Abstract
Bone marrow transplantation (BMT) potentially represents a novel therapy for the amelioration and even cure for multiple sclerosis (MS). It has important advantages over immunosuppressive drug treatments because, while effecting broad-based ablation of the immune system and autoreactive cells, it provides an important means for overcoming the resultant immunodeficiency, while possibly restoring self-tolerance. However, both of these benefits are predicated on a functional thymus that undergoes profound age-induced atrophy from puberty. Reversal of thymic atrophy has been achieved by several procedures, including removal of sex steroids by surgical or chemical (LHRH agonist) castration. Using a murine model of MS, experimental autoimmune encephalomyelitis (EAE), we combined BMT with androgen depletion to induce immune regeneration, and investigated the kinetics of increased thymic function on immune reconstitution and disease reduction. We show that androgen depletion significantly increased the efficacy of BMT to ameliorate the clinical signs of EAE while concurrently restoring the periphery with increased naive and regulatory lymphocytic populations. Upon rechallenge, mice with a regenerated thymus had a slower onset of clinical symptoms compared with mice undergoing BMT only. These results suggest that thymic regeneration strategies may be used as a complement to conventional BMT protocols for the treatment of MS.
Introduction
Multiple sclerosis (MS) is a devastating autoimmune disease of the central nervous system, if not initiated, certainly mediated as a consequence of an immune response against myelin antigens. MS is the most common cause of neurologic disability in young adults, displaying a relapsing-remitting course, with most patients entering a chronic progressive phase of steady decline in neurologic function.1 The etiology of this disease is still unclear, but based on altered immune responses in MS patients, the pathology of MS lesions, and its animal model, experimental autoimmune encephalomyelitis, the inflammatory element of MS pathogenesis is believed to be primarily T cell–mediated, possibly as the result of a breakdown in immune tolerance, in particular peripheral mechanisms.2 Recent thymic emigrants that escape central tolerance can still be susceptible to peripheral tolerance checkpoints such as regulatory T cells (Tregs). Tregs, which are also thymic dependent, increasingly appear to be pivotal in the maintenance of self-tolerance, with their elimination leading to the development of autoimmune diseases.3
Due to the heterogeneity of the disease, current immunosuppressive and modulatory treatments have shown only partial efficacy in ameliorating the course of MS. Bone marrow transplantation (BMT) therapies that aim to eliminate pathogenic immune cells by broad based immune ablation regimens decrease the severity and progression of disease in some MS patients who no longer responded to conventional treatments.4,5 The elimination of autoreactive lymphocytes is facilitated by conditioning regimens such as radiation therapy, chemotherapy, and/or specific immune cell depletion. More recently, monoclonal antibody–mediated blocking of B-cell activity has been clinically effective in MS patients.6 Not surprisingly, high-dose conditioning regimens (eg, 10 Gy total body irradiation) are more efficient at preventing spontaneous relapses than suboptimal irradiation (7 Gy total body irradiation),7 but with increased immune ablation, the need to regenerate lymphoid and myeloid compartments becomes critical. Whereas most lymphoid cell types such as natural killer T (NKT), CD8, and B cells recover 3 to 6 months after BMT in adult patients, the most vital CD4 naive T-cell population can take up to 2 years to recover, if at all.8,9 This prolonged period of immunosuppression can lead directly to opportunistic infections, contributing to some mortality associated with BMT.10 Consequently, although BMT and high-dose cytoreductive therapies are necessary for many cancer patients, it is currently of too high risk for a primarily nonfatal disease. It is therefore crucial that improvements be made to increase T-cell recovery in current BMT therapies, thereby enabling broader clinical applications.
Several studies have suggested that the delayed immune recovery in adults is attributed to the profound (loss of ∼90% of function) age-related thymic atrophy,11,12 the onset of which is most evident from puberty, concomitant with the associated increase in sex steroids.13 This leads to a severe reduction in the thymic output of naive T cells, which becomes most clinically relevant in postpubertal patients after immunodepleting diseases or therapies. Recently, our approach to overcoming thymic atrophy and improving bone marrow function has been achieved by the removal of sex steroids, via surgical and/or chemical castration with a known luteinizing hormone-releasing hormone agonist (LHRH-a).14 Such intervention has been shown to lead to a faster and more effective immune rebound after immunodepletion. As demonstrated by the assessment of peripheral organs from castrated C57Bl/6 mice, after intrathymic FITC injections as well as the analysis of T-cell receptor excision circle (TREC) of elderly males undergoing sex steroid ablation therapy for prostatic carcinomas, androgen ablation results in increased recent thymic emigrants.15 Significantly, this treatment has also been found to increase the rate of engraftment and reconstitution of donor cells after BMT.16 More recently, we have also shown this to be effective in humans.1
Thus, to determine whether androgen depletion can increase the efficacy of BMT in the treatment of MS, chronic experimental autoimmune encephalomyelitis (EAE; an animal model of MS) was induced in male C57Bl/6 mice by immunization with an encephalogenic myelin oligodendrocyte glycoprotein (MOG) peptide. EAE mice were treated at disease onset with conventional BMT coupled with androgen depletion by surgical castration (Cx).
We report that androgen depletion by surgical castration markedly improves conventional BMT by significantly reducing and slowing the clinical disease and progression of EAE. Although regeneration of the thymic and splenic compartments occurred, no increase in autoreactivity was observed. The marked increase in peripheral naive cells, combined with the increased presence of regulatory T cells, is thought to have contributed to this reduction in autoreactive proliferation and subsequent disease development. Furthermore, Cx/BMT mice displayed a slower onset of clinical symptoms after repriming with the MOG35-55 peptide, compared with mice treated with BMT only. Based on these findings, immune regeneration should be considered as an important addition to BMT protocols currently used for the treatment of MS.
Methods
Animals
Male 8- to 12-week-old C57Bl/6 mice were used throughout this study, with male 8- to 12-week-old B6.SJL-Ptprc (Ly5.1 congenic) mice used as donors for the BMT experiments and male C57Bl/6 mice for the adoptive transfer experiment. Mice were bred and maintained at the Monash University Central Animal Services and Precinct Animal Center under specific pathogen-free (SPF) facilities and obtained food and water ad libitum. All animal experiments were carried out in accordance with institutional animal ethics guidelines as set by the Alfred Medical Research and Education Precinct (AMREP) animal ethics committee.
Induction and scoring of EAE
EAE was actively induced by administration of 100 μL subcutaneously on the inner side of both hind flanks. The sterile inoculum consisted of 200 μg MOG35-55 peptide (GL Biochem, Shanghai, China) in PBS, emulsified with 100 μL complete Freund adjuvant (BD Biosciences, San Jose, CA) with 0.4 mg heat-inactivated Mycobacterium tuberculosis (strain H37 RA; Difco Laboratories, Detroit, MI). Mice were injected intravenously with 350 ng pertussis vaccine (Sapphire Biosciences, Sydney, Australia) in 100 μL sterile PBS immediately thereafter and 48 hours later. Clinical signs were scored as follows: 1 indicates flaccid tail; 2, hind limb weakness; 3, hind limb paralysis and/or forelimb weakness; 4, forelimb paralysis; and 5, moribund or dead, as described previously.17,18 For ethical reasons, all mice reaching a score of 4 were humanely killed. Mice were rechallenged 44 days after initial immunization, following the same procedure. Mice were monitored daily with fluid administration and mashed chow on the base of cages for all mice displaying a clinical score of 3.
