induction of T-cell activation vs tolerance in vivo,61 indicating that a nonproductive APC/T-cell interaction in PKC-θ KO mice may lead to impaired signal transduction of anergic T cells.62 PKC-θ and Rap1, in cultured cells and in animal studies, both play critical roles in regulating the functions of T lymphocytes64,65 and in maintaining normal immune system function. An important conclusion from the present study is that PKC-θ causes increased IL-2 promoter activation30,44 as well as increased LFA-1 adhesiveness, which together additively set the threshold for an effective and sustained immune response in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. L. Bos and F. Zwartkruis for Rap1A and RapGEF2 cDNA. We are grateful to Drs H. Dietrich, N. Krumböck, and G. Böck (all from Innsbruck Medical University, Innsbruck, Austria) for animal housekeeping, expert technical assistance, and FACS analysis, respectively.
This work was supported by grants from the Fonds zur Förderung der wissenschaftlichen Forschung (FWF) Austrian Science Fund (SFB-021, DK-MCBO, P19505-B05, P17807-B05), the Tyrolean Zukunftsstiftung, the OeNB/Jubiläumsfonds (12196), the Hertha Firnberg fellowship (T264-B13; C.P.-O.), and La Fondation pour la Recherche Médicale (S.K.).
Authorship
Contribution: T.L. performed research and analyzed data; V.K., S.K., and C.L.-N. contributed to experimental work; F.F. performed mutagenesis and expression analysis of recombinant RapGEF2; M.L. contributed PKC-α, -β and -ζ KO breeder pairs; G.J.O., C.P.-O., and S.K. designed and performed experiments of all OT-II imaging data; N.H.-K. contributed the statistical analysis of all data; and G.B. performed overall conception of the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gottfried Baier, Innsbruck Medical University, Schoepfstraße 41, A-6020 Innsbruck, Austria; e-mail: Gottfried.Baier@i-med.ac.at.

![Figure 3. PKC-θ physically interacts with and phosphorylates RapGEF2. (A) PKC-θ/RapGEF2 complex formation was first observed in the LexA Y2H library screen with the COOH-terminal CD of PKC-θ as bait. In retransformation analysis, the PKC-θ CD, but not the PKC-θ RD or the PKC-α CD or RD domain, interacted with RapGEF2 in parallel assays. Values are mean plus or minus SE of at least 3 independent experiments. (Inset) Immunoblot of the distinct subfragments of the cloned bait and prey proteins, the latter minimally representing the NH2-terminal region (amino acids [aa] 1-173) of RapGEF2, obtained from the Y2H system. (B) Coimmunoprecipitation analysis of Jurkat T cells transiently cotransfected with hemagglutinin (HA)–tagged RapGEF2 and PKC-θ or GFP expression control. Twenty-four hours after transfection, RapGEF2 wt and SS959/960AA mutant IPs were immunostained with the PKC-θ–specific antibody. (Bottom panel) The RapGEF2 input control. No PKC-θ coimmunoprecipitated with control serum (MOCK), indicating the specificity of the interaction. Similar results were obtained in 3 independent experiments. (C) Jurkat T cells were stimulated with anti-CD3 antibodies or with PDBu/ionomycin, as indicated. Then, RapGEF2 was immunoprecipitated and immunostained with a broadly reactive (p)Ser (top panel) or RapGEF2 (bottom panel) antibody. *A nonspecific upper protein band, recognized by the pan RapGEF2 antiserum. (D) Similarly, a phosphopeptide antibody to Ser960 reacted with immunoprecipitates of RapGEF2 in an epitope-specific manner on CD3 stimulation of transfected Jurkat cells. (E) RapGEF2 is selectively phosphorylated by PKC-θ but not PKC-α in vitro. Recombinant RapGEF2 wt or neutral exchange SS959/960AA mutant protein was incubated with γ32P-ATP as indicated. The reaction was stopped, and 32Pi incorporation was analyzed by SDS-PAGE and autoradiography (top panel). Equal loading of RapGEF2 GST fusion proteins was validated by an anti-GST immunoblot (middle panel). Comparable enzymatic activities of these baculo-derived PKC-α and PKC-θ preparations were verified with an MBP substrate phosphorylation reaction (bottom panel). Experiments were repeated at least 3 times, with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2007-11-121111/7/m_zh80230827370003.jpeg?Expires=1769816830&Signature=h43aEgysSoWcehUVfySszx7Zh7xPxdPJTTDpPb~wcxDvz32MRG11ZiM0rlMgBQ5QTnTkUyMXsUqoIkByYbGLcSpINNqHcCBwZo1fCYkgsPVNtCcjscX5UuJ6j5Klj7n34BAer3ZeRwj441ytbuz23j-SV2cEEqBN-2WZRJyXWtPQmMAyyloEBC9rBSX7ZCDt70cAWB9x1xe9yXQlNBV0NMdOQ3XzqGHQGoGUmuLH9eP7SkYUqnms3RG-xZR4L5Tm-1oCuh-N9Qca8t3AzXPgmcQGz~Pha1cZQyO5zJRDn5tGFZs5fOg1vs~v3HISf5ClTnIiQstvYLq6E2Eq3i7jbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)