Abstract
CD40 and CD27, members of the tumor necrosis factor receptor (TNFR) family, are critical regulators of lymphocyte growth and differentiation. In B-cell precursor acute lymphoblastic leukemia (BCP-ALL), we prospectively assessed the impact of CD40 and CD27 on outcome in 121 children treated according to the CoALL06-97 protocol. Expression of both CD40 and CD27 was found to be significantly higher in low- than in high-risk patients as defined by standard clinical risk parameters such as age and white blood cell count. In addition, in multivariable analysis, a very high percentage of CD40+ blasts at diagnosis was identified as an independent favorable prognostic factor for relapse-free survival. Of note, high CD40 expression particularly protected against late relapse. In B cells, CD40 is known to enhance both antigen-presenting capacity and sensitivity to proapoptotic signals. Yet, although CD40 ligation does result in significant up-regulation of CD80/CD86 in our cohort, it is up-regulation of the death receptor CD95 that significantly correlates with the percentage of CD40+ blasts. Thus very high expression of CD40 on BCP-ALL blasts is an independent prognostic marker indicative of superior relapse-free survival that may in part be due to CD40-dependent death receptor up-regulation.
Introduction
Although the overall prognosis in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is good, identification of new prognostic factors may result in improved risk classification and treatment outcome. Long-term survival is critically influenced by blast sensitivity toward chemotherapeutic agents. Immunologic control mechanisms are also thought to play a role.1-3 In normal B cells, homeostasis is maintained by an intricate regulatory network with the nerve growth factor/tumor necrosis factor (NGF/TNF) receptor/ligand family of proteins playing a pivotal role in T cell/B cell interactions.4-8 As adequate stimulation of effector cells as well as susceptibility of the target cells to apoptotic signals are required for optimal immunologic control, the capacity of B cells to respond to CD40 ligand (CD40L) stimulation is critical. In normal B cells, activation via the CD40 receptor promotes differentiation and results in increased expression of costimulatory molecules and the CD95 death receptor, another member of the TNF receptor (TNFR) family.7,9-11 To various degrees, the CD40 receptor is also expressed on acute lymphoblastic leukemia (ALL) of the B-cell lineage.12 In analogy to mature B cells, activation via CD40 turns leukemic blasts into efficient antigen-presenting cells mediated by up-regulation of MHC, adhesion, and costimulatory molecules.13-17 In addition to promoting their antigen-presenting ability, in chronic lymphoblastic leukemia (CLL) it has been shown that CD40 ligation also induces CD95 expression,18,19 although little is known about CD40-dependent CD95 modulation in BCP-ALL.
CD27, another member of the TNF receptor family, also plays an important role in lymphoid differentiation and apoptosis. Induced on normal B lymphocytes after antigenic challenge, it is a marker of memory B cells. Recently, increased expression of the membrane-bound form of CD27 on ALL blasts has been reported and ligation of CD27 has been shown to induce apoptosis.20-26 Although CD27 interaction with its ligand CD70 complements CD40-dependent modulation of B-cell responses, in ALL there are currently no data on the prognostic impact of TNFR expression.
These findings prompted us to initiate a prospective analysis investigating the influence of both CD40 and CD27 on outcome in a cohort of patients with BCP-ALL from the CoALL06-97 (cooperative study group for childhood ALL) study. This is the first report describing in detail the expression profile of these 2 TNF receptors as well as their influence on treatment outcome in pediatric BCP-ALL.
