Abstract
We report the outcomes of 24 patients with high-risk hematologic malignancies or bone marrow failure (BMF) who received haploidentical bone marrow transplantation (BMT) after ex vivo induction of alloantigen-specific anergy in donor T cells by allostimulation in the presence of costimulatory blockade. Ninety-five percent of evaluable patients engrafted and achieved full donor chimerism. Despite receiving a median T-cell dose of 29 ×106/kg, only 5 of 21 evaluable patients developed grade C (n = 4) or D (n = 1) acute graft-versus-host disease (GVHD), with only one attributable death. Twelve patients died from treatment-related mortality (TRM). Patients reconstituted T-cell subsets and immunoglobulin levels rapidly with evidence of in vivo expansion of pathogen-specific T cells in the early posttransplantation period. Five patients reactivated cytomegalovirus (CMV), only one of whom required extended antiviral treatment. No deaths were attributable to CMV or other viral infections. Only 1 of 12 evaluable patients developed chronic GVHD. Eight patients survive disease-free with normal performance scores (median follow-up, 7 years). Thus, despite significant early TRM, ex vivo alloanergization can support administration of large numbers of haploidentical donor T cells, resulting in rapid immune reconstitution with very few viral infections. Surviving patients have excellent performance status and a low rate of chronic GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) offers a curative approach for many patients with hematologic malignancies and certain nonmalignant hematologic conditions. Although the majority of such patients will lack fully HLA-matched related donors, and many will also lack available HLA-matched unrelated donors, almost all will have available HLA-mismatched related donors.1 However, HLA-mismatched HSCT is associated with increased graft failure, graft-versus-host disease (GVHD), and a higher risk of treatment failure.2 GVHD is mediated predominantly by alloreactive donor T cells, which expand in vivo posttransplantation. Nonselective T-cell depletion (nsTCD) of donor grafts is an efficient method for reducing alloreactivity and is very effective at preventing GVHD. Although nsTCD has been combined with high CD34+ stem cell doses to achieve engraftment without severe GVHD in haploidentical HSCT,3,4 the overall success of such strategies may be limited by delayed immune reconstitution, increased infectious complications, and higher relapse rates potentially associated with the loss of pathogen- and tumor-specific T cells.5-7
Various experimental strategies to selectively deplete alloreactive T cells within the donor graft to prevent GVHD while preserving pathogen- and tumor-specific immunity have been developed with the aim of improving immune reconstitution after HLA-mismatched HSCT. Most of these approaches use a common mechanistic platform of ex vivo donor T-cell stimulation by recipient alloantigens and subsequent removal or destruction of alloreactive T cells, identified by expression of activation markers, proliferation, or metabolic activity.8-12
An alternative to selective allodepletion is induction of allospecific anergy (hyporesponsiveness to subsequent restimulation with alloantigens) in donor T cells before HSCT. T cells require at least 2 signals to become activated: cognate antigen/MHC binding to the T-cell receptor (signal 1) and positive costimulatory signals from antigen presenting cells (APCs; signal 2).13 The predominant positive costimulatory signal to human CD4+ T cells is delivered via the CD28 receptor, (constitutively expressed on the surface of 95% of human CD4+ T cells).14 This signal may be blocked by fusion proteins (such as CTLA4-Ig) or monoclonal antibodies (such as anti-B7.1 and -B7.2) that bind to the CD28 ligands B7.1 and B7.2 on APCs. T cells stimulated with signal 1 without signal 2 enter a state of antigen-specific hyporesponsiveness. Thus, recipient allospecific donor T cells can be rendered anergic by ex vivo stimulation with recipient alloantigens in the presence of costimulatory blockade (CSB).15
We previously reported early results of a clinical trial using ex vivo induction of alloanergy in donor T cells within bone marrow (BM) grafts via CTLA4-Ig–mediated CSB.16 This technique permitted large doses of HLA-mismatched donor T cells to be infused at the time of BMT with reliable engraftment and establishment of rapid full donor chimerism without excess severe aGVHD. We now report the immune reconstitution, infection, acute and chronic GVHD characteristics, and long-term follow-up of a much larger cohort of patients with high-risk hematologic malignancies or BMF who received HLA-mismatched BMT after ex vivo CSB to induce alloanergy.
Methods
Patient and donor characteristics
Between March 1996 and March 2001, 24 patients entered phase 1 studies of haploidentical BMT with ex vivo CSB using CTLA4-Ig (n = 19) or anti–B7-1 and – B7-2 antibodies (n = 5) on Dana-Farber Cancer Institute Institutional Review Board-approved protocols. Informed consent was obtained from all patients or their guardians in accordance with the Declaration of Helsinki. Follow-up is reported through June 2008. Patients with high-risk hematologic malignancies (a group subsequently expanded to include patients with congenital or acquired BMF other than Fanconi anemia) were eligible as previously described.16 Patient and donor details are summarized in Table 1. The median age of adult patients was 26 years (range, 19-50, n = 7) and that of those aged less than 18 years was 6.5 years (range, 0.5-16, n = 17). Of the 21 patients with hematologic malignancy, only 7 patients were in complete remission (CR; CR2 = 6, CR3 = 1) and 14 had progressive disease (PD; 5 having never attained remission) at time of BMT. UPNs 002 and 006 had failed prior autologous HSCT. All 3 patients with BMF were transfusion-dependent having failed prior nontransplantation therapy (if available). The haploidentical family donors consisted of parents (n = 16), full siblings (n = 5), half-siblings (n = 2), and children (n = 1). Several different tissue-typing techniques were used.