Adoptive transfer of EAE
C57Bl/6 mice were actively induced with EAE. Nine days after immunization, spleens were removed, dissociated, red cell lysed with sterile lysis buffer (Sigma-Aldrich, St Louis, MO), and cultured with 25 μg/mL MOG35-55 peptide at 2 × 106 cells/mL in stimulation media (RPMI 1640 [Sigma-Aldrich] supplemented with 10% heat-inactivated fetal calf serum [Sigma-Aldrich], 100 U/mL penicillin [Sigma-Aldrich], 100 μg/mL streptomycin [Sigma-Aldrich], 2 mM l-glutamine [Invitrogen], and 50 mM 2-ME [Invitrogen]) and stimulated for 72 hours at 37°C with 5% CO2. During the last 48 hours of incubation, IL-2 and IL-12 were added at concentrations of 100 U/mL and 50 U/mL, respectively. After 72 hours, cells were harvested and transferred at 50 × 106 or more cells in 200 μL PBS intraperitoneally into recipient mice.19
Surgical androgen depletion
Twelve days after EAE immunization, a small scrotal incision was performed to reveal the testes in anesthetized mice. Testes were tied off with suture thread and removed with scissors.20 BMT-alone mice received the same procedure, without the removal of the testes. All mice undergoing surgery were given fluids subcutaneously to aid in recuperation.
Bone marrow transplantation
Donor bone marrow from Ly5.1 congenic mice was removed aseptically from femurs and tibias. On the same day as the castration surgery, mice were preconditioned with total body irradiation (1300 cGy 137Cs source) as split dose with 3 hours in between to reduce gastrointestinal toxicity. Mice were subsequently injected intravenously with 5 × 106 congenic bone marrow cells. Mice that underwent transplantation were given 3.2 mg/mL enrofloxacin antibiotics (Bayer, Pymble, Australia) in their autoclaved drinking water, for 2 weeks, and housed in sterilized microisolator cages.
Isolation of mononuclear cells from thymus, spleen, and central nervous system
After CO2 asphyxiation, mice were perfused with PBS and thymus, spleen, brain, and spinal cord were carefully removed. Cells from the thymi and spleens were gently dissociated into sterile fluorescence-activated cell sorting (FACS) buffer (PBS/2% BSA/0.02% azide). Splenic cells were incubated with sterile red cell lysis buffer (Sigma-Aldrich) for 1 minute and washed twice in FACS buffer. Infiltrating cells were isolated from the brain and spinal cord by gently pushing through a 100-μm mesh filter into FACS buffer. Cells were suspended in 4 mL of 30% Percoll (GE Healthcare, Uppsala, Sweden) in RPMI 1640 (SAFC Biosciences, Lenexa, KS), layered over 3.5 mL of 37% Percoll, and concentrated onto a 3.5-mL cushion of 70% Percoll by centrifugation at 100g for 20 minutes at 4°C. Cells were collected from the 37% to 70% interface and washed twice.
Flow cytometric analysis
For flow cytometric analysis, cells were washed in FACS buffer and stained with 30 μL primary antibody for 20 minutes. Cells were washed and where applicable were resuspended in 30 μL secondary antibody for 20 minutes. All staining procedures were carried out on ice, to retain viability of the cells. Cells were washed a final time and resuspended in 200 μL FACS buffer ready for acquisition on a 4–fluorescent channel FACScalibur (Becton Dickinson, Lincoln Park, NJ). Between 2 × 105 and 106 cells were collected for analysis using CellQuest software (Becton Dickinson). For all staining, an FcR block (clone 2.4G2) was used to prevent nonspecific binding of our antibodies to the Fc receptor. The following primary antibodies were used: FITC-conjugated anti-CD45.1 (clone A20; Pharmingen, San Diego, CA), Cy-5–conjugated anti-CD4 (clone RM4-5; Pharmingen), APC-conjugated anti-CD25 (clone PC61; Pharmingen), PE-conjugated anti-FoxP3 (clone FJK-16s; eBiosciences, San Diego, CA), APC-conjugated anti-CD45 (clone 30-11; Pharmingen), PE-conjugated anti-CD11b (clone M1/70; Pharmingen), APC-conjugated anti-CD62L (clone Mel-14; Pharmingen), CY-5–conjugated anti-CD44 (clone 1M7; Pharmingen), PE-conjugated anti-CD19 (clone 1D3; Pharmingen), purified mouse anti-GalC IgG (clone Icol), and rabbit immunoaffinity purified IgG anti-PDGFRα (Upstate Cell Signaling, Lake Placid, NY). The following secondary antibodies were used where applicable: Alexa Fluor 594 donkey anti–mouse IgG (Molecular Probes, Eugene, OR) and Alexa Fluor 568 goat anti–rabbit IgG (Molecular Probes).
Histology
Histologic analysis of CNS tissues was performed as previously described.21,22 Briefly, brain and spinal cord were dissected from PBS-perfused mice 42 days after immunization and fixed in 10% formalin (Ajax Chemicals, Sydney, Australia). Paraffin-embedded sections (5 μm) were cut from the brain, brain stem, and spinal cord. Sections were stained with hematoxylin-eosin (H&E), Luxol fast blue (LFB), and Bielschowsky silver impregnation to assess inflammation, demyelination, and axonal damage, respectively. Sections were scored blindly for semiquantitative histologic analysis of inflammation and demyelination. Microscopy was performed with an Olympus BX60 microscope (Olympus Imaging Australia, North Ryde, Australia) equipped with a 60×/1.4 NA objective. Images were recorded with a Sony DKC-500 digital camera (Tokyo, Japan) and transferred to Adobe Photoshop (Adobe, San Jose, CA) for minimizing and figure collation.
Proliferation and direct suppression assays
Spleens were removed from mice 42 days after immunization and splenocytes were cultured for 72 hours in triplicate in 96-well plates at a concentration of 2 × 105 cells/well in stimulation media and in the presence of 10 μg/mL of either anti-CD3e (145-2c11) and anti-CD28 (37.51), MOG35-55, guinea pig myelin basic protein, human myelin basic protein, or proteolipid protein. For the direct suppression assay, 2 × 104 FACS-purified (FACSVantage with DIVA options; BD Biosciences) CD4+CD25− cells were stimulated in triplicate with either 1 μg/mL anti-CD3e (145-2c11) or 10 μg/mL MOG35-55 peptide with FACS-purified CD4+CD25+ T regulatory cells added at graded Treg/responder ratios. To this culture, 105 irradiated (700 cGy) T cell–depleted splenocytes were added. Splenic T cells were depleted by incubation with a rat anti–mouse CD2 (RM2-1) for 20 minutes at 4°C. Cells were washed and resuspended in rat anti–mouse IgG (Fc)–conjugated immunomagnetic beads (Dynal, Lake Success, NY) at a target cell : bead ratio of 1:4 and incubated at 4°C for 30 minutes. Dynabeads with labeled cells attached were isolated using a Dynal magnetic particle concentrator and the unbeaded negative fraction was retained. For both assays, cells were stimulated for 72 hours at 37°C with 5% CO2, with the addition of 1 μCi (0.037 MBq)/well [3H] thymidine during the last 18 hours of culture. Cells were harvested onto filter mats and incorporated radioactive nucleic acid was counted on a Top Count Harvester (Packard Biosciences, Waltham, MA). Data are expressed as the mean plus or minus SEM SI values of triplicate measurements.