Methods
Patient cohort
One-hundred twenty-one children aged 1 to 17 years with B-cell precursor ALL diagnosed between April 1998 and August 2003 and treated according to the CoALL06-97 study protocol at the 2 main study centers (Hamburg and Duesseldorf) were assessed prospectively for the prognostic impact of CD40 expression at study entry after informed consent was obtained from the parents or legal guardians for the scientific substudy at the time of enrollment. Additional information on CD27 expression was obtained in a smaller subgroup of 108 patients only for logistic/technical reasons. The CoALL06-97 protocol was approved by the ethics committee (IRB) in Hamburg as well as the local ethics committee (IRB) in Duesseldorf including the conductance of research projects and in accordance with the Declaration of Helsinki. Risk classification of patients with BCP-ALL in the CoALL study is defined by immunophenotype, initial leukocyte count, age, and in vitro resistance to the critical chemotherapeutic agents prednisolone, vincristine, and asparaginase (PVA score).27,28 The patient cohort analyzed for TNFR expression is representative of the CoALL study population with regard to risk factor distribution, relapse rate, and survival (Table 1).27,29 Patient characteristics are summarized in Table 1. The median follow-up was 4 years (range, 2-7 years).
Flow cytometric analysis
A standard panel of murine fluorescein-conjugated monoclonal antibodies (mAbs) and respective isotype controls (Beckman Coulter, Fullerton, CA) was used for diagnosis of BCP-ALL. Analysis of the TNF receptor expression on blasts was performed at diagnosis by double-staining with a directly fluorescein-coupled anti-CD19 or anti-CD10 mAb and a directly labeled anti-CD27, anti-CD40, anti-CD80, anti-CD86, or anti-CD95 antibody (Beckman Coulter). Staining and flow cytometry were performed on a FACScan (Becton Dickinson, Franklin Lakes, NJ) according to standard protocol using the Cell Quest software (Becton Dickinson) for analysis. Results were calculated as the percentage of positive blasts. Samples expressing the respective markers on more than 10% of blasts were considered positive.
Stimulation of leukemic blasts by CD40 cross-linking coculture
For ligation of the CD40 receptor, 106 CD40+ ALL blasts were cultured in 1 mL RPMI supplemented with 10% FCS on genetically modified mouse fibroblasts expressing human CD40 ligand (CD40L) in trans for 3 to 4 days. Prior to use, fibroblasts were treated with mitomycin C (0.01 mg/mL) at 37°C in 5% CO2 for 2 hours and were washed twice.
Statistical analysis
Surface expression of TNFR and costimulatory molecules on the blasts of different patient groups were compared by Mann-Whitney test. For comparison of percentage positive blasts before and after stimulation, the t test for dependent variables was used. Relapse-free survival was calculated and survival curves were generated according to the Kaplan-Meier method. To focus on late relapses, a landmark analysis was performed excluding any patient who was not alive without relapse at 2 years after study entry. The Cox regression model was applied to determine the impact of the different risk parameters on survival. All statistical analyses were performed with the SPSS program version 12.0 (Chicago, IL). A P value of less than .05 was considered significant.
Results
Expression of CD27 and CD40 receptor on BCP-ALL blasts
Almost all of the patients in our cohort showed CD40 expression with a broad range of CD40+ blasts at diagnosis (median, range: 94%, 3%-100%), whereas only half of the patients were positive for CD27 beyond the defined threshold of more than 10% (median, range: 10%, 0%-98%). When focusing only on those blasts positive for CD27, expression of CD27 was still highly variable (median, range: 70%, 10%-98%). Of note, none of the blasts that were positive for CD27 lacked CD40 expression. When assessing TNFR expression in low-risk (LR) and high-risk (HR) patients as classified by standard-risk parameters, overall CD27 expression was significantly higher in low-risk ([LR] median, range: 32%, 0%-98%) than in high-risk ([HR] median, range: 5%, 0%-95%; P = .017) patients (Figure 1A). In addition, the percentage of CD40+ BCP-ALL blasts was found to be significantly higher in the low-risk than in the high-risk group (median, range: LR: 98%, 3%-100% vs HR: 84%, 3%-100%; P = .007; Figure 1B).