Patient, donor, and graft characteristics
| Patient . | Donor . | HLA mismatches* . | Infused cell doses ×106/kg . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Age . | Sex . | Disease . | Status† . | GVH . | HVG . | CD34+ . | CD3+ . | CD4+ . | CD8+ . | |
| 001 | 4 | F | B-ALL | CR2 | Mother | 4 | 4 | 6.5 | 55 | 41 | 18 |
| 002 | 26 | M | DLBC | PD | Half-brother | 1 | 2 | 2.0 | 17 | 13 | 07 |
| 003 | 6 | M | T-ALL | CR2 | Father | 5 | 5 | 2.1 | 16 | 11 | 06 |
| 004 | 15 | M | B-ALL | PD‡ | Sister | 4 | 4 | 2.3 | 32 | 21 | 10 |
| 005 | 7 | F | AML | PD‡ | Father | 4 | 4 | NE§ | 26 | 10 | 09 |
| 006 | 23 | M | T-NHL | PD | Mother | 4 | 4 | 1.3 | 12 | 04 | 07 |
| 007 | 7 | F | B-ALL | CR2 | Mother | 3 | 3 | 1.3 | 12 | 09 | 02 |
| 008 | 20 | F | T-ALL | CR3 | Father | 3 | 3 | 3.7 | 52 | 28 | 20 |
| 009 | 12 | M | AML | PD‡ | Father | 4 | 4 | 1.8 | 42 | 16 | 10 |
| 010 | 1.5 | F | AMT | N/A | Sister | 2 | 2 | 3.9 | 20 | 11 | 07 |
| 011 | 0.5 | F | AML | PD‡ | Father | 4 | 3 | 6.0 | 47 | 22 | 22 |
| 012 | 16 | M | T-ALL | PD‡ | Father | 4 | 4 | NE§ | 31 | 16 | 10 |
| 013 | 4 | F | SCN | N/A | Mother | 4 | 4 | 6.3 | 66 | 27 | 37 |
| 014 | 19 | M | B-ALL | PD | Half-brother | 4 | 4 | NE§ | 23 | 14 | 11 |
| 015 | 45 | F | B-ALL | CR2 | Brother | 2 | 2 | 1.4 | 20 | 15 | 08 |
| 016 | 50 | F | AML | PD | Son | 3 | 2 | 3.5 | 48 | 32 | 23 |
| 017 | 6 | F | AML | PD | Father | 2 | 2 | NE§ | 34 | 19 | 2.4 |
| 018 | 42 | M | MDS | PD | Sister | 2 | 2 | 1.9 | 15 | 08 | 09 |
| 019 | 16 | F | AML | PD | Mother | 4 | 3 | 1.1 | 07 | 04 | 02 |
| 020 | 12 | F | B-ALL | PD | Brother | 4 | 5 | 4.2 | 129 | 18 | 95 |
| 021 | 6 | M | AMT | N/A | Father | 2 | 3 | 3.1 | 68 | 35 | 31 |
| 022 | 6 | F | B-ALL | PD | Father | 4 | 4 | 12.3 | 11 | 12 | 31 |
| 023 | 4 | M | B-ALL | CR2 | Father | 4 | 4 | 3.8 | 21 | 18 | 12 |
| 024 | 4 | F | B-ALL | CR2 | Father | 4 | 4 | 9.8 | 43 | 24 | 13 |
| Median cell doses | 3.5 | 29 | 16 | 11 | |||||||
| Patient . | Donor . | HLA mismatches* . | Infused cell doses ×106/kg . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Age . | Sex . | Disease . | Status† . | GVH . | HVG . | CD34+ . | CD3+ . | CD4+ . | CD8+ . | |
| 001 | 4 | F | B-ALL | CR2 | Mother | 4 | 4 | 6.5 | 55 | 41 | 18 |
| 002 | 26 | M | DLBC | PD | Half-brother | 1 | 2 | 2.0 | 17 | 13 | 07 |
| 003 | 6 | M | T-ALL | CR2 | Father | 5 | 5 | 2.1 | 16 | 11 | 06 |
| 004 | 15 | M | B-ALL | PD‡ | Sister | 4 | 4 | 2.3 | 32 | 21 | 10 |
| 005 | 7 | F | AML | PD‡ | Father | 4 | 4 | NE§ | 26 | 10 | 09 |
| 006 | 23 | M | T-NHL | PD | Mother | 4 | 4 | 1.3 | 12 | 04 | 07 |
| 007 | 7 | F | B-ALL | CR2 | Mother | 3 | 3 | 1.3 | 12 | 09 | 02 |
| 008 | 20 | F | T-ALL | CR3 | Father | 3 | 3 | 3.7 | 52 | 28 | 20 |
| 009 | 12 | M | AML | PD‡ | Father | 4 | 4 | 1.8 | 42 | 16 | 10 |
| 010 | 1.5 | F | AMT | N/A | Sister | 2 | 2 | 3.9 | 20 | 11 | 07 |
| 011 | 0.5 | F | AML | PD‡ | Father | 4 | 3 | 6.0 | 47 | 22 | 22 |
| 012 | 16 | M | T-ALL | PD‡ | Father | 4 | 4 | NE§ | 31 | 16 | 10 |
| 013 | 4 | F | SCN | N/A | Mother | 4 | 4 | 6.3 | 66 | 27 | 37 |
| 014 | 19 | M | B-ALL | PD | Half-brother | 4 | 4 | NE§ | 23 | 14 | 11 |
| 015 | 45 | F | B-ALL | CR2 | Brother | 2 | 2 | 1.4 | 20 | 15 | 08 |
| 016 | 50 | F | AML | PD | Son | 3 | 2 | 3.5 | 48 | 32 | 23 |
| 017 | 6 | F | AML | PD | Father | 2 | 2 | NE§ | 34 | 19 | 2.4 |
| 018 | 42 | M | MDS | PD | Sister | 2 | 2 | 1.9 | 15 | 08 | 09 |
| 019 | 16 | F | AML | PD | Mother | 4 | 3 | 1.1 | 07 | 04 | 02 |
| 020 | 12 | F | B-ALL | PD | Brother | 4 | 5 | 4.2 | 129 | 18 | 95 |
| 021 | 6 | M | AMT | N/A | Father | 2 | 3 | 3.1 | 68 | 35 | 31 |
| 022 | 6 | F | B-ALL | PD | Father | 4 | 4 | 12.3 | 11 | 12 | 31 |
| 023 | 4 | M | B-ALL | CR2 | Father | 4 | 4 | 3.8 | 21 | 18 | 12 |
| 024 | 4 | F | B-ALL | CR2 | Father | 4 | 4 | 9.8 | 43 | 24 | 13 |
| Median cell doses | 3.5 | 29 | 16 | 11 | |||||||
UPN indicates unique patient number; HVG, host-versus-graft; GVH, graft-versus-host; F, female; M, male; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; DLBC diffuse large B-cell non-Hodgkin lymphoma; SCN, severe congenital neutropenia: MDS, myelodysplastic syndrome; AMT, amegakaryocytic thrombocytopenia; CR, complete remission; PD, progressive disease; N/A, not assessable; NE, Nonevaluable.
Number of mismatches between donor and recipient at A, B, Cw (where known), DR, and DQ loci at serologic typing level.
Disease status summary: 7/21 CR, 14/21 PD, 3 N/A (BM failure patients).
Patients who had failed to attain remission with induction therapy.
Peripheral blood leukaphereses of UPN 005, 012, 014, and 017 were contaminated with CD34+ leukemic blasts rendering infused CD34+ cell count determination nonevaluable.
Conditioning regimen, ex vivo manipulation of BM, and GVHD prophylaxis
Before conditioning, patient peripheral blood mononuclear cells (PBMCs) obtained by leukapheresis were cryopreserved for subsequent use as APCs during ex vivo coculture. Patients received 1400 cGy total body irradiation (175 cGy twice daily D-6-D-3) and cyclophosphamide 1800 mg/m2 on D-2 and D-1. The first 8 patients also received cytosine arabinoside 3 g/m2 every 12 hours for 6 doses. All patients also received methylprednisolone every 12 hours for 4 doses ending no later than 2 hours before BMT. The method of ex vivo alloanergy induction with CSB with CTLA4-Ig (Repligen, Waltham, MA) was as pre-viously described.16 Briefly, donor BM was harvested on D-2 and cocultured ex vivo with irradiated recipient PBMC for 36 to 40 hours in the presence of CTLA4-Ig, washed and reinfused on D0. For the last 5 patients, 10 μg/mL humanized murine IgG2 monoclonal anti-B7.1 and -B7.2 antibodies (Wyeth, Madison, NJ), replaced CTLA4-Ig in ex vivo cocultures. All patients received cyclosporine and short-course methotrexate, and 23 received folinic acid after some or all methotrexate doses.
