Abstract
Neurofibromatosis type 1 (NF1) is a common genetic disorder caused by mutations in the NF1 locus, which encodes neurofibromin, a negative regulator of Ras. Patients with NF1 develop numerous neurofibromas, which contain many inflammatory mast cells that contribute to tumor formation. Subsequent to c-Kit stimulation, signaling from Ras to Rac1/2 to the MAPK pathway appears to be responsible for multiple hyperactive mast cell phenotypes; however, the specific effectors that mediate these functions remain uncertain. p21-activated kinase 1 (Pak1) is a downstream mediator of Rac1/2 that has been implicated as a positive regulator of MAPK pathway members and is a modulator of cell growth and cytoskeletal dynamics. Using an intercross of Pak 1−/− mice with Nf1+/− mice, we determined that Pak1 regulates hyperactive Ras-dependent proliferation via a Pak1/Erk pathway, whereas a Pak1/p38 pathway is required for the increased migration in Nf1+/− mast cells. Furthermore, we confirmed that loss of Pak1 corrects the dermal accumulation of Nf1+/− mast cells in vivo to levels found in wild-type mice. Thus, Pak1 is a novel mast cell mediator that functions as a key node in the MAPK signaling network and potential therapeutic target in NF1 patients.
Introduction
Mutations at the NF1 locus cause neurofibromatosis type 1 (NF1), a common human genetic disorder that affects approximately 1 in 3500 live births.1 The NF1 gene encodes neurofibromin, a tumor suppressor that functions at least in part as a GTPase-activating protein (GAP) for p21ras (Ras)2 and accelerates the hydrolysis of active Ras-GTP to inactive Ras-GDP.3
NF1 is characterized by the development of tumors called neurofibromas,4 which are composed of Schwann cells, fibroblasts, endothelial cells, and large numbers of degranulating mast cells.5 Previous work in a genetically engineered murine model of NF1 that closely recapitulates the spontaneous tumor progression observed in patients has established that nullizygosity of Nf1 in tumorigenic Schwann cells is necessary but not sufficient for plexiform neurofibroma formation.6 Furthermore, haploinsufficiency of Nf1 in at least a subset of lineages within the tumor microenvironment is required to promote neurofibroma development.6
Besides mediating innate immune responses and allergic hypersensitivity, there is an emerging understanding that mast cells in the tumor microenvironment have relevance in the promotion of neoplastic development in multiple murine disease models.7-12 We have previously shown that bone marrow–derived mast cells (BMMCs) haploinsufficient at Nf1 have hyperactivated Ras-GTP, as well as increases in Ras-dependent functions such as proliferation and migration after stem cell factor (SCF) stimulation.13
Previous work has established that the excess proliferation in Nf1+/− BMMCs is the result of Ras activation of the class1A-PI-3 kinase (PI-3K) pathway.14 Genetic and pharmacologic blockade of PI-3K activity in Nf1+/− cells leads to reduction of phospho-Erk levels to that of wild-type control cells, implying a downstream interaction between these pathways.14 Identification of the specific mediator of this cross-talk from PI-3K to the classic MAPK pathway is critical for understanding the biochemical mechanisms underlying the abnormal mast cell phenotypes in NF1.
The p21-activated kinases (Paks) are serine/threonine kinases known to act downstream of Rac1/2 to regulate a broad range cellular functions,15 including the Ras-MAPK pathway signaling. Expression of a kinase-deficient Pak1 prevented Ras-mediated oncogenic cell transformation.16 Further experiments identified Pak kinase activity on Raf117 and Mek118 as essential for Erk activation in transformed cell lines. Importantly, Nf1+/− BMMCs have increased levels of Rac activation and Pak1 kinase activity after SCF stimulation.14 Together, these studies point toward a role for Pak to coordinate PI-3K–dependent activation of MAPK pathway members. Furthermore, Pak is known to interact with the p38 MAPK19-21 to positively regulate its activity. p38 signaling has been linked to BMMC migration toward SCF22 and has been shown to regulate the actin cytoskeleton in macrophages.19
By intercrossing Nf1+/− mice with the recently described Pak1−/− mice (J.D.A., Z.M.J., J.C., D.W.C., manuscript submitted, April 2008), we tested the role of Pak1 in modulating MAPK output in the context of hyperactivated Ras. For the first time in a primary cell system, we demonstrate that genetic disruption of Pak1 contributes to both Erk and p38 MAPK signaling and corrects the hyperactivation of Ras-MAPK pathways found in Nf1+/− mast cells. The increases in both proliferation and migration of Nf1+/− mast cells are corrected in cells that are disrupted at both the Nf1 and Pak1 loci. We provide cellular and biochemical evidence that Pak1 regulates these gains-in-function by activating Mek/Erk to influence proliferation and by activating p38 MAPK to control migration. Further, the increase in polymerized F-actin content associated with the gain-in-function of Nf1+/− mast cell migration23 is dependent on Pak1 and p38. In addition, we demonstrate that the SCF-mediated subcutaneous accumulation and degranulation of mast cells in vivo are dependent on Pak1. Given the high density of mast cells in the neurofibroma microenvironment5,24 and their ability to remodel extracellular matrix and initiate neoangiogenesis, the correction of Nf1+/− mast cell phenotypes reported here identify Pak1 as a potential molecular target for the treatment of neurofibromas.
Methods
Animals
Nf1+/− mice were obtained from Tyler Jacks at the Massachusetts Institute of Technology (Cambridge, MA) on a C57BL/6J strain. Pak1−/− mice were generated in Dr Jonathan Chernoff's Laboratory (Fox Chase Cancer Center) and were backcrossed 6 generations to be on a C57Bl/6 strain. A Pak1−/− mouse was generated by targeted disruption of the Pak1 allele in embryonic stem cells. The resultant allele contains a neomycin cassette and is lacking 2 kb of genomic DNA encoding the p21-binding domain. The Pak1 allele was genotyped by polymerase chain reaction using the following primers: Pak 1 forward: GCCCTTCACAGGAGCTTAATGA; Pak 1 reverse: GAAAGGACTGAATCTAATAGCA; neoReverse: CATTTGTCACGTCCTGCACGA set up in 2 separate reactions (one for wild-type [WT] and one for knockout [KO] band). Polymerase chain reaction program Pak1: 94 at 2 minutes, 94 at 20 seconds (92 for KO reaction), 52 (58 for KO reaction) at 20 seconds × 35 cycles, 71 at 2 minutes, 71 at 7 minutes, and 4 at 48 hours. WT reaction yields a 240-bp band; KO reaction yields a 360-bp band. Multiple F0 founders were used to generate the 4 Nf1 and Pak1 genotypes used in these experiments as outlined. F0: Nf1+/−; Pak1+/+ × Nf1+/+; Pak1−/−. F1: Nf1+/−; Pak1+/− × Nf1+/+; Pak1+/−. F2: Nf1+/−; Pak1−/−, Nf1+/+; Pak1−/−, Nf1+/−; Pak1+/+, Nf1+/+; Pak1+/+. These studies were conducted under a protocol approved by the Indiana University Laboratory Animal Research Center.