Surface expression of TNF receptor molecules on BCP-ALL blasts and their modulation upon CD40 ligation. (A,B). The percentage of CD40+ and CD27+ ALL blasts at diagnosis is higher in low-risk patients. The percentage of BCP-ALL cells positive for CD27 (A) and CD40 (B) surface expression was assessed by flow cytometry in patients (pts) allocated to the low-risk (LR) versus the high-risk (HR) group by standard clinical criteria such as age, leukocyte count, and chemoresistance. (C,D) Ligation of CD40 modulates the expression of other TNF receptor molecules on BCP-ALL blasts. Expression of the TNF receptor molecules in CD40-activated blasts was assessed by flow cytometry after culture of ALL cells on CD40 ligand–expressing feeder cells for 3 to 4 days. CD40 activation is further accompanied by significant down-regulation of CD27 surface expression on blasts both in the LR and HR patient group (C). In accordance with the higher percentage of CD40+ blasts in LR patients, this patient group reveals an enhanced capacity to up-regulate CD95 upon CD40 ligation compared with HR patients (P = .013; D). In the box plot diagrams the boxes represent the interquartile range with the line in the middle indicating the 50th percentile. The error bars, or whiskers, represent the highest and lowest values that are not outliers or extreme values.
Surface expression of TNF receptor molecules on BCP-ALL blasts and their modulation upon CD40 ligation. (A,B). The percentage of CD40+ and CD27+ ALL blasts at diagnosis is higher in low-risk patients. The percentage of BCP-ALL cells positive for CD27 (A) and CD40 (B) surface expression was assessed by flow cytometry in patients (pts) allocated to the low-risk (LR) versus the high-risk (HR) group by standard clinical criteria such as age, leukocyte count, and chemoresistance. (C,D) Ligation of CD40 modulates the expression of other TNF receptor molecules on BCP-ALL blasts. Expression of the TNF receptor molecules in CD40-activated blasts was assessed by flow cytometry after culture of ALL cells on CD40 ligand–expressing feeder cells for 3 to 4 days. CD40 activation is further accompanied by significant down-regulation of CD27 surface expression on blasts both in the LR and HR patient group (C). In accordance with the higher percentage of CD40+ blasts in LR patients, this patient group reveals an enhanced capacity to up-regulate CD95 upon CD40 ligation compared with HR patients (P = .013; D). In the box plot diagrams the boxes represent the interquartile range with the line in the middle indicating the 50th percentile. The error bars, or whiskers, represent the highest and lowest values that are not outliers or extreme values.
Modulation of other TNF receptor molecules after CD40 ligation
As in normal B cells, CD40 activation is known to regulate cell surface expression of immunomodulatory molecules, we therefore examined whether the percentage of CD40+ blasts at diagnosis corresponds to the capacity to modulate expression of the CD95 death receptor and the costimulatory molecules CD80/CD86. To this end, in a subset of patients (n = 34), blasts were cultured on feeder cells transgenically expressing CD40 ligand. First, we noted that CD40 activation reduced CD27 expression on the blasts of LR patients indicating that CD40 can indeed down-regulate the expression of other TNF receptor molecules. This down-regulation results in comparable low-level CD27 expression in both LR (median, range: 9%, 0%-36%) and HR (median, range: 8%, 2%-56%) patients alike (Figure 1C). In contrast, we found that CD95, expressed to negligible levels on native ALL blasts of both LR (median, range: 4%, 0%-26%) and HR (median, range: 6%, 0%-14%) patients, is significantly up-regulated following stimulation of blasts via CD40 (P < .01). In keeping with a higher percentage of CD40+ blasts in LR patients, this patient group also revealed an enhanced capacity to up-regulate CD95 upon CD40 ligation (median, range: LR: 77%, 17%-94% vs HR: 42%, 9%-95%; P = .012; Figure 1D). Accordingly, CD95 up-regulation strongly correlates with CD40 baseline expression (P = .009). When assessing modulation of the costimulatory molecules CD80 and CD86 upon CD40 activation, a significant increase was observed, yet there was no difference between the low- and high-risk groups either in baseline expression of CD80 (median, range: LR: 10%, 2%-19% vs HR: 8%, 1%-32%) and CD86 (median, range: LR: 33%, 2%-99% vs HR: 30%, 6%-79%) or in CD40-mediated up-regulation of the costimulatory molecules (median, range: CD80: LR: 46%, 5%-100% vs HR: 35%, 3%-89%; P = .287; CD86: LR: 80%, 5%-99% vs HR 83%, 15%-98%; P = .760).