Measurement of residual alloprecursor frequency
Residual alloprecursor frequencies in alloanergized BM were determined in some patients by helper T lymphocyte precursor frequency assay (hTLPf), as previously described.16
Supportive care
Patients received oral, nonabsorbable antibiotics from admission until neutrophil engraftment, fluconazole for fungal prophylaxis, and Pneumocystis carinii prophylaxis with trimethoprim-sulfamethoxazole or pentamidine during conditioning and after D +30 or discharge. Patients received acyclovir prophylaxis at 100 mg/m2 every 12 hours if they were seropositive for HSV or at 250 mg/m2 every 8 hours if the donor or recipient was CMV-seropositive. Intravenous immunoglobulin (IVIg) was given weekly (400-500 mg/kg) until trough levels were self-sustaining at IgG levels greater than 500 mg/dL. Patients were monitored for CMV reactivation at weekly intervals by detection of CMV antigenemia. Patients who became CMV antigenemic received intravenous ganciclovir therapy.
Chimerism and engraftment
Hematopoietic chimerism was determined on unfractionated blood or BM samples by fluorescence in situ hybridization in recipients with sex-mismatched donors or by polymerase chain reaction amplification of sequence specific primers or oligonucleotide probes for HLA class I and class II donor and recipient antigens.
Definitions of acute and chronic GVHD and performance status
Patients were evaluable for aGVHD if they successfully engrafted (graded with the International Bone Marrow Transplant Registry [IBMTR] Severity Index) and cGVHD if they reached D +100 without relapse (graded with consensus criteria).17,18 Wherever possible, histologic confirmation of clinical diagnoses of aGVHD was sought. Performance status of surviving patients was assessed using Karnofsky or Lansky scoring, appropriate to age.19,20
Immune reconstitution
Patients surviving beyond D +100 without relapse were eligible for immune reconstitution analysis. T-, B-, and NK-cell subsets were quantified at 1, 2, 3, 4, 6, 9, and 12 months after BMT by flow cytometric analysis of PBMCs using FITC/PE/PC5-labeled antibodies against CD3, CD4, CD8, CD19, CD56, CD45RA/RO and CD62L antibodies (Becton Dickinson, San Jose, CA). Serum immunoglobulin levels were determined by standard rate nephelometry (Dade Behring, Deerfield, IL). CMV (HLA-A2 NLVPMVATV) multimer analysis was performed on patients with HLA A*0201+ and *0206+ donors, as this multimer binds both HLA-A*0201– and HLA-A*0206–restricted CMV-specific T cells.21 PBMCs were costained with CD8-PC5, CD3-FITC and isotype-PE control antibody or PE-conjugated multimers (Proimmune, Oxford, United Kingdom), previously titrated for optimal staining. PBMCs from HLA A2− healthy donors served as additional negative controls. EBV (HLA-A2 GLCTLVAML) multimers were also used. A minimum of 100 multimer-positive events was acquired. The percentage of multimer-positive cells in the CD3+/CD8+ lymphocyte gate was expressed as a proportion of the CD8+ cells (with the negative control value subtracted).
Definitions and statistical aspects
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 10.0 software (SPSS, Chicago, IL). Associations between categorical variables was assessed using the χ-square test. Overall survival was estimated using the method of Kaplan and Meier.22 Transplant-related mortality (TRM) and relapse were calculated by the cumulative-incidence method using NCSS 2007 software (NCSS, Kaysville, UT) with relapse and TRM as the competing risks, respectively.23 To summarize the overall kinetics of immune reconstitution, the area under the curve (AUC) for each lymphocyte subset was calculated using the trapezoidal rule. To analyze categorical factors affecting immune reconstitution, mean AUC at 4, 6, and 12 months was compared between groups using a 2-sample t test and GraphPad Prism, version 4 (GraphPad Software, La Jolla, CA). P values less than .05 were considered significant.
Results
Engraftment and chimerism
Cell doses infused are detailed in Table 1. Two patients failed to engraft with one dose of ex vivo manipulated BM. UPN019 failed to engraft after receiving ex vivo manipulated BM from an initial BM harvest of only 0.7 × 106 CD34+ cells/kg. An additional dose of ex vivo alloanergized BM was administered on D +40 from a second BM harvest from the same donor (0.6 × 106 CD34+ cells/kg), but no engraftment occurred. This patient subsequently underwent nsTCD CD34-selected peripheral blood HSCT at another center with the other parent as donor, engrafted without aGVHD, but developed fatal disseminated zoster infection. UPN004 received 2.3 × 106 CD34+ cells/kg but had not engrafted by D +30. An additional dose of ex vivo alloanergized BM (0.6 × 106 CD34+ cells/kg) was administered from a second BM harvest from the same donor on D +35 without reconditioning and engraftment occurred at D +54. Two patients died (D +8 and D +22) too early to evaluate for engraftment. Of the remaining 20 patients, neutrophil engraftment occurred in all at a median D +21 (range, D +13-29) and 11 (55%) achieved an unsupported platelet count greater than 20 000-μL at a median D +46 (range, D +19-204) with the remaining patients dying before platelet recovery. All patients who engrafted achieved 100% donor chimerism at the first point of testing.
Toxicity
Twelve patients died of TRM. Two patients died of fungal infection, 2 from identified bacterial septicemia, and 1 from a syndrome consistent with bacterial sepsis (organism unidentified). Four of these infection-related deaths occurred in patients with PD at BMT, whereas only 1 occurred in patients in CR. Five patients died from noninfectious toxicity; 2 from multiorgan failure (1 of whom also had histologic evidence of cerebral toxoplasmosis postmortem), 1 from diffuse alveolar hemorrhage, and 1 from hepatic veno-occlusive disease. One patient, for whom aGVHD was considered the primary cause of death, died with radiologic evidence of fungal sinus infection while receiving immunosuppressive therapy. One patient was found dead at home; the cause of death was undetermined. Median time of TRM was D +53. Three-quarters of toxicity-related deaths occurred before D +100 and all before D +200 (Table 2). Cumulative incidence of TRM was 50%. Recipient age (above median) was the only factor significantly associated with overall TRM. Median duration of hospitalization for the 13 patients who survived to discharge was 70 days (range, 31-100).