Bone marrow mast cell culture
BMMCs were cultured in RPMI (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, UT), 1% glutamine (Lonza Walkersville, Walkersville, MD), 1.5% 1 M N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Lonza Walkersville), 2% penicillin/streptomycin (Lonza Walkersville), and 5 ng/mL recombinant murine interleukin-3 (IL-3; PeproTech, Rocky Hill, NJ). All cellular and biochemical assays used BMMCs that had been in culture between 4 and 8 weeks. All experiments were conducted using at least 3 independent lines from each genotype.
Immunoblotting
Whole cell protein extracts were obtained from Kit-L–stimulated BMMCs in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, pH 8.0, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM Na3VO4, 10% glycerol, and complete protease inhibitor), and equivalent amounts of protein were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membranes (GE Healthcare, Little Chalfont, United Kingdom), and detected by Western blotting using the ECL Plus system (GE Healthcare). Antibodies used were phospho-p44/42 MAPK (Thr202/Tyr204) (197G2) rabbit mAb (Cell Signaling Technology, Danvers, MA), p44/42 MAP kinase antibody (Cell Signaling Technology) phospho-MEK1/2 (Ser217/221) antibody (Cell Signaling Technology), anti-MEK1, NT (Upstate Biotechnology, Charlottesville, VA), phospho-MEK1/2 (Ser298) antibody (BioSource International, Camarillo, CA), phospho-p38 MAP kinase (Thr180/Tyr182) antibody (Cell Signaling Technology), and p38 MAP kinase antibody (Cell Signaling Technology).
Proliferation assay
Proliferation assays were performed as described previously.13 Briefly, BMMCs were deprived of growth factors overnight and treated with 10 μM Mek1 inhibitor PD98059 (BioSource International), 10 μM p38 MAPK inhibitor SB203580 (BioSource International), or dimethyl sulfoxide (DMSO) for 2 hours before stimulation. A total of 3 × 105 cells was plated in 24-well dishes in 1 mL RPMI containing 10% fetal calf serum, 1% glutamine (Lonza Walkersville), 1.5% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Lonza Walkersville), 2% penicillin/streptomycin (Lonza Walkersville), and 25 ng/mL recombinant murine SCF (PeproTech), or no growth factors as indicated, in a 37°C, 5% CO2 humidified incubator. After 72 hours, cells were counted using a hemocytometer. Cell viability was determined by a trypan blue exclusion assay. Assays were performed in triplicate.
Migration assay
To evaluate mast cell migration, a transwell haptotaxis assay was used as previously described.25,26 A total of 2.5 × 105 cells was resuspended in 100 μL RPMI with 10 μM Mek1 inhibitor PD98059 (BioSource International), 10 μM p38 MAPK inhibitor SB203580 (BioSource International), or DMSO for 2 hours before stimulation. These cells were loaded onto transwell filters (8-mm pore filter Transwell, 24-well cluster; Corning Life Sciences, Acton, MA) that were coated with recombinant fibronectin fragment (Retronectin CH296, Takara, Kyoto, Japan), which then were placed in wells containing 600 μL serum-free RPMI supplemented with 25 ng/mL recombinant murine SCF. After 4 hours of incubation at 37°C in 5% CO2, nonmigratory cells on the upper membrane surface were removed with a cotton swab, and migrated cells attached to the bottom surface of the membrane were stained with 0.1% crystal violet in 0.1 M borate, pH 9.0, 2% ethanol for 10 minutes at room temperature. The average number of migrated cells per higher-power field was counted with an inverted microscope using a 20× objective lens. Assays were performed in triplicate.
F-actin quantitation
To evaluate F-actin content, BMMCs were pretreated with inhibitors and stimulated as described above in “Migration assay.” After 30 minutes of incubation at 37°C in 5% CO2 cells were removed from the upper chamber of the transwell and placed into 3.7% formaldehyde solution for fixation. Fixed cells were treated with 0.01% Triton X-100 (Sigma-Aldrich, St Louis, MO); in phosphate-buffered saline (PBS) for 5 minutes at 25°C, washed, and then incubated with 160 nM Alexa Fluor 488 Phalloidin (Invitrogen) for 20 minutes at 25°C before fluorescence-activated cell sorter analysis by fluorescence cytometry. A minimum of 10 000 mast cell events were recorded, and the results are reported as the fold increase over WT mean channel fluorescence. Assays were performed in triplicate.
Confocal microscopy
To evaluate F-actin organization, BMMCs were pretreated with inhibitors and stimulated as described in “Migration assay.” After 30 minutes of incubation at 37°C in 5% CO2, cells were removed from the upper chamber of the transwell and were placed into cytospin chambers for centrifugation onto microscope slides. The slides were placed into 3.7% formaldehyde solution for fixation. Fixed cells were treated with 0.01% Triton X-100 (Sigma-Aldrich); in PBS for 5 minutes at 25°C, washed, and then incubated with 160 nM Alexa Fluor 488 Phalloidin (Invitrogen) for 20 minutes at 25°C before mounting with 4,6-diamidino-2-phenylindole (DAPI). Confocal images were captured with the Zeiss UV LSM-510 microscope system (Carl Zeiss, Thornwood, NY).
