High levels of granulocyte/macrophage–colony-stimulating factor (GM-CSF) autoantibodies are thought to cause pulmonary alveolar proteinosis (PAP), a rare syndrome characterized by myeloid dysfunction resulting in pulmonary surfactant accumulation and respiratory failure. Paradoxically, GM-CSF autoantibodies have been reported to occur rarely in healthy people and routinely in pharmaceutical intravenous immunoglobulin (IVIG) purified from serum pooled from healthy subjects. These findings suggest that either GM-CSF autoantibodies are normally present in healthy people at low levels that are difficult to detect or that serum pooled for IVIG purification may include asymptomatic persons with high levels of GM-CSF autoantibodies. Using several experimental approaches, GM-CSF autoantibodies were detected in all healthy subjects evaluated (n = 72) at low levels sufficient to rheostatically regulate multiple myeloid functions. Serum GM-CSF was more abundant than previously reported, but more than 99% was bound and neutralized by GM-CSF autoantibody. The critical threshold of GM-CSF autoantibodies associated with the development of PAP was determined. Results demonstrate that free serum GM-CSF is tightly maintained at low levels, identify a novel potential mechanism of innate immune regulation, help define the therapeutic window for potential clinical use of GM-CSF autoantibodies to treat inflammatory and autoimmune diseases, and have implications for the pathogenesis of PAP.
Introduction
Granulocyte/macrophage–colony-stimulating factor (GM-CSF) is a pleiotropic cytokine regulator of myeloid and other immune and nonimmune cells that is required for terminal differentiation of alveolar macrophages in the lungs and regulates the basal functional capacity of circulating neutrophils in mice and humans.1,,,,,–7 The paracrine,3,8 autocrine,9 and endocrine10 effects of GM-CSF are mediated via heterologous cell-surface receptors11 reported to stimulate myeloid cell survival at low GM-CSF concentrations, and survival, proliferation, differentiation, and antimicrobial functions at high concentrations.12 Normally, GM-CSF is present at very low or undetectable levels in the serum and tissues in both mice and humans.5,13 Nonetheless, these low levels are critical because GM-CSF–deficient mice have impaired myeloid cell functions, increased mortality from microbial infections, and a lung phenotype characterized by progressive surfactant accumulation as a result of impaired alveolar macrophage surfactant catabolism.3,5,14,,–17
Autoimmune pulmonary alveolar proteinosis (PAP) is a human disease characterized by high levels of GM-CSF autoantibodies and respiratory insufficiency as a result of pulmonary surfactant accumulation4,18,19 with features similar in nearly every respect to those seen in GM-CSF knockout mice.3 Disease pathogenesis is thought to be mediated by GM-CSF autoantibodies, which eliminate GM-CSF bioactivity20 and impair GM-CSF–dependent myeloid cell functions.5
Sustained elevation of GM-CSF also seems to be detrimental because transgenic mice nonspecifically overexpressing GM-CSF develop a fatal syndrome of myeloproliferation and inflammation-related tissue destruction.21 Furthermore, increased local expression of GM-CSF occurs in rheumatoid arthritis in humans, and neutralization of GM-CSF ameliorates disease development in animal models of rheumatoid arthritis22 and multiple sclerosis,23 indicating that GM-CSF may be involved in the pathogenesis of inflammatory and autoimmune diseases.24 These findings strongly suggest that GM-CSF is tightly maintained at very low but critical levels in both humans and mice.
GM-CSF autoantibodies are consistently detected and comprise the major anti-cytokine activity in pharmaceutical intravenous immunoglobulin (IVIG) prepared from pooled serum of healthy subjects.25 In contrast, GM-CSF autoantibodies have been rarely detected in the serum of healthy persons25 and, when present, levels were far lower than in patients with PAP.26 These seemingly paradoxical findings suggest that the pooled serum used to prepare pharmaceutical IVIG may include serum from perons who seem healthy but have high levels of serum GM-CSF autoantibodies. This possibility is consistent with the recent report that 31% of people with autoimmune PAP were asymptomatic.27 Alternatively, GM-CSF autoantibodies may normally be present in healthy people but at low levels and/or in a form not detected in typical immunoassays. Other cytokine autoantibodies have been reported, although their significance remains unclear.28,29
We hypothesized that GM-CSF autoantibodies are ubiquitous in humans and function by scavenging and neutralizing free GM-CSF, thereby reducing nonspecific endocrine signaling and myeloid cell priming. Our experiments address the question of why high levels of GM-CSF autoantibody are virtually 100% specific and sensitive for a diagnosis of autoimmune PAP,4,19,27,30 yet the level of autoantibody does not correlate with the severity of the disease.31 We tested the hypothesis that GM-CSF autoantibodies rheostatically reduce myeloid cell functions and, above a critical threshold, eliminate GM-CSF signaling altogether. Results provide an estimate of this critical threshold and its association with PAP, help define the therapeutic window for potential future use of GM-CSF autoantibodies to treat inflammatory or autoimmune diseases, and describe a previously unrecognized potential mechanism of innate immune regulation.
Methods
Participants
The institutional review board of the Cincinnati Children's Hospital Medical Center approved this study. All participants or their legal guardians gave written informed consent; minors gave assent in accordance with the Declaration of Helsinki. Volunteers were enrolled into the study as healthy subjects. This group included 57 women and 15 men; mean (± SE) age was 30 plus or minus 0.63 years. All were nonsmokers, were disease-free without a history of major illness, and were symptom-free at the time of enrollment in the study. Patients with autoimmune PAP were recruited from the Rare Lung Diseases Clinic at the University of Cincinnati Medical Center, the Cincinnati Children's Hospital Medical Center, or Niigata Medical and Dental University. The diagnosis of autoimmune PAP was based on clinical and radiographic findings, an open lung biopsy, transbronchial lung biopsy, or cytologic analysis of bronchoalveolar lavage cells and fluid4 and a positive GM-CSF autoantibody test.5 This group included 12 women and 11 men; mean age ( ± SE) was 38 plus or minus 4.0. Of these, 2 (1 man, 1 woman) were in remission (defined as being asymptomatic at the time of enrollment and not requiring treatment of PAP lung disease in the preceding 5 years). All others had active PAP lung disease (defined as having symptoms typical of PAP [eg, dyspnea] and an ongoing requirement for treatment for PAP lung disease at the time of enrollment into the study).
Reagents
Phycoerythrin-conjugated anti–human CD11b antibodies (347557)5 and fluorescein isothiocyanate–conjugated anti–human CD16 antibodies (555406)5 were from BD Biosciences (San Jose, CA). Antibodies against STAT5 (SC-835) and actin (SC-1616) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against STAT5A (71-2400) and STAT5B (71-2500) were from Zymed Laboratories (South San Francisco, CA). The phospho-STAT5 antibody (05-495) was from Millipore (Billerica, MA). Horseradish peroxidase (HRP)–conjugated goat anti–human IgG antibodies (A-2290) and rabbit anti–bovine serum albumin (BSA; B-7276)32 were from Sigma-Aldrich (St Louis, MO). Rabbit anti–human GM-CSF polyclonal antibodies (AB-9667) were from Abcam (Cambridge, MA). Biotinylated goat anti–human GM-CSF polyclonal antibodies (BAF215) were from R&D Systems (Minneapolis, MN). Recombinant human GM-CSF was from Berlex Laboratories (Wayne, NJ; yeast-derived, glycosylated form [Leukine]), Invitrogen (Carlsbad, CA; Escherichia coli–derived, unglycosylated form), or PerkinElmer Life and Analytical Sciences (E coli–derived, 125I-labeled). The GM-CSF–dependent cell line TF-1 (CRL-2003) was from ATCC. Recombinant IL-8 was from R&D Systems. Protein G columns (17-0404-01) and HiTrap NHS-activated affinity chromatography columns (17-0716-01) were from GE Healthcare (Chalfont St Giles, United Kingdom). Microcon YM-100 filters (42 424) were from Millipore. Nile red–labeled fluorescent microspheres (FP-2056-2) were from Spherotech (Lake Forest, IL). Diethylenetriamine pentaacetic acid (D6518) and ExtrAvidin HRP solution (E2886) were from Sigma-Aldrich. The RIPA Lysis Buffer Kit (24948) was from Santa Cruz Biotechnology.