Toxicity and outcomes
| UPN . | Age . | Status . | EBV . | CMV . | GVHD . | Relapse . | OS . | Day . | COD . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D/R . | PTLD . | D/R . | Reactivation . | Disease . | Acute* . | Chronic . | |||||||
| 001 | 4 | CR2 | +/+ | No | +/+ | No | No | N/A | N/A | No | Dead | +22 | DAH |
| 002 | 26 | PD | UNK/+ | No | −/− | No | No | N/A | N/A | No | Dead | +8 | Sepsis |
| 003 | 6 | CR2 | +/+ | No | −/− | No | No | B | No | D+1440 | Dead | +1758 | T-ALL |
| 004 | 15 | PD | +/+ | No | +/+ | D+64 | No | No | No | No | Dead | +102 | MOF |
| 005 | 7 | PD | +/+ | No | −/− | No | No | C | N/A | No | Dead | +39 | Fungus |
| 006 | 23 | PD | +/+ | No | −/− | No | No | No | N/A | No | Dead | +31 | MOF |
| 007 | 7 | CR2 | +/+ | No | −/+ | No | No | No | No | No | Alive | +3909 | — |
| 008 | 20 | CR3 | +/+ | No | −/− | No | No | No | N/A | No | Dead | +24 | Fungus |
| 009 | 12 | PD | +/+ | No | −/+ | D+32,150†† | No | No | Yes† | No | Alive | +3708 | — |
| 010 | 1.5 | N/A | −/− | No | −/− | No | No | No | No | No | Alive | +3694 | — |
| 011 | 0.5 | PD | +/+ | No | −/− | No | No | B | N/A | D+60 | Dead | +70 | AML |
| 012 | 16 | PD | +/+ | No | −/− | No | No | No | No | No | Alive | +3520 | — |
| 013 | 4 | N/A | +/+ | No | +/− | No | No | No | No | No | Alive | +3394 | — |
| 014 | 19 | PD | +/+ | No | +/− | No | No | No | N/A | No | Dead | +35 | Sepsis |
| 015 | 45 | CR2 | +/+ | No | −/− | No | No | C | No | No | Dead | +176 | UNK |
| 016 | 50 | PD | +/+ | No | −/− | No | No | B | N/A | D+50 | Dead | +149 | AML |
| 017 | 6 | PD | +/+ | No | +/+ | No | No | B | N/A | No | Dead | +38 | VOD |
| 018 | 42 | PD | +/+ | No | +/+ | D+37, +103 | No | D | No | No | Dead | +159 | aGVHD§ |
| 019 | 16 | PD | +/− | No | −/− | No | No | N/A¶ | N/A | D+90 | Dead | +563 | AML |
| 020 | 12 | PD | +/+ | No | −/− | No | No | C | No | No | Alive | +2966 | — |
| 021 | 6 | N/A | +/+ | No | −/+ | D+32 | No | No | No | No | Alive | +2890 | — |
| 022 | 6 | PD | +/− | No | −/− | No | No | No | N/A | No | Dead | +71 | Sepsis |
| 023 | 4 | CR2 | +/+ | No | −/+ | No | No | No | N/A | No | Dead | +31 | MOF |
| 024 | 4 | CR2 | +/+ | No | +/+ | D+44 | No | C | No | No | Alive | +2647 | — |
| Total | 9/21‖, 5/21** | 1/12 | 4/24 | 8/24 | |||||||||
| UPN . | Age . | Status . | EBV . | CMV . | GVHD . | Relapse . | OS . | Day . | COD . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D/R . | PTLD . | D/R . | Reactivation . | Disease . | Acute* . | Chronic . | |||||||
| 001 | 4 | CR2 | +/+ | No | +/+ | No | No | N/A | N/A | No | Dead | +22 | DAH |
| 002 | 26 | PD | UNK/+ | No | −/− | No | No | N/A | N/A | No | Dead | +8 | Sepsis |
| 003 | 6 | CR2 | +/+ | No | −/− | No | No | B | No | D+1440 | Dead | +1758 | T-ALL |
| 004 | 15 | PD | +/+ | No | +/+ | D+64 | No | No | No | No | Dead | +102 | MOF |
| 005 | 7 | PD | +/+ | No | −/− | No | No | C | N/A | No | Dead | +39 | Fungus |
| 006 | 23 | PD | +/+ | No | −/− | No | No | No | N/A | No | Dead | +31 | MOF |
| 007 | 7 | CR2 | +/+ | No | −/+ | No | No | No | No | No | Alive | +3909 | — |
| 008 | 20 | CR3 | +/+ | No | −/− | No | No | No | N/A | No | Dead | +24 | Fungus |
| 009 | 12 | PD | +/+ | No | −/+ | D+32,150†† | No | No | Yes† | No | Alive | +3708 | — |
| 010 | 1.5 | N/A | −/− | No | −/− | No | No | No | No | No | Alive | +3694 | — |
| 011 | 0.5 | PD | +/+ | No | −/− | No | No | B | N/A | D+60 | Dead | +70 | AML |
| 012 | 16 | PD | +/+ | No | −/− | No | No | No | No | No | Alive | +3520 | — |
| 013 | 4 | N/A | +/+ | No | +/− | No | No | No | No | No | Alive | +3394 | — |
| 014 | 19 | PD | +/+ | No | +/− | No | No | No | N/A | No | Dead | +35 | Sepsis |
| 015 | 45 | CR2 | +/+ | No | −/− | No | No | C | No | No | Dead | +176 | UNK |
| 016 | 50 | PD | +/+ | No | −/− | No | No | B | N/A | D+50 | Dead | +149 | AML |
| 017 | 6 | PD | +/+ | No | +/+ | No | No | B | N/A | No | Dead | +38 | VOD |
| 018 | 42 | PD | +/+ | No | +/+ | D+37, +103 | No | D | No | No | Dead | +159 | aGVHD§ |
| 019 | 16 | PD | +/− | No | −/− | No | No | N/A¶ | N/A | D+90 | Dead | +563 | AML |
| 020 | 12 | PD | +/+ | No | −/− | No | No | C | No | No | Alive | +2966 | — |
| 021 | 6 | N/A | +/+ | No | −/+ | D+32 | No | No | No | No | Alive | +2890 | — |
| 022 | 6 | PD | +/− | No | −/− | No | No | No | N/A | No | Dead | +71 | Sepsis |
| 023 | 4 | CR2 | +/+ | No | −/+ | No | No | No | N/A | No | Dead | +31 | MOF |
| 024 | 4 | CR2 | +/+ | No | +/+ | D+44 | No | C | No | No | Alive | +2647 | — |
| Total | 9/21‖, 5/21** | 1/12 | 4/24 | 8/24 | |||||||||
CMV risk summary: 13/24 were low-risk (donor and recipient CMV-seronegative); 11/24 were high-risk (donor, recipient, or both CMV-seropositive).
D/R indicates donor/recipient serostatus; EBV, Epstein-Barr virus; PTLD, posttransplant lymphoproliferative disorder; CMV, cytomegalovirus; TRM, treatment-related mortality; GVHD, graft-versus-host disease; OS, overall survival; COD, cause of death; DAH, diffuse alveolar hemorrhage; VOD, veno-occlusive disease; MOF, multiorgan failure; and —, not applicable. Other abbreviations as in Table 1.
IBMTR Severity Index Criteria.
cGVHD resolving after 6 months immunosuppression.
Diagnosed on histologic appearance of GI biopsies postmortem.
UPN018 died of probable fungal infection (radiologic evidence) while on immunosuppression for aGVHD.
UPN019 failed to engraft despite two infusions on alloanergized BMT.
IBMTR grades B-D.
IBMTR grades C-D.
UPN 009 had a history of prior CMV disease and was given extended anti-CMV treatment.
GVHD
Of 21 evaluable patients, 8 (38%) had clinical findings consistent with aGVHD, clinically graded B (n = 3), C (n = 4), and D (n = 1), diagnosed at a median time of D +38 (range, 20-64). In addition, UPN017 developed diarrhea in the day before death from VOD, and postmortem GI biopsies were consistent with aGVHD. Eight patients received treatment with systemic corticosteroids, with only 2 requiring additional treatment. All patents responded with improvement of symptoms attributable to aGVHD. UPN 018 developed a presumed sinus fungal infection and died while on immunosuppressive treatment for aGVHD. No other death was attributable to aGVHD (Table 3). There was no significant difference in median infused cell doses of CD34+, CD3+, CD4+, or CD8+ cells in assessable patients who developed aGVHD and those who did not. Nine patients had alloreactive cell frequencies estimated by hTLPf assay before and after ex vivo manipulation of BM, 7 of whom were assessable for aGVHD. There was no significant difference in median hTLPf (or dose) in those who developed aGVHD and those who did not. Only 1 of 12 evaluable patients (8%) developed de novo chronic GVHD (with GI tract and skin involvement), on D +145 after discontinuing aGVHD prophylaxis on D +122, resolving after receiving slowly tapering immunosuppression until D +355. The cumulative incidence of cGVHD at 8 years was only 8%.