Studies in vivo
Adapting a method described previously,27 adult Nf1+/+/Pak1+/+, Nf1+/−/Pak1+/+, Nf1+/+/Pak1−/−, and Nf1+/−/Pak1−/− mice received a continuous infusion of various doses of SCF or vehicle (PBS) from Alzet 1007D microosmotic pumps (Durect, Alzet, Cupertino, CA) placed under the dorsal (back) skin. Osmotic pumps were surgically placed under light avertin anesthesia. SCF or vehicle was released over 7 days at a rate of 0.5 μL/hour, and osmotic pumps were surgically removed after death on day 7 by cervical dislocation. Before removal of the pump, the dorsal skin was stained with a drop of Davidson Marking System green tissue dye at the point of exit of SCF from the osmotic pump for accurate identification of cutaneous sections for quantitation of changes in mast cell numbers in response to SCF; 3-cm sections of skin marked with tissue dye were removed, fixed in buffered formalin, and processed in paraffin-embedded sections. Specimens were stained with hematoxylin and eosin to assess routine histology and with Giemsa to identify mast cells. Cutaneous mast cells were quantitated in a blinded fashion by counting 2-mm2 sections in proximity to the tissue dye stain. Cells were considered degranulated if there was a change from their normal compacted and granular appearance resulting in an extensive dispersion of more than 15 extruded vesicles localized near the cell, or when there was extensive loss of granule staining, giving the cell a “ghostly” or “hollow” look.28
Results
Loss of Pak1 does not affect expression of mast cell maturation markers
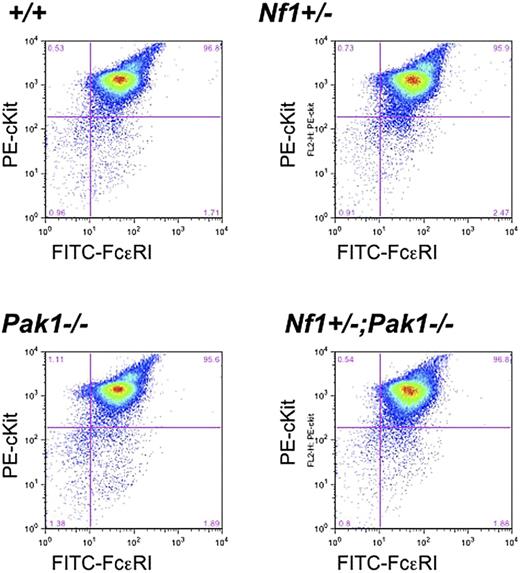
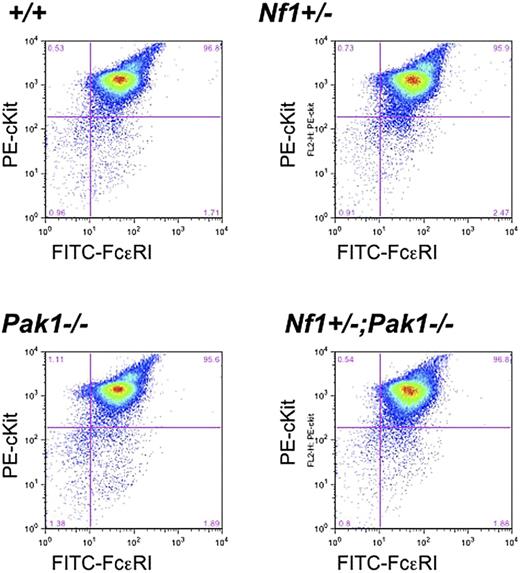
To verify that genetic disruption of Pak1 does not influence mast cell development in intercrossed mast cells, low-density mononuclear bone marrow cells in the presence of serum and IL-3 were cultured for 4 to 5 weeks, and expression of the c-kit receptor and FcϵRI receptor was examined. Fluorescence cytometry analysis of the F2 progeny demonstrated equivalent expression of both c-kit and FcϵRI receptors in all genotypes (Figure 1). Further, the cells had typical cellular morphology and staining of cytoplasmic granules on Alcian blue/Saffranin-O staining (data not shown). Collectively, the fluorescence cytometry and histologic data indicate that alterations in Pak1 expression do not influence c-kit expression or mast cell development in cells that are WT or haploinsufficient at the Nf1 locus.
Loss of Pak1 does not affect expression of important mast cell maturation markers. Mast cells were cultured for 4 weeks, and expression of c-kit and FcϵRI was measured by incubation with anti-IgE followed by FITC-conjugated anti–mouse IgG, as well as PE-conjugated anti–c-kit antibodies. Double-positive cells (upper right quadrant) are mature mast cells, expressing both c-kit and FcϵRI. Data shown are representative of 6 independent lines from each genotype.
Loss of Pak1 does not affect expression of important mast cell maturation markers. Mast cells were cultured for 4 weeks, and expression of c-kit and FcϵRI was measured by incubation with anti-IgE followed by FITC-conjugated anti–mouse IgG, as well as PE-conjugated anti–c-kit antibodies. Double-positive cells (upper right quadrant) are mature mast cells, expressing both c-kit and FcϵRI. Data shown are representative of 6 independent lines from each genotype.
Hyperproliferation of Nf1+/− BMMCs is dependent on a Pak1/MAPK pathway
Given previous work demonstrating elevated Pak kinase activity in Nf1+/− mast cells, as well as a pathologic increase in Nf1+/− mast cell proliferation through Ras-MAPK signals,14 a combination of genetic and pharmacologic experiments was conducted to test the functional consequences of Pak1 loss on BMMCs. Proliferation was assessed by Trypan blue exclusion at the time SCF was added (day 0) and after 72 hours in culture. As anticipated, Nf1+/− BMMCs showed a greater proliferation in response to SCF compared with wild-type cells (Figure 2A closed bars). Genetic disruption of Pak1 resulted in a significant decrease in proliferation at 72 hours in Nf1+/− cells (P < .005), implicating Pak1 as a critical mediator of Nf1 haploinsufficient BMMC hyperproliferation. Further, Pak1−/− BMMCs had reduced proliferation (∼ 60%) at 72 hours compared with wild-type cells (P < .05), suggesting that Pak1 is important for regulating proliferation in the setting of normal Ras activity as well.
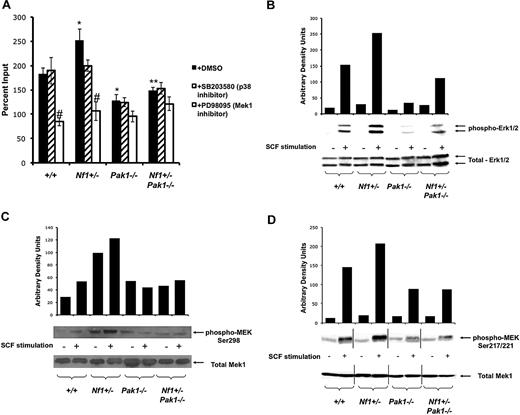
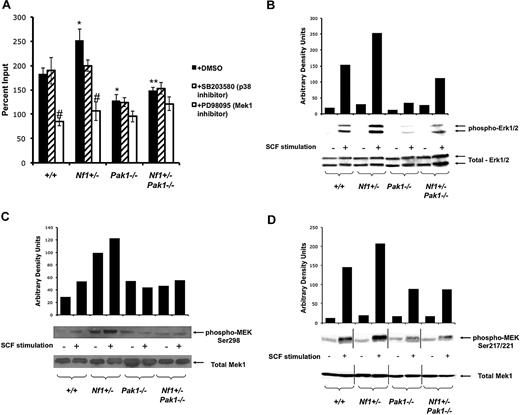
A Pak/MAPK pathway regulates Nf1 haploinsufficient mast cell hyperproliferation. (A) Mast cells were starved overnight in RPMI and plated in a 24-well plate at 3 × 105 per well in triplicate samples after treatment with DMSO (control; ■), 10 μM of selective p38 inhibitor SB203580 ( ), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.
), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.
A Pak/MAPK pathway regulates Nf1 haploinsufficient mast cell hyperproliferation. (A) Mast cells were starved overnight in RPMI and plated in a 24-well plate at 3 × 105 per well in triplicate samples after treatment with DMSO (control; ■), 10 μM of selective p38 inhibitor SB203580 ( ), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.
), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.
