Abstract
Thrombopoietin (Tpo), acting through the c-Mpl receptor, promotes the survival and proliferation of hematopoietic stem and progenitor cells and drives megakaryocyte differentiation. The proproliferation and survival signals activated by Tpo must therefore be tightly regulated to prevent uncontrolled cell growth. In this work, we determined the mechanisms that control Tpo-stimulated c-Mpl internalization and defined the processes leading to its degradation. Stimulation of BaF-Mpl cells with Tpo leads to rapid, clathrin-dependent endocytosis of the receptor. Using small interfering RNA (siRNA), we found that inhibition of adaptor protein 2 (AP2), which mediates endocytosis of transmembrane proteins, strongly attenuates Tpo-stimulated c-Mpl internalization. AP2 interacts with YXXΦ motifs and we identified 2 such motifs in c-Mpl (Y8RRL and Y78RRL) and investigated Tpo-stimulated internalization of receptors bearing point mutations at these sites. After Tpo stimulation, internalization was greatly reduced in c-Mpl Y78F and c-Mpl Y8+78F, and these cell lines also exhibited increased proliferation and increased strength and duration of Jak2, STAT5, AKT, and ERK1/2 activation in response to Tpo. We also found that the Y8RRL motif regulates Tpo-stimulated lysosomal degradation of c-Mpl. Our data establishes that c-Mpl cytoplasmic YRRL motifs are responsible for both Tpo-mediated internalization via interactions with AP2 and lysosomal targeting after endocytosis.
Introduction
Thrombopoietin (Tpo) is critical for the maintenance of hematopoietic stem and progenitor cells and is also the primary regulator of megakaryocyte development.1,2 The binding of Tpo to its receptor, c-Mpl, causes associated Janus kinase 2 (Jak2) activation, which in turn phosphorylates (activates) several downstream effectors including signal transducers and activators of transcription (STAT) 3 and 5, mitogen-activated protein kinase (MAPK), phosphotidylinositol-3-kinase (PI3-K), and protein kinase C (PKC).3-7 Activation of these pathways promotes proliferation and survival in c-Mpl–expressing cell lines and hematopoietic progenitor cells, in addition to megakaryocyte lineage differentiation and maturation.8-11 It is critical that Tpo signal transduction is stringently controlled to prevent uncontrolled proliferation. Suppressors of cytokine signaling (SOCS) proteins, phosphatases, and negative regulators such FAK, Lnk, and Lyn have all been shown to down-modulate Tpo-induced signaling.12-15 However, the most effective method of regulating Tpo signaling is by controlling expression of c-Mpl on the plasma membrane. Tpo-mediated c-Mpl endocytosis, recycling, and degradation are rapid mechanisms to control signaling longevity and represent a mechanism that regulates Tpo signaling without new protein expression.
The principal mechanism of receptor-mediated endocytosis in eukaryotic cells is the clathrin-coated vesicle.16 Soluble clathrin molecules self-assemble and are recruited to the plasma membrane, where they form lattice structures and interact with transmembrane receptors via adaptor proteins (APs), such as AP2, to form clathrin-coated pits.17,18 These pits then further invaginate before finally budding from the membrane to form clathrin-coated vesicles. AP2 is a heterotetramer composed of α2, β2, μ2, and σ2 subunits. The α2 and β2 subunits localize AP2 to the membrane, recruit endocytic accessory proteins, and bind clathrin heavy chain.19-21 Transmembrane proteins are associated with the AP2-clathrin complex via the μ2 domain, which binds directly to cytoplasmic YXXΦ (where X = any amino acid and Φ = bulky hydrophobic residue) and [DE]XXXL[IL] motifs.22,23 To ensure that AP2 specifically associates with membrane-bound proteins, phosphorylation of 156Thr in the μ2 subunit results in a conformational change in AP2, dramatically increasing the affinity of μ2 for YXXΦ motifs.24,25 156Thr is phosphorylated by adaptor-associated kinase 1 (AAK1),26 the activity of which is maximized by its association with clathrin,27,28 ensuring that AP2–cargo protein interactions are initiated only at the plasma membrane. In addition to being an endocytic signal, YXXΦ motifs located between 6 to 9 amino acids from the transmembrane domain mediate targeting of cargo protein to the lysosome and lysosome-like organelles via interactions with AP3.29-32
c-Mpl contains 2 YXXΦ motifs in its cytoplasmic domain at Tyr8 (Y8RRL; numbering is from the first cytoplasmic domain residue) and Tyr78 (Y78RRL). Previous findings suggest that Y78 acts as a negative regulator in a truncated (T-83) form of c-Mpl,33 although the function of this residue in a full-length receptor remains unclear. A role for Y8 in c-Mpl signaling has not been identified. In addition, c-Mpl contains 2 dileucine/isoleucine repeats at L54L55 and I57L58 in the cytoplasmic box2 region. Using a series of truncated receptors and point mutations, Dahlen and colleagues34 reported that c-Mpl requires L54L55 and I57L58 in addition to Y112 for Tpo-dependent internalization. In this work, we demonstrate the importance of AP2 in controlling c-Mpl internalization and determine the roles of the 2 YRRL motifs in the receptor after Tpo stimulation. We found that AP2 is essential for Tpo-stimulated clathrin-mediated c-Mpl internalization by reducing expression of the AP2α2 subunit using small interfering RNA (siRNA). By generating cells stably expressing c-Mpl with a Y-to-F point mutation at Y8 and Y78, we found that Y78RRL but not the Y8RRL motif mediated the AP2-Mpl interactions and is essential for receptor internalization. In addition, cells expressing the Y78F mutation exhibited increased proliferation and Jak2 and AKT phosphorylation in response to Tpo. Interestingly, although c-MplY8 appears not to be significantly involved in c-Mpl internalization, receptors bearing this mutation are not degraded as rapidly as wild-type forms and appear not to be targeted to the lysosome. Our findings provide novel insights into the regulation of Tpo signaling, and potentially that of other hematopoietic growth factors, via receptor internalization and degradation.
