The presence of antibodies (Abs) in hemophilia A patients can potentially influence the therapeutic qualities of factor VIII (fVIII) administration. Much work has been focused on the presence of inhibitory antibodies, whereas the quantitation of noninhibitory anti-fVIII antibodies has been largely undetermined. Our objective was to develop a sensitive and specific fluorescence-based immunoassay (FLI) for the quantitation of anti-fVIIIAbs in human plasma. Affinity-purified human anti-fVIIIAb, isolated from a hemophilia A subject, was used as a calibrator with a detectability limit of 40 (±1.5) pM. The calibrator and the human plasma anti-fVIIIAb were captured on recombinant fVIII (rfVIII)– coupled microspheres and probed with mouse anti–human Ig–R-phycoerythrin. Plasma samples from 150 healthy donors and 39 inhibitor-negative hemophilia A subjects were compared with 4 inhibitor-positive hemophilia A plasma samples with inhibitor titers of 1 BU/mL (94.6 ± 0.8 nM), 11 BU/mL (214.3 ± 7.1 nM), 106 BU/mL (2209.4 ± 84.9 nM), 140 BU/mL (2417.7 ± 3.8 nM) as measured by the Nijmegen method. We also describe the validation of a mouse anti–human fVIIIAb as a surrogate calibrator. Four healthy individuals (3%) showed detectable anti-fVIIIAb in the range of 0.6 to 6.2 nM, whereas 13 (33%) of the 39 inhibitor-free hemophilia A subjects were positive for anti-fVIIIAb in the range of 0.5 to 20 nM. The method may be useful for therapeutic management of hemophilia A patients.
Introduction
Hemophilia A is a clinically heterogeneous bleeding disorder characterized by the absence of functional fVIII and is the most common hemorrhagic disease, affecting 0.01% to 0.02% of the male population.1 Hemophilia is clinically classified into 3 groups: severe, with less than 1% fVIII activity; moderate, with 1% to 5% fVIII activity; and mild, with 6% to 40% fVIII activity.2 After the reported successful cloning of the fVIII gene in 19843,–5 and the favorable outcome of its product protein in clinical settings, recombinant fVIII (rfVIII) as well as plasma-derived fVIII (pdfVIII) continue to be the most effective and prominent lifelong treatment for this hemostatic deficiency. The most severe complication from exposure to exogenous fVIII therapy is the development of alloantibodies that neutralize the effect of the therapeutic agent. It has been reported that the inhibitory antibodies develop in the range of 15% to 50% of hemophilia A patients.6,–8 The development of inhibitors is a major medical obstacle affecting the quality of life of treated individuals, with possible grave consequences, and is associated with an increased treatment cost.9
Various risk factors have been suggested to be associated with the immune response to the infused fVIII. Host-related factors include the severity of the disease, the genotype, and ethnicity.8 The majority (90%) of inhibitor-positive cases are observed in severe hemophilia A, and 3% to 13% of cases in mild-to-moderate hemophilia A.10,,–13 Defects in the fVIII gene such as large deletions and stop mutations, mutations Arg593Cys in the A2 domain and Trp2229Cys in the C2 domain, which are speculated to result in conformational changes of the fVIII protein, rendering it immunogenic, have been shown to be related to an increased risk.12,14,15 However, familial risk factors extend beyond the fVIII gene defect with associations reported to be linked with polymorphisms associated with the IL-10 and CTLA-4 genes.16,17 Data also suggest that African-Americans with severe hemophilia A are twice as likely as whites to develop inhibitors.18 Treatment-related risk factors include the intensity of treatment during the child's earliest exposures to fVIII concentrate, with high doses being associated with increased risk, and the administration of prophylaxis, associated with decreased risk.19 The association between the type of fVIII product and inhibitor risk is controversial.20,–22
The standard method for quantitation of fVIII inhibitors in human plasma is by the Nijmegen method, a modification of the Bethesda assay.23 The Bethesda unit (BU) is defined as the amount of antibody from patient plasma that neutralizes 50% of normal plasma fVIII activity.24 Titers of 0.6 BU/mL or greater are considered to be inhibitor positive. Low inhibitor profiles are classified as titers below 5 BU/mL and high titer inhibitors are above 5 BU/mL.25,26 Patients are categorized as low responders if their titer is below 5 BU/mL and do not show an immune response upon reexposure to fVIII. Some high responders with antibody titers above 5 BU/mL demonstrate an immune response upon reexposure and are considered for treatment with fVIII-bypassing therapy to control bleeds. The existence of noninhibitory antibodies and low titer inhibitory antibodies, unrecognized by the Bethesda assay, has been widely accepted, and their possible relevance acknowledged. Immunoassays have been used to detect anti-fVIII antibodies, but report only the presence of antibody relative to controls or quantitation based on recombinant constructs as calibrators.27,,,,,,–34 An assay for the detection of such antibodies with a suitable calibrator for absolute quantitation has been unavailable.