Purification of GM-CSF autoantibodies
IgG was isolated from serum or commercial IVIG using protein G affinity chromatography as directed by the manufacturer. To remove bound GM-CSF, purified IgG was subjected to ultrafiltration under acidic conditions, pH 2.8, using Microcon YM-100 filters as directed by the manufacturer.29 IgG recovered from the retentate cup in phosphate-buffered saline (PBS), pH 7.4, was further purified by GM-CSF affinity chromatography on GM-CSF–coupled NHS HiTrap columns as described previously.5,20 In brief, purified, ultrafiltered IgG was loaded onto the GM-CSF affinity column, and the effluent and 10 mL wash buffer was collected (unbound fraction). GM-CSF–bound proteins were then eluted using 10 mL 100 mmol/L glycine-HCl (pH 2.8; bound fraction).
Far-Western blotting
Bound or unbound immunoglobulin fractions from GM-CSF affinity chromatography were fractionated by sodium dodecyl sulfate (SDS)–polyacrylamide electrophoresis (PAGE) on 2% to 15% gradient gels under nonreducing conditions (30 mA, 150 minutes). Fractionated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes by electroblotting (12 volts, 75 minutes). Membranes were incubated with blocking solution (PBS containing 1% (wt/vol) BSA and 0.1% (vol/vol) Tween 20; 4°C, overnight), washed, and then incubated with 125I–GM-CSF (0.16 nM, room temperature, 1 hour), washed, and subjected to autoradiography to localize bound GM-CSF.
Liquid chromatography/tandem mass spectroscopy
GM-CSF–bound proteins were fractionated by gel electrophoresis as above. The single visible bands corresponding in molecular mass to that of IgG were cut out of the gel, minced, digested with trypsin (37°C, overnight), and evaluated with Micromass Quadrupole Time-Of-Flight II mass spectrometer (Waters, Milford, MA). Results were analyzed using the Mascot search engine.33,34 The top 50 best matching proteins for each subject were analyzed. All matches had a probability-based molecular weight search (Mowse) score over 64 that indicated a specific, nonrandom match (P value < .05).
ELISA
IgG subclasses.
The concentration of IgG subclasses in affinity-purified GM-CSF autoantibodies was measured using a commercial enzyme-linked immunosorbent assay (ELISA [99-1000; Zymed Laboratories]).
GM-CSF autoantibody.
Serum GM-CSF autoantibody levels were measured using a sandwich ELISA as described previously5,20 with slight modifications. In brief, microtiter plates (96-well, Maxisorp; Nalge Nunc International, Rochester, NY) were coated with recombinant human GM-CSF (1 μg/mL in PBS, 4°C, overnight), washed (PBS containing 0.1% Tween 20), blocked with Stabilcoat (room temperature, 1 hour; Surmodics, Eden Prairie, MN). Serum samples were diluted 1/100 (for healthy subjects) or 1/3000 (for patients with PAP) in sample dilution buffer (PBS, 1% [wt/vol] BSA, 0.1% [vol/vol] Tween 20), and 50 μL were incubated in duplicate wells (room temperature, 40 minutes). Plates were washed and incubated with ammonium acetate (10 mM, pH 5.0, room temperature, 15 minutes) to remove nonspecific binding. Bound IgGs were detected with goat anti–human IgG-HRP diluted 1/3000 with sample dilution buffer (room temperature, 30 minutes) and imaged with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (50 μL; T4444; Sigma-Aldrich) followed by addition of 1 N H2SO4 and read at 450 nm wavelength with Benchmark ELISA plate reader (Bio-Rad Laboratories, Hercules, CA). The accuracy and precision of the assay were determined (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Free GM-CSF.
Serum concentrations of free GM-CSF (ie, not bound to GM-CSF autoantibody) were measured using Quantikine HS Human GM-CSF ELISA kits (R&D Systems) according to the manufacturer's instructions. The sensitivity of the assay was 0.26 pg/mL.
Total GM-CSF.
Serum concentrations of total GM-CSF (ie, autoantibody-bound plus free GM-CSF) were measured using a novel sandwich ELISA based on a method developed for measurement of HIV-1 p24 antigen.35 Microtiter plates were coated with capture antibody (PBS containing 1 μg/mL rabbit anti–human GM-CSF antibody, 4°C, overnight), and blocked with PBS containing 1% (wt/vol) BSA. To evaluate the detection of free GM-CSF and autoantibody-bound GM-CSF in serum, a set of standard samples composed of recombinant human GM-CSF (Leukine) ranging from 0 to 30 ng/mL was prepared in mouse serum in the absence or presence of purified human GM-CSF autoantibodies (30 μg/mL). Aliquots (10 μL) of serum or standard control samples were mixed with 20 μL pretreatment buffer (SDS-HD buffer; PBS, 1% SDS, 1.5 mM diethylenetriamine pentaacetic acid, pH 7.4, and incubated (95°C, 5 minutes). After chilling briefly on ice, 270 μL PBS containing 1% (wt/vol) BSA was added to quench residual SDS. Duplicate aliquots (50 μL) of samples were applied to wells, and the plates were incubated (room temperature, 90 minutes), washed, and then incubated with detection antibody solution (PBS containing 1% [wt/vol] BSA, 500 ng/mL biotin-conjugated goat anti–human GM-CSF antibody; room temperature, 90 minutes). Detection was enhanced by incubation with ExtrAvidin HRP solution (diluted 1/250; room temperature, 30 minutes), imaged by TMB solution followed by addition of 1 N H2SO4 and read at a wavelength of 450 nm with Benchmark ELISA plate reader.
Phospho-STAT5 immunoblotting
Neutrophils were isolated on Ficoll gradients, followed by red blood cell lysis. More than 97% of the isolated cells consist of granulocytes. Isolated neutrophils were resuspended (5 × 106 cells/mL) in assay buffer (Hank balanced salt solution [HBSS] containing 10% fetal bovine serum, 25 mmol/L HEPES, pH 7.4) and incubated with various concentrations of purified GM-CSF autoantibodies (0, 0.01, 0.1, 0.5, 1.0 μg/mL) and GM-CSF (Leukine, 10 ng/mL) for 15 minutes at 37°C. After incubation, cells were collected by centrifugation, washed with PBS, and suspended in 200 μL RIPA buffer, which consisted of 0.05 mol/L Tris-HCl, pH 8.0, 0.15 M NaCl (Tris-buffered saline [TBS]), 1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] SDS, 0.004% [wt/vol] sodium azide) containing 2% (vol/vol) proteinase inhibitor cocktail, phenylmethylsulfonyl fluoride, and sodium orthovanadate as directed by the manufacturer. Samples were kept on ice for 30 minutes, and soluble lysate was collected after removing insoluble debris (9000g, 4°C, 15 minutes). Neutrophil lysate was then fractionated on SDS-PAGE gels (4%-12% Tris Glycine Gel; Invitrogen) and transferred to PVDF membranes by electroblotting. After blocking the membrane with TBS, 5% [wt/vol] dry milk, 0.1% [vol/vol] Tween 20 (4°C, overnight), phosphorylated-STAT5 was detected with murine anti–phospho-STAT5 antibody (diluted 1/500) followed by the incubation with HRP-conjugated sheep anti–murine IgG and imaged with ECL-Plus (GE Healthcare) as directed by the manufacturer. This procedure was used for measuring STAT5A, STAT5B, total STAT5, and actin in the same samples with the appropriate antibodies. Band intensity was quantified using KODAK Image Station 440FC equipped with KODAK 1D Image Analysis Software (Carestream Health, Rochester, NY), and the ratio of phosphorylated STAT5 to total STAT5 was calculated.
Serum GM-CSF–neutralizing capacity (TF-1 cell) assay
GM-CSF neutralization activity was evaluated using the GM-CSF–dependent cell line, TF-1 as described previously.20 In brief, cells were cultured in the absence or presence of GM-CSF autoantibodies purified from equal volumes (30 μL) of serum or IVIG (600 μg/well). After 4 days in culture, cell proliferation was measured using the TACS 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay kit (R&D Systems) according to the manufacturer's instructions.
Neutrophil CD11b stimulation index assay
The neutrophil stimulation assay was performed as described previously.5 In brief, aliquots of heparinized fresh whole blood were incubated with GM-CSF, and then cell-surface CD11b levels were quantified by flow cytometry. The CD11b stimulation index is calculated as the mean fluorescent intensity of stimulated neutrophils minus the mean fluorescent intensity of unstimulated neutrophils divided by mean fluorescent intensity of unstimulated neutrophils and multiplied by 100.
Neutrophil chemotaxis assay
Neutrophils were isolated as above and suspended (4 × 106 cells/mL) in HBSS supplemented with 0.1% (wt/vol) BSA, 1 mM CaCl2, and 1 mM MgCl2 with or without various doses of GM-CSF autoantibodies. Neutrophil chemotactic capacity was evaluated using Transwell (Corning Life Sciences, Acton, MA) with 3-μm pore size with 10 ng/mL rhIL-8 as a chemoattractant. One hundred microliters of cell suspension was transferred to upper chamber and incubated for 2 hours at 37°C, 5% CO2. After incubation, neutrophils migrating into the lower chamber were enumerated using a hemocytometer.