Methods
Chemicals and reagents
Pharmacologic inhibitors JakI, LY294002, SU6656, and U0126 where all purchased from Calbiochem (San Diego, CA). The clathrin inhibitor monodansylcadaverine (MDC), the proteosome inhibitor Z-LLL-al (ZL), and cycloheximide were purchased from Sigma-Aldrich (St Louis, MO). Extracellular-specific polyclonal rabbit anti–human c-Mpl was provided by Amgen Pharmaceuticals (Thousand Oaks, CA), and intracellular-specific polyclonal rabbit anti–human c-Mpl was purchased from Upstate (Lake Placid, NY). Monoclonal mouse anti-AP2α2 antibody was purchased from BD Biosciences (La Jolla, CA). Antibodies specific to phosphospecific and total Jak2, STAT5, AKT, and ERK1/2 were purchased from Cell Signaling Technologies (Danvers, MA). Monoclonal mouse antiactin was purchased from Sigma-Aldrich. Secondary antibodies goat anti–rabbit–horseradish peroxidase (HRP) and rabbit anti–mouse–HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and fluorescently labeled goat anti–rabbit-488 was purchased from Invitrogen (Carlsbad, CA). Membrane-impermeable N-hydroxysulfo-succinimidobiotin (sulfo-NHS-biotin) and neutravidin-coupled agarose beads were purchased from Pierce (Rockford, IL). Recombinant human (rh) Tpo was a gift from Don Foster (Zymogenetics, Seattle, WA).
Plasmid construction
Wild-type human c-Mpl was cloned into the retroviral expression vector pMX-puro using EcoRI and XhoI cloning sites. The c-Mpl intracellular domain point mutations (c-Mpl Y8F, c-Mpl Y78F, and c-Mpl Y8+78F) were introduced using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) with the following oligonucleotides: Y8F forward primer, 5′-CCTGCACACTTCAGGAGACTGAGGCAT-3′; Y8F reverse primer, 5′-ATGCCTCAGTCTCCTGAAGTGTGCAGG-3′; Y78F forward primer, 5′-CCAGATGGACTTCCGAAGATTGCAGCCTT-3′; and Y78F reverse primer, 5′-AAGGCTGCAATCTTCGGAAGTCCATCTGG-3′. Mutations were confirmed by sequencing.
Cell lines and culture conditions
The interleukin-3 (IL-3)–dependent prolymphoid cell line BaF3 and BaF3-Mpl clones were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS) supplemented with IL-3 (2 μL/mL conditioned medium from IL-3–producing baby hamster kidney cells). The retroviral packaging cell line Platinum-E (Plat-E) was maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS. To generate BaF3-Mpl clones, subconfluent Plat-E cells were transfected with pMX-puro-c-Mpl constructs using FuGENE 6 transfection reagent (Roche Applied Sciences, Indianapolis, IN) for 48 hours before viral supernatant collection. BaF3 parental cells were then incubated in viral supernatant supplemented with IL-3 for 48 hours before selection with puromycin (2 μg/mL). Clonal populations were generated by limiting dilution. Total c-Mpl protein expression was determined by Western blot and cell-surface expression by flow cytometry.
RNA interference
The previously described AP2α2 subunit siRNA target sequence AAGAGCAUGUGCACGCUGGCCA35 and the DY547-labeled negative control were purchased from Dharmacon (Chicago, IL). BaF3-Mpl cells were transfected using an Amaxa nucleofector (Gaithersburg, MD) according to the manufacturer's protocol. Briefly, 4 × 106 BaF3-Mpl cells were resuspended in solution V (Amaxa) containing 2 μg of either negative control or AP2α2 siRNA and electroporated using the program X-001. SiRNA efficiency was determined by Western blot analysis for AP2α2 subunit after 24, 48, and 72 hours. Maximal knockdown occurred 48 hours after transfection.
Western blotting
After chemical treatment or Tpo stimulation, BaF3-Mpl cell lines were washed 3 times with cold phosphate-buffered saline (PBS) and lysed in NP-40 buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and 1 mM NaF) containing 1% protease inhibitors (Sigma-Aldrich). Denatured proteins were fractionated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and transferred to PVDF membranes. Protein expression was detected by incubating with specific antibodies and visualized by chemiluminescent detection reagent (ECL-plus; GE Healthcare, Little Chalfont, United Kingdom).
Internalization assays
BaF3-Mpl clones were starved of IL-3 for 2 hours before stimulation with 50 ng/mL rhTpo for 0, 10, 30, and 60 minutes. Cells were washed 3 times in cold PBS before biotinylation of the extracellular domains of membrane-bound proteins with 5 mg/mL sulfo-NHS-biotin for 30 minutes at 4°C, a cell-impermeable biotinylation reagent. Further protein biotinylation was prevented by washing the cells 3 times with cold PBS containing 100 mM glycine. Cells were lysed in NP-40 buffer, and protein concentrations were determined using the BCA protein assay kit (Pierce). Biotinylated proteins were pulled down using neutravidin-conjugated agarose beads, proteins were denatured by adding SDS-loading dye and boiling for 5 minutes, and c-Mpl expression was analyzed by Western blotting.