Anti-MOG antibody determination
Antibody reactivity to MOG35-55 was measured by enzyme-linked immunosorbent assay (ELISA) as previously described.23 In brief, sera collected at day 42 after immunization were tested at a dilution of 1/100 in 96-well plates (Nunc, Roskilde, Denmark) coated with either 5 μg/mL scrambled MOG peptide (H-Gly-Arg-Ser-Val-Leu-Asn-Val-Pro-Ser-Arg-Met-Glu-Trp-Arg-His-Phe-Tyr-Gly-Lys-Val-Tyr-OH; GL Biochem Shanghai) as a control antigen or 5 μg/mL MOG 35-55 as the test antigen, in 0.1 M carbonate buffer (pH 9.6). Specific antibody binding was revealed using an anti–mouse IgG biotin conjugate and streptavidin–horseradish peroxidase (Sigma-Aldrich). O-phenylenediamine (Sigma-Aldrich) was used as a substrate and the OD was measured at 490 nm. The results are expressed as the mean plus or minus sem of specific optical density of triplicate measurement.
Cytometric bead assay
For cytokine analysis, 2 × 105 splenocytes were stimulated with either 10 μg/mL CD3e (145-2c11) and anti-CD28 (37.51), or 10 μg/mL MOG35-55 peptide for 72 hours. Supernatant was removed and incubated with IL-2, IL-4, IL-5, IFN-γ, TNF, IL-10, and IL-17 mouse cytokine capture beads (Th1/Th2 Cytometric bead array kit; BD Biosciences) for 2 hours at room temperature. Data were acquired using the FACSCanto II (BD Biosciences) and analyzed using the BD CBA software.
Statistics
Statistical analysis was performed using the nonparametric, unpaired Mann-Whitney U test, Pearson correlations, and Fisher z test for correlations, with a P value less than .05 considered statistically significant.
Results
Androgen depletion significantly reduces the clinical signs of EAE
As a prelude to these experiments, EAE mice underwent surgical castration (Cx) at the onset of the disease to ascertain the direct effect of androgen depletion on disease progression. In agreement with a previous study,24 no difference in disease onset (13.0 ± 1.2 vs 13.0 ± 2.0), maximum clinical score (2.3 ± 0.3 vs 2.8 ± 0.3), cumulative clinical score (8.1 ± 0.9 vs 9.1 ± 1.0), and duration of the disease (9.0 ± 1.2 vs 9.0 ± 2.0) was observed between 5 castrated and 5 sham-castrated mice, respectively.
To assess the combined effect of surgical androgen depletion with BMT on the development of EAE, 3 groups of C57Bl/6 mice were immunized with MOG35-55 peptide. At disease onset, mice underwent BMT and Cx (Cx/BMT), underwent BMT in combination with a sham surgery to control for procedural stress (BMT), or remained untreated.
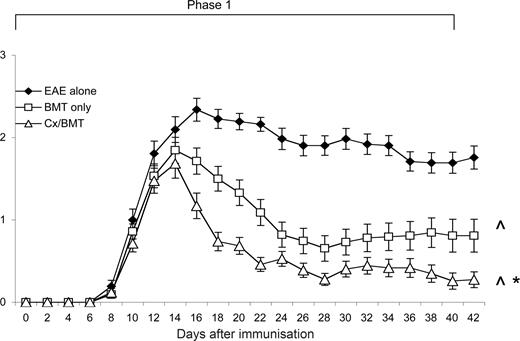
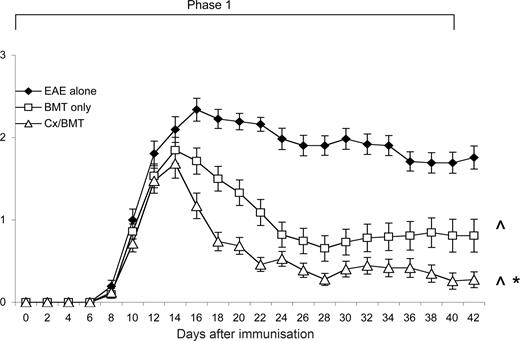
After EAE induction, mice were evaluated daily for clinical signs of disease. As shown in Figure 1, mice treated with either BMT alone or in combination with castration, displayed a significant reduction in clinical signs from 6 days after treatment compared with untreated EAE mice. It was also evident that androgen depletion increased the efficacy of BMT, with castrated mice displaying a further reduction in clinical signs (P = .032) and had a significantly reduced cumulative clinical score compared with both the BMT alone (18.54 ± 15.7 vs 32.38 ± 27.7; P < .05) and EAE-untreated groups (18.54 ± 15.7 vs 62.91 ± 20.3; P < .001; Table 1). Furthermore, in contrast to the 3 out of 43 mice treated with BMT only, none of the castrated mice suffered from treatment-related mortality.
Castration significantly increases the efficacy of BMT in reducing clinical disease. Young 8- to 12-week-old C57Bl/6 treated at day 12 after EAE induction with BMT only (n = 43) showed a significant reduction in clinical disease compared with untreated EAE mice (n = 31), whereas the mice treated with BMT in combination with castration (n = 43) displayed even further amelioration of disease. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus BMT only; ∧P < .05 versus EAE untreated.
Castration significantly increases the efficacy of BMT in reducing clinical disease. Young 8- to 12-week-old C57Bl/6 treated at day 12 after EAE induction with BMT only (n = 43) showed a significant reduction in clinical disease compared with untreated EAE mice (n = 31), whereas the mice treated with BMT in combination with castration (n = 43) displayed even further amelioration of disease. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus BMT only; ∧P < .05 versus EAE untreated.