Prognostic relevance of a very high percentage of CD40+ blasts at diagnosis
We also investigated whether in the entire cohort studied the level of CD27 and CD40 expression has an impact on clinical outcome. A very high percentage of CD40+ blasts at diagnosis above the median (94%) was predictive of superior clinical outcome (RFS; CD40 > median 0.86 ± 0.07 vs CD40 < median 0.72 ± 0.07; P = .049; Figure 2A). In contrast, a high expression level of CD27 at diagnosis, again defined as the level below or above the median of CD27+ blasts (70%), had no impact on relapse-free survival (RFS; CD27 > median 0.89 ± 0.06 vs CD27 < median 0.80 ± 0.06; P = .44; Figure 2B). However, even in the smaller subgroup of patients with a low percentage of CD27+ blasts, high CD40 expression remained a prognostic marker indicative of superior RFS (RFS in patients with CD27 < median; CD40 > median 0.96 ± 0.04 vs CD40 < median 0.70 ± 0.08; P = .03; Figure 2C). When multivariable Cox regression analysis was applied to determine the relative prognostic importance of the standard-risk parameters such as age, leukocyte count, PVA score, and CD40 (Table 2), a very high percentage of CD40+ blasts at diagnosis was independently associated with superior RFS (P = .018). In addition, the prognostic impact of CD40 was independent of CD27 when included in the multivariable analysis (P = .01).
A very high percentage of blasts positive for CD40 but not for CD27 is associated with improved relapse-free survival. Whereas there is no difference in relapse-free survival between patients with high and low CD27 expression (B), patients whose ALL blasts exhibit a very high percentage of CD40 expression at diagnosis as assessed by flow cytometry display significantly better relapse-free survival than patients with low CD40 expression (A). Even in the smaller subgroup of patients with a low percentage of CD27+ blasts at diagnosis, high CD40 expression remains a significant prognostic marker indicative of superior RFS (C). In each case the cutoff chosen for patient allocation to the TNFR “high” and “low” groups is the median of CD27+ or CD40+ blasts at diagnosis, respectively.
A very high percentage of blasts positive for CD40 but not for CD27 is associated with improved relapse-free survival. Whereas there is no difference in relapse-free survival between patients with high and low CD27 expression (B), patients whose ALL blasts exhibit a very high percentage of CD40 expression at diagnosis as assessed by flow cytometry display significantly better relapse-free survival than patients with low CD40 expression (A). Even in the smaller subgroup of patients with a low percentage of CD27+ blasts at diagnosis, high CD40 expression remains a significant prognostic marker indicative of superior RFS (C). In each case the cutoff chosen for patient allocation to the TNFR “high” and “low” groups is the median of CD27+ or CD40+ blasts at diagnosis, respectively.