MTT proliferation assay
BaF3-Mpl clones in log-phase growth were washed 3 times with PBS to remove IL-3, resuspended in RPMI 1640 supplemented with 2% FBS, and plated into 96-well plates at a concentration of 1000 cells per well before rhTpo was added at concentrations ranging from 1 pg/mL to 10 ng/mL. Cells were incubated for 48 hours before treatment with 1 mg/mL MTT reagent (Sigma-Aldrich) and incubation at 37°C for 3 hours. Cells were lysed, formazen crystals were dissolved in 100 μL MTT lysis solution (dH2O, 20% SDS, 40% dimethyl formamide (DMF), 2% acetic acid, and 0.2% HCl) for 6 hours at 37°C, and absorbance was read on a colorimetric plate reader at 570 nm. Each data point is expressed as a percentage of proliferation stimulated by a maximal dose of murine IL-3 (4 μL/mL murine IL-3 supernatant). Each experiment was performed in triplicate with 3 different clones for each wild-type or mutant Mpl construct.
Flow cytometry
Tpo-stimulated BaF3-Mpl cells were washed in cold labeling buffer (PBS with 0.5% bovine serum albumin) and fixed in cold 2% paraformaldehyde for 5 minutes. Cells were then labeled with primary antibody specific to the extracellular domain of c-Mpl for 30 minutes at 4°C. Unbound antibody was removed by washing, and cells were incubated for 30 minutes at 4°C with goat anti–rabbit-488 fluorescent secondary antibody. Cells were then washed and analyzed using a FACSCalibur flow cytometer and CellQuest Pro analysis software (BD Biosciences).
Results
Tpo-induced internalization of c-Mpl is mediated by SFK signaling
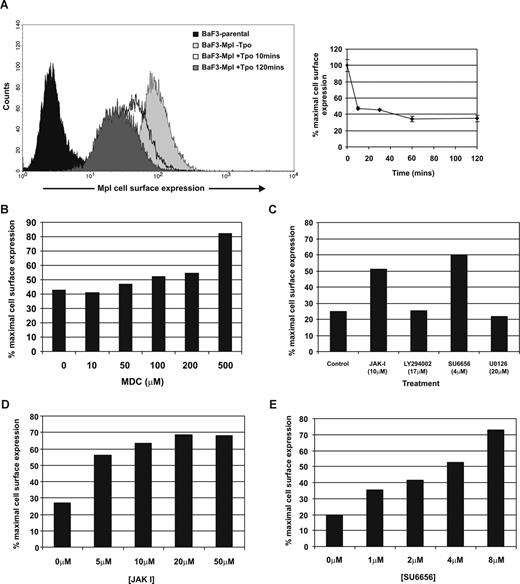
Using BaF3 cells expressing full-length human c-Mpl (BaF3-Mpl), we studied the kinetics of receptor internalization after stimulation with Tpo. Flow cytometric analyses demonstrated that c-Mpl internalization was near maximal (reduction to 46% [± 1.2] of normal cell-surface expression) 10 minutes after Tpo stimulation and maximal after 60 minutes (to 34.3% [± 3.2] of baseline; Figure 1A). These kinetics are comparable with those previously published.34 To determine whether c-Mpl internalization was dependent on clathrin-mediated endocytosis, cells were pretreated with an inhibitor of clathrin-coated pit formation, MDC.36 MDC addition inhibited c-Mpl internalization in a concentration-dependent manner, with maximal attenuation achieved at 500 μM (Figure 1B), indicating that Tpo-induced receptor internalization is dependent on the formation of clathrin-coated pits. To further determine the intracellular signaling mechanisms responsible for c-Mpl internalization, we used pharmacologic inhibitors to several kinases activated by Tpo, including Jak2, Jak and Src family kinases (SFKs), PI3-K, and ERK1/2. Inhibitors to Jak2 and SFKs (JAK I and SU6656, respectively) greatly reduced Tpo-stimulated c-Mpl internalization, while inhibitors to PI3-K and ERK1/2 had no effect (Figure 1C). A concentration-dependent inhibition of c-Mpl internalization by preincubation with JAK I and SU6656 was further demonstrated (Figure 1D,E).
Tpo-mediated internalization of c-Mpl is dependent on clathrin, Jak2 and SFKs. (A) BaF-Mpl cells were stimulated with 50 ng/mL rhTpo for increasing periods of time, and cell-surface expression of c-Mpl was analyzed by flow cytometry. Error bars represent SE. Receptor internalization was determined in the presence of inhibitors to clathrin (B) and a panel of protein kinases (C). (D,E) The effects of JAKI and SU6656 were further determined by concentration response. The percentage of baseline c-Mpl cell-surface expression is determined by comparing Tpo-treated cells with unstimulated cells. All data are representative of at least 3 independent experiments using 2 BaF-Mpl clones.
Tpo-mediated internalization of c-Mpl is dependent on clathrin, Jak2 and SFKs. (A) BaF-Mpl cells were stimulated with 50 ng/mL rhTpo for increasing periods of time, and cell-surface expression of c-Mpl was analyzed by flow cytometry. Error bars represent SE. Receptor internalization was determined in the presence of inhibitors to clathrin (B) and a panel of protein kinases (C). (D,E) The effects of JAKI and SU6656 were further determined by concentration response. The percentage of baseline c-Mpl cell-surface expression is determined by comparing Tpo-treated cells with unstimulated cells. All data are representative of at least 3 independent experiments using 2 BaF-Mpl clones.