Acute GVHD
| UPN . | Day . | Organ . | Grade* . | Treatment . | |||
|---|---|---|---|---|---|---|---|
| Primary . | Response . | Secondary . | Response . | ||||
| 003 | +64 | GI | B | Corticosteroids | Complete | — | — |
| 005 | +20 | GI | C | Corticosteroids | Complete | — | — |
| 011 | +49 | GI | B | Corticosteroids | Complete | — | — |
| 015 | +27 | Skin, liver | C | Corticosteroids | Partial | Dacluzimab, MMF | Complete |
| 016 | +39 | Skin | B | Corticosteroids | Complete | — | — |
| 017 | +38 | GI† | B | N/A | N/A | — | — |
| 018 | +22 | GI, liver | D | Corticosteroids | Partial | Sirolimus, tacrolimus | Died‡ |
| 020 | +25 | GI | C | Corticosteroids | Complete | — | — |
| 024 | +38 | GI | C | Corticosteroids | Complete | — | — |
| UPN . | Day . | Organ . | Grade* . | Treatment . | |||
|---|---|---|---|---|---|---|---|
| Primary . | Response . | Secondary . | Response . | ||||
| 003 | +64 | GI | B | Corticosteroids | Complete | — | — |
| 005 | +20 | GI | C | Corticosteroids | Complete | — | — |
| 011 | +49 | GI | B | Corticosteroids | Complete | — | — |
| 015 | +27 | Skin, liver | C | Corticosteroids | Partial | Dacluzimab, MMF | Complete |
| 016 | +39 | Skin | B | Corticosteroids | Complete | — | — |
| 017 | +38 | GI† | B | N/A | N/A | — | — |
| 018 | +22 | GI, liver | D | Corticosteroids | Partial | Sirolimus, tacrolimus | Died‡ |
| 020 | +25 | GI | C | Corticosteroids | Complete | — | — |
| 024 | +38 | GI | C | Corticosteroids | Complete | — | — |
GI indicates gastrointestinal; MMF, mycophenolate mofetil; and —, not applicable. Other abbreviations as in Table 1.
IBMTR grading criteria.
Diagnosed on histologic appearance of GI biopsies postmortem.
UPN018 died of probable fungal infection (radiologic evidence) while on immunosuppression for aGVHD.
Viral reactivation and infection
Of 11 at-risk patients (donor and/or recipient seropositive for CMV), 5 patients experienced a total of 7 episodes of CMV reactivation (45%; Table 2). UPNs 004, 021, and 024 reactivated on single occasions at D +64, D +32, and D +44, respectively. All cleared CMV antigen with 3 days of antiviral treatment. UPN 018 reactivated on D +37 and again on D +103, clearing CMV antigen rapidly after treatment with ganciclovir on both occasions. UPN 009, who had a history of CMV-associated acute hepatic necrosis requiring intensive care immediately before BMT reactivated on D +32 and cleared CMV antigen after 14 days of treatment with Foscarnet, reactivating again on D +150 and clearing CMV antigen by D +154 after further Foscarnet treatment. No CMV disease and no other clinically significant viral infections or posttransplantion lymphoproliferative disorder (PTLD) occurred.
Immune reconstitution
Absolute lymphocyte count (ALC) recovery, determined in all 21 patients surviving beyond D +30, occurred rapidly (Figure 1A). The median ALC at D +30 (ALCD+30) was 0.42 × 109/L. Although no pre- or post-BMT factors were statistically significantly associated with the ALCD+30 there was a trend toward a higher ALCD+30 in surviving patients compared with those that died of any cause (P = .10, paired t test).
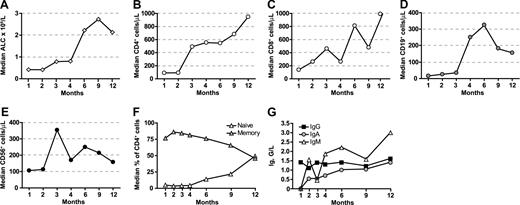
Immune reconstitution after alloanergized haploidentical bone marrow transplantation. (A) Median absolute lymphocyte count (ALC) recovery (×109/L). (B) CD4+ T-cell reconstitution. (C) CD8+ T cells. (D) CD19+ B cells. (E) NK cells. Median absolute cell counts/μL are shown for panels B through E. (F) Proportion of naive (CD45RA+) and memory (CD45RO+) cells in reconstituting CD4+ T cells. (G) Median trough immunoglobulin levels for IgG, IgA, and IgM G/L (grams/liter).
Immune reconstitution after alloanergized haploidentical bone marrow transplantation. (A) Median absolute lymphocyte count (ALC) recovery (×109/L). (B) CD4+ T-cell reconstitution. (C) CD8+ T cells. (D) CD19+ B cells. (E) NK cells. Median absolute cell counts/μL are shown for panels B through E. (F) Proportion of naive (CD45RA+) and memory (CD45RO+) cells in reconstituting CD4+ T cells. (G) Median trough immunoglobulin levels for IgG, IgA, and IgM G/L (grams/liter).
Eight of 9 evaluable patients surviving without relapse beyond D +100 were assessed for T-cell subset reconstitution. CD4+ T-cell reconstitution (Figure 1B) was rapid with a median CD4+ count of 90/μL at 1 month and almost 500/μL at 3 months. Three of 8 patients achieved CD4+ T cell counts above 200/μL by 2 months, 5/8 had CD4+ counts above 200/μL by 4 months and all 8 patients achieved this by 9 months. CD8+ T-cell reconstitution was also rapid, with 5/8 patients achieving CD8+ counts greater than 200/μL at 2 months, 7/8 by 4 months, and all by 12 months (Figure 1C). B-cell reconstitution was less rapid, although the median CD19 count was greater than 200/μL by 4 months (Figure 1D). We saw an early peak in CD56+ NK-cell immune reconstitution at 3 months (Figure 1E). Reconstituting CD4+ T cells were predominantly memory cells and emergence of naive CD4+ T cells was not seen until 6 months (Figure 1F). In 2 patients in which CD8 memory cell subsets were measured by dual-color CD45RA and CD62L expression, the majority of reconstituting memory CD8 cells had a CD45RA−CD62L− effector memory phenotype (data not shown). Endogenous immunoglobulin production recovered rapidly (Figure 1G), and IVIg replacement was discontinued at a median of 4 months post-BMT.