Neutrophil phagocytosis assay
The phagocytic capacity of neutrophils in whole blood (hereafter called the phagocytic capacity) was measured by flow cytometry as described previously.5 In brief, triplicate samples of heparinized human blood were incubated with IgG-opsonized fluorescent microspheres32 with gentle orbital rotation. Uptake of microspheres by neutrophils was evaluated with flow cytometry. Phagocytic capacity was calculated as the percentage of neutrophils containing internalized microspheres multiplied by the mean fluorescence intensity of phagocytic neutrophils and multiplied by the neutrophil count in blood. This assay was validated using isolated neutrophils (Figure S2).
Alveolar macrophage phagocytosis assay
Human alveolar macrophages were obtained from lung bronchoalveolar or whole lung lavage fluid and then washed, resuspended, and seeded into 35-mm culture dishes as described previously.1 Cells were incubated with heat-killed E coli, Staphylococcus aureus, zymosan, or 0.1-μm latex beads, each fluorescently labeled with Texas Red (Invitrogen), fixed and examined by fluorescence microscopy36 as described previously1 to evaluate alveolar macrophage phagocytosis.
Statistical analysis
Numerical data were evaluated for a normal distribution using the Kolmogorov-Smirnov test and for equal variance using the Levene median test; parametric data are presented as means (± SE), and nonparametric data are presented as medians and interquartile ranges. Categorical data are presented as a percentage of the total or numerically, as appropriate. Statistical comparisons of parametric data were made with the Student t test for 2-group comparisons and with Kruskal-Wallis one-way analysis of variance with post-hoc analysis according to the Holm-Sidak method for multiple-group comparisons. Nonparametric data were compared with the use of the Kruskal-Wallis rank-sum test. Correlation coefficients were obtained using the Spearman correlation method. All tests were 2-sided, and P values of less than .05 were considered to indicate statistical significance. Regression analysis was performed with Spearman rank order correlation using SigmaPlot software (version 8.0; Systat Software, San Jose, CA). All experiments were repeated at least twice, with similar results.
Results
Circulating GM-CSF autoantibodies in healthy subjects
To determine whether GM-CSF autoantibodies are normally present in healthy persons, we used a novel method29 based on the prior removal of potentially bound GM-CSF. GM-CSF-binding immunoglobulins similar in molecular weight to IgG were detected in all healthy subjects evaluated, in pharmaceutical IVIG, and in a patient with PAP (as a positive control; Figure 1A). Liquid chromatography and tandem mass spectroscopy confirmed the authenticity of the autoantibodies in healthy subjects, demonstrating they were composed exclusively of immunoglobulin (Figure 1B). IgG subtyping further demonstrated they were composed primarily of IgG1 and IgG2 with only small amounts of IgG3 and IgG4 and had a similar composition in healthy persons, patients with PAP, and IVIG (Figure 1C). Using a sensitive and specific ELISA (Figure S1A-C), GM-CSF autoantibodies were detected in all samples evaluated, including the serum from 72 healthy persons, 21 patients with PAP, and IVIG (Figure 1D). The serum concentration of GM-CSF autoantibodies in healthy subjects was similar to that of pharmaceutical IVIG reconstituted at physiologic serum IgG concentration and was markedly lower than serum levels in patients with PAP (Figure 1D). GM-CSF autoantibody levels in healthy subjects were unaffected by sex (Figure 1E) or age (Figure 1F). These results demonstrate that GM-CSF autoantibodies are common or ubiquitous in healthy persons, albeit at levels far lower than in patients with PAP.
Presence of GM-CSF autoantibodies in healthy subjects. (A) Total IgG was isolated from the serum of healthy control subjects (HC) or patients with PAP (PAP) or from pharmaceutical grade IVIG by protein G chromatography and subjected to ultrafiltration under acidic conditions to remove bound GM-CSF. GM-CSF autoantibodies were then isolated by GM-CSF affinity chromatography and evaluated by far-Western analysis probed with 125I-GM-CSF. Shown are Coomassie Blue–stained electrophoresis gels (bottom panels) and corresponding far-Western blots (top panels). Each numbered lane represents the corresponding bound (left panels) and unbound (right panels) chromatography fractions from 1 subject or sample. (B) Serum GM-CSF–binding proteins from 6 healthy human subjects were fractionated individually on gels as in panel A, and proteins in the 180-kDa band from each were extracted, subjected to liquid chromatography and tandem mass spectroscopy, and the results evaluated by comparison to Mascot database as described under “Methods.” Only matches with a probability-based Mowse score greater than 64 (indicating a P value < .05) were considered in the analysis. The percentages of immunoglobulin (Ig) and non-Ig peptides among the top 50 peptide fragment matches identified for each sample are shown. (C) GM-CSF autoantibodies were isolated by GM-CSF affinity chromatography from serum of healthy subjects (HC; n = 10), patients with PAP (PAP, n = 4), or from pharmaceutical IVIG (n = 1 clinical grade vial), and the percentage of IgG subtypes was measured by ELISA. (D) Serum GM-CSF autoantibody concentrations in healthy subjects (HC), patients with PAP (PAP), or IVIG (reconstituted at 9.94 mg/mL in PBS). Serum GM-CSF autoantibody levels in healthy subjects (median (interquartile range [IQR]) = 1.04 [0.63-1.7] μg/mL, n = 72) were lower than in patients with PAP (median [IQR] = 59.8 (27.4–116.5) μg/mL; n = 21; P < .001). Median values (HC, PAP) are indicated by a horizontal bar. (E) Serum GM-CSF autoantibody levels in males (n = 15) and females (n = 57). Data are shown as whisker plots indicating the interquartile range (upper and lower borders of box), the 90th and 10th percentile (error bars), the 95th and 5th percentile (upper and lower open symbols), the median (solid horizontal line in box), and mean (dashed line in box) values of GM-CSF autoantibody levels. (F) Serum GM-CSF autoantibody levels in healthy women (△) and men ( □ ) of various ages (n = 72). Regression analysis did not reveal a significant correlation GM-CSF autoantibody levels with age (R2 = 0.08).
Presence of GM-CSF autoantibodies in healthy subjects. (A) Total IgG was isolated from the serum of healthy control subjects (HC) or patients with PAP (PAP) or from pharmaceutical grade IVIG by protein G chromatography and subjected to ultrafiltration under acidic conditions to remove bound GM-CSF. GM-CSF autoantibodies were then isolated by GM-CSF affinity chromatography and evaluated by far-Western analysis probed with 125I-GM-CSF. Shown are Coomassie Blue–stained electrophoresis gels (bottom panels) and corresponding far-Western blots (top panels). Each numbered lane represents the corresponding bound (left panels) and unbound (right panels) chromatography fractions from 1 subject or sample. (B) Serum GM-CSF–binding proteins from 6 healthy human subjects were fractionated individually on gels as in panel A, and proteins in the 180-kDa band from each were extracted, subjected to liquid chromatography and tandem mass spectroscopy, and the results evaluated by comparison to Mascot database as described under “Methods.” Only matches with a probability-based Mowse score greater than 64 (indicating a P value < .05) were considered in the analysis. The percentages of immunoglobulin (Ig) and non-Ig peptides among the top 50 peptide fragment matches identified for each sample are shown. (C) GM-CSF autoantibodies were isolated by GM-CSF affinity chromatography from serum of healthy subjects (HC; n = 10), patients with PAP (PAP, n = 4), or from pharmaceutical IVIG (n = 1 clinical grade vial), and the percentage of IgG subtypes was measured by ELISA. (D) Serum GM-CSF autoantibody concentrations in healthy subjects (HC), patients with PAP (PAP), or IVIG (reconstituted at 9.94 mg/mL in PBS). Serum GM-CSF autoantibody levels in healthy subjects (median (interquartile range [IQR]) = 1.04 [0.63-1.7] μg/mL, n = 72) were lower than in patients with PAP (median [IQR] = 59.8 (27.4–116.5) μg/mL; n = 21; P < .001). Median values (HC, PAP) are indicated by a horizontal bar. (E) Serum GM-CSF autoantibody levels in males (n = 15) and females (n = 57). Data are shown as whisker plots indicating the interquartile range (upper and lower borders of box), the 90th and 10th percentile (error bars), the 95th and 5th percentile (upper and lower open symbols), the median (solid horizontal line in box), and mean (dashed line in box) values of GM-CSF autoantibody levels. (F) Serum GM-CSF autoantibody levels in healthy women (△) and men ( □ ) of various ages (n = 72). Regression analysis did not reveal a significant correlation GM-CSF autoantibody levels with age (R2 = 0.08).