AP2 is essential for Mpl internalization
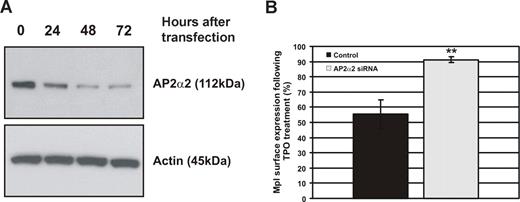
To determine whether AP2 is required for Tpo-induced c-Mpl internalization, BaF3-Mpl cells were transfected with siRNA specific to the α2 subunit of AP2. Western blot analysis of whole-cell lysates after treatment with siRNA demonstrated an approximate 4-fold decrease in AP2α2 protein expression after 48 hours (Figure 2A). For internalization studies, BaF3-Mpl cells were transfected with AP2α2 siRNA for 48 hours before stimulation with 50 ng/mL Tpo, before c-Mpl expression was analyzed by flow cytometry. AP2α2-depleted cells exhibited greatly reduced c-Mpl internalization in response to Tpo; c-Mpl expression was reduced to 55.3% (± 9.6%) of baseline expression in control cultures, whereas AP2α2 siRNA–treated cell c-Mpl expression was reduced only to 91.2% (± 1.9%) of baseline maximal expression (Figure 2B).
c-Mpl internalization is reduced in AP2α2-depleted cells. BaF-Mpl cells were transfected with siRNA specific to the AP2α2 subunit, which is essential for the function of AP2. (A) AP2α2 depletion was analyzed by Western blot and was maximal 48 hours after transfection (approximately 75% depletion). (B) Tpo-stimulated c-Mpl internalization was then determined by flow cytometry in AP2α2-depleted BaF-Mpl cells 48 hours after transfection (**P < .01). Error bars represent SD.
c-Mpl internalization is reduced in AP2α2-depleted cells. BaF-Mpl cells were transfected with siRNA specific to the AP2α2 subunit, which is essential for the function of AP2. (A) AP2α2 depletion was analyzed by Western blot and was maximal 48 hours after transfection (approximately 75% depletion). (B) Tpo-stimulated c-Mpl internalization was then determined by flow cytometry in AP2α2-depleted BaF-Mpl cells 48 hours after transfection (**P < .01). Error bars represent SD.
c-Mpl internalization is mediated via an AP2-Y78RRL motif interaction
The AP2μ2 domain has previously been shown to interact with YXXΦ motifs on transmembrane receptors and drive clathrin-mediated receptor endocytosis.22 To determine whether the 2 YRRL motifs in the cytoplasmic domain of c-Mpl are required for AP2-mediated internalization, we generated 4 expression-matched BaF3-Mpl cell lines stably expressing either wild-type or Y-to-F point mutations at cytoplasmic Y8, Y78, and both Y8 and Y78 (Figure 3A). In the absence of Tpo, there was no significant difference in cell-surface expression of c-Mpl between clones when analyzed by flow cytometry (data not shown).
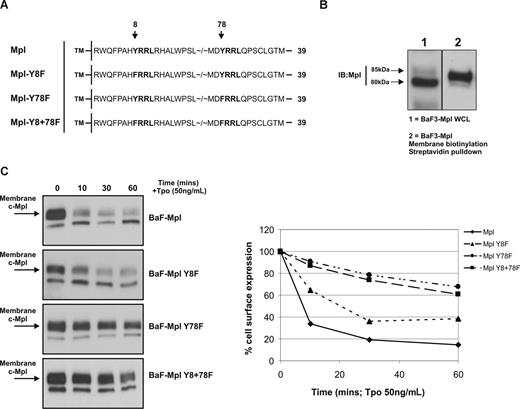
Cytoplasmic Y78 is essential for c-Mpl internalization. (A) Human c-Mpl cytoplasmic amino acid sequence is shown with the 2 potential AP2 interaction motifs highlighted in bold. A total of 3 constructs were made with Y → F point mutations at Y8, Y78, and both Y8 and Y78. (B) Biotinylation of membrane proteins demonstrates expression of the larger (approximately 85 kDa) mature form of c-Mpl at the at the cell surface (WCL indicates whole-cell lysate). (C) The effects of Tpo stimulation on receptor surface expression in each of the clones was determined by membrane biotinylation and Western blot analysis. Internalization was quantified by densitometry—comparing expression of membrane-localized c-Mpl (85 kDa) to Tpo-starved cells (0 minutes). The data are representative of 3 independent experiments.
Cytoplasmic Y78 is essential for c-Mpl internalization. (A) Human c-Mpl cytoplasmic amino acid sequence is shown with the 2 potential AP2 interaction motifs highlighted in bold. A total of 3 constructs were made with Y → F point mutations at Y8, Y78, and both Y8 and Y78. (B) Biotinylation of membrane proteins demonstrates expression of the larger (approximately 85 kDa) mature form of c-Mpl at the at the cell surface (WCL indicates whole-cell lysate). (C) The effects of Tpo stimulation on receptor surface expression in each of the clones was determined by membrane biotinylation and Western blot analysis. Internalization was quantified by densitometry—comparing expression of membrane-localized c-Mpl (85 kDa) to Tpo-starved cells (0 minutes). The data are representative of 3 independent experiments.