In this study we report the development of a quantitative immunoassay for the detection of anti-fVIII antibody, including those unrecognized by the Bethesda assay. The assay uses an affinity purified human anti-fVIII antibody as a calibrator and detects inhibitory as well as noninhibitory antibodies to fVIII. We also describe the validation of a mouse anti-human fVIII monoclonal antibody (mAb) as a suitable surrogate standard in lieu of the human calibrator. The antibody concentrations obtained represent the absolute amount of anti-fVIII immunoglobulin in the plasma samples. (Results of the pharmacokinetic study for 7 subjects, 5 products, and 29 injections will be presented in another manuscript.)
Methods
Proteins
Albumin-free rfVIII, (average mass 285 kDa, 3134 IU/mL, 5000 U/mg, extinction coefficient [E1cm0.1%] 1.3) was a gift from Dr R. Lundblad, Hyland Division, Baxter Healthcare (Deerfield, IL). The 7th International Standard fVIII Concentrate (IST fVIII; code 99/678) was purchased from the National Institute for Biological Standards and Control (NIBSC; Potters Bar, United Kingdom). Human von Willebrand factor (VWF) was obtained from Dr Richard Jenny (Haematologic Technologies, Essex Junction, VT). Albumin from chicken egg white grade 5 (ovalbumin), average mass 44 kDa, was purchased from Sigma-Aldrich (St Louis, MO). Mouse anti–human IgG (Fc)–R-phycoerythrin (R-PE), stock concentration 0.1 mg/mL, was purchased from SouthernBiotech (Birmingham, AL). Mouse anti-fVIII-68 mAb35 (mAb average mass 150 kDa, E1cm0.1% 1.4) was a gift from Dr Fass (Mayo Medical School, Rochester, MN). Mouse anti–fVIII-24 mAb was generated in our laboratory (average mass 150 kDa, E1cm0.1% 1.4). Goat anti–mouse IgG conjugated to R-PE was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Materials
Microsphere beads Luminex classification nos. 030 and 044 with predefined 658:712 nm emission ratios were purchased from Luminex (Austin, TX). Multiscreen filter plates containing 96 wells covered with 1.2 μm polyvinylidene difluoride (PVDF) membrane were purchased from Millipore part no. MABV-N1250 (Millipore, Billerica, MA). Luminex100 series instrument was used to analyze the fluorescence-based immunoassays (Luminex). Activated partial thromboplastin time (APTT) reagent was purchased from Trinity Biotech PLC (Bray, Ireland). The ST8 instrument used for determination of plasma clotting times was purchased from Diagnostica Stago (Parsippany, NJ). The Bethesda assay (fVIII inhibitor) kit was purchased from DiaPharma Group (West Chester, OH).
Human plasma
Samples of citrated human plasma from healthy donors were obtained from 2 sources: (1) the Red Cross at the Fletcher Allen Health Center, University of Vermont (n = 43), provided by Dr Russell Tracy (University of Vermont College of Medicine, Burlington, VT); and (2) Dr Anetta Undas (Department of Medicine, Jagiellonian University School of Medicine, Krakow, Poland; n = 107). Inhibitor-negative hemophilia A plasma was obtained from 3 sources with a total number of inhibitor-negative hemophilia A subjects of 39: (1) Centre Hospitalier Universitaire (CHU) Sainte-Justine (Montreal, Canada): 39 plasma samples from 25 subjects (identified as subjects 1-25) with some subjects undergoing phlebotomy at 2 separate times; (2) Children's Hospital Los Angeles (Los Angeles, CA): 7 inhibitor-negative hemophilia A subjects (identified as subjects 26-32) who underwent phlebotomy multiple times (cycles) during a study focused on the pharmacokinetics (PK) of various fVIII concentrates defined as A through E; (3) George King Bio-Medical (Overland Park, KS): 7 inhibitor-free plasma samples with less than 1% fVIII activity and 4 inhibitor-positive samples with less than 1% fVIII activity containing, respectively, 1, 11, 106, and 140 BU/mL fVIII inhibitors. FVIII/VWF double immuno-depleted plasma (ddfVIII/VWF) used for the determination of fVIII was purchased from Precision Biologics (Tampa, FL). Citrated plasma samples from 10 healthy donors were collected in-house to serve as controls. Human studies were approved by the Institutional Review Boards at the University of Vermont, CHU Sainte-Justine, and Children's Hospital Los Angeles, and donors' informed consent was obtained in accordance with the Declaration of Helsinki.