Circulating complexes of autoantibody and GM-CSF
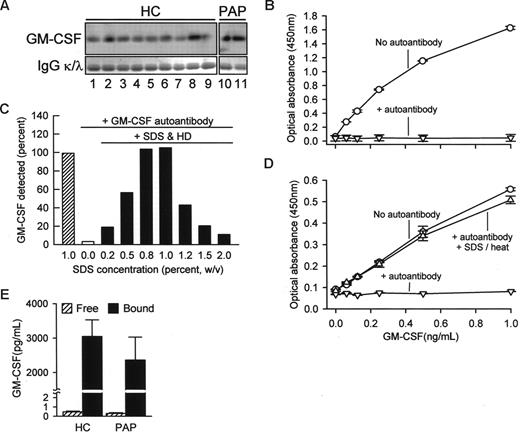
To determine whether GM-CSF was bound to circulating GM-CSF autoantibodies, potentially interfering with detection, total serum IgG was isolated on protein G, washed to remove proteins not directly bound to autoantibodies, eluted, and evaluated by Western blotting. GM-CSF bound to IgG was present in all healthy subjects and in patients with PAP evaluated, thus demonstrating the presence of GM-CSF-autoantibody complexes (Figure 2A). To determine whether immune complex formation interfered with detection of serum GM-CSF, we first measured the concentration of GM-CSF in the absence or presence of GM-CSF autoantibodies. GM-CSF autoantibodies completely abolished detection of GM-CSF using a commercially available ELISA (Figure 2B). We then developed a novel ELISA using a polyclonal capture antibody and pretreatment with SDS and heat denaturation that enabled detection of both free GM-CSF and GM-CSF bound to autoantibody (Figure 2C,D). Using this ELISA, the mean (± SE) total serum GM-CSF concentration in healthy subjects was 3047 plus or minus 484 pg/mL (Figure 2E). Similar levels of total serum GM-CSF levels were observed in patients with PAP. In contrast, levels of free GM-CSF measured using the commercial ELISA (see Figure 2B) were less than 1 pg/mL in both groups (Figure 2E). Thus, serum GM-CSF was more abundant than previously reported12,13 and existed almost exclusively in a form complexed to IgG in health and disease with less than 0.1% present in the unbound form.
Concentration of free and IgG-bound GM-CSF in human serum. (A) Total IgG was isolated individually from the sera of healthy subjects (HC) or patients with PAP (PAP) using protein G and evaluated by Western blotting to detect GM-CSF (top panels) or IgGκ (κ) and -λ (λ) light chains (as a loading control, bottom blots). Each lane represents one subject. (B) Detection of free GM-CSF and autoantibody-bound GM-CSF in serum. A set of “standard” samples composed of recombinant human GM-CSF (Leukine) at various concentrations ranging from 0 to 30 ng/mL were prepared in mouse serum in the absence (○) or presence (▽) of purified human GM-CSF autoantibodies (30 μg/mL). Standard samples were diluted 1/30 with 1% BSA in PBS and then GM-CSF was measured using a commercial human ELISA kit (R&D Systems) as directed by the manufacturer. (C) Use of a novel ELISA (SDS-HD ELISA, see “Methods”) to quantify GM-CSF in PBS in the absence or presence of GM-CSF autoantibody (1 μg/mL) and in the absence or presence of a pretreatment with SDS and heat denaturation. Each bar represents the mean of duplicate determinations for 1 of 4 separate experiments with similar results. (D) GM-CSF level evaluated using a novel human GM-CSF ELISA as described in “Methods.” Symbols represent the same samples and conditions as described in the legend to panel B above. GM-CSF was detectable in the absence of GM-CSF autoantibody (○), undetectable in the presence of GM-CSF autoantibody in the absence of SDS-HD pretreatment (▽), and detection was restored in the presence of GM-CSF autoantibody by SDS-HD pretreatment (△). (E) Free GM-CSF (▨) or total GM-CSF (free and autoantibody-bound; ■) were measured in sera of healthy subjects (HC) or patients with PAP (PAP) using a commercially available ELISA or the SDS-HD ELISA, respectively, as described in “Methods.” Total serum GM-CSF levels in HC and PAP were not different (3048 ± 484, n = 11; PAP 2360 ± 668, n = 5; respectively, P = .43).
Concentration of free and IgG-bound GM-CSF in human serum. (A) Total IgG was isolated individually from the sera of healthy subjects (HC) or patients with PAP (PAP) using protein G and evaluated by Western blotting to detect GM-CSF (top panels) or IgGκ (κ) and -λ (λ) light chains (as a loading control, bottom blots). Each lane represents one subject. (B) Detection of free GM-CSF and autoantibody-bound GM-CSF in serum. A set of “standard” samples composed of recombinant human GM-CSF (Leukine) at various concentrations ranging from 0 to 30 ng/mL were prepared in mouse serum in the absence (○) or presence (▽) of purified human GM-CSF autoantibodies (30 μg/mL). Standard samples were diluted 1/30 with 1% BSA in PBS and then GM-CSF was measured using a commercial human ELISA kit (R&D Systems) as directed by the manufacturer. (C) Use of a novel ELISA (SDS-HD ELISA, see “Methods”) to quantify GM-CSF in PBS in the absence or presence of GM-CSF autoantibody (1 μg/mL) and in the absence or presence of a pretreatment with SDS and heat denaturation. Each bar represents the mean of duplicate determinations for 1 of 4 separate experiments with similar results. (D) GM-CSF level evaluated using a novel human GM-CSF ELISA as described in “Methods.” Symbols represent the same samples and conditions as described in the legend to panel B above. GM-CSF was detectable in the absence of GM-CSF autoantibody (○), undetectable in the presence of GM-CSF autoantibody in the absence of SDS-HD pretreatment (▽), and detection was restored in the presence of GM-CSF autoantibody by SDS-HD pretreatment (△). (E) Free GM-CSF (▨) or total GM-CSF (free and autoantibody-bound; ■) were measured in sera of healthy subjects (HC) or patients with PAP (PAP) using a commercially available ELISA or the SDS-HD ELISA, respectively, as described in “Methods.” Total serum GM-CSF levels in HC and PAP were not different (3048 ± 484, n = 11; PAP 2360 ± 668, n = 5; respectively, P = .43).
Effects of circulating GM-CSF autoantibodies on GM-CSF signaling
To determine the significance of GM-CSF autoantibodies in healthy subjects, we evaluated their effects on GM-CSF signaling. Using a bioassay based on GM-CSF–dependent proliferation of TF-1 cells,20 autoantibody-dependent GM-CSF–neutralizing activity was detected in IgG isolated from all persons evaluated, and levels fell between that of serum from a patient with PAP as a positive control and culture media as a negative control (Figure 3A). IVIG reconstituted at physiologic IgG concentration also contained GM-CSF–neutralizing activity at levels similar to serum from healthy subjects. GM-CSF autoantibodies reduced GM-CSF signaling in a concentration-dependent fashion as shown by the decrease in GM-CSF–stimulated phosphorylation of signal transducer activation and transcription 5 (STAT5), a downstream signaling molecule (Figure 3B). Although low concentrations of free GM-CSF were highly bioactive, 5-fold higher concentrations of autoantibody-bound GM-CSF had practically no signaling activity (Figure 3C). GM-CSF stimulates increased levels of CD11b on neutrophils, a phenomenon we previously exploited in developing an assay to measure GM-CSF–neutralizing activity in whole blood.5 Low concentrations of GM-CSF–stimulated maximal CD11b levels in healthy persons but had no effect in patients with PAP (Figure 3D). The block in stimulation of CD11b levels caused by higher GM-CSF autoantibody levels in patients with PAP could be overcome by exposure to high concentrations of GM-CSF (Figure 3D inset). Highly purified GM-CSF autoantibodiesisolated from IVIG or patients with PAP blocked the increase in CD11b levels to a similar degree and in a concentration-dependent fashion (Figure 3E). Purified GM-CSF autoantibodies were specific and did not block an IL-8–stimulated increase in CD11b levels (Figure 3F). To determine the endogenous autoantibody level associated with complete loss of GM-CSF signaling in vivo, the CD11b stimulation index was measured in whole blood from healthy subjects and patients with PAP. The CD11b stimulation index correlated inversely with endogenous levels of serum GM-CSF autoantibody up to 5.7 μg/mL and was zero over a wide range of higher concentrations (Figure 3G). It is noteworthy that all positive results below this autoantibody level (referred to as the critical threshold for CD11b stimulation) were from healthy subjects and all negative results above it were from patients with PAP. Thus, GM-CSF autoantibodies seem to scavenge free GM-CSF in vivo and modulate the endocrine signaling capacity of GM-CSF in whole blood, suggesting that they may function to negatively regulate GM-CSF signaling in health and disease.