Castration reduces demyelination and axonal damage in the CNS
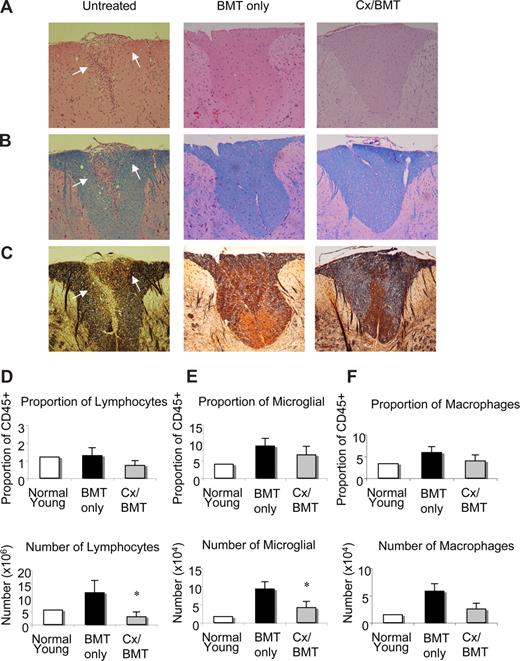
Histopathologic evaluation of the CNS tissues of untreated EAE mice at day 42 after immunization showed extensive inflammatory lesions, which were characterized by mononuclear cells, particularly abundant in the cerebellum and spinal cord (Figure 2A-C). Luxol fast blue and Bielschowsky staining revealed extensive pathology in the cerebellum and spinal cord, as indicated by the marked loss of myelin and severe axonal injury. By contrast, as illustrated in Figure 2A-C, histologic analysis of the CNS of mice treated with either BMT alone or in combination with castration showed a significant reduction in inflammatory lesions, with little or no demyelination and/or axonal damage or loss.
BMT leads to a reduction in infiltrates, demyelination, and axonal damage in the CNS. Representative spinal cord sections from untreated EAE mice, mice undergoing BMT, or mice undergoing BMT in combination with castration 12 days after EAE induction. Sections stained with (A) hematoxylin and eosin for infiltration, (B) Luxol fast blue for demyelination, and (C) Bielschowsky silver impregnation for axonal damage illustrating that EAE mice treated with either BMT only or BMT in combination with castration had little or no inflammatory infiltrates, demyelination, and axonal damage compared with untreated EAE mice. (D) Flow cytometric analysis of infiltrating lymphocytic population as described by CD45+CD4+CD11b− surface marker staining. Whereas proportions of infiltrating lymphocytes did not alter, their number significantly decreased in Cx/BMT mice. (E) Microglia cell analysis by flow cytometric analysis, as determined by CD45lo CD11b+ cell surface staining, demonstrated a significant reduction in the number of activated microglia cells in Cx/BMT-treated mice than the BMT-only mice, however proportions remain unchanged. (F) Macrophage and granulocyte populations in the CNS as examined by flow cytometric analysis of the CD45hiCD11b+ population. Graphed results indicate that there was no significant alteration between treatment groups in proportion and cellularity. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus BMT only. Original magnification ×60.
BMT leads to a reduction in infiltrates, demyelination, and axonal damage in the CNS. Representative spinal cord sections from untreated EAE mice, mice undergoing BMT, or mice undergoing BMT in combination with castration 12 days after EAE induction. Sections stained with (A) hematoxylin and eosin for infiltration, (B) Luxol fast blue for demyelination, and (C) Bielschowsky silver impregnation for axonal damage illustrating that EAE mice treated with either BMT only or BMT in combination with castration had little or no inflammatory infiltrates, demyelination, and axonal damage compared with untreated EAE mice. (D) Flow cytometric analysis of infiltrating lymphocytic population as described by CD45+CD4+CD11b− surface marker staining. Whereas proportions of infiltrating lymphocytes did not alter, their number significantly decreased in Cx/BMT mice. (E) Microglia cell analysis by flow cytometric analysis, as determined by CD45lo CD11b+ cell surface staining, demonstrated a significant reduction in the number of activated microglia cells in Cx/BMT-treated mice than the BMT-only mice, however proportions remain unchanged. (F) Macrophage and granulocyte populations in the CNS as examined by flow cytometric analysis of the CD45hiCD11b+ population. Graphed results indicate that there was no significant alteration between treatment groups in proportion and cellularity. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus BMT only. Original magnification ×60.
To determine the phenotype of infiltrating cells in the CNS tissue of mice treated with BMT only or in combination with androgen ablation, mononuclear cells were isolated from brain and spinal cord and analyzed by flow cytometry, 42 days after EAE induction. As shown in Figure 2D through F, the number of infiltrating CD45+CD4+CD11b− lymphocytes and proliferating CD45lo CD11b+ microglia cells in the CNS of androgen-ablated mice was reduced compared with BMT only. Proportions of lymphocytes and microglia cells did not change and no significant differences were noted in the number of CD45hi CD11b macrophages and granulocytes. These results demonstrated that androgen ablation combined with BMT significantly reduced the number of infiltrating cells in the CNS.
Androgen depletion modulates the cellular immune response
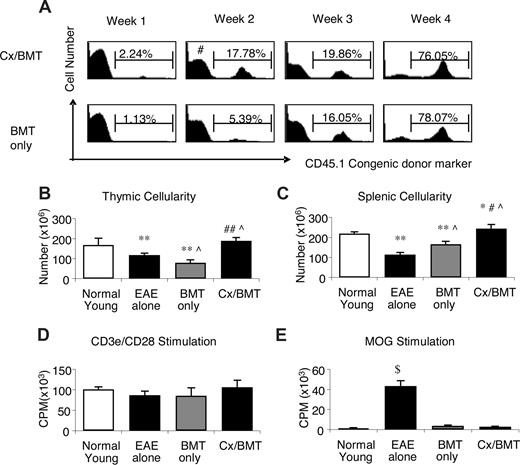
We next focused our attention on mechanisms potentially involved in the significant amelioration of EAE in the castrated mice after BMT. Weekly assessment of chimerism denoted by the congenic CD45.1 leukocyte cell surface antigen demonstrated that by 2 weeks after BMT, the castrated mice had a significantly higher proportion of donor cells in their periphery (16.3 ± 2.3 vs 6.4 ± 5.5; P = .032) than mice treated with BMT only. In these mice, similar levels of chimerism were reached only 4 weeks after BMT (78.6 ± 7.6 vs 81.4 ± 8.1; P = .69; Figure 3A). Along with the increased rate of donor reconstitution, castrated mice had increased thymic (1.9 × 108 ± 3.7 × 107 vs 7.9 × 107 ± 2.9 × 107; P = .032) and splenic (2.4 × 108 ± 4.6 × 107 vs1.7 × 108 ± 3.0 × 107; P = .032) cell numbers (Figure 3B and C, respectively).
Cx/BMT-treated mice display an increased rate of reconstitution and subsequent increased cellularity, with no alteration in autoreactivity. (A) Peripheral blood of Cx/BMT and BMT-only groups were analyzed by flow cytometry for the donor CD45.1 congenic cell surface marker expression. By week 2 after BMT, Cx/BMT mice demonstrate a significantly higher donor presence than BMT-alone groups and remained higher until week 4 after BMT, when the BMT-only mice achieved similar levels of chimerism. (B) Untreated EAE mice had a significantly reduced cellularity in the thymus, with irradiation conditioning in the BMT-only group inducing even further thymic atrophy. By contrast, castration overcame the degenerative effects of EAE and irradiation. (C) Similar results were seen in the spleen. (D,E) Whereas the general T-cell proliferative capacity did not differ between groups, MOG35-55–specific proliferation of mice treated with either BMT only or BMT in combination with castration was significantly reduced in comparison with untreated groups. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus normal young; #P < .05 versus BMT alone; **P < .01 versus normal young; ##P < .01 versus BMT alone; ∧P < .05 versus EAE alone; and $P < .001 versus all groups.