Fluorescence Luminex immunoassay for determination of fVIII in human plasma
The fVIII antigen detection assay was performed by fluorescence Luminex immunoassay (FLI) as previously described.36 Briefly, human plasma was reduced with 10 mM β-mercaptoethanol for 2 hours, diluted in ddfVIII-VWF plasma, and assayed. The reduction of plasma samples is necessary for fVIII analysis, as previously described.37 Various concentrations of rfVIII in ddfVIII-VWF plasma and human plasma samples were captured on microspheres coupled to mouse anti–human fVIII-68 mAb (light chain–specific), probed with biotinylated anti-fVIII-24 mAb (heavy chain–specific), and detected with streptavidine–R-PE. This immunoassay allows for the quantitation of fVIII antigen in normal human plasma as well as in hemophilia A plasma, with a detectability limit of 3 pM (0.4% of normal).
Activated partial thromboplastin time
The assay for activated partial thromboplastin time (APTT) was performed as previously described.38 Sample plasma was incubated with 100 μL substrate plasma (ddfVIII-VWF containing 10 μg/mL VWF) and 100 μL of the APTT reagent for 5 minutes at 37°C. Clotting was initiated by the addition of 100 μL of 25 mM CaCl2, and clotting times were determined using the ST8 instrument. The calibration curve was generated by adding various concentrations of rfVIII (21-630 pM) into the substrate plasma.
Bethesda assay
Two inhibitor-positive commercial plasmas (11 BU/mL and 106 BU/mL, with < 1% fVIII activity) and 2 inhibitor-negative commercial plasmas (GK1 and GK2) were tested for the presence of inhibitor, using the Bethesda assay kit according to the manufacturer's instructions.
Affinity purification of human anti-fVIII antibody calibrator
Citrated human plasma from a hemophilia A patient containing inhibitory anti-fVIII antibody (Ab), 64 BU/mL, was applied to a Sepharose-coupled rfVIII column. Bound immunoglobulin was eluted with 3 M sodium thiocyanate. The concentration of the purified human anti-fVIII Ab was determined by absorbance at 280 nm. The reduced and nonreduced antibody samples were then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 4%-12% acrylamide) stained with Coomassie blue. The apparent mobility of the isolated immunoglobulin was compared with previously purified and characterized human anti–streptokinase antibody and mouse anti–tissue factor-5 mAb.39,40
Inhibition of binding of purified human anti-fVIII antibody by rfVIII and International Standard fVIII Concentrate
The purified anti-fVIII Ab, quantitated by absorbance, was diluted to a concentration of 1 nM in phosphate-buffered saline (PBS) containing 150 mM sodium chloride, 5 mM sodium phosphate/0.05% Tween 20/0.1% ovalbumin (PBST/ovalbumin). Various rfVIII and IST fVIII concentrations (0.125-2.0 nM) were added to tubes containing 1 nM anti-fVIII Ab and incubated at room temperature (25°C) for 10 minutes. The reaction mixtures were then added to wells containing rfVIII coupled beads, and FLI was performed as described in the next paragraph for the detection of anti-fVIII antibody. The mean fluorescence intensity unit (MFIU) at each fVIII concentration was compared with the binding of 1 nM anti-fVIII Ab with no fVIII added and calculated as the percentage inhibition of anti-fVIII Ab binding to rfVIII beads. The inhibition pattern was used to confirm the concentration estimate for the purified anti-fVIII Ab, as determined by absorbance at 280 nm. Apparent equilibrium dissociation constants (Kd) were calculated from solution-phase fVIII concentration at 50% inhibition of antibody binding to coupled fVIII protein.