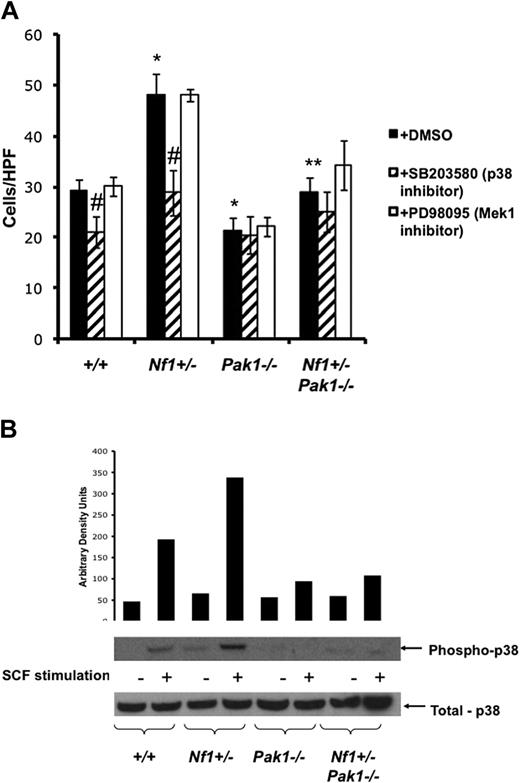
To link this phenotype to a particular MAPK pathway, concomitant proliferation experiments of cultured mast cells were performed in the presence or absence of 10 μM of PD98059, a selective inhibitor of Mek1 activity (Figure 2A open bars) or 10 μM of SB203580, a selective inhibitor of p38 MAPK (Figure 2A striped bars). Addition of p38 inhibitor SB203580 did not affect the proliferation of BMMCs of any genotype, indicating that p38 has little control over BMMC growth. Conversely, inhibition of Mek by PD98059 caused significant decreases in mast cell proliferation in the context of normal Pak1 (∼100% and ∼150% decreases for WT and Nf1+/− BMMCs, respectively). Importantly, Mek inhibition did not significantly affect proliferation of Pak1-null cells, regardless of Nf1 genotype. Collectively, these data imply that Pak1 interacts with the Mek/Erk MAPK pathway in Nf1+/− mast cells to selectively regulate proliferation.
Loss of Pak1 corrects MAPK hyperactivation in Nf1 haploinsufficient BMMCs
To establish the role of Pak1 signaling in the activation of the Ras-Raf-Mek-Erk signaling pathway and to identify the specific MAPK residues that are phosphorylated, BMMCs were stimulated with SCF and assayed for activated MAPK pathway members using phospho-specific antibodies after Western blotting. First, Erk1/2 phosphorylation was measured. Consistent with previous studies,13 Nf1+/− BMMCs have a 2.5-fold increase in phosphorylated (activated) Erk1/2 (Figure 2B). Pak1−/− mast cells had decreased Erk activation, and importantly genetic disruption of Pak1 fully corrects the hyperphosphorylation of Erk1/2 in Nf1+/− mast cells to that of wild-type controls, which correlates with the correction in proliferation (Figure 2A).
Frost et al previously established that Pak1 directly activates Mek at serine residue 298 in NIH3T3 cells.29 Immunoblotting of lysates from SCF stimulated Nf1+/− BMMCs revealed increased phosphorylation of Mek1 at this established target residue of direct Pak1 kinase activity compared with WT controls (Figure 2C). Nf1+/−;Pak1−/− mast cells showed greatly diminished phospho-S298 Mek1 levels compared with Nf1+/− cells (Figure 2C). Serine 298 phosphorylation is important for “priming” Mek1 activity and increasing the interaction between Mek1 and Raf1, resulting in increased Raf-1 directed phosphorylation of Mek1 at Ser217/221.18 Phosphorylation of Ser217/221 by Raf-1 is a necessary step for fully initiating Mek1 kinase activity. Interestingly, in Nf1+/− BMMCs, loss of Pak1 reduced Mek phosphorylation at S217/222, the site of Raf-1–dependent activation (Figure 2D). Raf-1 has been described in multiple systems as a direct substrate of Pak at serine residue 338,17,30,31 and our data provide indirect evidence to support this concept. Collectively, these studies establish that Pak1, a downstream mediator of PI-3 K/Rac signaling, is biochemically linked to the classic Ras/Raf/Mek/Erk pathway in primary cells. Together with the proliferation data displayed in Figure 2A, we establish a crucial role for Pak1 kinase activity in regulating the increased proliferation of Nf1+/− BMMCs by relaying signals to the Mek/Erk pathway.
A Pak1/p38 pathway regulates increased migration of Nf1+/− mast cells
The recruitment of mast cells from the peripheral blood to sites of neurofibroma formation is thought to be an early and required process in the formation of plexiform neurofibromas.6,23,32 Nf1+/− BMMCs have a PI3K-Rac–dependent gain in function in SCF-mediated haptotaxis compared with WT BMMCs.23 Therefore, we questioned whether loss of Pak1 would be sufficient to correct the pathologic increase in migration of Nf1+/− BMMCs toward SCF. To explore these questions, transwells were coated with recombinant fibronectin fragment CH296 (Takara) and migration assays were performed in response to 25 ng/mL of SCF. After 4 hours of incubation in the transwells, the number of mast cells that had migrated to the bottom surface of the fibronectin-coated membrane was counted after staining the cells with crystal violet. BMMCs that were stimulated with media alone showed fewer than 5 cells per high-power field had migrated to the lower side of the membrane (data not shown). As expected, increased numbers of Nf1+/− mast cells migrate toward SCF compared with WT mast cells (Figure 3A closed bars). In addition, loss of Pak1 causes a decrease in migration compared with WT BMMCs (P < .05). Notably, Nf1+/−;Pak1−/− BMMCs have decreases in the number of migrated mast cells compared with BMMCs haploinsufficient at the Nf1 locus, indicating that the increased migration in Nf1+/− mast cells is Pak1-dependent.
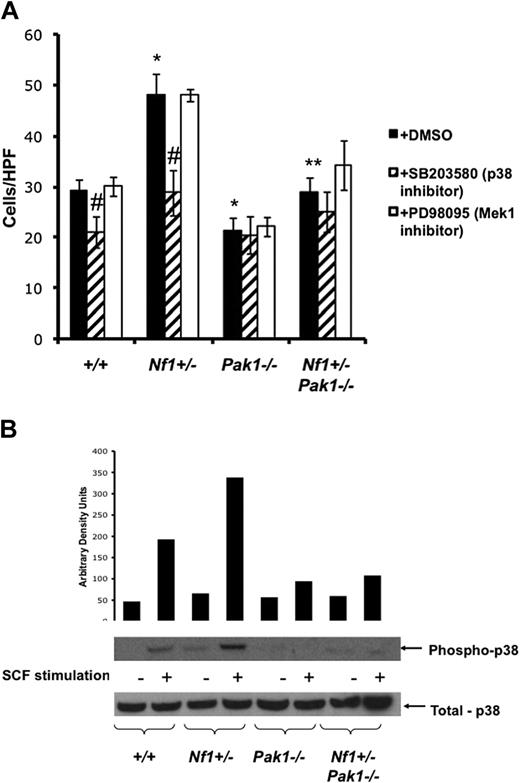
Increased migration of Nf1+/− mast cells is mediated through a Pak/p38 pathway. (A) Mast cells were starved overnight in RPMI without serum and plated in the upper well of a transwell chamber at 105 per well in triplicate samples after treatment with DMSO (control; ■), 10 μM of selective p38 inhibitor SB203580 ( ), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF in the lower chamber for 4 hours, and mast cells that had migrated to the bottom surface of the CH296-coated membrane in response to SCF were counted after staining the cells with crystal violet. Results are expressed as cells per 20× high-power field. Each value represents the mean; error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test. (B) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 5 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active p38 were determined by Western blotting using phospho-specific antibodies. Level of total p38 is shown as a loading control. Western blot of the results is shown and is representative of 3 independent experiments.
), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF in the lower chamber for 4 hours, and mast cells that had migrated to the bottom surface of the CH296-coated membrane in response to SCF were counted after staining the cells with crystal violet. Results are expressed as cells per 20× high-power field. Each value represents the mean; error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test. (B) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 5 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active p38 were determined by Western blotting using phospho-specific antibodies. Level of total p38 is shown as a loading control. Western blot of the results is shown and is representative of 3 independent experiments.
Increased migration of Nf1+/− mast cells is mediated through a Pak/p38 pathway. (A) Mast cells were starved overnight in RPMI without serum and plated in the upper well of a transwell chamber at 105 per well in triplicate samples after treatment with DMSO (control; ■), 10 μM of selective p38 inhibitor SB203580 ( ), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF in the lower chamber for 4 hours, and mast cells that had migrated to the bottom surface of the CH296-coated membrane in response to SCF were counted after staining the cells with crystal violet. Results are expressed as cells per 20× high-power field. Each value represents the mean; error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test. (B) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 5 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active p38 were determined by Western blotting using phospho-specific antibodies. Level of total p38 is shown as a loading control. Western blot of the results is shown and is representative of 3 independent experiments.
), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF in the lower chamber for 4 hours, and mast cells that had migrated to the bottom surface of the CH296-coated membrane in response to SCF were counted after staining the cells with crystal violet. Results are expressed as cells per 20× high-power field. Each value represents the mean; error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test. (B) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 5 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active p38 were determined by Western blotting using phospho-specific antibodies. Level of total p38 is shown as a loading control. Western blot of the results is shown and is representative of 3 independent experiments.
Previous studies have established that mast cells migrate in response to SCF in a p38-dependent manner.22,33,34 To test whether the gain-in-function phenotype for Nf1+/− mast cell migration was the result of communication from Pak1 to p38, BMMCs were treated with 10 μM of the selective inhibitor of p38 SB203580 (Figure 3A striped bars), 10 μM of the selective inhibitor of PD98059 (Figure 3A open bars), or the vehicle only and stimulated as described in Figure 2A. Interestingly, treatment of BMMCs with p38 inhibitor results in a significant decrease in migration of WT and Nf1+/− mast cells (by 28% and 41%, respectively) but does not further reduce migration of Pak1−/− or Nf1+/−;Pak1−/− mast cells, implying that SCF stimulates Nf1+/−BMMC hyperactived migration through a Pak1/p38 pathway. Treatment with the Mek1 inhibitor PD98059 did not affect the migration of BMMCs to SCF, as reported previously.22
Pak1 is required for the increase in p38 phosphorylation seen in Nf1 haploinsufficient mast cells
To establish a biochemical correlate to the functional results shown in Figure 3A, which implicate a Pak1/p38 axis in mediating the increased migration of Nf1+/− mast cells, BMMCs were stimulated with SCF and examined for levels of activated phospho-p38 (R180/Y182). Figure 3B shows that Nf1+/− BMMCs have increased activated p38, consistent with the results of Figure 3A. In addition, loss of Pak1 greatly diminishes phospho-p38 levels after SCF stimulation, providing biochemical evidence that SCF activates a Pak1/p38 pathway in mast cells. This pathway is hyperactivated in Nf1+/− mast cells and leads to increased migration. Therefore, molecular targeting of this pathway could potentially inhibit the recruitment of Nf1+/− mast cells to sites of growing neurofibromas and delay or prevent tumor development.
Pak1 regulates F-actin content and organization in a p38-dependent manner
Actin polymerization at the cell front leads to the early extension of plasma membrane, which is necessary for cell migration.35 c-Kit-mediated mast cell migration is highly dependent on alterations and activation of the actin cytoskeleton by Ras and PI-3K.26,36,37 In addition, other groups have used overexpression systems to describe a role for Pak1 in the regulation of actin dynamics and migration in murine embryonic fibroblasts,38 murine bone marrow-derived macrophages,19 and others. Mast cells deficient in Rac2 (a direct activator of Pak1) have diminished actin cytoskeleton-dependent functions.26 Based on the established association of increases in F-actin content with the increases in migration seen in Nf1+/− BMMCs,23 we hypothesized that the Pak1-dependent decrease in Nf1+/− BMMC migration (Figure 3A) stemmed from a disruption of the F-actin content and/or organization in these cells.
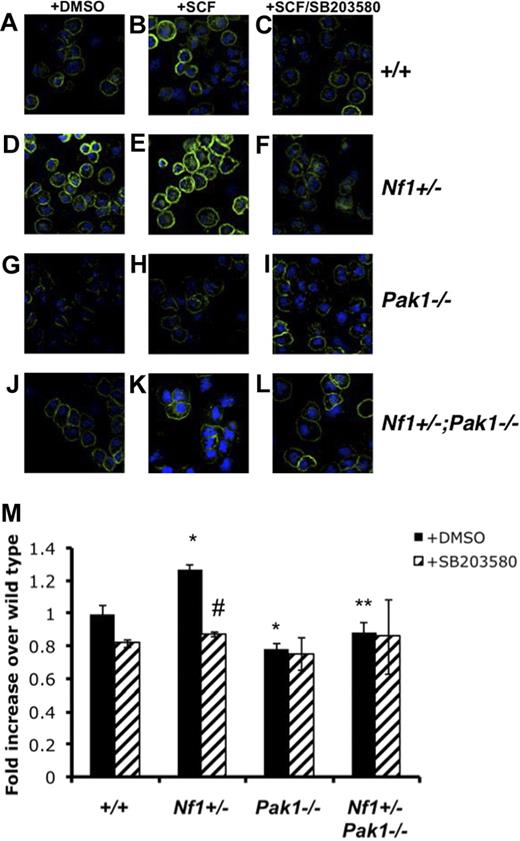
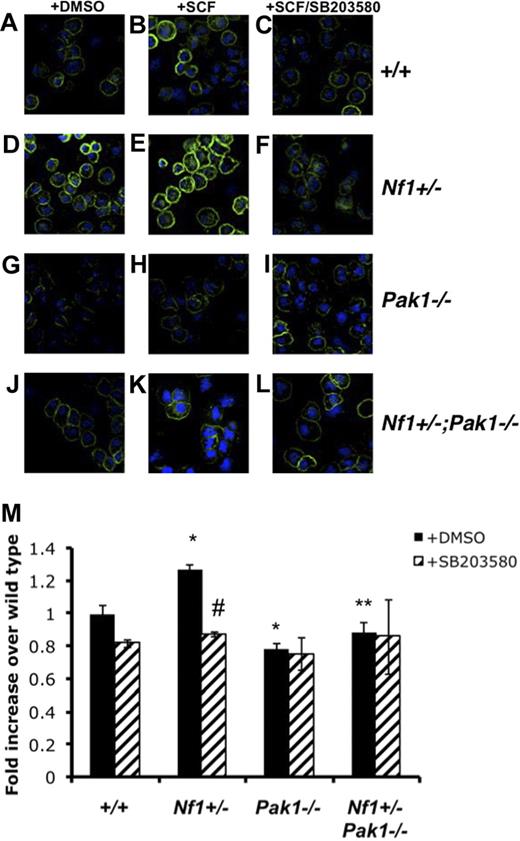
To determine whether Pak1 loss affects BMMC F-actin content, SCF-stimulated BMMCs were stained with phalloidin and analyzed using fluorescence cytometry and confocal microscopy. Figure 4A-L shows representative confocal images of phalloidin and DAPI-costained BMMCs. At a single cell level, confocal images reveal that, at baseline (Figure 4D) and after SCF stimulation (Figure 4E), Nf1+/− BMMCs have increased phalloidin staining compared with wild-type BMMCs (Figure 4A,B), whereas Pak1−/− cells (Figure 3A) have decreased F-actin compared with wild-type cells (Figure 4B) after SCF stimulation. Consistent with the functional migration data (Figure 3A), the Nf1+/−;Pak1−/− BMMCs (Figure 4K) have a reduction in F-actin levels compared with Nf1+/− cells (Figure 4E) after SCF stimulation. Figure 4M details a formal quantification of the phalloidin intensity from 6 independent experiments using fluorescence-activated cell sorter to quantitate F-actin content on SCF stimulated BMMCs.