Using a membrane-impermeable biotinylation reagent and a streptavidin pull-down strategy, we were able to accurately determine expression of c-Mpl on the cell surface after Tpo stimulation in the BaF3-Mpl wild-type and mutant clones. Previous findings suggest that posttranslational modifications of c-Mpl give rise to a more “mature” form of the receptor, which is expressed at the cell membrane.37 In accordance with this finding, Western blot analyses of human c-Mpl–bearing cell lines display 2 specific protein bands, one migrating at 80 kDa and a second at 85 kDa, the latter of which represents the mature cell-surface form of the c-Mpl receptor (Figure 3B). We measured the intensity of the 85-kDa band to evaluate Tpo-induced Mpl internalization in the BaF3-Mpl clones. Cells were stimulated with 50 ng/mL rhTpo for 10, 30, and 60 minutes before membrane biotinylation, lysis, and streptavidin pull-down. Levels of internalization were quantified by densitometry of the 85-kDa membrane-localized c-Mpl band in Tpo-stimulated cells compared with unstimulated controls. Wild-type c-Mpl was rapidly internalized in response to Tpo, with cell-surface expression reduced to approximately 33% of baseline after 10 minutes and to 14% after 60 minutes (Figure 3C). c-Mpl Y8F–expressing cells exhibited slightly reduced internalization in response to Tpo compared with wild-type receptor–bearing cells (surface expression reduced to 64% of baseline after 10 minutes and to 38% after 60 minutes). In contrast, Y78F c-Mpl–bearing cells exhibited greatly blunted internalization in response to Tpo, with cell-surface expression remaining at 90% of baseline after 10 minutes and 68% after 60 minutes. c-Mpl internalization kinetics of Mpl Y8+78F cells were similar to those of Mpl Y78F cells.
c-Mpl Y78F exhibits enhanced Tpo-mediated proliferation and signaling
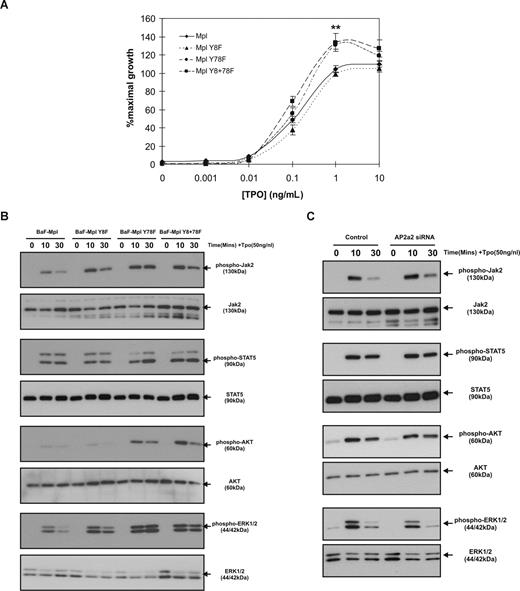
Our previous studies identified Y78 in the cytoplasmic domain of c-Mpl as a potential negative regulator of Tpo proliferation,33 although these findings were made using a truncated receptor, missing the distal 2 tyrosine residues. In the current study, we sought to determine the role of Y78 in the context of the full-length receptor and assess whether our findings regarding c-Mpl internalization had any effects on Tpo-mediated proliferation and signaling. MTT assays were performed using 3 clones from each receptor type: wild-type, Y8F, Y78F, and Y8+78F. All of the clones analyzed displayed comparable levels of c-Mpl expression as determined by Western blot analysis (data not shown). rhTpo induced a concentration-dependent increase in cell proliferation in all clones, although cells bearing c-Mpl with the Y78F mutation displayed significantly greater maximal proliferation. Using 1 ng/mL rhTpo, BaF3/Mpl cells proliferated at 104% (± 3.9%) of IL-3–stimulated controls, whereas c-Mpl Y78F cells proliferated to 133% (± 4.4%) of IL-3–stimulated controls (Figure 4A). c-Mpl Y8F was not significantly different from c-Mpl wild-type receptor–bearing cells, and c-Mpl Y8+78F was not significantly different from c-Mpl Y78F. To determine the effects of the Y8F and Y78F mutations on Tpo-induced signaling, cells expressing the different forms of the receptor were starved for 18 hours before stimulation with rhTpo (50 ng/mL) for 0, 10, and 30 minutes. Cells bearing the Y78F mutation showed moderately increased phosphorylation of Jak2 and STAT5 and increased longevity of signal and greatly increased phosphorylation of AKT and ERK1/2 (Figure 4B). Signaling in the c-Mpl Y8F mutation was similar to wild-type c-Mpl control cells. To determine whether the increased Tpo-stimulated signaling was as a result of reduced internalization of c-Mpl observed with the Y78F mutation, we treated wild-type c-Mpl–expressing cells with AP2α2 siRNA to inhibit endocytosis. Interestingly, we found that signaling was not affected by knocking down AP2α2 protein expression (Figure 4C), even to levels that blunted c-Mpl Y78F receptor internalization, suggesting that attenuating receptor internalization may not significantly affect Tpo signaling in these cells.
The Y78F mutation leads to increased Tpo-mediated proliferation and signaling. (A) MTT proliferation assays were performed on c-Mpl wild-type–, Y8F-, Y78F-, and Y8+78F-expressing cells. Data points represent the mean of 3 independent experiments using 3 different c-Mpl–expressing clones (**P < .01). Error bars represent SE. (B) Levels of phosphorylated Jak2, STAT5, AKT, and ERK1/2 in response to 50 ng/mL Tpo were determined by Western blot analyses. (C) Tpo-stimulated activation of Jak2, STAT5, AKT, and ERK1/2 in wild-type c-Mpl–expressing cells treated with control or AP2α2 siRNA. Data are representative of 3 independent experiments.