FLI for the detection of anti-fVIII antibody using human anti-fVIII antibody calibrator
Microsphere beads with Luminex classification no. 030 were coupled to rfVIII at 10 μg/mL (35 nM) according to the manufacturer's instructions (Luminex Corporation). Nonspecific binding control microsphere beads with Luminex classification no. 044 were coupled to ovalbumin at 1.5 μg/mL (35 nM). Fifty microliters of human anti-fVIII Ab at various concentrations, in PBST/ovalbumin buffer, was added to wells containing 50 μL rfVIII and ovalbumin coupled microspheres at 100 beads/μL each. The wells contained both rfVIII (bead region 030) and ovalbumin (bead region 044) at 5000 beads/well of each. The mixture was allowed to incubate at room temperature (25°C) on a shaker (500 rpm) for 2 hours. Wells were washed 3 times with 125 μL PBST (pH 7.4) buffer and probed with mouse anti–human IgG–R-PE at 5 μg/mL in PBST/ovalbumin (100 μL per well). After the mixture was incubated for 1 hour, the wells were washed 3 times with PBST, and the beads were resuspended in the same buffer and analyzed by the Luminex 100 instrument. Human plasma samples were diluted 10- and 50-fold in PBST/ovalbumin buffer and applied to wells containing rfVIII and ovalbumin coupled beads. Plasma samples with high inhibitor antibody titers were diluted as follows: 1 BU/mL and 11 BU/mL to 100- and 200-fold, 106 BU/mL and 140 BU/mL to 1000- and 2000-fold. The detection proceeded as described above for the human calibration. The readings were recorded as MFIU. Data were analyzed by the subtraction of the fluorescence intensity of the nonspecific control ovalbumin-coupled beads from the fluorescence intensity of the specific binding of human anti-fVIII Ab to rfVIII-coupled beads. A sample was considered positive for anti-fVIII antibody whenever the signal of binding to rfVIII beads reproducibly exceeded that of the binding to ovalbumin beads. The signals for the 4 healthy donors were as follows: subject 1 rfVIII = 47.2 plus or minus 2.4 MFIU versus ovalbumin = 34.0 plus or minus 1.4 MFIU; subject 2 rfVIII = 61.2 plus or minus 6.7 MFIU versus ovalbumin = 42.2 plus or minus 3.1 MFIU; subject 3 rfVIII = 88.7 plus or minus 10.9 MFIU versus ovalbumin = 49.5 plus or minus 10.6 MFIU; subject 4 rfVIII = 705.7 plus or minus 2.4 MFIU versus ovalbumin = 513.0 plus or minus 1.4 MFIU. Positive and negative controls were included in each assay plate analyzed. The negative control consisted of plasma pool from 10 healthy donors, in which the signal of binding to rfVIII beads was significantly lower than the signal of binding to ovalbumin beads (rfVIII = 230 ± 36 MFIU vs ovalbumin = 600 ± 43 MFIU). The positive control consisted of a commercial plasma sample with a fVIII inhibitor value of 1 BU/mL and < 1% fVIII activity. The signal of binding of the 1 BU/mL plasma to rfVIII beads was significantly higher than the signal of binding to ovalbumin-coupled beads (rfVIII = 1200 ± 112 MFIU vs ovalbumin = 400 ± 22 MFIU). The net signal of binding of the affinity pure anti-fVIII antibody calibrator at the low end of the calibration curve was 10.5 plus or minus 1.4 MFIU at 40 pM, 5.0 plus or minus 1.5 MFIU at 20 pM, and 1.8 plus or minus 1.0 MFIU at 10 pM. A net signal of a tested plasma sample below 5.0 MFIU was considered negative for anti-fVIII antibodies. Results were reproducibly obtained upon repeated analyses.
FLI for the detection of anti-fVIII antibody using mouse anti-fVIII antibody calibrator
The fluorescence-based assay using the mouse calibrator was performed as described above for human calibration with the following modifications. Various dilutions of mouse anti–fVIII-68 mAb were spiked into PBST/ovalbumin buffer and applied to beads. The binding of the mouse protein was detected with goat anti–mouse IgG–R-PE (5 μg/mL). The corresponding MFIU of each dilution of the mouse protein was analyzed and calculated against the various concentrations of the human calibrator. The concentration of the mouse antibody protein was therefore validated for use as a suitable standard for the analysis of human plasma samples.