Pak1 and p38 cooperate to regulate activation and organization of the F-actin cytoskeleton. Mast cells were starved overnight in RPMI and plated in the upper well of a transwell chamber at 105 per well in triplicate samples after treatment with DMSO or 10 μM of selective p38 inhibitor SB203580. Cells were then stimulated with 25 ng of SCF in the lower chamber for 30 minutes, and mast cells were removed from the upper chamber for phalloidin staining of the F-actin cytoskeleton. (A-L) Representative micrographs of phalloidin-stained mast cells analyzed with the Zeiss UV LSM-510 confocal microscope system equipped with a UV Argon laser (351, 364 nm excitation), a visible Argon laser (458, 488 nm excitation) and two Helium-Neon lasers (543, 633 nm excitation). The microscope was equipped with 4 epifluorescence detectors and 1 transillumination detector. The system was mounted on a Zeiss Axiovert 100 inverted microscope and software for image analysis was Zeiss LSM browser R 4.0 (all Carl Zeiss, Thornwood, NY). Green indicates phalloidin stain; blue, DAPI nuclear stain. Original magnification ×400. (M) Fluorescence intensity of phalloidin-stained mast cells, determined by fluorescence cytometry. Data are expressed as fold increases over WT levels; each value represents the mean, and error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test.
Pak1 and p38 cooperate to regulate activation and organization of the F-actin cytoskeleton. Mast cells were starved overnight in RPMI and plated in the upper well of a transwell chamber at 105 per well in triplicate samples after treatment with DMSO or 10 μM of selective p38 inhibitor SB203580. Cells were then stimulated with 25 ng of SCF in the lower chamber for 30 minutes, and mast cells were removed from the upper chamber for phalloidin staining of the F-actin cytoskeleton. (A-L) Representative micrographs of phalloidin-stained mast cells analyzed with the Zeiss UV LSM-510 confocal microscope system equipped with a UV Argon laser (351, 364 nm excitation), a visible Argon laser (458, 488 nm excitation) and two Helium-Neon lasers (543, 633 nm excitation). The microscope was equipped with 4 epifluorescence detectors and 1 transillumination detector. The system was mounted on a Zeiss Axiovert 100 inverted microscope and software for image analysis was Zeiss LSM browser R 4.0 (all Carl Zeiss, Thornwood, NY). Green indicates phalloidin stain; blue, DAPI nuclear stain. Original magnification ×400. (M) Fluorescence intensity of phalloidin-stained mast cells, determined by fluorescence cytometry. Data are expressed as fold increases over WT levels; each value represents the mean, and error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO-treated cells within a genotype using Student unpaired t test.
As shown in Figure 3A, inhibition of p38 MAPK leads to decreases in WT and Nf1+/− mast cell migration but does not affect the migration of Pak1−/− cells. To determine whether the observed differences in the cytoskeleton after SCF stimulation were linked to p38 activity, we examined the F-actin content and organization of BMMCs that were incubated with 10 μM of SB203580 for 2 hours before stimulation. As shown in Figure 4M, the total F-actin content in Nf1+/− BMMCs was decreased after SB203580 treatment (striped bar). Similar to the migration results in Figure 3A, inhibition of p38 MAPK did not affect the total F-actin content of Nf1+/−;Pak1−/− BMMC after SCF stimulation (Figure 4M), indicating that a hyperactive Pak1/p38 pathway regulates F-actin formation in Nf1+/− mast cells. Confocal analysis of phalloidin-stained BMMCs pretreated with SB203580 shows that the inhibition of p38 in wild-type (Figure 4C) and Nf1+/− mast cells (Figure 4F) disrupts the organization of F-actin to a pattern similar to that seen in BMMCs lacking Pak1 (Figure 4I,L).
Cutaneous expansion and degranulation of mast cells in Nf1+/− mice in response to SCF is Pak1-dependent
In vivo mast cell expansion in response to local injection of SCF occurs secondary to local proliferation.27 To determine whether our in vitro findings are relevant in a more physiologic system, we examined cutaneous mast cell accumulation in vivo after local administration of SCF. The progeny generated from the Nf1+/− and Pak1−/− genetic intercross were implanted with slow-release micro-osmotic pumps to deliver 10 μg/kg per day of SCF or PBS (as a vehicle control) continuously. Overlying skin sections were harvested 7 days later and stained with Giemsa to identify mast cells. No significant differences in mast cell numbers between genotypes were noted in control treated mice (data not shown).