To evaluate factors affecting the kinetics of T-cell reconstitution, AUC analysis was performed (Table 4). There was a significantly greater CD4+ T-cell AUC at 4 months in patients aged below the median (compared with those aged above), and there was also a trend toward greater CD4+ T-cell AUC in younger patients at 12 months and CD8+ T-cell AUC at 4 months. Neither malignant versus nonmalignant diagnosis nor administered CD34+, CD4+, or CD8+ cell doses had any effect on CD4+ or CD8+ T-cell AUC at 4, 6, or 12 months. Similarly, there was no significant difference in CD4+ or CD8+ T-cell AUC at 4, 6 or 12 months between patients who developed aGVHD and those who did not. However, although no significant difference was seen in CD4+ T-cell AUC at 3, 6, or 12 months, there was a trend toward greater CD8+ T-cell AUC at 4 months and 6 months and significantly greater CD8+ T-cell AUC at 12 months in those who reactivated CMV compared with those who did not.
Univariate analysis of factors influencing AUC for T-cell subset immune reconstitution
| Factor . | P (by 2-tailed t test between mean AUCs) . | |||||
|---|---|---|---|---|---|---|
| CD4+ T cells . | CD8+ T cells . | |||||
| 4 m . | 6 m . | 12 m . | 4 m . | 6 m . | 12 m . | |
| Age (below median vs above) | .02* | .19 | .05 | .05 | .13 | .17 |
| Diagnosis (malignancy vs BMF) | .76 | .54 | .56 | .68 | .70 | .77 |
| CD34+ cell dose (above median vs not above) | .59 | .82 | .47 | .84 | .71 | .86 |
| CD4+ or CD8+ T-cell dose (above median vs not above) | .32 | .58 | .74 | .19 | .08 | .16 |
| aGvHD (grades B-D vs less than grade B)† | .26 | .94 | .39 | .50 | .54 | .63 |
| CMV (reactivation vs no reactivation)‡ | .43 | .64 | .88 | .08 | .06 | .005* |
| Factor . | P (by 2-tailed t test between mean AUCs) . | |||||
|---|---|---|---|---|---|---|
| CD4+ T cells . | CD8+ T cells . | |||||
| 4 m . | 6 m . | 12 m . | 4 m . | 6 m . | 12 m . | |
| Age (below median vs above) | .02* | .19 | .05 | .05 | .13 | .17 |
| Diagnosis (malignancy vs BMF) | .76 | .54 | .56 | .68 | .70 | .77 |
| CD34+ cell dose (above median vs not above) | .59 | .82 | .47 | .84 | .71 | .86 |
| CD4+ or CD8+ T-cell dose (above median vs not above) | .32 | .58 | .74 | .19 | .08 | .16 |
| aGvHD (grades B-D vs less than grade B)† | .26 | .94 | .39 | .50 | .54 | .63 |
| CMV (reactivation vs no reactivation)‡ | .43 | .64 | .88 | .08 | .06 | .005* |
AUC indicates area under curve; other abbreviations as in Table 1.
Statistically significant.
Grade B-D aGVHD (n = 3) versus less than grade B aGVHD (n = 5).
CMV reactivation (n = 3) versus no CMV reactivation (n = 5).
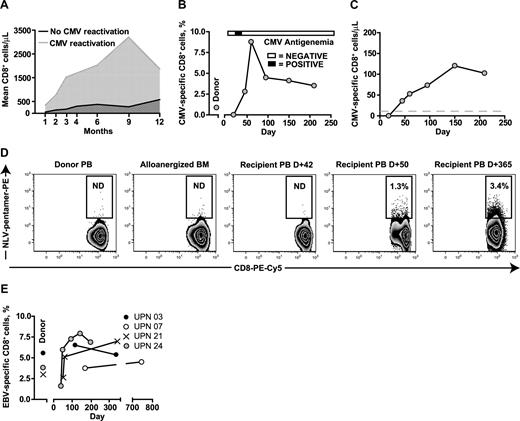
The more rapid expansion of CD8+ T cells in the 3 evaluated patients who reactivated CMV (UPNs 009, 021, and 024) compared with the 5 who did not is shown in Figure 2A. Serial frequencies of CMV-specific pentamer+ CD8+ T cells were determined in UPN 021 and 024, who both received BMT from HLA A2+ donors, to determine whether the CD8+ T-cell expansion included significant populations of CMV-specific T cells (shown for UPN 024 in relation to a single episode of CMV antigenemia in Figure 2B). Absolute levels of CMV-specific pentamer+ CD8+ T cells were detectable at levels above 10 cells/μL as early as D +50 and these levels were sustained (Figure 2C). A similar in vivo expansion of CMV-specific CD8+ T cells occurred in UPN 021, who in contrast to UPN024, received alloanergized BMT from an HLA A2+ CMV-seronegative donor. CMV-specific pentamer+ CD8+ T cells were not detectable in the donor peripheral blood pre-BMT or in the donor BM after alloanergization before infusion, but became detectable after a single episode of CMV antigenemia at D +37. A sustained expansion of CMV-specific pentamer+ CD8+ T cells was seen, and no further episodes of CMV antigenemia occurred (Figure 2D).
Pathogen-specific immune reconstitution after alloanergized haploidentical bone marrow transplantation. (A) CD8+ T-cell reconstitution in patients with and without CMV reactivation. Mean values for absolute CD8+ cells/μL are shown. (B) Expansion of CMV-specific pentamer+ CD8 cells in vivo in UPN 24 in relation to a single episode of CMV antigenemia. (C) Absolute levels of CMV-specific pentamer+ CD8 cells in UPN 024 (gray dashed line indicates 10 cells/μL). (D) Primary CMV-specific response in a CMV-seropositive patient (UPN 021) who received alloanergized BMT from an HLA A2+ CMV-seronegative donor. CMV-specific pentamer+ CD8 cells were not detectable in the donor pretransplantation or in the donor bone marrow after alloanergization before infusion, but became detectable after a single episode of CMV antigenemia at D +37. A sustained expansion of CMV-specific pentamer+ CD8 cell was seen and no further episodes of CMV antigenemia occurred. Numbers in gated regions show HLA A2-restricted peptide (NLV) -pentamer+ cells, expressed as percentage of CD3+CD8+ T cells. BM, bone marrow; PB, peripheral blood; ND; not detectable above negative control frequency in pentamer-stained HLA A2− donor cells. (E) Rapid and early expansion of EBV-specific pentamer+ CD8 cells in 4 patients who received alloanergized BMT from HLA A2+ EBV-seropositive donors.
Pathogen-specific immune reconstitution after alloanergized haploidentical bone marrow transplantation. (A) CD8+ T-cell reconstitution in patients with and without CMV reactivation. Mean values for absolute CD8+ cells/μL are shown. (B) Expansion of CMV-specific pentamer+ CD8 cells in vivo in UPN 24 in relation to a single episode of CMV antigenemia. (C) Absolute levels of CMV-specific pentamer+ CD8 cells in UPN 024 (gray dashed line indicates 10 cells/μL). (D) Primary CMV-specific response in a CMV-seropositive patient (UPN 021) who received alloanergized BMT from an HLA A2+ CMV-seronegative donor. CMV-specific pentamer+ CD8 cells were not detectable in the donor pretransplantation or in the donor bone marrow after alloanergization before infusion, but became detectable after a single episode of CMV antigenemia at D +37. A sustained expansion of CMV-specific pentamer+ CD8 cell was seen and no further episodes of CMV antigenemia occurred. Numbers in gated regions show HLA A2-restricted peptide (NLV) -pentamer+ cells, expressed as percentage of CD3+CD8+ T cells. BM, bone marrow; PB, peripheral blood; ND; not detectable above negative control frequency in pentamer-stained HLA A2− donor cells. (E) Rapid and early expansion of EBV-specific pentamer+ CD8 cells in 4 patients who received alloanergized BMT from HLA A2+ EBV-seropositive donors.