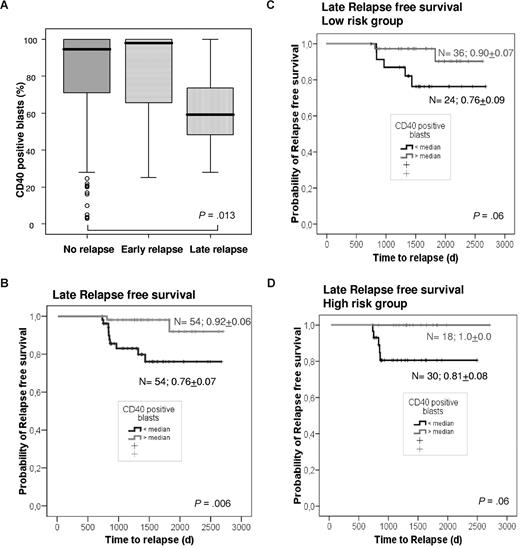
Also when testing CD40 expression above and below the median against the association to the clinically defined low- and high-risk group rather than the standard-risk factors age, leukocytes, and PVA score separately, CD40 expression (P = .07) but not risk group affiliation (P = .71) was predictive for relapse-free survival (Table 2). In contrast, for overall survival risk group affiliation (P = .06) but not CD40 expression (P = .41) impacts on outcome. Next we assessed the impact of high and low CD40 expression in high- and low-risk patients separately. In Kaplan-Meier analysis of relapse-free survival, an additional prognostic impact of CD40 expression was observed in the clinically defined high-risk group with superior relapse-free survival in patients with CD40 “high” versus “low” expression (P = .08). The impact of CD40 expression became even more apparent when focusing the analysis on late relapse. Of note, CD40 expression was significantly lower in patients who relapse late (> 2 years after diagnosis) than in patients without relapse (P = .013), whereas no difference was observed for patients who relapse early (Figure 3A). Moreover, when focusing the Kaplan-Meier analysis on patients with late relapse performing a landmark analysis excluding any patient who was not alive without relapse at 2 years after study entry, a high percentage of CD40+ blasts at diagnosis significantly protected against late relapse (P = .006; Figure 3B) even when analyzing high- and low-risk patients separately (P = .06 in both cases; Figure 3C,D). The same holds true in multivariable analysis: high CD40 expression selected for patients with a reduced risk for late relapse (P = .018), although clinical risk group association had no impact (P = .97; Table 2).
A very high percentage of blasts positive for CD40 is indicative of a specific decrease of late relapse. The percentage of BCP-ALL cells positive for CD40 surface expression as assessed by flow cytometry was found to be significantly different in patients with late relapse (> 730 days after diagnosis) compared with those remaining in remission (P = .013) with no difference for patients with early relapse (A). In a landmark analysis excluding any patient who is not alive without relapse at 2 years after study entry, high CD40 expression at diagnosis is associated with significantly better relapse-free survival (B). Even in the smaller, clinically defined subgroups of low-risk (LR; C) and high-risk (HR; D) patients, high CD40 expression remains a strong prognostic marker when focusing on late relapse. In the box plot diagrams the boxes represent the interquartile range with the line in the middle indicating the 50th percentile. The error bars, or whiskers, represent the highest and lowest values that are not outliers or extreme values.
A very high percentage of blasts positive for CD40 is indicative of a specific decrease of late relapse. The percentage of BCP-ALL cells positive for CD40 surface expression as assessed by flow cytometry was found to be significantly different in patients with late relapse (> 730 days after diagnosis) compared with those remaining in remission (P = .013) with no difference for patients with early relapse (A). In a landmark analysis excluding any patient who is not alive without relapse at 2 years after study entry, high CD40 expression at diagnosis is associated with significantly better relapse-free survival (B). Even in the smaller, clinically defined subgroups of low-risk (LR; C) and high-risk (HR; D) patients, high CD40 expression remains a strong prognostic marker when focusing on late relapse. In the box plot diagrams the boxes represent the interquartile range with the line in the middle indicating the 50th percentile. The error bars, or whiskers, represent the highest and lowest values that are not outliers or extreme values.
Thus, a very high percentage of CD40+ cells is an independent prognostic parameter that identifies a group of pediatric ALL patients with superior relapse-free survival. Conversely, patients with low or intermediate CD40 expression are at increased risk to suffer a relapse. Taken together, our observations underscore the notion that CD40 positivity of BCP-ALL blasts at diagnosis may serve as a predictor for a differential response to immune surveillance protecting against late relapse in particular.