Regulation of GM-CSF signaling by GM-CSF autoantibodies in healthy subjects and patients with PAP. (A) The serum GM-CSF–neutralizing capacity of GM-CSF autoantibodies was measured using the TF-1 cell-proliferation assay.20 Equal volumes (30 μL) of serum from healthy subjects (△) or a PAP patient (■) (as a positive control), IVIG reconstituted at physiologic concentration (◇), or culture media (●) (as a negative control) were evaluated. The neutralizing capacity of purified GM-CSF autoantibodies from HC serum or IVIG (dashed line) was intermediate between that of autoantibodies isolated from serum the patient with PAP, which contains high concentrations of GM-CSF autoantibody, and control media, which contains none. (B) Neutrophils isolated from healthy subjects were incubated with various concentrations of GM-CSF affinity-purified autoantibodies isolated from IVIG or with control antibody (1 μg/mL) and stimulated with 10 ng/mL GM-CSF for 15 minutes and total and phosphorylated STAT5 (pSTAT5) was measured by immunoblotting. (C) The signaling activity of free and autoantibody-bound GM-CSF was measured by quantifying the level of STAT5 phosphorylation in isolated neutrophils by immunoblotting (shown as the ratio of phosphorylated STAT5 to total STAT5) as described in “Methods.” The signaling activity of GM-CSF complexed to autoantibody was markedly lower than free GM-CSF (0.142 ± 0.1 vs 2.192 ± 0.2 pg/mL, respectively; n = 3 each; *P < .001). (D) The typical pattern of GM-CSF–stimulated increase in CD11b levels on neutrophils in whole blood (CD11b stimulation index) is shown for a healthy subject (HC) and a patient with PAP (PAP). The amount of exogenous GM-CSF (dashed lines) required to stimulate an increase in neutrophil CD11b levels to the threshold value (dotted line) was lower in HCs than in patients with PAP. The median (IQR) GM-CSF concentration required to reach this stimulation threshold (inset) was significantly higher in patients with PAP than in healthy subjects (120 [80-347] ng/mL, n = 5; and 3.96 [1.07-4.86] ng/mL; n = 12; respectively; P < .002, Mann-Whitney). (E) Concentration-dependent reduction in the CD11b stimulation index by GM-CSF autoantibody purified from IVIG ( ) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 (
) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 ( ) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).
) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).
Regulation of GM-CSF signaling by GM-CSF autoantibodies in healthy subjects and patients with PAP. (A) The serum GM-CSF–neutralizing capacity of GM-CSF autoantibodies was measured using the TF-1 cell-proliferation assay.20 Equal volumes (30 μL) of serum from healthy subjects (△) or a PAP patient (■) (as a positive control), IVIG reconstituted at physiologic concentration (◇), or culture media (●) (as a negative control) were evaluated. The neutralizing capacity of purified GM-CSF autoantibodies from HC serum or IVIG (dashed line) was intermediate between that of autoantibodies isolated from serum the patient with PAP, which contains high concentrations of GM-CSF autoantibody, and control media, which contains none. (B) Neutrophils isolated from healthy subjects were incubated with various concentrations of GM-CSF affinity-purified autoantibodies isolated from IVIG or with control antibody (1 μg/mL) and stimulated with 10 ng/mL GM-CSF for 15 minutes and total and phosphorylated STAT5 (pSTAT5) was measured by immunoblotting. (C) The signaling activity of free and autoantibody-bound GM-CSF was measured by quantifying the level of STAT5 phosphorylation in isolated neutrophils by immunoblotting (shown as the ratio of phosphorylated STAT5 to total STAT5) as described in “Methods.” The signaling activity of GM-CSF complexed to autoantibody was markedly lower than free GM-CSF (0.142 ± 0.1 vs 2.192 ± 0.2 pg/mL, respectively; n = 3 each; *P < .001). (D) The typical pattern of GM-CSF–stimulated increase in CD11b levels on neutrophils in whole blood (CD11b stimulation index) is shown for a healthy subject (HC) and a patient with PAP (PAP). The amount of exogenous GM-CSF (dashed lines) required to stimulate an increase in neutrophil CD11b levels to the threshold value (dotted line) was lower in HCs than in patients with PAP. The median (IQR) GM-CSF concentration required to reach this stimulation threshold (inset) was significantly higher in patients with PAP than in healthy subjects (120 [80-347] ng/mL, n = 5; and 3.96 [1.07-4.86] ng/mL; n = 12; respectively; P < .002, Mann-Whitney). (E) Concentration-dependent reduction in the CD11b stimulation index by GM-CSF autoantibody purified from IVIG ( ) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 (
) or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 ( ) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).
) that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).
Effects of circulating GM-CSF autoantibodies on myeloid cell functions
The functional significance of GM-CSF autoantibodies in healthy subjects was evaluated by correlating levels with endogenous neutrophil functions. We recently developed a method to measure neutrophil phagocytosis in whole blood and then showed that transfer of GM-CSF autoantibodies into healthy human blood reduced neutrophil phagocytosis in a concentration-dependent fashion.5 Here, we verified that the assay can detect differences in the baseline phagocytic capacity of neutrophils among healthy subjects (Figure S2) and then measured the endogenous, phagocytic capacity of unstimulated neutrophils in whole blood from healthy subjects and patients with PAP. Neutrophil phagocytic capacity decreased with increasing endogenous GM-CSF autoantibody levels up to 3.2 μg/mL in healthy persons and reached a trough level and was unchanging over a wide range of higher concentrations above 39 μg/mL in patients with active PAP (Figure 4A). Two patients in remission of PAP lung disease who had lower serum GM-CSF autoantibody levels (5.7 and 10.4 μg/mL) had a neutrophil phagocytic capacity in the normal range, indicating the critical threshold of serum GM-CSF autoantibodies associated with reduced neutrophil phagocytosis might lie between 10.4 and 39 μg/mL. GM-CSF autoantibodies, at levels similar to those present in healthy subjects, reduced interleukin 8–stimulated neutrophil chemotaxis in vitro in a concentration-dependent fashion by a mechanism not attributable to chemokinesis or nonspecific effects (Figure 4B). Together, these results suggest that the low levels of GM-CSF autoantibodies present in healthy subjects may regulate GM-CSF–dependent neutrophil functions rheostatically and that the critical threshold level of GM-CSF autoantibodies associated with loss of GM-CSF priming of neutrophil functions in patients with PAP is between 10.4 and 39 μg/mL.
Correlation of GM-CSF autoantibody level and basal neutrophil function in vivo, and effects of GM-CSF autoantibody level on neutrophil function in vitro. (A) The phagocytic capacity of unstimulated neutrophils in whole blood was measured in healthy subjects (○), patients with PAP with active disease (◇), and patients with PAP in clinical remission of the lung disease (♦) by quantifying the uptake of IgG-opsonized latex microspheres as described under “Methods.” The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-39 μg /mL, ▨). Each symbol represents the results for triplicate determinations for one subject. The mean (IQR) free serum GM-CSF in healthy subjects was 0.00 (0.00-0.267) pg/mL serum and did not correlate with the neutrophil phagocytic capacity (P > .05), whereas GM-CSF autoantibody levels (1.07 [0.74-1.66] μg/mL) correlated with neutrophil phagocytic capacity (R2 = −0.70, P = .001) (Spearman rank order correlation). (B) Neutrophil chemotaxis was measured as described in “Methods.” In brief, neutrophils were placed in the upper chamber of a transwell culture plate and IL-8 (10 ng/mL) was placed in the lower chamber, both the upper and lower chambers (chemokinesis [CK] control) or was omitted (No IL-8) and GM-CSF autoantibody (0.5, or 1.0 μg/mL) or isotype control antibody (0.5 or 1.0 μg/mL) was placed in the upper chamber. Each bar represents the mean (± SE) for results from 3 determinations. Compared with the respective isotype antibody controls, increasing concentrations of GM-CSF autoantibody reduced neutrophil chemotaxis in rheostatic fashion (*P < .05; **P < .005).