Cx/BMT-treated mice display an increased rate of reconstitution and subsequent increased cellularity, with no alteration in autoreactivity. (A) Peripheral blood of Cx/BMT and BMT-only groups were analyzed by flow cytometry for the donor CD45.1 congenic cell surface marker expression. By week 2 after BMT, Cx/BMT mice demonstrate a significantly higher donor presence than BMT-alone groups and remained higher until week 4 after BMT, when the BMT-only mice achieved similar levels of chimerism. (B) Untreated EAE mice had a significantly reduced cellularity in the thymus, with irradiation conditioning in the BMT-only group inducing even further thymic atrophy. By contrast, castration overcame the degenerative effects of EAE and irradiation. (C) Similar results were seen in the spleen. (D,E) Whereas the general T-cell proliferative capacity did not differ between groups, MOG35-55–specific proliferation of mice treated with either BMT only or BMT in combination with castration was significantly reduced in comparison with untreated groups. Results are expressed as mean plus or minus SEM from 2 or more independent experiments. *P < .05 versus normal young; #P < .05 versus BMT alone; **P < .01 versus normal young; ##P < .01 versus BMT alone; ∧P < .05 versus EAE alone; and $P < .001 versus all groups.
Although increased thymic and splenic cellularity is normally beneficial for immune regeneration after BMT,16 increased development and/or expansion of autoreactive T cells could be deleterious. To address this, lymphocytes from mice treated with either BMT alone or in combination with castration were stimulated with either anti-CD3e/CD28 antibodies or the MOG peptide. As shown in Figure 3D,E, both experimental groups responded equally well to specific and nonspecific stimuli and castration did not increase the proliferative capacity of the newly generated peripheral cells toward MOG35-55 (CD3e/CD28: Cx/BMT 106.4 × 103 ± 6.9 × 103 CPM vs BMT 85.3 × 103 ± 7.5 × 103 CPM; P = .545; MOG: Cx/BMT 2.7 × 103 ± 2.1 × 102 CPM vs BMT 3.7 × 103 ± 2.5 × 102 CPM; P = .121). Taken together, these data indicate that although androgen depletion alters the thymic-derived and peripheral lymphocyte pool, it does not appear to expand potentially autoreactive lymphocytes.
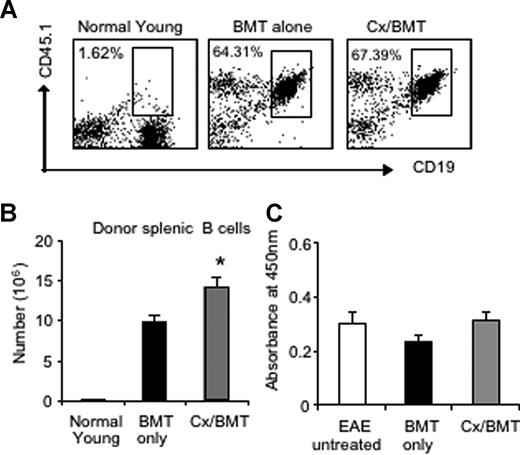
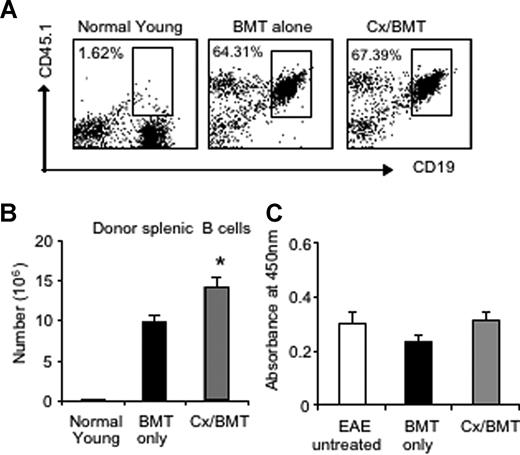
Given the pathogenic role of anti-MOG antibodies in MOG EAE and MS,17 we assessed the potential variations in the number of donor splenic B cells by flow cytometry as well as serum levels of anti-MOG antibodies by ELISA. Flow cytometric results revealed similar proportions of B cells between mice treated with BMT only or in combination with androgen depletion. However, castrated mice housed a significantly higher number of donor B cells in the periphery than BMT alone (BMT 9.78 × 106 ± 1.99 × 106 vs Cx/BMT 14.20 × 106 ± 2.70 × 106; P = .032; Figure 4A,B), thus contributing to the increased splenic cellularity as reported earlier. The significant increase in donor B cells did not influence antibody production, however, with all 3 groups—untreated EAE, BMT only, or BMT in combination with androgen depletion—displaying similar levels of autoantibodies (Figure 4C). These results suggest that the reduction in disease severity observed after castration and BMT was not due to a reduction in antibody production.
Androgen depletion increases the number of donor B cells but does not alter autoantibody production to MOG35-55. (A,B) Splenic donor B cells were analyzed by flow cytometry for CD19 and CD45.1 marker expression. Results showed that there was no significant difference in proportion between Cx/BMT- and BMT-alone–treated groups. CX/BMT-treated mice displayed significantly higher numbers of donor B cells. (C) This significant increase did not influence antibody production to MOG35-55. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments. *P < .05 versus BMT only.
Androgen depletion increases the number of donor B cells but does not alter autoantibody production to MOG35-55. (A,B) Splenic donor B cells were analyzed by flow cytometry for CD19 and CD45.1 marker expression. Results showed that there was no significant difference in proportion between Cx/BMT- and BMT-alone–treated groups. CX/BMT-treated mice displayed significantly higher numbers of donor B cells. (C) This significant increase did not influence antibody production to MOG35-55. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments. *P < .05 versus BMT only.