A rapid and sustained expansion of EBV-specific pentamer+ CD8+ T cells was also observed in all 4 evaluated patients with HLA A2+ EBV-seropositive donors (Figure 2E).
Outcomes
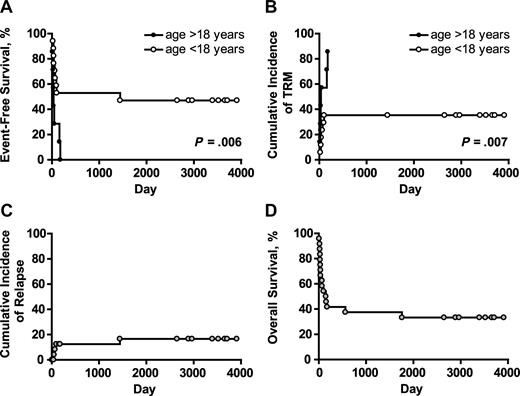
Actuarial event-free survival (EFS) was 33% at 10 years for the whole patient cohort. EFS was significantly higher in patients aged less than 18 years than in those aged more than 18 years. The difference in EFS in pediatric and adult patients was due to increased early TRM in adult patients (Figure 3A,B). No other factors statistically significantly affected EFS. Three of the 4 patients with hematologic malignancies who relapsed/progressed did so before D +100. Of the 7 patients transplanted in second (or higher) CR, one relapsed at 48 months, 4 remained disease-free until the time of death from TRM (median, D +48, range, D +21-176) and 2 survive in CR at D +3909 and D +2647 (UPN 007 and 0024, respectively). Fourteen patients had PD at the time of BMT; 3 progressed at D +50, +60, and +90 (UPNs 016, 011, and 019, respectively); 8 died in clinical remission/without evidence of disease progression at a median of D +37 (range, 8-159) and 3 survive in CR at D +3708, +3520, and +2966 (UPNs 009, UPN 012, and UPN020, respectively). Three of 4 patients who relapsed/progressed had PD at time of BMT. All 3 patients transplanted for BMF are free of disease at a median of D +3394 (range, 2890-3694). The cumulative incidence of relapse/progression (competing risk death in CR/without progression) was 17% (Figure 3C). Eight patients survive with a median follow-up of 7.2 years (Table 2). All are free of disease, have normal peripheral blood counts and immunoglobulin levels, and have demonstrated humoral responses to post-BMT vaccinations. None is on immunosuppressive or anti-infectious medications, and all have normal performance scores. Actuarial overall survival at 10 years was 33% (Figure 3D).
Long-term outcome after alloanergized haploidentical bone marrow transplantation. (A) Actuarial event-free survival (EFS) in patients aged above and below 18 years. The difference in EFS in pediatric and adult patients was due to an increased incidence of early treatment-related mortality (TRM) in adult patients. (B) P values for panels A and B are for the log-rank test. Cumulative incidence of relapse/progression (competing risk death without relapse/progression) was low at 17% for the whole patient cohort (C), and actuarial overall survival at 10 years was 33% (D).
Long-term outcome after alloanergized haploidentical bone marrow transplantation. (A) Actuarial event-free survival (EFS) in patients aged above and below 18 years. The difference in EFS in pediatric and adult patients was due to an increased incidence of early treatment-related mortality (TRM) in adult patients. (B) P values for panels A and B are for the log-rank test. Cumulative incidence of relapse/progression (competing risk death without relapse/progression) was low at 17% for the whole patient cohort (C), and actuarial overall survival at 10 years was 33% (D).
Discussion
In both of these phase 1 clinical studies, myeloablative haploidentical BMT containing large numbers of alloanergized donor T cells resulted in acceptable engraftment and less severe aGVHD than historical reports using unmanipulated haploidentical BM. Moreover, alloanergized BMT was associated with rapid T-cell reconstitution, in vivo expansion of pathogen-specific CD8+ T cells, and early recovery of immunoglobulin production. Although substantial early TRM was observed in these very high risk patients, no TRM was related to viral infection, and long-term disease-free survival uncomplicated by cGVHD was seen in one-third of patients.
Alloanergization did not appear to impair immune reconstitution. The median ALCD+30 in our study (0.42 × 109/L), evaluated for all patients who survived to engraftment, was similar to that reported after conventional unmanipulated BMT from HLA-matched sibling donors (0.49 × 109/L).24 Both ALC and CD4+ T-cell counts rose rapidly by 3 months. The presence of CD4+ cells with a predominantly memory phenotype during the initial period post-BMT, when rapid recovery of T-cell counts occurred, was consistent with peripheral expansion of mature donor T cells contained in the BM graft. In contrast naive CD4+ T cells were not detectable in the periphery until 6 months post-BMT. Although the favorable prognostic value of early ALC recovery reported after adult and pediatric matched sibling HSCT25,26 has not been established after haploidentical BMT, and cannot be inferred from our limited data, the observed recovery of ALC suggests donor lymphocytes maintained their capacity to expand in vivo after alloanergization. Indeed, rapid reconstitution of T-cell subsets occurred in patients evaluable for immune reconstitution (all of whom were less than 18 years of age). The median time to achieve a CD4+ T-cell count of 300 μL was 3 months, faster than the 8 months observed after haploidentical CD34-selected BMT in children reported by Eyrich et al27 and comparable to the 4 months observed after CD34-selected HSCT followed by infusion of 105 selectively allodepleted donor T cells/kg reported by Amrolia et al.28
In contrast to a recent study of immune reconstitution after haploidentical HSCT,29 our AUC analysis demonstrated that CD4+ and CD8+ T-cell recovery was not adversely affected by the presence of aGVHD, probably reflecting the modest dose and duration of corticosteroid treatment in most patients who developed aGVHD. Conversely, the absence of marked acceleration of T-cell recovery in patients with aGVHD suggests that alloantigen-driven expansion of alloanergized donor T cells in vivo did not occur (or, if it did, more rapid expansion was not associated with clinically apparent aGVHD). However, the marked acceleration of CD8+ reconstitution seen in patients with CMV reactivation may have been driven at least in part by CMV antigens. Indeed, the expansion of CMV-specific CD8+ T cells seen in patients with HLA A2+ donors (both CMV-seropositive and -seronegative) demonstrates that alloanergized donor T cells retained their capacity to mount both effective memory and primary CMV responses in vivo after episodes of CMV reactivation. In both cases, absolute numbers of CMV-specific CD8+ T cells above the level shown to be protective against CMV (10-μL) after HSCT30 were rapidly attained and sustained. No repeat CMV reactivation occurred in these patients. Although we did not prospectively monitor patients for EBV reactivation, we saw no cases of PTLD despite transplantation of donor BM replete with B cells from 22 EBV seropositive donors. EBV-related PTLD remains a significant problem after nsTCD haploidentical HSCT, especially after transplantation of grafts containing significant numbers of donor B cells.4,31 A protective effect against EBV+ PTLD may have been conferred by expansion of donor EBV-specific CD8+ T cells, as directly demonstrated in patients with HLA A2+EBV+ donors, consistent with previously reported efficacy of donor lymphocyte infusions in treating EBV+ PTLD.32