Results
fVIII antigen concentration and activity in hemophilia A subjects
The fVIII antigen concentration and activity were evaluated in inhibitor-negative hemophilia A subjects, as determined by the Bethesda assay. The results are shown in Table 1 for 25 subjects from CHU Sainte-Justine (subjects 1-25) and Table 2 for 7 subjects obtained from Children's Hospital Los Angeles (subjects 26-32). fVIII antigen concentration and activity were determined as previously reported.36,38 fVIII antigen concentration and activity levels are reflective of hemophilia A2 and vary from below the detectability in both assays (FLI 3 pM or 0.4% of normal plasma) to 140 pM or 20% of normal plasma.
Affinity isolation of human anti-fVIII antibody from human plasma
The purification of human anti-fVIII Ab from human plasma resulted in a homogenous immunoglobulin preparation, as judged by Coomassie blue staining (data not shown). At reducing conditions, 2 bands are observed, 1 at an apparent molecular mass of approximately 50 kDa, representing the heavy chain, and a second band at an apparent mobility of 30 kDa, representing the light chain. The nonreduced gel profile demonstrates a band at approximately 150 kDa, representing an intact immunoglobulin molecule. The concentration of the purified protein was established by scatter corrected absorbance at 280 nm (E1cm0.1% 1.4, mass 150 kDa).
Generation of human anti-fVIII antibody calibrator in FLI
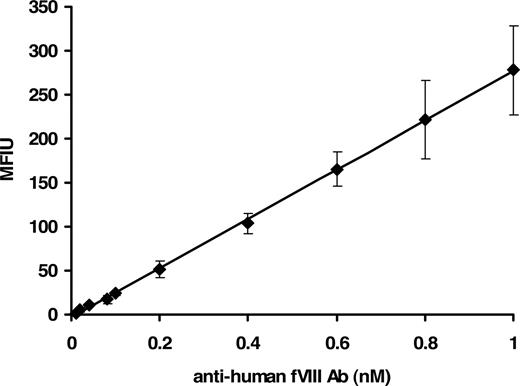
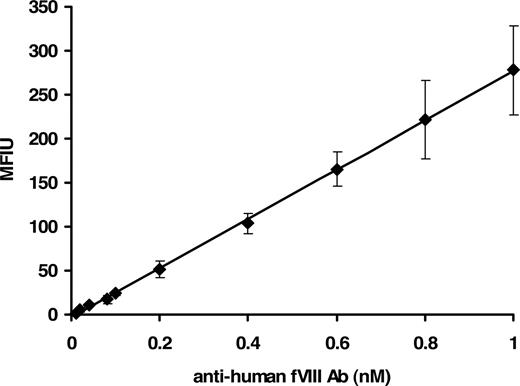
The concentration range of the assay was between 0.01 and 1.0 nM with a reliable detection limit of 40 plus or minus 1.5 pM. Figure 1 shows a concentration-dependent immunoreactivity of the antibody to rfVIII coupled beads. The captured antibody was probed with mouse anti–human Ig–R-PE, the fluorophore detected by the Luminex instrument. Thus a suitable and physiologically applicable calibrator for the quantitation of anti-fVIII Ab in human plasma was established and used for the assessment of antibodies in plasma from healthy donors and donors with hemophilia A.
Fluorescence Luminex immunoassay of human anti-fVIII antibody calibrator. Various molar concentrations (0.01-1.0 nM) of purified human immunoglobulin in PBS/0.1% albumin/0.05% Tween 20 were added to wells containing rfVIII-coupled beads and ovalbumin-coupled beads (nonspecific binding control). Binding was detected with mouse anti–human Ig–R-PE. Each point is a mean of 8 independent determinations plus or minus standard deviation (SD). The calibrator was generated by subtraction of the MFIU signal of the nonspecific binding to ovalbumin from the MFIU of specific binding to rfVIII at each concentration of anti-fVIII Ab.
Fluorescence Luminex immunoassay of human anti-fVIII antibody calibrator. Various molar concentrations (0.01-1.0 nM) of purified human immunoglobulin in PBS/0.1% albumin/0.05% Tween 20 were added to wells containing rfVIII-coupled beads and ovalbumin-coupled beads (nonspecific binding control). Binding was detected with mouse anti–human Ig–R-PE. Each point is a mean of 8 independent determinations plus or minus standard deviation (SD). The calibrator was generated by subtraction of the MFIU signal of the nonspecific binding to ovalbumin from the MFIU of specific binding to rfVIII at each concentration of anti-fVIII Ab.