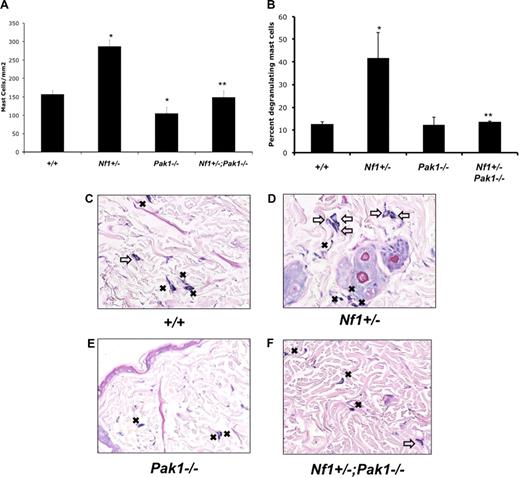
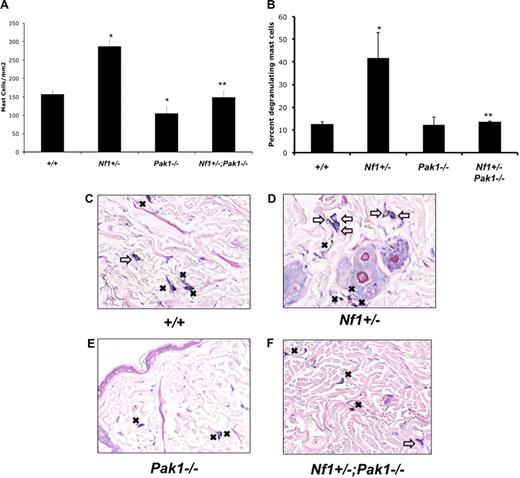
Representative histologic sections from animals treated with SCF and stained with Giemsa are shown in Figure 5C-F. Figure 5A shows quantitative results scoring the number of mast cells in the sections per mm2. Pak1 null mice had a significant decrease in cutaneous mast cells compared with WT mice after SCF delivery (Figure 5A). This result was not the result of a decrease in myeloid or mast cell progenitors in Pak1−/− mice, which are comparable with wild-type controls (data not shown). Nf1+/− mice had a greater accumulation (> 80% increase) of mast cells at the site of SCF infusion compared with wild-type control mice (Figure 5A). In keeping with the in vitro proliferation data (Figure 2A), this excess expansion of cutaneous mast cells in Nf1+/− mice was corrected in Nf1+/−;Pak1−/− mice. SCF infusion into Nf1+/− mice causes increased degranulation of local mast cells compared with wild-type mice.25 We reproduced this result (Figure 5B,D, open arrows) and also found that loss of Pak1 corrects this phenotype by significantly reducing the percentage of degranulating mast cells in the skin of Nf1+/−;Pak1−/− mice. Our observations suggest that the biochemical mechanisms identified in vitro resulting from genetic disruption of Pak1 in mast cells are biologically operative in vivo.
Effect of genetic inactivation of Pak1 on accumulation of cutaneous Nf1+/− mast cells in response to local administration of SCF in vivo. SCF was delivered in vivo via a micro-osmotic pump on the middorsum at 10 μg/kg per day. Skin sections at the site of SCF administration were fixed and stained with hematoxylin and eosin to assess routine histology along with Giemsa to identify mast cells. (A) Cutaneous mast cells were quantitated in a blinded fashion by counting 2-mm2 sections. (B) The percentage of degranulating mast cells present per 2-mm2 section was calculated. Representative sections are displayed in panels C to F. Resting mast cells in panels C to F are marked with ■; degranulating mast cells in panels C to F are marked with an open arrow. Values in panels A and B represent the mean of 3 independent experiments each using 3 mice per genotype, and error bars represent SEM. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control using Student unpaired t test. Images in panels C through F were obtained using a Nikon Eclipse 80i microscope (Tokyo, Japan) using a 10×/0.30 DIC L/N1 magnification and lens in an air medium with a QCapture 2.90.1 camera (QImaging, Surrey, BC).
Effect of genetic inactivation of Pak1 on accumulation of cutaneous Nf1+/− mast cells in response to local administration of SCF in vivo. SCF was delivered in vivo via a micro-osmotic pump on the middorsum at 10 μg/kg per day. Skin sections at the site of SCF administration were fixed and stained with hematoxylin and eosin to assess routine histology along with Giemsa to identify mast cells. (A) Cutaneous mast cells were quantitated in a blinded fashion by counting 2-mm2 sections. (B) The percentage of degranulating mast cells present per 2-mm2 section was calculated. Representative sections are displayed in panels C to F. Resting mast cells in panels C to F are marked with ■; degranulating mast cells in panels C to F are marked with an open arrow. Values in panels A and B represent the mean of 3 independent experiments each using 3 mice per genotype, and error bars represent SEM. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control using Student unpaired t test. Images in panels C through F were obtained using a Nikon Eclipse 80i microscope (Tokyo, Japan) using a 10×/0.30 DIC L/N1 magnification and lens in an air medium with a QCapture 2.90.1 camera (QImaging, Surrey, BC).
Discussion
Mast cells carry out their functions by migrating toward signals emanating from local microenvironments, followed by local proliferation and subsequent release of inflammatory mediators (including proteases). In these studies, we used a disease model (NF1) where Ras-related gains in function in mast cells have a well-known association with the pathologic complications of this disorder.13,39-41 These cellular phenotypes and increased Ras activity have also been observed in NF1 patients (S.C., F.C. Yang, D.W.C., unpublished results, October 2007). We have evaluated the role of Pak1 in modulating Nf1-related phenotypes in primary mast cells generated from intercrossing Nf1+/− mice with Pak1−/− mice.
In this report, we use pharmacologic, genetic, and biochemical approaches to demonstrate that the hyperproliferation of Nf1+/− mast cells is the result of Pak1 signaling to the MEK/Erk pathway. Although multiple studies link Pak1 to MAPK signaling in overexpression systems,16,18,31,42,43 in this report we establish for the first time in primary cells using a knockout model that Pak1 loss leads to decreased activation of MEK and Erk. Importantly, subcutaneous insertion of micro-osmotic pumps continuously releasing SCF in Nf1+/−;Pak1−/− mice corrected the increased accumulation of mast cells in the dermis seen in Nf1+/− mice to WT levels, correlating our in vivo results in a physiologically relevant system (Figure 5) with the mechanisms identified in vitro (Figure 2). Furthermore, we also demonstrate that the increased release of preformed mediators associated with Nf1 haploinsufficiency seen in vivo is corrected when Pak1 is genetically disrupted. Interestingly, although Nf1+/− mast cells have prolonged survival in response to SCF stimulation13 and Pak1 has been implicated as a potential antiapoptotic mediator in some cell types, we found that loss of Pak1 had no significant effect on apoptosis or survival in mast cells (data not shown).
A basic tenet of mast cell biology is that the mast cell progenitors circulate in the peripheral blood after development in the bone marrow and migrate from the bloodstream to peripheral tissues where they complete their developmental maturation. Nf1 null Schwann cells (the tumorigenic cell of the neurofibroma) secrete a 6- to 7-fold higher concentration of SCF compared with WT cells,23 which stimulates a pathologic gain in migration and proliferation of Nf1+/− but not WT mast cells. Hyperactive Nf1+/− mast cells at the site of developing tumors could enhance the tumor-forming ability of Schwann cells by their described role as potential inducers of neurofibroma pathogenesis via effects on the microenvironment.41 Work submitted from our group confirms this hypothesis after we observed that adoptive transfer of Nf1+/− mast cells, but not WT mast cells, into mice that have Nf1 conditionally deleted in the Schwann cell compartment (Krox20Cre;Nf1flox/flox) is necessary and sufficient to allow plexiform neurofibromas to develop in vivo.44 In this report, we demonstrate that loss of Pak1 reduces Nf1+/− mast cell migration by approximately 40% in response to SCF and that this reduction is p38-dependent. In addition, we present experimental evidence that SCF-mediated increases in the F-actin cytoskeleton in Nf1+/− BMMCs are dependent on a Pak1-p38 pathway.