We next assessed whether the androgen depletion had any effect on the cytokine profiles of mice undergoing both the castration and BMT. To that effect, supernatants from nonstimulated and MOG35-55-stimulated splenocytes were analyzed using a cytometric bead assay. Splenocyte stimulation with MOG35-55 revealed no significant alterations in levels of IL-2, IL-4, IL-5, IFN-γ, and TNF expressed in mice treated with only BMT or BMT in combination with castration (data not shown). This indicated that the amelioration of EAE was not due to a skewing response toward a TH2 anti-inflammatory phenotype that had previously been described as protective in EAE.25
Androgen depletion significantly increases the regulatory T-cell population
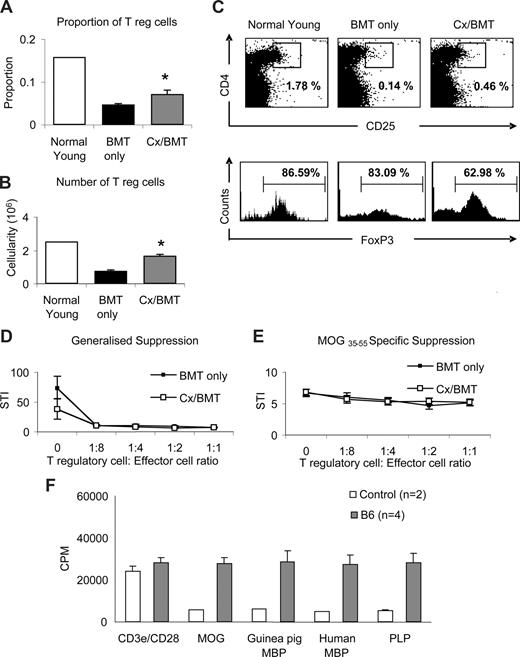
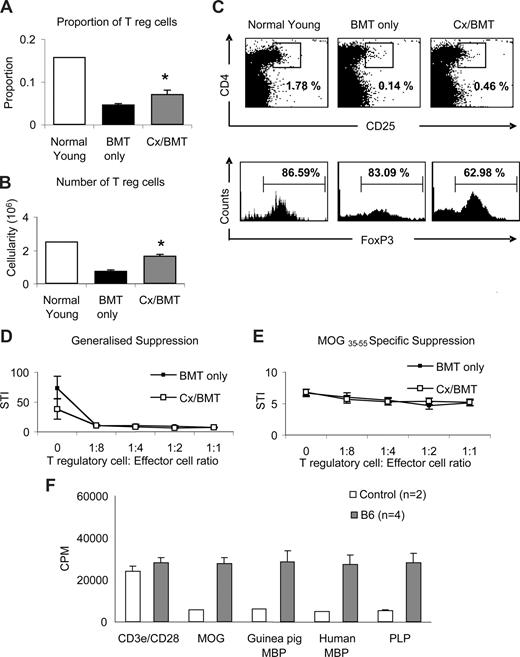
To further ascertain whether the dramatic recovery of clinical signs in Cx/BMT-treated mice was mediated by regulatory T cells, splenocytes were analyzed by flow cytometry for the presence of CD4+CD25+FoxP3+ Tregs. Castration significantly increased the proportion (Cx/BMT 0.07 ± 0.02 vs BMT 0.05 ± 0.01; P = .008) and number (1.7 × 105 ± 0.2 × 105 vs 0.8 × 105 ± 0.1 × 105; P = .008) of CD4+CD25+FoxP3+ Tregs in the spleen population of mice undergoing BMT combined with androgen depletion compared with mice treated with BMT only (Figure 5A,B). Interestingly, mice treated with BMT in combination with androgen depletion had fewer FoxP3+ cells in the CD4+CD25+ population than the BMT-only group (Figure 5C). Direct suppression assay with splenocytes from mice undergoing either BMT only or BMT in combination with androgen depletion showed no difference in generalized or MOG-specific suppression.
Castration increases the regulatory T-cell population but does not alter MOG-specific suppression. (A-C) Splenic regulatory T-cell populations were analyzed by flow cytometric expression of CD4+CD25+FoxP3+. Mice treated with BMT and castration displayed a significantly higher proportion and cellularity of FoxP3+ T regulatory cells than mice treated with BMT only. (D,E) Direct suppression assays on splenocytes were used to analyze the generalized (CD3e/CD28 stimulated) and specific (MOG35-55 stimulated) suppressive capabilities between mice treated with BMT or BMT in combination with castration. Although Cx/BMT mice had proportionally fewer FoxP3+ cells in their CD4+CD25+ population, they were equally capable of suppressing the generalized and MOG-specific activation of splenocytes. (F) Spleen cells from C57Bl6 mice adoptively transferred with MOG35-55–specific cells proliferated strongly in the presence of all antigens. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments, unless otherwise stated. *P < .01 versus BMT only.
Castration increases the regulatory T-cell population but does not alter MOG-specific suppression. (A-C) Splenic regulatory T-cell populations were analyzed by flow cytometric expression of CD4+CD25+FoxP3+. Mice treated with BMT and castration displayed a significantly higher proportion and cellularity of FoxP3+ T regulatory cells than mice treated with BMT only. (D,E) Direct suppression assays on splenocytes were used to analyze the generalized (CD3e/CD28 stimulated) and specific (MOG35-55 stimulated) suppressive capabilities between mice treated with BMT or BMT in combination with castration. Although Cx/BMT mice had proportionally fewer FoxP3+ cells in their CD4+CD25+ population, they were equally capable of suppressing the generalized and MOG-specific activation of splenocytes. (F) Spleen cells from C57Bl6 mice adoptively transferred with MOG35-55–specific cells proliferated strongly in the presence of all antigens. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments, unless otherwise stated. *P < .01 versus BMT only.
To further examine the possibility that bystander suppression26 could be operating in our experimental paradigm, we assessed the potential of epitope spreading to MOG, myelin basic protein (MBP), and proteolipid protein (PLP). Spleen cells from MOG35-55–immunized C57Bl/6 mice were passively transferred into naive C57Bl/6 mice. One week after transfer, splenocytes were prepared and stimulated with MOG, guinea pig or human myelin basic protein (MBP), or proteolipid protein. As shown in Figure 5F, cells from adoptively transferred C57Bl/6 mice proliferated strongly in the presence of all antigens (MOG: 27.6 × 103 ± 3.0 × 103 CPM, guinea pig MBP: 28.7 × 103 ± 5.3 × 103 CPM, human MBP: 27.2 × 103 ± 4.6 × 103 CPM; PLP: 28.2 × 103 ± 4.6 × 103 CPM). Given the evidence of epitope spreading between MOG and other antigens, generalized suppression is preferential in dampening the CNS-directed immune response.