Concentration-dependent inhibition of binding of anti-fVIII antibody by fVIII in FLI
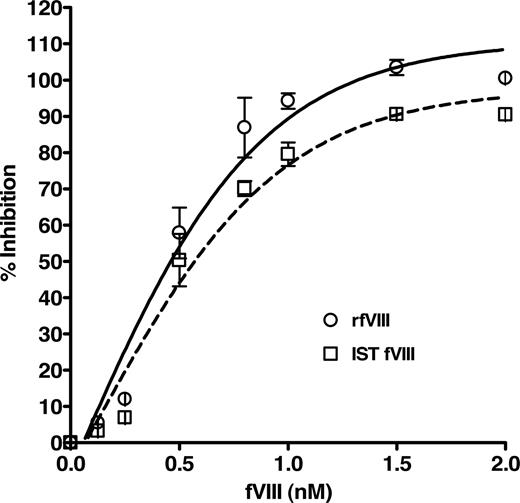
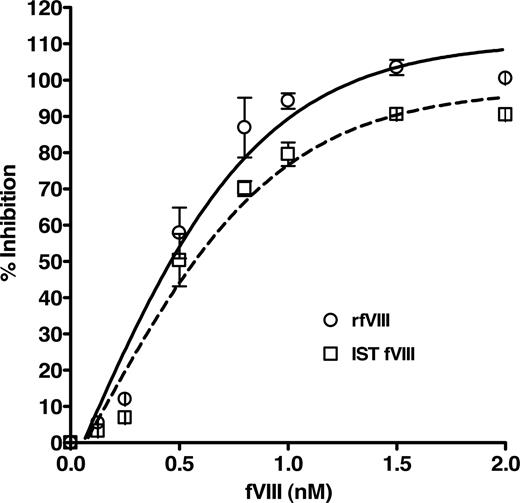
The specific binding of the purified human anti-fVIII Ab to fVIII was determined by a competition immunoassay, which demonstrated a concentration-dependent inhibition of anti-fVIII Ab binding to rfVIII coupled beads. Figure 2 shows the dose-dependent and specific inhibition pattern of the antibody by rfVIII and IST fVIII material. The immunoreactivity of the antibody with various concentrations of fVIII was compared with the total binding of 1 nM purified anti-fVIII Ab to bead-coupled rfVIII without the presence of solution-phase rfVIII. The inhibitory capacity of rfVIII increased with its concentration where concentrations of fVIII between 1 and 2 nM inhibit 1nM anti-fVIII Ab binding in the range of 90 to 103%. As the fVIII concentration decreased below 1 nM so did the inhibitory capacity. Our results indicate that the purified antibody reacts with various preparations of fVIII, quantitated by assay, in a concentration dependent manner and is therefore suitable for use as the standard for the quantitation of total anti-fVIII Ab determination in human plasma. The apparent Kd of the human antibody for fVIII was determined to be 0.4 plus or minus 0.1 nM for rfVIII and 0.5 plus or minus 0.1 nM for IST fVIII.
Inhibition of binding of purified human anti-fVIII Ab to rfVIII-coupled beads by various concentrations of fVIII. Human Ab (1 nM) was incubated with concentrations of rfVIII (○) and IST fVIII (□) in the range of 0.125 to 2.0 nM and applied to FLI for detection. Observed is the dose-dependent and specific inhibition of binding of the human antibody by fVIII protein. The apparent affinity of the antibody for rfVIII was calculated to be 0.4 plus or minus 0.1 nM and for IST fVIII 0.5 plus or minus 0.1 nM.
Inhibition of binding of purified human anti-fVIII Ab to rfVIII-coupled beads by various concentrations of fVIII. Human Ab (1 nM) was incubated with concentrations of rfVIII (○) and IST fVIII (□) in the range of 0.125 to 2.0 nM and applied to FLI for detection. Observed is the dose-dependent and specific inhibition of binding of the human antibody by fVIII protein. The apparent affinity of the antibody for rfVIII was calculated to be 0.4 plus or minus 0.1 nM and for IST fVIII 0.5 plus or minus 0.1 nM.