The recovery of B-cell counts and independently sustained immunoglobulin levels demonstrated rapid functional B-cell recovery in vivo. Unfortunately, a limitation of our immune reconstitution data is the absence of data similarly demonstrating functional pathogen-specific CD8+ responses. However, the low frequency of CMV reactivation and absence of both CMV disease and EBV+ PTLD strongly suggest that the expanded populations of CMV- and EBV-specific CD8+ cells we observed retained function in vivo. In common with other reports of graft manipulation techniques to selectively reduce alloreactivity, we also lack data demonstrating antigen-specific CD4+ responses. However, we have recently demonstrated that CMV-specific CD4+ T cells are retained after alloanergization in vitro. If such cells are also retained after alloanergized BMT, they might be capable of providing pathogen-specific memory responses needed to maintain CMV immunity in vivo post-BMT.33
The optimum dose of alloanergized T cells able to improve pathogen-specific immune reconstitution without significant GVHD remains to be determined, and may be significantly lower than the doses in this study. Although administration of alloanergized donor T cells was associated with aGVHD in 43% of patients, three-quarters of those with aGVHD responded to brief courses of corticosteroids. Steroid-refractory aGVHD only occurred in 2 patients (9%), despite a median T-cell dose 2 logs greater than the dose (8-20 × 104/kg) at which 30% of children undergoing nsTCD haploidentical HSCT were observed to develop steroid-refractory GVHD and 3 logs above a published threshold dose below which severe aGVHD was reliably prevented in the haploidentical setting.34,35 AGVHD did not occur more often in those patients receiving CD3+, CD4+, or CD8+ T cell doses above median levels, and although alloreactive precursor frequency data were available on a relatively small proportion of patients, we also did not see an association between residual in vitro CD4+ alloresponses and aGVHD occurrence. A potential explanation for this observation might be that residual alloresponses after alloanergization are mediated in vivo by CD28− donor T cells (which are predominantly CD8+), γδ T cells, or successfully alloanergized CD4+ donor T cells that had their anergic state temporarily reversed by high levels of cytokines in vivo.36,37 We also observed an unusual distribution of organ involvement in patients with aGVHD, predominantly limited to the GI tract, suggesting that donor T cells with residual alloreactivity preferentially targeted the gut. Murine GI-specific alloresponses mediated by CD8+ cells are not abrogated by CD28-blockade; such cells may receive CD28-independent positive costimulatory signals via CD134.38,39 Alternatively, GI tract aGVHD may have been disproportionately driven by nonallospecific mechanisms.
Our strategy resulted in a low cumulative incidence of cGVHD (8%) comparable to nsTCD haploidentical HSCT,4,40 and surviving patients were therefore free of cGVHD-associated morbidity and immunosuppression. In contrast, recent studies of T-replete G-CSF–stimulated haploidentical BMT have reported up to 70% cGVHD (and nearly 50% extensive cGVHD) in both adults and children.41
Recent series of nsTCD haploidentical HSCT have reported significant rates of late TRM in both children and adults, predominantly from delayed T-cell immune reconstitution and viral infections, resulting in overall TRM of 40% to 60%.4,40,42,43 In contrast we saw no significant morbidity or mortality related to viral infection and no TRM beyond 6 months post-BMT. Although the incidence of CMV reactivation in our study was lower than that in recent reports of nsTCD haploidentical HSCT, many such studies used detection techniques with greater sensitivity.42,44-46 However, the CMV reactivation observed was associated with high rates of fatal CMV disease in many such studies; up to 60% of TRM was related to CMV infection and up to 16% of all patients transplanted died from human herpesvirus–related infections, the majority from CMV.4,43 In our studies, there was no CMV-related TRM. Furthermore, we saw no mortality from adenovirus. In contrast, adenovirus-related deaths occurred in 10% to 18% of children with acute leukemia receiving nsTCD haploidentical HSCT by either addition of alemtuzumab or CD34-selection of donor grafts.40,47
We are encouraged that alloanergized BMT was associated with low incidences of steroid-refractory aGVHD, cGVHD and viral infection, and with rapid T-cell reconstitution, a key area of therapeutic weakness in many other current strategies for haploidentical HSCT. The adoptive transfer of nonalloreactive donor T-cell doses as low as 105/kg has also been associated with improved viral-specific immune reconstitution after haploidentical CD34-selected HSCT, although relapse remained a significant problem, occurring in 7 of 16 patients.28 While early TRM and small patient numbers preclude any conclusions regarding long-term disease control, cumulative incidence of relapse/progression in our study was low at 17% with long follow-up.
The major limitation of our strategy, however, was significant early TRM, particularly in adult patients. While the engraftment rate was comparable to alternate strategies of haploidentical HSCT,4,40,48-50 the period of neutropenia was relatively long after alloanergized BMT (median 21 days). This may have contributed to infection-related TRM, all of which was caused by bacterial or fungal pathogens. Although the only factor significantly associated with risk of overall TRM was patient age, infection-related TRM occurred in 4 of 14 patients with hematologic malignancy with PD, compared with only 1 of 10 patients in CR or with BMF, suggesting that patients with PD, most of whom had been heavily pretreated, were at greater risk of infection-related TRM. Strategies using high doses of CD34+ HSCs to achieve more rapid neutrophil recovery4 and reduced-intensity conditioning29 to minimize regimen-related toxicity have been successfully used in haploidentical HSCT and could reduce early TRM in the context of strategies using alloanergization.
Encouraged by the rapid immune reconstitution after alloanergized BMT but mindful of the early TRM, we are undertaking a new study using alloanergized donor T cells in haploidentical HSCT, modifying the strategy with megadose CD34+ HSCT and reduced intensity conditioning in older patients to minimize early TRM, followed by dose-escalating delayed infusion of alloanergized donor T cells to define the optimal dose of donor T cells able to improve immune reconstitution without causing severe GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the hospital staff who provided exceptional medical care and especially the patients and families who chose to participate in the studies.
These studies were supported by grants from the National Institutes of Health (Bethesda, MD; P01 AI41584 and P50 HL54785) and the Frank J. Hanna Jr Fund (Dana-Farber Cancer Institute, Boston, MA). J.K.D. is a recipient of a Career Development Award from the Leukemia & Lymphoma Society (White Plains, NY).
National Institutes of Health
Authorship
Contribution: J.K.D. performed immune reconstitution assays, collected data, performed statistical analysis and wrote the manuscript; J.G.G. designed the clinical study, provided patient care, and critically reviewed the manuscript; L.L.B. provided patient care and collected data; D.Y. performed immune reconstitution assays; L.M.N. developed and provided important reagents and laboratory resources and critically reviewed the manuscript; and E.C.G. conceived and designed the clinical study, provided patient care, and wrote the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eva C. Guinan MD, Associate Director, Center for Clinical and Translational Research, Associate Professor of Pediatrics, Harvard Medical School, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail: eva_guinan@dfci.harvard.edu.