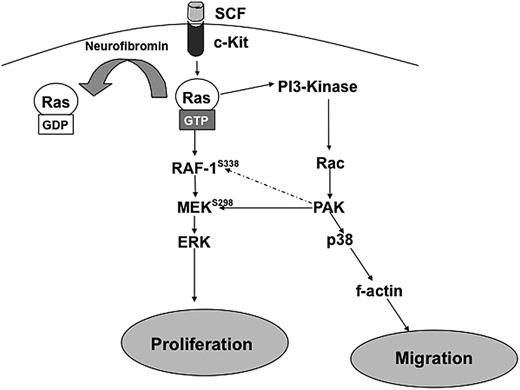
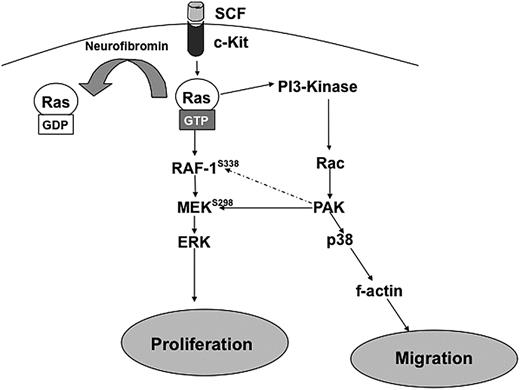
Figure 6 outlines a proposed biochemical mechanism to explain the results described in these studies. In Nf1+/− BMMCs, after stimulation by SCF, Ras becomes hyperactivated because of the lack of neurofibromin GAP activity. This leads to increased PI-3K/Rac activation13,25 and subsequent increases in Pak1 activity.14 Hyperactivated Pak1 transmits mitogenic signals through selective activation of MEK and Erk and migratory signals through selective activation of p38 and the actin cytoskeleton Therefore, we propose that Pak1 acts as a significant “hub” of hyperactivated Ras signaling and that disruption of Pak1 can reduce the pathologic increases in proliferation and migration in Nf1 haploinsufficient BMMCs. The role of Pak1 in other lineages in the tumor microenvironment and in Schwann cells also is an area of active investigation.
Schematic representation of hypothesized pathways explaining how Nf1 haploinsufficient-associated increases in proliferation and migration are dependent on Pak1 signaling. Hatched arrows represent potential downstream effectors of Pak1.
Schematic representation of hypothesized pathways explaining how Nf1 haploinsufficient-associated increases in proliferation and migration are dependent on Pak1 signaling. Hatched arrows represent potential downstream effectors of Pak1.
Greater than 25% of NF1 patients have plexiform neurofibromas surrounding spinal nerve roots,45 which can cause significant morbidity and premature death.46 These tumors present major challenges to surgical treatment, and no effective medical therapies for these tumors are available.41 Further, plexiform neurofibromas have a propensity to transform into malignant peripheral nerve sheath tumors. Lack of existing medical therapies has encouraged investigations of the cellular interactions in the neurofibroma microenvironment and molecular pathways regulating them in an attempt to identify potential pharmacologic targets for clinical use. Emerging evidence designating non-Schwann cell components as key moderators of the pathophysiology at the site of neurofibroma development provides attractive targets for pharmacologic interventions.
In addition to mast cells, haploinsufficiency at Nf1 results in tumor-promoting gain-in-function responses in other microenvironmental cells within neurofibromas, specifically fibroblasts, endothelial cells, and pericytes. Fibroblasts and their secreted extracellular matrix compose more than 50% of the dry weight of neurofibromas,47 and our group has previously demonstrated that Nf1+/− fibroblasts have increased invasiveness and extracellular matrix remodeling capability in response to Nf1+/− mast cell conditioned media.48 In addition, the highly vascular nature of plexiform neurofibromas is thought to be the result of hyperactive Ras-dependent increases in angiogenesis found in Nf1+/− endothelial cells.49,50
If molecular therapeutics designed for correcting hyperactive pathways in Nf1+/− cells located in the tumor microenvironment are developed, plexiform neurofibroma formation may be delayed or even prevented. For example, in recently submitted studies using the Krox20Cre;Nf1flox/− murine tumor model, we have effectively used the small molecule imatinib (which inhibits c-kit and other RTKs) to reduce plexiform neurofibroma mass and significantly extend survival.44 Currently, we are using this agent in a phase 2 clinical trial in pediatric NF1 patients with plexiform neurofibromas. This report outlines the critical function that Pak1 carries out in pathogenic Nf1+/− mast cell gains in function and identifies the Pak1 signaling axis in mast cells as a potential therapeutic target for treating NF1. Indeed, recent reports have used the histone acytelase inhibitor FK228, which leads to a reduction in Pak1 kinase activity, to inhibit the growth of Nf1-deficient xenografts.51 Based on this and our work in mast cells, the identification of specific inhibitors of Pak1 could be useful in the treatment or prevention of neurofibromas.
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Briana M. Baker for administrative support in the preparation of this manuscript.
This work was supported by the National Institutes of Health, National Cancer Institute (R01 CA74177 to D.W.C.), National Cancer Institute (R01 CA117884 to J.C.), National Institute of Neurological Disorders and Stroke (P50 NS052606 to D.W.C. and F30 NS060322-01 to A.S.M.), and National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK007519-23 to A.S.M.). A.S.M. is a PhD candidate at Indiana University, and this work is submitted in partial fulfillment of the requirement for the doctoral degree.
National Institutes of Health
Authorship
Contribution: A.S.M., S.-J.P., E.G.M., S.J.B., S.C., and W.K.B. collected data; A.S.M., J.D.A., S.-J.P., D.A.I., and D.W.C. analyzed and interpreted data; Z.M.J., C.H., and J.C. contributed vital new reagents; A.S.M. and D.W.C. designed the experiments; J.D.A., D.A.I., and D.W.C. revised and edited the manuscript; and A.S.M. produced the figures and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: D. Wade Clapp, Indiana University School of Medicine, Cancer Research Institute, 1044 W Walnut Street, R4 402, Indianapolis, IN 46202; e-mail: dclapp@iupui.edu.


 ), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.
), or 10 μM of selective Mek1 inhibitor PD98059 (□). Cells were then stimulated with 25 ng of SCF for 72 hours, and viable cells were measured by trypan blue exclusion. Results are expressed as percentage of input number of cells at 72 hours after stimulation. Each value represents the mean, and the error bars represent the SEM of 6 independent experiments. *P < .05 compared with WT control. **P < .05 compared with Nf1+/− control. #P < .05 compared with DMSO treated cells within a genotype using Student unpaired t test. (B-D) Mast cells were serum starved overnight, stimulated with SCF, and cell lysates isolated at 0 and 2 minutes after stimulation. A total of 100 μg of protein was used for each time point. Levels of active Erk1 (B) and Mek1 (C,D) were determined by Western blotting using phospho-specific antibodies. Levels of total Erk1 and Mek1 are shown as loading controls. Western blot of the results is shown and is a representative of 3 independent experiments. Vertical lines in panel D have been inserted to indicate repositioned gel lanes for consistency with other blots.