Generation of mouse anti-fVIII antibody calibrator in FLI
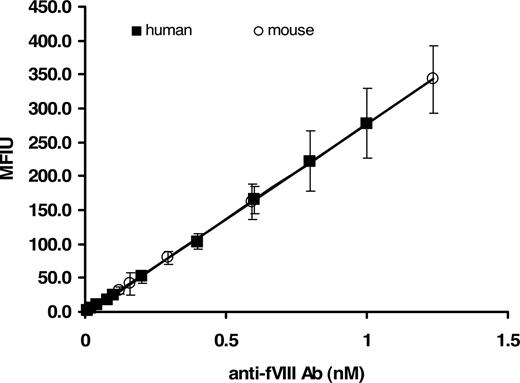
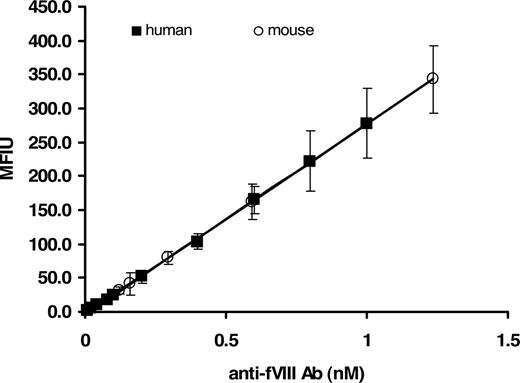
Because the supply of human alloantibody to fVIII is limited, we developed a surrogate murine monoclonal calibrator. The mouse monoclonal anti–fVIII-68 antibody was calibrated against the human anti-fVIII antibody standard. The binding of the mouse antibody to rfVIII coupled beads was detected by goat anti–mouse Ig–R-PE. The MFIU of various dilutions of the mouse protein were related to the MFIU of the human calibrator at determined concentrations. The established stock concentration of the mouse anti-fVIII Ab, based on the human calibrator, was then used to generate a standard curve validated as a surrogate for the human protein. Figure 3 demonstrates the concentration-dependent binding of the mouse and human antibody to rfVIII coupled beads. Only the human calibrator was used for the analyses described in this study.
Fluorescence Luminex immunoassay of mouse and human anti-fVIII Ab calibrator. Various molar concentrations (0.01-1.0 nM) of immunoglobulin in PBS/0.1% albumin/0.05% Tween 20 were added to wells containing rfVIII-coupled beads and ovalbumin-coupled beads (nonspecific binding control). Binding of mouse Ab was detected with goat anti–mouse Ig–R-PE, whereas binding of human Ab was detected with mouse anti–human Ig–R-PE. Each point is a mean of 8 independent determinations plus or minus SD. The figure demonstrates the validation of a mouse anti-fVIII Ab as a suitable calibrator for the determination of antibodies to fVIII in human plasma.
Fluorescence Luminex immunoassay of mouse and human anti-fVIII Ab calibrator. Various molar concentrations (0.01-1.0 nM) of immunoglobulin in PBS/0.1% albumin/0.05% Tween 20 were added to wells containing rfVIII-coupled beads and ovalbumin-coupled beads (nonspecific binding control). Binding of mouse Ab was detected with goat anti–mouse Ig–R-PE, whereas binding of human Ab was detected with mouse anti–human Ig–R-PE. Each point is a mean of 8 independent determinations plus or minus SD. The figure demonstrates the validation of a mouse anti-fVIII Ab as a suitable calibrator for the determination of antibodies to fVIII in human plasma.
Detection of anti-fVIII antibody in healthy donors
Among the 150 control samples of normal plasma (Table 3), 4 were determined as positive for anti-fVIII Ab. Two healthy donor controls had antibody levels of 0.6 plus or minus 0.2 nM and 0.7 plus or minus 0.1 nM. The other 2 controls fell within the 1 to 10 nM range with antibody concentrations of 1.4 plus or minus 0.5 nM and 6.2 plus or minus 0.5 nM. The absolute concentration of the antibody in the 4 individuals from the healthy population is well below that quantitated in a 1-BU/mL inhibitor plasma sample (94.6 ± 0.8 nM). Our data demonstrate the prevalence of detectable anti-fVIII Ab (> 40 pM) in the healthy controls to be approximately 3%. The lowest concentration of anti-fVIII Ab detected in the healthy controls that were determined as fVIII Ab positive was 0.6 nM, which is well above the detection limit of the assay of 0.040 nM. Healthy donor plasma tested for the presence of anti-fVIII Ab demonstrated reproducible concentrations of antibody at 10- and 50-fold plasma dilution (data not shown). The endogenous fVIII concentration, mean value of 0.7 nM, in the healthy subjects did not interfere with the detection of the anti-fVIII autoantibody as observed by the equivalent measurement of the antibody at the 2 dilutions. The apparent affinity of the human anti-fVIII antibody calibrator for the fVIII used in our study is approximately 0.5 nM with fVIII antigen levels in healthy donors being in the range of 0.7 nM. The endogenous fVIII would not therefore interfere with the detection of autoantibodies to fVIII at the dilutions of plasma used in the assay.