Correlation of GM-CSF autoantibody level and basal neutrophil function in vivo, and effects of GM-CSF autoantibody level on neutrophil function in vitro. (A) The phagocytic capacity of unstimulated neutrophils in whole blood was measured in healthy subjects (○), patients with PAP with active disease (◇), and patients with PAP in clinical remission of the lung disease (♦) by quantifying the uptake of IgG-opsonized latex microspheres as described under “Methods.” The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-39 μg /mL, ▨). Each symbol represents the results for triplicate determinations for one subject. The mean (IQR) free serum GM-CSF in healthy subjects was 0.00 (0.00-0.267) pg/mL serum and did not correlate with the neutrophil phagocytic capacity (P > .05), whereas GM-CSF autoantibody levels (1.07 [0.74-1.66] μg/mL) correlated with neutrophil phagocytic capacity (R2 = −0.70, P = .001) (Spearman rank order correlation). (B) Neutrophil chemotaxis was measured as described in “Methods.” In brief, neutrophils were placed in the upper chamber of a transwell culture plate and IL-8 (10 ng/mL) was placed in the lower chamber, both the upper and lower chambers (chemokinesis [CK] control) or was omitted (No IL-8) and GM-CSF autoantibody (0.5, or 1.0 μg/mL) or isotype control antibody (0.5 or 1.0 μg/mL) was placed in the upper chamber. Each bar represents the mean (± SE) for results from 3 determinations. Compared with the respective isotype antibody controls, increasing concentrations of GM-CSF autoantibody reduced neutrophil chemotaxis in rheostatic fashion (*P < .05; **P < .005).
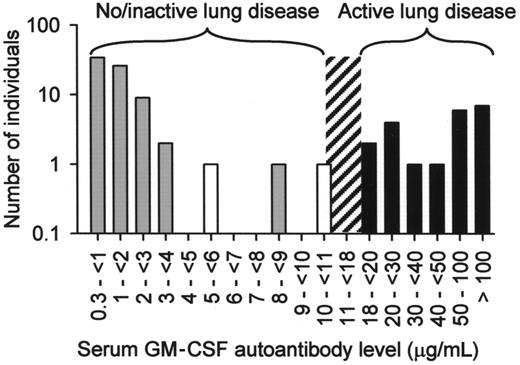
Because GM-CSF deficiency in mice impairs alveolar macrophage functions, including phagocytosis of E coli, S aureus, Mycobacterium tuberculosis, yeast particles (zymosan), and latex beads,1,32,37,38 we evaluated phagocytic function of alveolar macrophages from healthy subjects and patients with autoimmune PAP. Compared with alveolar macrophages from healthy subjects, alveolar macrophages from patients with PAP had impaired phagocytosis of E coli, S aureus, zymosan, and latex beads (Figure S3) and impaired clearance of surfactant (not shown). Although high levels of GM-CSF autoantibodies are thought to cause lung disease in PAP by blocking the paracrine stimulation of alveolar macrophages by GM-CSF secreted from adjacent respiratory epithelial cells,4,19,39 the level of GM-CSF autoantibodies associated with development of lung disease is unknown. Therefore, we measured serum GM-CSF autoantibody concentrations in healthy persons and in patients with active PAP lung disease. The critical threshold of serum GM-CSF autoantibodies associated with active PAP lung disease was between 8.5 and 19 μg/mL (Figure 5). It is noteworthy that 2 patients in remission of PAP lung disease at the time of evaluation had serum GM-CSF autoantibody levels of 5.7 and 10.4 μg/mL. Thus, the minimum serum level of GM-CSF autoantibodies associated with the presence of active lung disease in PAP lies between 10.4 and 19 μg/mL.
Histogram showing the frequency distribution of serum GM-CSF autoantibody levels in healthy subjects ( , n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).
, n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).
Histogram showing the frequency distribution of serum GM-CSF autoantibody levels in healthy subjects ( , n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).
, n = 72), patients with PAP with active lung disease (■, n = 21), and patients with PAP in clinical remission of the lung disease (□, n = 2). Clinical remission was defined as formerly diagnosed patients with PAP who were currently presenting no respiratory insufficiency and had normal chest X-ray images. The range of serum GM-CSF autoantibody levels separating subjects with no or active lung disease from those without active disease is indicated (10.4-19 μg/mL, ▨).
Discussion
Here, we demonstrate that GM-CSF autoantibodies are normally present in healthy human subjects, albeit at levels lower than in patients with PAP. GM-CSF autoantibody levels correlated inversely with neutrophil functions in vivo and reduced neutrophil functions rheostatically in vitro. GM-CSF was far more abundant in healthy human serum than previously reported12,13 ; however, more than 99% was bound and inactivated by GM-CSF autoantibodies. Although the majority of serum GM-CSF was undetectable with a commercial ELISA kit, it was readily detected with a novel ELISA developed to measure both free and autoantibody-bound GM-CSF. We measured the critical threshold of GM-CSF autoantibodies associated with the presence of PAP. We conclude that GM-CSF autoantibodies scavenge free GM-CSF in vivo and may negatively modulate myeloid cell functions in health and disease.
The observation that GM-CSF autoantibodies are ubiquitous in healthy subjects has implications for the pathogenesis of PAP, suggesting it is caused by a pathologic increase in the level of preexisting GM-CSF autoantibodies rather than a new adaptive antibody response. This is supported by several findings, including that: (1) low levels of GM-CSF autoantibodies were present in all healthy persons evaluated, were inversely correlated with the CD11b stimulation index and basal neutrophil phagocytic function in vivo, and reduced the CD11b stimulation index and neutrophil chemotaxis in vitro; (2) GM-CSF autoantibodies from healthy subjects (from IVIG) and from patients with PAP reduced GM-CSF signaling in vitro to a similar degree; (3) at levels above a critical threshold, GM-CSF autoantibodies were associated with multiple simultaneously impaired GM-CSF–dependent myeloid functions; (4) GM-CSF autoantibodies were specific; (5) other anti-cytokine or noncytokine autoantibodies have not been reported in patients with PAP20 ; and (6) PAP does not occur as a complication of other more common autoimmune diseases.27,40 Our experimental measurement of the critical threshold of GM-CSF autoantibodies associated with the presence of PAP lung disease showed it lies between 10.4 and 19 μg/mL. This agrees well with our prior estimate of 8-22 μg/mL26 calculated from data for 1258 healthy subjects,25 425 patients with autoimmune diseases but without PAP,40 and 158 patients with autoimmune PAP.4 GM-CSF autoantibodies were distinct from the recently identified soluble GM-CSF receptor,41 which differs in molecular weight and was not detected by far-Western blotting or by liquid chromatography and mass spectroscopy of the GM-CSF-binding band seen in the serum of healthy subjects or patients with PAP. Our study does not rule out that GM-CSF autoantibodies in healthy subjects and patients with PAP may differ in ways important to PAP pathogenesis. Examples of such potential differences include the GM-CSF epitopes targeted, the binding affinity of GM-CSF autoantibodies,20 or the relative composition of neutralizing and non-neutralizing GM-CSF autoantibodies in healthy subjects and patients with PAP. Future studies focused on these questions and the mechanism of immune dysregulation responsible for increasing the GM-CSF autoantibody level in autoimmune PAP will be important in furthering our understanding of its pathogenesis.
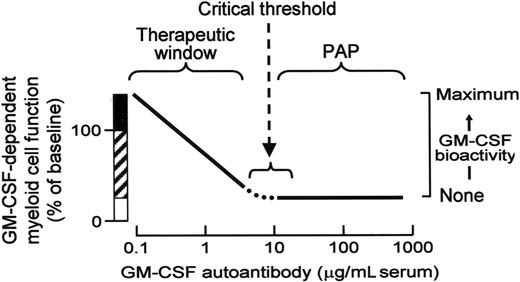
These results predict a novel mechanism of innate immune regulation (Figure 6). In healthy persons, low levels of endogenous GM-CSF autoantibodies determine the ambient level of GM-CSF bioactivity, which determines the basal level of myeloid cell functions. Endogenous myeloid cell priming varies inversely with autoantibody concentration up to the critical threshold. Myeloid cell functions likely regulated by this mechanism include: surfactant catabolism, cell adhesion, phagocytosis, microbial killing, pathogen receptor expression, Toll-like receptor 4 signaling, and others.1,5 In patients with PAP, very high levels of GM-CSF autoantibodies reduce GM-CSF bioactivity to zero,20 thereby eliminating GM-CSF priming of myeloid cell functions. This model is consistent with reports that administration of exogenous GM-CSF antibody rheostatically reduces myeloid cell functions in vivo in mice42 and ex vivo in human blood.5 It is also consistent with the observation that GM-CSF bioactivity is undetectable in patients with PAP.20 Because surfactant clearance by alveolar macrophages requires GM-CSF and GM-CSF bioactivity is eliminated at all autoantibody concentrations above the critical threshold, this model explains the lack of correlation between the level of GM-CSF autoantibody and the severity of lung disease in patients with PAP.31 It also supports the interesting hypothesis of Bendtzen and colleagues (Svenson et al,25 Ross et al,43 and Metcalf et al44 ), who first proposed that the therapeutic effects of IVIG may be due to GM-CSF autoantibodies that may function by reducing GM-CSF stimulated myeloid cell reactivity.