Discussion
Leukemogenesis is facilitated by deregulated survival signals and loss of immunologic control mechanisms. Among the TNF receptor/ligand family of regulatory proteins,4-6,23,30,31 CD40 and CD27 are critically involved in B-cell maturation. Here we assessed the impact of these key regulators of B-cell responses in BCP-ALL in a prospective analysis including 121 pediatric patients from the CoALL06-97 study and identified a very high percentage of CD40+ blasts at diagnosis as an independent prognostic marker for superior relapse-free survival in children with BCP-ALL.
When analyzing distribution of CD40 expression in BCP-ALL, we found that despite a broad range of expression, almost all patients were CD40+, whereas CD27 was expressed on only half of the ALL samples. Patients classified as low risk by standard clinical parameters exhibited a significantly higher percentage of CD40+ as well as CD27+ blasts at diagnosis than high-risk patients. Although in the past, elevated expression of surface-bound CD27 as well as increased serum levels of soluble CD27 in BCP-ALL were reported,20 here we describe for the first time that high CD27 expression is associated with allocation to the clinically defined low-risk group. However, despite its correlation with clinical risk groups, CD27 expression did not have any impact on relapse-free survival, whereas very high percentage of CD40+ blasts seemed to protect against relapse. Indeed even in the absence of CD27 expression, very high CD40 expression is a favorable prognostic marker with regard to relapse-free survival. One explanation why high CD27 surface expression on ALL cells at diagnosis, although associated with low-risk classification, is not a strong indicator for improved outcome may be the fact that CD27 expression is highly modulated: Indeed, we could demonstrate that just as in mature normal and malignant B cells such as CLL32 and follicular lymphoma,33 CD40 activation of ALL blasts down-regulates CD27 expression. Moreover, CD27 expression on malignant B cells has been found to oscillate during different phases of the disease as shown by elevated levels in the acute phase of low-grade lymphoma.34 In addition, increased levels of soluble CD27 were detected in different B-cell malignancies, including ALL, which might suggest that shedding of the receptor may influence CD27 surface expression.20 Such variability during the course of disease might be another reason why, despite its pertinent regulatory role in B-cell differentiation, cell growth, and death,23,26,35-37 cell surface expression of CD27 at diagnosis does not serve as a parameter for outcome. Thus, when including CD27 and CD40 into the multivariable analysis analyzing the impact of TNFR and the classical clinical risk factors such as age, leukocyte count, and the PVA score, only a very high percentage of CD40+ blasts at diagnosis presents itself as an independent prognostic marker for relapse-free survival in children with BCP-ALL.
The prognostically favorable impact of high CD40 expression on relapse-free survival was also apparent when interrogating CD40 expression above and below the median against the clinically defined high- versus low-risk group rather than the component parts. In contrast, overall survival is not affected by CD40, as CD40 expression does not influence treatment-related complications. Instead, overall survival is determined primarily by affiliation to the clinically defined risk groups as risk-adapted treatment intensity, whereas reducing the relapse risk is associated with a higher rate of chemotherapy-related adverse events in high-risk patients.
Of note, the favorable prognosis in patients with high CD40 expression is due to a specific decrease in late relapse. Thus, the lowest percentage of CD40+ blasts at diagnosis in our cohort is observed in those patients who relapse late. Moreover, when performing a landmark analysis excluding any patient who was not alive without relapse at 2 years after study entry for calculation of relapse-free survival, a high percentage of CD40+ blasts at diagnosis significantly protects against late relapse even when analyzing high- and low-risk patients separately. Consequently, in multivariable analysis CD40 expression rather than risk group association features as an independent prognostic marker. These observations confirm the differential impact of risk group affiliation and CD40 expression on overall and relapse-free survival and suggest that response to immune surveillance mediated by CD40 expression may well contribute to a favorable prognosis.
