Abstract
Residual vein thrombosis (RVT) indicates a prothrombotic state and is useful for evaluating the optimal duration of oral anticoagulant treatment (OAT). Patients with a first episode of deep vein thrombosis, treated with OAT for 3 months, were managed according to RVT findings. Those with RVT were randomized to either stop or continue anticoagulants for 9 additional months, whereas in those without RVT, OAT was stopped. Outcomes were recurrent venous thromboembolism and/or major bleeding. Residual thrombosis was detected in 180 (69.8%) of 258 patients; recurrent events occurred in 27.2% of those who discontinued (25/92; 15.2% person-years) and 19.3% of those who continued OAT (17/88; 10.1% person-years). The relative adjusted hazard ratio (HR) was 1.58 (95% confidence interval [CI], 0.85-2.93; P = .145). Of the 78 (30.2%) patients without RVT, only 1 (1.3%; 0.63% person-years) had a recurrence. The adjusted HR of patients with RVT versus those without was 24.9 (95% CI, 3.4-183.6; P = .002). One major bleeding event (1.1%; 0.53% person-years) occurred in patients who stopped and 2 occurred (2.3%; 1.1% person-years) in those who continued OAT. Absence of RVT identifies a group of patients at very low risk for recurrent thrombosis who can safely stop OAT. This trial was registered at http://www.ClinicalTrials.gov as no. NCT00438230.
Introduction
Long-term anticoagulant treatment is highly effective in preventing recurrent venous thromboembolism (VTE), but the optimal duration of this therapy remains uncertain.1-4 After oral anticoagulant therapy (OAT) withdrawal, the recurrence of risk is greatest in the first year and gradually diminishes,5 while the increased bleeding risk may offset the benefits of prolonged OAT.1-4,6,7
In patients with a first episode of deep vein thrombosis (DVT) of the lower limbs, the current standard for establishing the duration of OAT is based on the nature of DVT (at least 3-6 months for idiopathic and 3 months for provoked thrombosis).8 New parameters have, however, been proposed to optimize OAT duration; among them, D-dimer assay was shown to be effective in selecting patients with idiopathic DVT who may benefit from a prolonged anticoagulation.9
In earlier prospective studies conducted in patients with symptomatic DVT, the presence of a residual thrombus was associated with an increased risk of thrombotic recurrence either in idiopathic or provoked venous thrombosis.10,11 Recurrent events occurred not only in the previously affected veins but also in other sites, which suggests that residual vein thrombosis (RVT) may indicate an underlying prothrombotic state.10,11
To test the hypothesis that RVT may be used for establishing the OAT duration, we performed a randomized study in patients with a first episode of symptomatic DVT.
Methods
Patients with a first episode of documented idiopathic and provoked proximal DVT were eligible for the study if they had completed 3 months of OAT (target INR [international normalized ratio], 2.5; range, 2.0-3.0). Provoked DVT was defined as a thrombosis episode associated with pregnancy or puerperium, recent (ie, less than 3 months) fracture or plaster casting of a leg, immobilization with confinement to bed for 3 consecutive days, and surgery with general anesthesia lasting more than 30 minutes. Idiopathic DVT was defined as a thrombosis episode occurring in apparently healthy individuals. Patients with active cancer, limited life expectancy, antiphospholipid antibody syndrome, or other known thrombophilic states (such as deficiencies of antithrombin and protein C and S, homozygous for FV Leiden or FII 20210G>A mutations, or combined heterozygosity for the same), serious liver disease, or renal failure, and, finally, those who lived too far from the recruiting center were excluded from the study. The Duration of Anticoagulation based on Compression UltraSonography (DACUS) study was approved by the institutional review boards of participating centers (Palermo, Catania, and Trapani, Italy). All enrolled patients provided written informed consent. Informed consent was obtained in accordance with the Declaration of Helsinki.