The Y78F mutation leads to increased Tpo-mediated proliferation and signaling. (A) MTT proliferation assays were performed on c-Mpl wild-type–, Y8F-, Y78F-, and Y8+78F-expressing cells. Data points represent the mean of 3 independent experiments using 3 different c-Mpl–expressing clones (**P < .01). Error bars represent SE. (B) Levels of phosphorylated Jak2, STAT5, AKT, and ERK1/2 in response to 50 ng/mL Tpo were determined by Western blot analyses. (C) Tpo-stimulated activation of Jak2, STAT5, AKT, and ERK1/2 in wild-type c-Mpl–expressing cells treated with control or AP2α2 siRNA. Data are representative of 3 independent experiments.
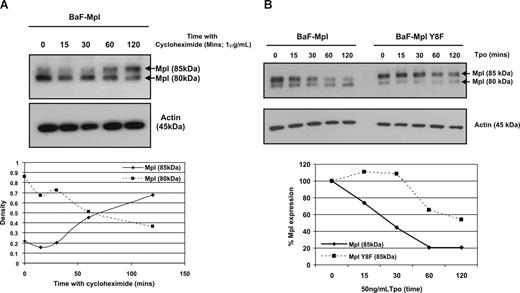
c-Mpl lysosome targeting is mediated by the Y8RRL motif
It has previously been demonstrated that YXXΦ motifs localized between 6 and 9 residues from the transmembrane domain mediate lysosomal targeting of internalized receptors.31,32 Consequently, we assessed whether the c-Mpl Y8RRL motif was involved in targeting the internalized receptor for lysosomal degradation. To accurately determine the effects on receptor degradation, we treated BaF-Mpl cells with the protein synthesis inhibitor cycloheximide (1 μg/mL) for a range of time periods from 0 to 120 minutes; c-Mpl expression was then analyzed by Western blot and the ratio of 80-kDa (“immature”) to 85-kDa (“mature”) Mpl determined by densitometry (Figure 5A). In the absence of cycloheximide, the majority (approximately 80%) of Mpl exists as an 80-kDa form. In the presence of cycloheximide, the mature form (85 kDa) becomes increasingly predominant with time. In the subsequent experiments, cells were treated with cycloheximide for 60 minutes, having determined that after this period of treatment, synthesis of new protein had been prevented without significant cell death (data not shown). Cycloheximide-treated cells were stimulated with rhTpo (50 ng/mL) for 0 to 120 minutes before the level of c-Mpl degradation was determined by Western blot analysis and densitometry. In wild-type c-Mpl–expressing cells, Tpo stimulation resulted in a steady degradation of the receptor (Figure 5B), which was maximal after 60 minutes (reduction to approximately 20% of normal c-Mpl expression). However, in Y8F c-Mpl–expressing cells, receptor degradation only occurred after 30 minutes of Tpo treatment; after 60 minutes, the level of degradation was significantly lower than for wild-type c-Mpl (reduced to only approximately 65% of normal c-Mpl expression). These findings strongly suggest a role for Y8RRL in mediating c-Mpl degradation.
Cytoplasmic Y8 is important in Tpo-induced c-Mpl degradation. (A) Wild-type c-Mpl–expressing cells were treated for increasing periods of time with 1 μg/mL cycloheximide to inhibit the synthesis of new protein. The bottom panel represents densitometry of the 2 different forms of c-Mpl compared with actin. The data are representative of 2 independent experiments. (B) Wild-type and c-Mpl Y8F–expressing cells were pretreated with 1 μg/mL cycloheximide for 60 minutes before stimulation with Tpo (50 ng/mL) for increasing periods of time. Degradation of the mature 85-kDa form of c-Mpl was determined by Western blot and density compared with unstimulated cells. The data are representative of 4 independent experiments.
Cytoplasmic Y8 is important in Tpo-induced c-Mpl degradation. (A) Wild-type c-Mpl–expressing cells were treated for increasing periods of time with 1 μg/mL cycloheximide to inhibit the synthesis of new protein. The bottom panel represents densitometry of the 2 different forms of c-Mpl compared with actin. The data are representative of 2 independent experiments. (B) Wild-type and c-Mpl Y8F–expressing cells were pretreated with 1 μg/mL cycloheximide for 60 minutes before stimulation with Tpo (50 ng/mL) for increasing periods of time. Degradation of the mature 85-kDa form of c-Mpl was determined by Western blot and density compared with unstimulated cells. The data are representative of 4 independent experiments.