Schematic of the proposed mechanism of innate immune regulation by GM-CSF autoantibodies showing the relationship between endogenous GM-CSF autoantibody level (abscissa), GM-CSF–dependent myeloid cell functions, and GM-CSF bioactivity (ordinate). More than a range of low autoantibody levels present in healthy subjects, myeloid cell functions vary inversely with level of GM-CSF autoantibodies (ordinate, ▨) and increased levels of GM-CSF (eg, present at inflammatory sites or from exogenous administration) increase myeloid cell functions above baseline levels by a mechanism known as “GM-CSF priming” (ordinate, ■). At and above GM-CSF autoantibody levels sufficient to completely neutralize GM-CSF bioactivity (eg, the critical threshold), GM-CSF–stimulated myeloid cell functions are minimal or zero (ordinate, □) and the risk of PAP is increased.
Schematic of the proposed mechanism of innate immune regulation by GM-CSF autoantibodies showing the relationship between endogenous GM-CSF autoantibody level (abscissa), GM-CSF–dependent myeloid cell functions, and GM-CSF bioactivity (ordinate). More than a range of low autoantibody levels present in healthy subjects, myeloid cell functions vary inversely with level of GM-CSF autoantibodies (ordinate, ▨) and increased levels of GM-CSF (eg, present at inflammatory sites or from exogenous administration) increase myeloid cell functions above baseline levels by a mechanism known as “GM-CSF priming” (ordinate, ■). At and above GM-CSF autoantibody levels sufficient to completely neutralize GM-CSF bioactivity (eg, the critical threshold), GM-CSF–stimulated myeloid cell functions are minimal or zero (ordinate, □) and the risk of PAP is increased.
Although multiple lines of evidence support the conclusion that GM-CSF autoantibodies are present in healthy subjects, their potential role in immune regulation is less certain. Our current and reported observations strongly support a role for GM-CSF in the constitutive regulation of myeloid cell functions in vivo and suggest that GM-CSF autoantibodies may be important in negatively modulating GM-CSF signaling in health and disease.1,,–4,20,23,24,26 The virtually complete binding and neutralization of GM-CSF suggests that GM-CSF autoantibodies may function to scavenge and inactivate GM-CSF released at sites of inflammation, thus preventing detrimental distal endocrine effects. This is consistent with the rapid, receptor-mediated clearance of GM-CSF reported in mice45 and also with the low levels of free GM-CSF present in human serum.5,13 Alternatively, GM-CSF present in the form of inactive circulating immune complexes may be released at sites of infection (perhaps by a drop in pH), where it could stimulate myeloid functions by locking the GM-CSF receptor into its high STAT5-mediated signaling state,12 thereby amplifying local innate immunity on a microscopic scale. This is consistent with the observation that anti-cytokine antibody binding prolongs the half-life of interleukin-4 in vivo.46
Several observations have identified GM-CSF as a molecular target for therapeutic development,22,23,47,–49 including the failure of GM-CSF knockout mice to develop the typical lesions of arthritis or multiple sclerosis in experimental models of these diseases as well as the pathogenic implications of increased local levels of GM-CSF at sites of disease in rheumatoid arthritis. Administration of GM-CSF autoantibody at doses below the critical threshold may rheostatically reduce myeloid cell activity,5,42 potentially reducing the severity of inflammatory and autoimmune disorders.24 However, doses exceeding the critical threshold may result in excessive myeloid cell suppression and unwanted clinical manifestations, including alveolar proteinosis and impaired antimicrobial host defenses. Thus, our estimate of the critical threshold helps to define the therapeutic window for the safe use of GM-CSF autoantibodies in potential new clinical therapies. The close agreement of estimates based on various myeloid functions suggests that serum GM-CSF autoantibody levels below 10 μg/mL may not result in adverse clinical manifestations. However, it is also possible that different critical threshold values may exist for distinct GM-CSF–regulated myeloid cell functions (eg, surfactant catabolism, antimicrobial host defense functions), different modes of GM-CSF signaling (eg, endocrine, autocrine, and paracrine), or various tissue compartments (eg, lung versus blood). For example, a lower concentration of GM-CSF is required for maintenance of alveolar macrophage-mediated surfactant clearance than for normal alveolar macrophage immune functions (B.C.C. and B.C.T., unpublished observations). Separately, it is possible that clinically safe levels of very highly purified GM-CSF autoantibodies may be lower than levels predicted from data based on endogenous autoantibody levels because autoantibody potency is increased by removal of GM-CSF. This is consistent with the observation that estimates of the critical threshold obtained by correlating levels of endogenous GM-CSF autoantibody and neutrophil functions in unstimulated whole blood (Figures 3G and 4A) are higher than estimates from experiments in which highly purified GM-CSF autoantibodies are incubated with healthy human blood.5 It is important to note that GM-CSF autoantibody levels are relative values linked to the autoantibody standard used in their measurement. Thus, standardization of these methods will be useful to ensure comparability of future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jonathan Puchalski, John Howington, and Michael Reed (University of Cincinnati Medical Center, Cincinnati, OH) for help with the care of patients with PAP and clinical sample collection, and Drs. Jeffrey Whitsett, Fred Finkelman, and Christopher Karp (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) for their critical reading of the manuscript.
This work was supported by the National Institutes of Health (Bethesda, MD; HL085453 to B.C.T.), National Center for Research Resources (Bethesda, MD; RR019498 to B.C.T.), and the Japan Society for the Promotion of Science (Tokyo, Japan; B19390403 to Y.Y.).
National Institutes of Health
Authorship
Contribution: K.U., K.N., M.W., and B.C.T. designed research; K.U., T.S., D.E.K., and D.C.B. performed research; M.L. provided vital reagents; C.A.S. served as research coordinator and obtained all the clinical samples from normal volunteers; J.P.K., K.U., and B.C.T. analyzed the data; and K.U., K.N., B.C.C., L.A.D., N.K., Y.Y., and B.C.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce C. Trapnell, MD, Division of Pulmonary Biology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Avenue, Cincinnati, OH 45229-3039; e-mail: bruce.trapnell@cchmc.org

![Figure 1. Presence of GM-CSF autoantibodies in healthy subjects. (A) Total IgG was isolated from the serum of healthy control subjects (HC) or patients with PAP (PAP) or from pharmaceutical grade IVIG by protein G chromatography and subjected to ultrafiltration under acidic conditions to remove bound GM-CSF. GM-CSF autoantibodies were then isolated by GM-CSF affinity chromatography and evaluated by far-Western analysis probed with 125I-GM-CSF. Shown are Coomassie Blue–stained electrophoresis gels (bottom panels) and corresponding far-Western blots (top panels). Each numbered lane represents the corresponding bound (left panels) and unbound (right panels) chromatography fractions from 1 subject or sample. (B) Serum GM-CSF–binding proteins from 6 healthy human subjects were fractionated individually on gels as in panel A, and proteins in the 180-kDa band from each were extracted, subjected to liquid chromatography and tandem mass spectroscopy, and the results evaluated by comparison to Mascot database as described under “Methods.” Only matches with a probability-based Mowse score greater than 64 (indicating a P value < .05) were considered in the analysis. The percentages of immunoglobulin (Ig) and non-Ig peptides among the top 50 peptide fragment matches identified for each sample are shown. (C) GM-CSF autoantibodies were isolated by GM-CSF affinity chromatography from serum of healthy subjects (HC; n = 10), patients with PAP (PAP, n = 4), or from pharmaceutical IVIG (n = 1 clinical grade vial), and the percentage of IgG subtypes was measured by ELISA. (D) Serum GM-CSF autoantibody concentrations in healthy subjects (HC), patients with PAP (PAP), or IVIG (reconstituted at 9.94 mg/mL in PBS). Serum GM-CSF autoantibody levels in healthy subjects (median (interquartile range [IQR]) = 1.04 [0.63-1.7] μg/mL, n = 72) were lower than in patients with PAP (median [IQR] = 59.8 (27.4–116.5) μg/mL; n = 21; P < .001). Median values (HC, PAP) are indicated by a horizontal bar. (E) Serum GM-CSF autoantibody levels in males (n = 15) and females (n = 57). Data are shown as whisker plots indicating the interquartile range (upper and lower borders of box), the 90th and 10th percentile (error bars), the 95th and 5th percentile (upper and lower open symbols), the median (solid horizontal line in box), and mean (dashed line in box) values of GM-CSF autoantibody levels. (F) Serum GM-CSF autoantibody levels in healthy women (△) and men ( □ ) of various ages (n = 72). Regression analysis did not reveal a significant correlation GM-CSF autoantibody levels with age (R2 = 0.08).