Study design
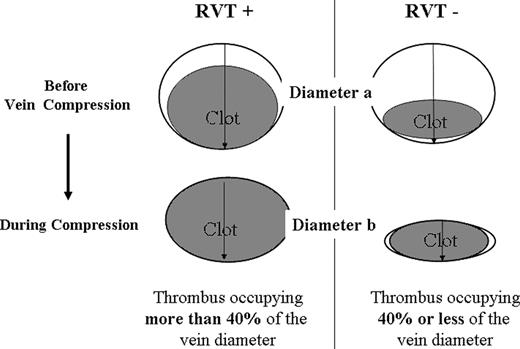
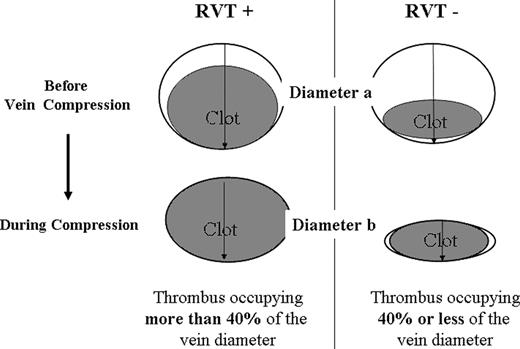
This multicenter prospective study was conducted in patients with a first episode of symptomatic proximal DVT, detected by compression ultrasonography (C-US), and who received OAT for 3 months (warfarin [Bristol- Myers Squibb, New York, NY] or acenocoumarol [Novartis Pharma, East Hanover, NJ]). Those who agreed to participate in the study underwent an examination to assess baseline clinical conditions and to exclude contraindications. C-US of the affected leg was performed and images were obtained in transverse section only. Lumen compressibility was then evaluated by gentle pressure of the probe; RVT diameter was taken by measuring the distance between the anterior and posterior walls of the vein, on freeze-frame B-mode images, during compression with the ultrasound probe.10,11 The examination was performed with the patient in the supine position with the leg externally rotated and slightly flexed at the knee. Measurements were taken at the common femoral vein, 1 cm below the inguinal ligament and at popliteal vein, at the most prominent crease in the midpopliteal fossa. RVT calculation was made as follows: RVT = vein diameter during compression (diameter b) × 100/vein diameter before compression (diameter a; Figure 1). RVT was arbitrarily scored as “absent” when the figure was 40% or less of the vein diameter.10 Patient was considered as having RVT when a persisting thrombus was shown to be present in at least 1 of the 2 examined vein segments.
Evaluation of residual vein thrombosis. RVT calculation = vein diameter during compression (diameter b) × 100/vein diameter before compression (diameter a). RVT indicates residual vein thrombosis.
Evaluation of residual vein thrombosis. RVT calculation = vein diameter during compression (diameter b) × 100/vein diameter before compression (diameter a). RVT indicates residual vein thrombosis.
Patients with RVT were randomized to either stop or continue OAT (INR, 2.0-3.0) for 9 additional months (groups A2 and A1, respectively). Those without RVT did not continue anticoagulation (group B; Figure 2). We discouraged study investigators from performing D-dimer testing after OAT discontinuation or at any point during the course of the study. C-US tests were performed at the study onset or at any other time when a recurrence was suspected.
Study design. A1 indicates patients with RVT who continued OAT for 12 months (in total); A2 indicates patients with RVT randomized to stop OAT after 3 months; and B indicates patients without RVT who stopped OAT after 3 months. DVT indicates deep vein thrombosis; RVT, residual vein thrombosis; C-US, compression ultrasonography; and OAT, oral anticoagulant therapy.
Study design. A1 indicates patients with RVT who continued OAT for 12 months (in total); A2 indicates patients with RVT randomized to stop OAT after 3 months; and B indicates patients without RVT who stopped OAT after 3 months. DVT indicates deep vein thrombosis; RVT, residual vein thrombosis; C-US, compression ultrasonography; and OAT, oral anticoagulant therapy.
A different randomization sequence for each different study site was computer generated and encapsulated in a randomization computer program. The sequences were balanced in blocks of 10.
Study outcomes and follow-up
Patients were followed for at least 1 year after OAT discontinuation (Figure 2). Study outcomes were recurrent VTE and/or major bleeding. Patients were instructed to contact the clinical center if symptoms developed suggestive of VTE or bleeding. In cases of recurrence, results of C-US were compared with those of the previous examination. Diagnosis of recurrent DVT was made if a previously fully compressible segment (contralateral or ipsilateral) became no longer compressible or if an increase of 4 mm or more in the diameter of the residual thrombus during compression was detected12 ; in undetermined cases, repetition of the test (after 5-7 days) or contrast venography was performed. In patients with suspected pulmonary embolism, diagnosis of VTE recurrence was based on objective algorithms.13,14 Major bleeding was defined as a decrease in hemoglobin level of 20 g/L (2.0 g/dL) or more, intracranial or retroperitoneal bleeding, bleeding needing surgical intervention or blood transfusion, or any other bleeding considered clinically relevant by the physician in charge requiring suspension of anticoagulation and the use of hemostatic approaches. Minor bleedings were all the other bleeding events. All suspected events were evaluated by a central adjudication committee whose members were unaware of the patient name, the center, and the group assignment.
Statistical analysis
The sample size was calculated taking into account, for the group with the worst prognosis, an incidence of 20% for recurrent thrombotic events and a 5% incidence of complications for the group with the best prognosis. An overall sample size of 300 patients (100 for each study group) was calculated to achieve a power of 80% to document a difference of at least 15% in at least one of the different head-to-head comparisons, based on the Bonferroni method for distributing type I error (.05) among multiple comparisons. To monitor the safety of the trial, an interim analysis was planned after the enrollment of two-thirds of the total patient population.