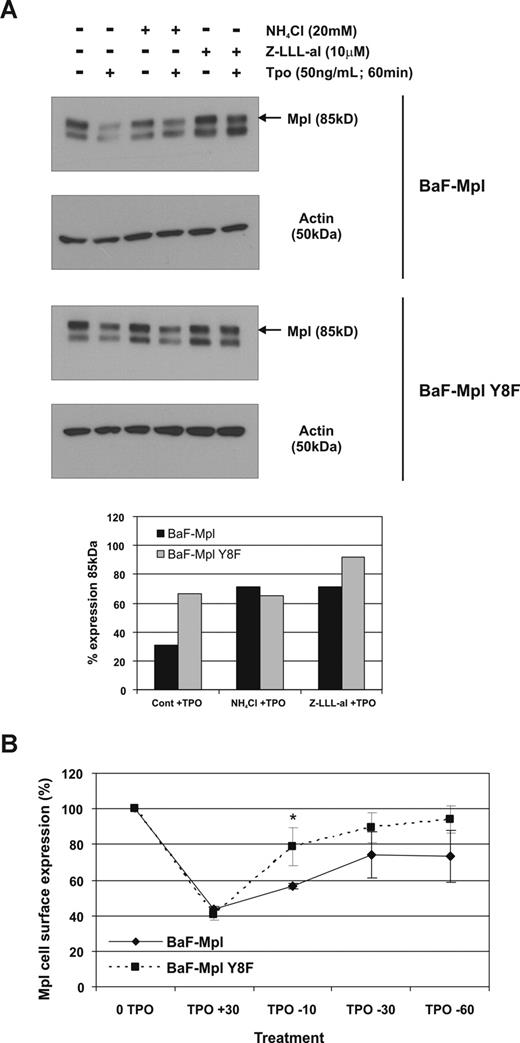
To determine whether the Y8RRL motif is involved in lysosomal targeting and degradation rather than proteasomal degradation, cycloheximide-treated wild-type and Y8F c-Mpl–expressing cells were pretreated with lysosome (NHCl4) and proteasome (Z-LLL-al) inhibitors before Tpo stimulation. Both NHCl4 and Z-LLL-al inhibited receptor degradation after Tpo stimulation in wild-type c-Mpl–expressing cells (Figure 6A). However, in Y8F Mpl–expressing cells, NHCl4 had no effect on degradation, while Z-LLL-al treatment alone almost completely aborted c-Mpl degradation, indicating that Y8RRL is involved in lysosome targeting but not in proteasomal degradation. If the mutation in Y8F c-Mpl–bearing cells destroys the lysosome targeting motif, the receptor would be expected to build up in the early endosome, and be available for rapid recycling. To determine whether this hypothesis was correct, Y8F c-Mpl cells were stimulated with Tpo for a limited period to induce receptor internalization before Tpo was removed to promote recycling of the receptor back to the membrane. Y8F c-Mpl recycled back to the membrane significantly more rapidly than wild-type (Figure 6B), further indicating a failure of Y8F-Mpl to target to lysosomes.
The Y8F mutation inhibits lysosome targeting of c-Mpl. (A) Cells expressing wild-type or c-Mpl Y8F were pretreated with cycloheximide for 30 minutes with or without lysosome or proteasome inhibitors for an additional 30 minutes before Tpo stimulation for 60 minutes. Receptor expression was determined by Western blot and degradation was quantified by comparing the density of cells with or without Tpo. The data represent 3 independent experiments. (B) Flow cytometry for surface c-Mpl expression of wild-type and c-Mpl Y8F–expressing cell lines after Tpo stimulation, followed by Tpo removal. The data represent mean of 3 independent experiments. Error bars represent SD.
The Y8F mutation inhibits lysosome targeting of c-Mpl. (A) Cells expressing wild-type or c-Mpl Y8F were pretreated with cycloheximide for 30 minutes with or without lysosome or proteasome inhibitors for an additional 30 minutes before Tpo stimulation for 60 minutes. Receptor expression was determined by Western blot and degradation was quantified by comparing the density of cells with or without Tpo. The data represent 3 independent experiments. (B) Flow cytometry for surface c-Mpl expression of wild-type and c-Mpl Y8F–expressing cell lines after Tpo stimulation, followed by Tpo removal. The data represent mean of 3 independent experiments. Error bars represent SD.
Discussion
In this study, we demonstrate a role for AP2 in mediating Tpo-stimulated c-Mpl internalization and determined that Y78RRL is an AP2μ2-cytoplasmic binding motif in the receptor. In addition, we have shown that an identical sequence located close to the transmembrane domain (Y8RRL) appears to target the receptor for lysosomal degradation after Tpo stimulation. Consistent with previous studies, we have shown that the Y78F mutation results in increased Tpo-induced proliferation and further demonstrate that this mutation also leads to increased activity of Jak2 and AKT, which indicates a possible mechanism for the increase in cell numbers. These findings suggest a critical role for Y8 and Y78 in the cytoplasmic domain of c-Mpl in controlling surface expression and receptor degradation after Tpo stimulation, which is highly significant considering the importance of tightly regulating the response to Tpo.
Although we have been able to show the importance of AP2 and the predicted AP2-binding motif in controlling c-Mpl internalization, we have been unable to demonstrate direct protein-protein interactions between the receptor and the AP2μ2 subunit by coimmunoprecipitation (data not shown). Interestingly, this finding is similar to previous studies with the transferrin receptor (TfR). Both AP2 and the YTRF motif in the cytoplasmic domain of the TfR are critical to its internalization, but no direct interaction between AP2 and TfR could be detected.38,39 Although we were unable to show a direct interaction, having demonstrated that AP2 and Y78RRL are both vital for c-Mpl internalization, we hypothesize that the receptor interacts with AP2 at this site to mediate Tpo-induced endocytosis. It is possible that the AP2–c-Mpl interaction is too weak and/or transient to be detected by coimmunoprecipitation, and it may be feasible to identify these interactions by other methods, for example, fluorescence resonance energy transfer (FRET) with confocal microscopy.