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-05-155689/5/m_zh80240828630001.jpeg?Expires=1769160869&Signature=MubU84jiZdYHDdUTfI5J1uy9tvN7QO0JzRP2D6z9zEe0BaysTqgbTzmqb~Z4tPpYyH1O8pu0EviDjedKeUmhX0O5V1EEu27xtjooA~xUEXBfXN5O~LdNUeTLVIelFfONIoFvbaxipdKprUGho-kMKOGkKfElZ3XuE~CuRwZ2snfoqjdK3dzQPUOcV4Kd2V68jeRIYTwWLqzccZsASgMJYvEfdsV0q~0LqUmMrJ2o35WRtIzxpNvA4Q9-jzfAtJiEdynott85wRzNl-buuVVvITVf2gyhyfLEJQsystm7xcaT0s58qiwuREAEGqKnV4ScLbmaHpnJbDsEqC5rJAVpkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Regulation of GM-CSF signaling by GM-CSF autoantibodies in healthy subjects and patients with PAP. (A) The serum GM-CSF–neutralizing capacity of GM-CSF autoantibodies was measured using the TF-1 cell-proliferation assay.20 Equal volumes (30 μL) of serum from healthy subjects (△) or a PAP patient (■) (as a positive control), IVIG reconstituted at physiologic concentration (◇), or culture media (●) (as a negative control) were evaluated. The neutralizing capacity of purified GM-CSF autoantibodies from HC serum or IVIG (dashed line) was intermediate between that of autoantibodies isolated from serum the patient with PAP, which contains high concentrations of GM-CSF autoantibody, and control media, which contains none. (B) Neutrophils isolated from healthy subjects were incubated with various concentrations of GM-CSF affinity-purified autoantibodies isolated from IVIG or with control antibody (1 μg/mL) and stimulated with 10 ng/mL GM-CSF for 15 minutes and total and phosphorylated STAT5 (pSTAT5) was measured by immunoblotting. (C) The signaling activity of free and autoantibody-bound GM-CSF was measured by quantifying the level of STAT5 phosphorylation in isolated neutrophils by immunoblotting (shown as the ratio of phosphorylated STAT5 to total STAT5) as described in “Methods.” The signaling activity of GM-CSF complexed to autoantibody was markedly lower than free GM-CSF (0.142 ± 0.1 vs 2.192 ± 0.2 pg/mL, respectively; n = 3 each; *P < .001). (D) The typical pattern of GM-CSF–stimulated increase in CD11b levels on neutrophils in whole blood (CD11b stimulation index) is shown for a healthy subject (HC) and a patient with PAP (PAP). The amount of exogenous GM-CSF (dashed lines) required to stimulate an increase in neutrophil CD11b levels to the threshold value (dotted line) was lower in HCs than in patients with PAP. The median (IQR) GM-CSF concentration required to reach this stimulation threshold (inset) was significantly higher in patients with PAP than in healthy subjects (120 [80-347] ng/mL, n = 5; and 3.96 [1.07-4.86] ng/mL; n = 12; respectively; P < .002, Mann-Whitney). (E) Concentration-dependent reduction in the CD11b stimulation index by GM-CSF autoantibody purified from IVIG () or patients with PAP (■) and incubated with fresh whole blood at various concentrations. Each bar represents the results of 3 separate determinations. *Significant decrease (P < .001) from baseline determined in the absence of GM-CSF autoantibody. (F) Specificity of purified GM-CSF autoantibody. Neutrophils were incubated in the presence of GM-CSF (10 ng/mL) or IL-8 (100 ng/mL) and in the absence or presence of 1 μg GM-CSF autoantibody or control IgG as indicated. Data represent the level of CD11b in stimulated cells—the level in unstimulated cells. GM-CSF autoantibodies markedly inhibited the GM-CSF–stimulated (■), but resulted in levels of inhibition by IL-8 () that were significantly lower and similar to control (IgG, □). *A significant difference (P < .001) compared with inhibition of the GM-CSF–stimulated increase by GM-CSF autoantibody (■). (G) The CD11b stimulation index was measured in fresh blood from healthy control subjects (○), patients with PAP with active disease (◇), or patients with PAP in clinical remission of the lung disease (♦). The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-24 μg/mL, ▨). Each symbol represents the results of triplicate determinations for one subject. The median (IQR) free serum GM-CSF level in healthy subjects was 0.00 (0.00-0.390) pg/mL and did not correlate with the CD11b stimulation index (P > .05), whereas the median (IQR) GM-CSF autoantibody level (0.90 [0.58-1.19] μg/mL) correlated with CD11b stimulation index (R2 = 0.46, P = .03) (Spearman rank order correlation).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-05-155689/5/m_zh80240828630003.jpeg?Expires=1769160869&Signature=3IGfhK5k8QL8efQHqvhYUmaipFNofhdg8Y-zqfdJGk1l4Pd1LwaDRDVGeDwquFGRtecD41oMTf2kQp4xZ5NIjSc8QLifdYvQH4SHRfyJZvPtLdF~aXsFCqcPFgCkOiLpdSKYPFcxJNCbjePMRlA5SexdiF7q36mvQsoe4EnV1nAqensfEAzlaCgx9EbIPgZABGdu9-vJuueFKFSl-C66PNijSAMkc24zn~-6~oFvd9Gl-MGVCf84GOy9xbwUclErdbf2cmrC9rWSnvtu2DV6kaZvusddZfkF~S79bdMVYzz3RY-GDxWNB1Rl6NAEEtoD8kGynfhZLwgviEYxTSA1GA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Correlation of GM-CSF autoantibody level and basal neutrophil function in vivo, and effects of GM-CSF autoantibody level on neutrophil function in vitro. (A) The phagocytic capacity of unstimulated neutrophils in whole blood was measured in healthy subjects (○), patients with PAP with active disease (◇), and patients with PAP in clinical remission of the lung disease (♦) by quantifying the uptake of IgG-opsonized latex microspheres as described under “Methods.” The range of serum GM-CSF autoantibody levels separating healthy subjects and patients with PAP with active disease evaluated with this assay is indicated (3.2-39 μg /mL, ▨). Each symbol represents the results for triplicate determinations for one subject. The mean (IQR) free serum GM-CSF in healthy subjects was 0.00 (0.00-0.267) pg/mL serum and did not correlate with the neutrophil phagocytic capacity (P > .05), whereas GM-CSF autoantibody levels (1.07 [0.74-1.66] μg/mL) correlated with neutrophil phagocytic capacity (R2 = −0.70, P = .001) (Spearman rank order correlation). (B) Neutrophil chemotaxis was measured as described in “Methods.” In brief, neutrophils were placed in the upper chamber of a transwell culture plate and IL-8 (10 ng/mL) was placed in the lower chamber, both the upper and lower chambers (chemokinesis [CK] control) or was omitted (No IL-8) and GM-CSF autoantibody (0.5, or 1.0 μg/mL) or isotype control antibody (0.5 or 1.0 μg/mL) was placed in the upper chamber. Each bar represents the mean (± SE) for results from 3 determinations. Compared with the respective isotype antibody controls, increasing concentrations of GM-CSF autoantibody reduced neutrophil chemotaxis in rheostatic fashion (*P < .05; **P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/11/10.1182_blood-2008-05-155689/5/m_zh80240828630004.jpeg?Expires=1769160869&Signature=E7Jpu5amPvuNNhHuioEl-SpjGrNsNVh4gR3oZ9xYb7-NVsgqAXi17WXIuWm8NYw1gpKK0riT6m97Nf9w~FX6vSCO1vNSevPd65q09ahKq15iS1rUCGbBP9cWB~xgQ4r2xzAOdS9PrnjCt5Xqb5gvePSnRSju-uWpYEC6rXOgUWFvecNegrAz1zGx50SzWa5KhUUGXbHit~awl2fRqqUIEih8wSVfUHMriOpnZ4lkXTx5kuiWIw3~w5pEI0fjXDgIjcXRG6edJvTToyBwIvVJ~ieSBh~-xata-VTFjxZQEfmzAvizssJeni3zG2p2VXdUFqqxzVb4AS7iTJduJlwiXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)