Before the study onset, we set up an internal quality control system to assess interexaminer and intraexaminer reproducibility for RVT analysis among operators by the use of the unweighted Cohen kappa (κ) test.
Baseline differences between groups were assessed by the chi-square test (Yates correction) for categorical variables and t test or Kruskal-Wallis test for parametric and nonparametric analyses, as appropriate. Data were analyzed on an intention-to-treat basis. Kaplan-Meier curves were plotted to estimate the cumulative incidence of symptomatic recurrent thrombosis. Patients who during the study developed clinical conditions interfering with study outcomes (such as ischemic heart disease, cancer, stroke, or superficial vein thrombosis) and left the assigned group were regularly followed and included in the analyses. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model. Data were analyzed using Epi Info software (version 6.0; Centers for Disease Control and Prevention, Atlanta, GA) and SPSS Software (version 14.0; SPSS, Chicago, IL). All P values were 2-sided, and P values less than .05 were considered to indicate statistical significance.
Results
Of the 312 patients enrolled as of March 2005, 17 were excluded because they did not provide informed consent and 37 because a long-term oral anticoagulation was indicated. At the interim analysis, the safety committee board decided to stop inclusion of additional patients because withdrawing OAT, especially in patients with RVT randomized to receive short-anticoagulation, was considered potentially dangerous. A total of 258 patients were included in the study. No patient was lost to follow-up. Baseline patient characteristics and follow-up data are reported in Table 1.
RVT was present in 180 (69.8%) of 258 patients; they were randomized to continue (88 patients; group A1) or to stop anticoagulation (92 patients; group A2). RVT was absent in 78 (30.2%) of 258 patients (group B). Patients with RVT were more likely to have idiopathic DVT in comparison with those without RVT, and the latter patients were also younger (Table 1).
Outcomes
Tables 2 and 3 show the outcomes of follow-up. Thrombosis recurred in 23.3% (42/180) of patients with RVT in comparison with 1.3% of those without (Table 2; Figure 3); most of these events were venous thromboses and more than 20% (9/42) were contralateral. The rate of outcome in patients with provoked DVT was 8 of 92 (8.7%; 95% CI, 3.0-14.4) and the number/100 person-years was 8 of 182.5 (4.4%; 95% CI, 0.3-8.5). Among patients with idiopathic DVT, the outcome rate was 35 or 166 (21.1%; 95% CI, 14.8-27.4) and the number/100 person-years was 35 of 307.9 (11.4%; 95% CI 6.5-16.3).
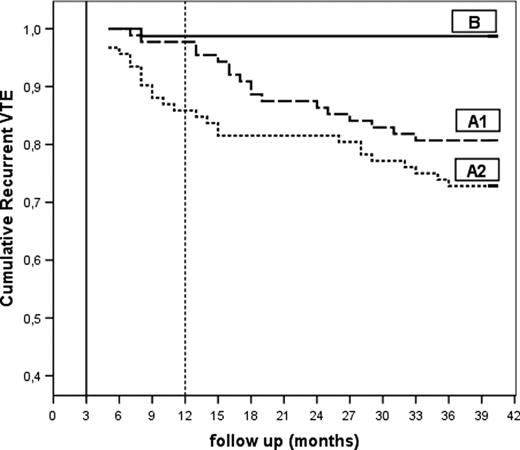
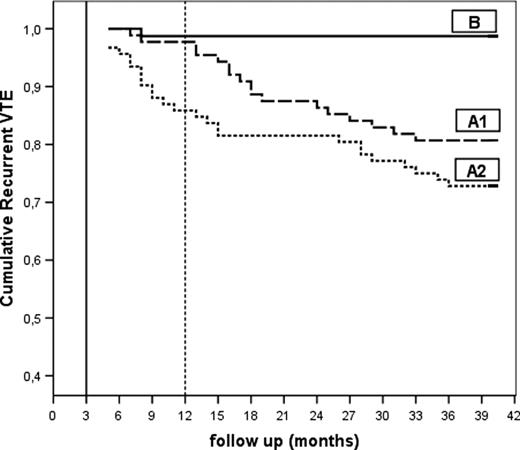
Kaplan-Meier curve for recurrent VTE in the 3 groups. A1 indicates patients with RVT who continued OAT for 12 months (in total); A2 indicates patients with RVT randomized to stop OAT after 3 months; and B indicates patients without RVT who stopped OAT after 3 months. VTE indicates venous thromboembolism.