The position of the YXXΦ motif in the cytoplasmic domain is thought to be of great importance to its function. Purely endocytic YXXΦ sequences are commonly considered to lie within 10 to 40 residues from the transmembrane domain.22 The c-Mpl endocytic motif at Y78RRL is therefore considerably further away than in other transmembrane receptors. Whether this is exclusive to c-Mpl, or whether it also occurs in other hematopoietic growth factor receptors, is currently unknown.
Regulation of AP2 localization and binding is mediated by PI(4,5)P240 and AAK-1 kinase activity.26,27 PI(4,5)P2 interacts via 2 binding sites on AP2, one on the α-subunit to dock the complex at the plasma membrane,19 and the other on the μ-subunit.41 AAK-1 increases the affinity of the μ-subunit to bind its target cargo by phosphorylation of 156Thr. We have shown that Tpo is able to directly cause the internalization of its receptor via activation of Jak2 and SFK signaling cascades, although the downstream effectors remain unclear. Interestingly, a previous finding that c-Mpl Y112 mediates receptor internalization34 could also support a potential role for SFKs in regulating c-Mpl endocytosis, as this residue has previously been shown to regulate Tpo-stimulated Lyn kinase activity.42 The role of SFKs in regulating receptor internalization has been studied previously with differing results. For example, the SFK inhibitor SU6656 fails to attenuate internalization of epidermal growth factor receptor (EGFR),43 but previous studies on the stem cell factor receptor c-Kit suggest that SFKs are vital for internalization and degradation of the receptor.44,45 We have demonstrated that similar to c-Kit, SFKs are important in regulating c-Mpl internalization. In addition, we cannot rule out the possibility that c-Mpl Y78 is involved in the activation of another signaling pathway that mediates receptor internalization, in addition to interacting with AP2. Whether the Tpo-mediated stimulation of certain signaling pathways causes changes in AP2 structure and/or phosphorylation, for example by increasing AAK-1 kinase activity, is currently being investigated.
Although we demonstrate significant Tpo signaling alterations in the cells expressing the c-Mpl Y78F mutation, our finding that signaling was not affected after AP2α2 siRNA treatment strongly suggests that the differences in signaling intensity and duration between wild-type c-Mpl and c-Mpl Y78F are due to effects not directly linked with receptor endocytosis. Relatively little is known about the role of the Y78 residue in mediating Tpo signaling, although our data strongly support the previous findings that identified a potential negative regulatory role.33 Considering the increase in the extent of protein phosphorylation and longevity of activation, which was particularly evident in AKT and ERK1/2, it is possible that the c-MplY78F mutation affects the activity of one or more phosphatases, for example the protein tyrosine phosphatases SHP1 or SHP2, which is activated in response to Tpo.6 Future experiments aimed at identifying Y78-interacting proteins and the role of Y78 in the activation of specific protein phosphates will elucidate the exact nature of this negative regulation.
As well as identifying a novel c-Mpl internalization motif, we have also described a potential lysosome-targeting motif at Y8RRL. The location of this motif is consistent with previous findings that dileucine or YXXΦ motifs located 6 to 9 residues from the transmembrane domain can target proteins to the lysosome for degradation.22,29 Studies of lysosome-associated membrane proteins (LAMPs) and lysosome integral membrane proteins (LIMPs) have highlighted AP3 as the prime candidate for association with lysosome-targeting motifs to mediate the transport of protein to the lysosomes.32,46 Therefore, internalized c-Mpl is likely routed to the lysosomes via interactions between AP3 and Y8RRL, and this interaction may be responsible for the rapid degradation of c-Mpl after Tpo stimulation. Although recent colocalization studies suggested that c-Mpl is not present in lysosomes,47 we have found that the Tpo-stimulated rapid degradation of the receptor can be significantly inhibited by NHCl4, strongly suggesting that there is in fact a role for lysosomal degradation in controlling c-Mpl expression.
This work is the first to describe the YXXΦ motif for receptor internalization and lysosome-targeting in the hematopoietic growth factor receptor (HGFR) family. Importantly, we have recently identified that potential AP2-interaction motifs are also present in a number of other receptors. For example, the erythropoietin receptor (EpoR), which shares significant homology with c-Mpl, contains one [DE]XXXL[IL] motif (D78EGPLL) and several YXXΦ motifs (starting at Y93, Y153, Y181, and/or Y183). Interestingly, granulocyte colony-stimulating factor receptor (G-CSFR) contains 2 YXXΦ motifs, one of which is deleted by a common truncation in severe congenital neutropenia.48 Future study of these and other potential HGFR internalization motifs may well prove important in understanding receptor clearance and degradation.
Our studies provide a novel insight into the mechanisms that regulate Tpo signaling. Interest surrounding the function of c-Mpl in controlling hematopoietic progenitor cell expansion and megakaryopoiesis has recently intensified after the discovery of the Jak2V617F mutation and its role in the myeloproliferative diseases polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) (reviewed in Levine et al49 ). Given the emerging link between uncontrolled receptor signaling and disease states, it has become even more important to determine the precise mechanisms controlling the clearance and degradation of c-Mpl and other HGFRs.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by National Institutes of Health grants R01DK49855, R01 CA31615, and P01 HL078784-04.
National Institutes of Health
Authorship
Contributions: All authors have substantially contributed to the content of the paper and have agreed to the submission in its current format. I.S.H. designed and performed research, analyzed data, and wrote the manuscript; M.M.C. and J.R.K. performed research and analyzed data; and K.K. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ian S. Hitchcock, Department of Medicine, University of California–San Diego, 9500 Gilman Drive, La Jolla, CA 92093; e-mail: ihitchcock@ucsd.edu.