There is growing evidence that for immunologic control, both adequate stimulation of effector cells as well as susceptibility of leukemic target cells to apoptotic signals are required. In normal B cell/T cell interactions, the CD40 receptor/ligand pair plays a pivotal role in both these aspects: In B cells, CD40 activation results in up-regulation of the costimulatory molecules CD80 and CD86, thus enhancing their antigen-presenting capacity.7,9,13,14,38 In addition, the CD95 TNF receptor molecule is up-regulated sensitizing activated B cells to apoptotic stimuli.7,10,11 It is well recognized that the same mechanisms take effect in malignant B cells. Thus in chronic lymphoblastic leukemia (CLL) it has been shown that CD40 ligation promotes the antigen-presenting capacity of leukemic blasts while at the same time inducing the death receptor CD95 (Fas).17-19 In a subgroup of our patients with CD40+ BCP-ALL, ligation of the CD40 receptor also results in significantly increased expression of the costimulatory molecules CD80 and CD86. This is in keeping with previous reports by us and others, documenting that in BCP-ALL the T-cell stimulatory capacity is enhanced following CD40 activation.3,39,40 Yet the capacity to up-regulate these costimulatory molecules following cross-linking of CD40 did not correlate with affiliation to the high- versus low-risk group. Instead there was a significant correlation between CD40 receptor–positive blasts at diagnosis and the capacity to up-regulate the death receptor CD95 following CD40 activation. Furthermore, we could demonstrate that the modulatory impact of CD40 on CD95 expression was significantly higher in low-risk patients reaching CD95 expression levels as high as found in normal CD40-activated B cells. Superior relapse-free survival in patients with very high percentage of CD40+ blasts may therefore result in part from enhanced death receptor expression with increased susceptibility to proapoptotic stimuli. Indeed, we have recently shown in BCP-ALL that CD40-induced up-regulation of CD95 results in significantly enhanced blast sensitivity to death receptor–induced apoptosis compared with nonstimulated blasts, even if sensitization does not reach the same levels of normal B cells due to concomitant modulation of antiapoptotic molecules.41
In summary, in this prospective analysis, we showed that in children and adolescents with BCP-ALL treated according to the CoALL06-97 protocol, a very high percentage of CD40+ blasts at diagnosis served as an independent prognostic marker to identify patients with a very favorable outcome. The observation that improved relapse-free survival in our patient cohort is particularly due to a specific decrease in late relapse underscores the notion that a differential response to immune surveillance may underlie the beneficial effects of high CD40 expression on prognosis. Although the capacity to up-regulate costimulatory molecules following CD40 activation might well contribute to the observed improvement in survival, enhanced up-regulation of the CD95 receptor seems to be the leading mechanism mediating sensitization toward apoptotic stimuli.41 The protective role of a high percentage of CD40+ blasts will need to be confirmed in a second cohort of ALL patients prior to using this marker to further refine treatment stratification. In addition, a better insight into the mechanisms involved in CD40-dependent modulation of blast maturation, cell growth, and death in ALL may facilitate the development of new targeted treatment strategies such as CD40-directed agonistic antibodies or CD40L-based immunotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Borkhardt for critical review. We also thank U. Wieczorek and S. Furlan for excellent technical assistance.
This study was supported by the Elterninitiative Kinderkrebs-klinik e.V. Duesseldorf (Duesseldorf, Germany).
Authorship
Contribution: A.T. designed research, analyzed data, and wrote the paper; L.G. designed and performed research, and analyzed data; B.A. performed research and analyzed data; G.E. provided vital materials and wrote the paper; R.M. supported statistical analysis; H.H. performed phenotyping; G.E.J.-S. provided vital materials and wrote the paper; M.L.B., R.P., and U.G. wrote the paper; H.-J.L. performed phenotyping and wrote the paper; and D.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anja Troeger, Clinic for Pediatric Oncology, Hematology and Clinical Immunology, University Hospital, Moorenstr. 5, 40225 Duesseldorf, Germany; e-mail: troeger@med.uni-duesseldorf.de.