Androgen depletion induces a shift toward a naive lymphocyte pool
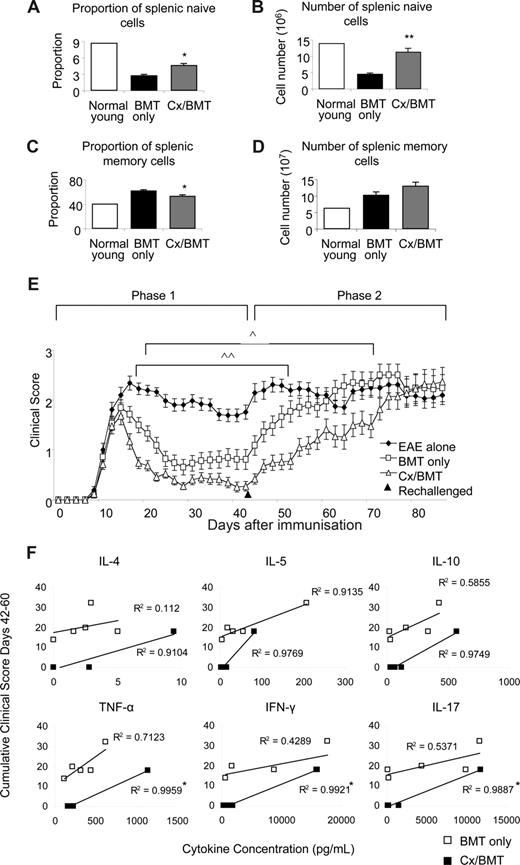
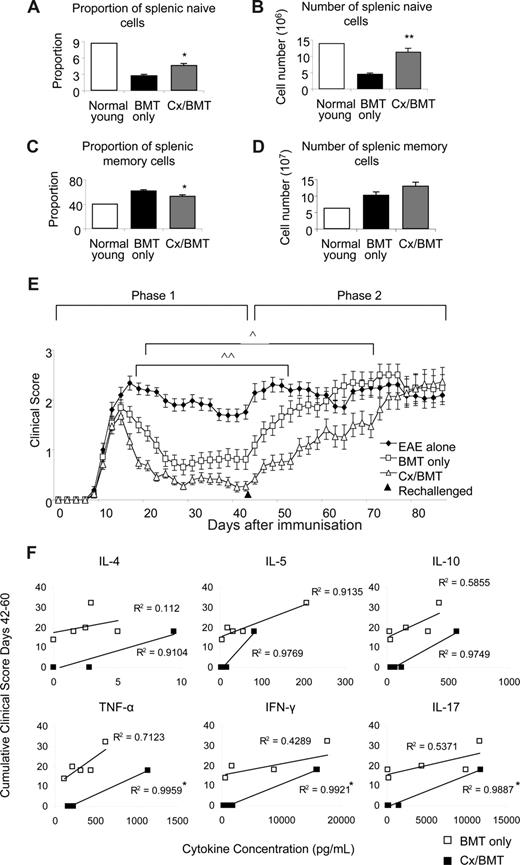
The clinical benefits of androgen depletion–enhanced BMT was investigated further by assessing the memory and naive spleen populations, taken 42 days after EAE induction. CD62LhiCD44− naive and CD44hiCD62Llo memory populations were analyzed by flow cytometry. Androgen depletion significantly increased naive cell proportions (Cx/BMT 4.7 ± 0.7 vs BMT 2.8 ± 0.4; P = .008) and numbers (11.4 × 106 ± 2.3 × 106 vs 4.6 × 106 ± 0.6 × 106; P = .008), while significantly reducing the memory cell proportion (Cx/BMT 53.9 ± 3.4 vs BMT 62.5 ± 3.1; P = .008) compared with mice treated with BMT only (Figure 6A-D). After rechallenge with the MOG35-55 peptide, the BMT-only group quickly progressed to EAE scores similar to those of untreated control EAE mice. By contrast, mice treated with BMT in combination with androgen depletion took twice as long to reach equivalent disease scores (Figure 6E). Cytokine profiles of rechallenged mice were assessed to determine whether androgen ablation initiated development of a more severe myelin-reactive Th1 or Th17 response.27 Supernatants from nonstimulated, CD3e/CD28-stimulated, and MOG35-55–stimulated splenocytes were analyzed using a cytometric bead assay. Splenocyte stimulation with MOG35-55 revealed a direct Pearson correlation between cumulative clinical score and the levels of IL-4, IL-5, IL-10, TNF-α, IFN-γ, and IL-17 in the Cx/BMT-treated mice, with correlations differing significantly compared with the BMT-only treated mice for the 3 latter cytokines assayed (Figure 6F). Overall, however, there was no significant difference in the total level of cytokine expression between the BMT-only and CX/BMT-treated mice for the cytokines assayed, suggesting IL-17 and Th1 responses failed to be enhanced in the rechallenged androgen-ablated mice. Collectively, these data imply that the switch toward a naive and regulatory pool after androgen depletion does not initiate a TH-1 or TH-17 inflammatory response, and may be important in delaying the time for such treated mice to succumb to severe disease.
Castration significantly increases the naive population. Naive and memory splenic cells as denoted by CD62LhiCD44− and CD62LloCD44hi, respectively, were analyzed by flow cytometry. (A,B) Results indicated a significant increase in proportion and number of naive cells in the Cx/BMT group, (C,D) and a significant reduction in the proportion of memory cells. (E) In contrast to BMT only–treated mice, Cx/BMT-treated mice displayed a significant delay in developing severe EAE. (F) Androgen ablation did not enhance a TH-1 or TH-17 immune response as assayed by cytometric bead analysis of MOG35-55–stimulated splenocyte supernatant. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments. **P < .01 versus BMT only; *P < .01 versus BMT only; ∧P < .05 BMT only versus Cx/BMT; and ∧∧P < .01 BMT only versus EAE untreated.
Castration significantly increases the naive population. Naive and memory splenic cells as denoted by CD62LhiCD44− and CD62LloCD44hi, respectively, were analyzed by flow cytometry. (A,B) Results indicated a significant increase in proportion and number of naive cells in the Cx/BMT group, (C,D) and a significant reduction in the proportion of memory cells. (E) In contrast to BMT only–treated mice, Cx/BMT-treated mice displayed a significant delay in developing severe EAE. (F) Androgen ablation did not enhance a TH-1 or TH-17 immune response as assayed by cytometric bead analysis of MOG35-55–stimulated splenocyte supernatant. Results are expressed as mean plus or minus SEM of 5 to 10 mice from 2 or more independent experiments. **P < .01 versus BMT only; *P < .01 versus BMT only; ∧P < .05 BMT only versus Cx/BMT; and ∧∧P < .01 BMT only versus EAE untreated.
Discussion
Autologous BMT is one of the therapeutic options for the treatment of progressive MS. So far, initial clinical trials have proven inconclusive, with the risk of mortality possibly outweighing its benefits.11,28 Here we report for the first time that the efficacy of conventional BMT in ameliorating EAE in mice can be significantly enhanced by sex steroid ablation, a procedure inducing thymic regeneration.