Kaplan-Meier curve for recurrent VTE in the 3 groups. A1 indicates patients with RVT who continued OAT for 12 months (in total); A2 indicates patients with RVT randomized to stop OAT after 3 months; and B indicates patients without RVT who stopped OAT after 3 months. VTE indicates venous thromboembolism.
The HRs for recurrent events adjusted for age and sex were (a) A1 versus A2: 1.58 (95% CI, 0.85-2.93; P = .145); (b) A1 versus B: 15.7 (95% CI, 2.1-118.0; P = .007); and (c) A2 versus B 24.9 (95% CI, 3.4-183.6; P = .002). The HRs for recurrent events adjusted for idiopathic versus provoked DVT were (d) A1 versus A2: 1.58 (95% CI, 0.86-2.94; P = .141); (e) A1 versus B: 15.2 (95% CI, 1.9-121.8; P = .01); and (f) A2 versus B: 19.3 (95% CI, 2.5-147.2; P = .004).
Three deaths occurred during follow-up: one was due to acute myocardial infarction, and the other 2 were related to active cancer. No deaths due to thrombotic recurrences or bleeding were recorded. All deaths occurred in patients with RVT.
The reproducibility of RVT measurement, performed before the study, was conducted on a consecutive series of 64 noncompressible venous segments (in popliteal and common femoral veins). The result of interoberver and intraobserver variation assessment among operators proved to be adequate (κ, 0.7403; 95% CI, 0.70-0.86).
Discussion
Our results suggest that RVT assessment is useful for evaluating the features of a DVT of the lower limb. In fact, RVT assessment not only identifies a subset of patients at lower risk of recurrence but also allows us to gauge the individual risk of recurrence after a first DVT episode.
Absence of RVT was used to identify a substantial subset of patients (at least 30% of patients with unprovoked DVT) characterized by a low risk of recurrent VTE who require a short-term antithrombotic treatment. This fact has evident advantages in clinical practice: it reduces the clinical burden for both the patient and the health care system and substantially reduces the overall risk for bleeding inherent with OAT treatment.
The difference between patients with and without RVT is not a minor one, especially if considered in terms of recurrences/person-year, which, in those with a persistent thrombus, was found to be 20 times higher. As expected, many patients without RVT had a provoked DVT, but no differences were found in terms of outcomes in comparison with those with idiopathic DVT (Table 3). Further, in patients with RVT, the recurrence rate was high even when the index DVT was provoked.
Of interest is the fact that at least 20% of the recurrent thromboses occurred in the controlateral leg, a finding that supports the hypothesis that RVT can indicate the presence of an underlying prothrombotic state triggering a sustained hypercoagulability. Worthy of note is the fact that a prolonged OAT only delayed recurrences,15 which further supports this hypothesis.
In patients with RVT, the recurrence rate was high and relapses occurred soon after OAT suspension (Figure 3); this evidence, in agreement with that of a previous study,15 suggests that RVT-positive patients probably need prolonged anticoagulation treatment. In fact, our findings show that, in this group, even 1 year of OAT does not significantly reduce the risk of recurrent thrombosis.
The most relevant features of our study were that we used a simple and reproducible imaging method for both idiopathic and provoked DVTs. This strategy is important in practical terms as it can be easily and broadly applied. At any rate, we acknowledge that the results of our study need to be evaluated with some caution because the trial could not be blinded.
D-dimer assay has been proven to be effective in similar clinical settings,9 and it would be of interest to know whether RVT assessment is comparable, in terms of efficiency, to this assay. RVT assessment, however, has the advantage of being insensitive to other changes involving blood coagulation (such as those triggered by infections or cancer); another advantage is the fact that it can be performed without interrupting anticoagulation.9 This comparison should be addressed in properly designed clinical trials.
To conclude, our results indicate that absence of RVT identifies patients at low risk for recurrent thrombotic events. This ability is important in driving a management strategy for both the prevention of recurrences and the selection of a DVT patient population that may benefit from a short period of anticoagulation. Further trials are needed to assess the optimal OAT duration in RVT-positive patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.S. and G. Mariani designed and organized the study; A.M. gathered the data; S.S., G. Mariani, and A.C. analyzed the data and vouch for the results of analysis; S.S., G. Mariani, A.M., and A.C. contributed to writing the paper; R.A., V.C., G. Milio, C.A., M.B., M.T.A., O.C., M.P., A.D., and G.B. contributed to collecting data and to enrolling patients at the participating centers; and S.S., G. Mariani, G. Milio, M.B., and O.C. constituted the safety board.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sergio Siragusa, Cattedra e Unità di Ematologia con trapianto, Università degli Studi di Palermo, Via del Vespro 127, I-90127 Palermo, Italy; e-mail: sergio.siragusa@unipa.it.