Controversy surrounds the precise role of sex steroids in the pathogenesis and incidence of autoimmunity, with many groups suggesting that sex hormones, due to their strong immunosuppressive qualities, are actually protective in MS and EAE.21,29-31 It is also well documented that although females have an increased susceptibility to MS (2-3:1), male patients can actually suffer a more severe disease.32 However, a recent pilot study has indicated that testosterone administration over a 12-month period to relapsing-remitting male patients had little if any effect on the clinical symptoms of MS.33 Likewise, our study shows that castration by itself had no effect on EAE susceptibility, a finding in agreement with the work reported by Palaszynski et al24 for C57Bl/6 mice. These results are at variance with other published studies, particularly those of Voskuhl and Palaszynski,34 who reported that castrated SJL mice developed a more severe EAE disease.34 The reason for these discrepancies is not clearly apparent but could possibly be explained by strain differences. Given that the susceptibility of MS is in part under environmental and genetic influences, it would be of interest to ascertain whether the putative beneficial effect of androgen ablation is dependent on the EAE model used and/or the species and strain of animals used.24,35,36
The thymus supports the development of T cells from hematopoietic progenitor cells migrating from the bone marrow. During the first years of life thymic activity is highest, but progressively declines, resulting in a diminished naive T-cell output in adults. Underlying causes of thymic involution may be degeneration of the stromal thymic network that provides survival and differentiation factors for developing T cells, or insufficiency of the progenitor cells to home and/or develop in the aged thymus.37 In young people, the reduced thymic output is insignificant, with the peripheral T-cell compartment under compensatory homeostatic control. However, immunocompromised individuals, including the aged and patients depleted of T cells after HIV infection or immunosuppressive conditioning regimens, require a functioning thymus to replenish the peripheral T-cell compartment and overcome the reduced levels of naive T cells and lack of CD4 T lymphocytes needed to fight opportunistic infections.38,39
As reported in this study, the combination of conventional BMT with androgen depletion can ultimately reduce the time frame of immunosuppression through an increased rate of thymic reconstitution. Previous studies have shown increases in lymphocyte numbers in both thymic and splenic organs after castration.40 Our work corroborates and extends these observations by demonstrating that the significant increases in lymphoid cellularity are due to an enhanced naive lymphocyte pool, with no evidence of increased autoreactivity. These observations are clinically relevant in view of the current BMT predicament whereby reduced naive lymphocytes available to fight conditioning regimen–associated infections often leads to mortality. The expansion of naive splenocytes also abrogated the problem of irradiation-induced lymphopenia, often associated with secondary autoimmunity.41 Furthermore, the significant skewing toward a naive phenotype with androgen depletion contributed to the slower onset of severe disease in Cx/BMT upon rechallenge with MOG35-55. It was also noted that androgen ablation did not skew these naive T cells toward differentiating into effector TH-1 or TH-17 responding T cells, a subset of T cells reported to be pathogenic in the progression of EAE.42 Our results take particular significance in view of recent studies, demonstrating that the in vitro differentiation of Th-17 cells from naive CD4+ T cells occurs via an IL-6– and TGF-β–dependent pathway.43,44 It is noteworthy that TGF-β has been shown to initiate the differentiation of regulatory T cells, a cell population that, as reported here, is significantly altered after androgen ablation.
We found that, in addition to immune reconstitution, regulatory T cells may also be involved in the reduction of EAE disease observed after sex steroid ablation. Notably, castration increased both the number and proportion of Tregs (CD4+CD25+Foxp3+) in those who underwent Cx/BMT compared with BMT alone. In addition, there were more CD4+ T cells expressing CD25, indicating an activated phenotype. Using a direct suppression assay, we show that sorted CD4+CD25+ splenocytes from Cx/BMT were equally able to suppress the MOG-specific and CD3e/CD28-activated spleen cells, despite there being proportionally fewer Tregs within this subset. This would imply that androgen depletion not only increased the number of Tregs but may also have increased their suppressive capacity on a per cell basis. These results are in line with several studies demonstrating the important role of regulatory T cells in controlling autoimmunity and more specifically EAE.4,45,46 A recent report47 showed that type II monocytes play a major role in the differentiation of naive T cells into T regula-tory cells, and that the in vivo transfer of these cells into mice with EAE reversed paralysis. Whether such subset of CD8+CD122+ regulatory cells48 is in part responsible for the amelioration of EAE after BMT and androgen depletion is not known but is currently the focus of another investigation. In this context, it is of relevance to note that to promote this differentiation, a TH2 polarization is required49 as seen with the administration of Glatiramer acetate, a synthetic copolymer prescribed for the treatment of relapsing-remitting MS.50 Importantly, our results showed no skewing from a TH1 to a TH2 phenotype. Studies by Kuchroo and colleagues (Korn et al51 ) have shown that the presence of T regulatory cells in the CNS of EAE mice in the presence of inflammatory cytokines, such as IL-6 and TNF, does not always necessarily correlate with disease amelioration. Here we show that androgen depletion significantly reduced the number of infiltrating lymphocytes in the CNS of mice treated with BMT combined with androgen depletion.
The treatment of multiple sclerosis is emerging as requiring a multifaceted approach, including the dampening of the inflammatory immune response, the reinduction of tolerance, and the regeneration of damaged tissue. BMT combined with retroviral-mediated autoantigen encoding gene transfer has the potential to reestablish tolerance to target autoantigens through induced peripheral antigen gene expression in the thymus and subsequent negative selection.52 Mezey et al53 demonstrated the presence of donor neuronal cells in BMT-treated leukemic patients, with the youngest patient (2 years of age at the time of BMT) having the greatest proportion of donor neuronal cells. This raises the question of whether immune regeneration by sex steroid ablation can increase the proportion of donor neuronal cells in an adult transplantation setting and subsequently enhance repopulation and regeneration of the damaged CNS. Cell replacement therapy is another attractive approach for myelin repair, with Schwann cells, oligodendrocyte progenitors, olfactory ensheathing cells, and neural stem cells having been shown to form myelin after transplantation into the demyelinated CNS.54 Moreover, the injection of mesenchymal stem cells (MSCs) before and at the onset of EAE has been shown to reduce clinical signs, postulated to be due to the immunomodulatory effects of MSCs.55,56 Together these combined tolerance-inducing and regenerating therapies, enhanced by temporary androgen ablation, may eventually lead to the treatment of autoimmune diseases in general and that of MS in particular.
In summary, the present study demonstrated that sex steroid ablation enhanced BMT therapy in ameliorating disease in EAE-induced mice. Androgen depletion significantly regenerated the cellular components of the primary lymphoid organs after immunoablation, while controlling the activity of MOG-specific autoreactivity by increasing the Foxp3+ Treg population. Importantly, we have shown that sex steroid ablation can increase the rate of donor reconstitution, therefore reducing the period of immunosuppression and subsequent risk of infections that currently plague BMT therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Hugh Reid, Christopher Siatskas, and Anne Fletcher for their insightful comments and help with this study. We also thank Dr Shunhe Wang, Mark Malin, Jade Barbuto, Jade Crabb, Katerina Vlahos, and Tamara Lowen for expert technical assistance.
This work was supported by grants from the National Health and Medical Research Council of Australia (Canberra, Australia), Norwood Immunology (Victoria, Australia), the Australian Stem Cell Centre, the Baker Foundation (Melbourne, Australia), and the national Multiple Sclerosis Society of New York (New York, NY).
Authorship
Contribution: A.L.B. performed experiments, analyzed results, and wrote the paper; A.P.C. helped with results analysis and drafting of the paper; and C.C.B and R.L.B. designed the research and helped with results analysis and drafting of the paper.
Conflict-of-interest disclosure: R.L.B. is Chief Scientific Officer of and A.P.C. is a consultant to Norwood Immunology. All other authors declare no competing financial interests.
Correspondence: Richard Boyd, Thymic Regeneration Laboratory, Monash Immunology and Stem Cell Laboratories, Level 3 STRIP Building 75, Monash University, Wellington Road, Clayton, Victoria, Australia 3800; e-mail: richard.boyd@med.monash.edu.au.
References
Author notes
*C.C.B and R.L.B. contributed equally to this study.