Evaluation of commercial plasma samples with < 1% fVIII activity with and without inhibitors in FLI
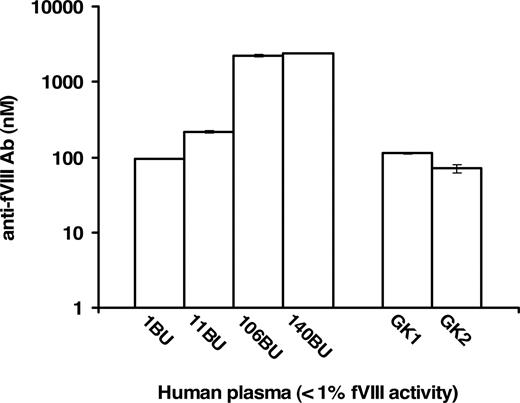
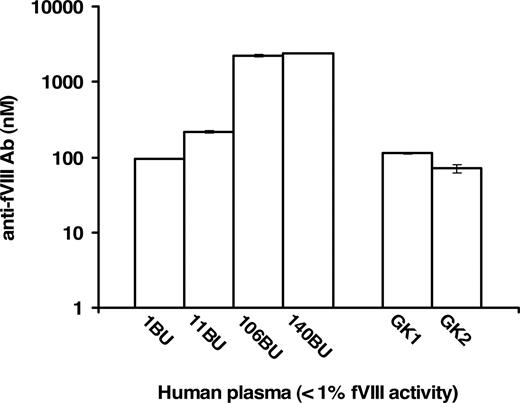
Commercial human plasma samples specified as having less than 1% fVIII activity and fVIII inhibitor titers of 1, 11, 106, and 140 BU/mL were analyzed in FLI for the quantitation of anti-fVIII Ab in absolute (molar) terms. These analyses, presented in Figure 4, quantitated the presence of anti-fVIII Ab. The increase in molar concentration of each sample corresponded to the increase in the Bethesda Unit titer. The presence and quantity of anti-fVIII Ab was also evaluated in 7 commercial inhibitor-free plasma samples reported to contain less than 1% fVIII activity. Two, labeled as GK1 and GK2 (George King sample 1 and 2), of the 7 plasmas were found to contain anti-fVIII Ab (Figure 4). The measured antibody concentrations in both the inhibitor-positive and inhibitor-negative samples were dilution independent, that is, identical antibody concentrations were obtained for each dilution tested. The following antibody concentrations and corresponding standard deviations were obtained for 4 inhibitor-positive and 2 inhibitor-negative plasma samples at different dilutions (100- to 2000-fold): 1 BU/mL = 94.6 plus or minus 0.8 nM, 11 BU/mL = 214.3 plus or minus 7.1 nM, 106 BU/mL = 2209.4 plus or minus 84.8 nM, 140 BU/mL = 2417.7 plus or minus 3.8 nM, GK = 112.1 plus or minus 1.9 nM, GK2 = 71.1 plus or minus 8.5 nM. These results indicate that each plasma antibody conformed (colinear) to the same standard curve, leading to the conclusion that the assay can be applied across a reasonable spectrum of plasmas containing antibodies to fVIII.
Quantitation of anti-fVIII Ab in human plasma samples with less than 1% fVIII activity. Plasma samples with predetermined anti-fVIII antibody titers of 1, 11, 106, and 140 BU/mL had their molar concentrations of antibody determined to be 94.6 plus or minus 0.08, 214.3 plus or minus 7.1, 2209.4 plus or minus 84.9, 2417.7 plus or minus 3.8 nM, respectively. Seven plasma samples designated as inhibitor-free were also analyzed and 2 of the 7, presented as samples GK1 and GK2, were measured to contain 112.9 plus or minus 1.9 nM and 71.1 plus or minus 8.5 nM anti-fVIII Ab.
Quantitation of anti-fVIII Ab in human plasma samples with less than 1% fVIII activity. Plasma samples with predetermined anti-fVIII antibody titers of 1, 11, 106, and 140 BU/mL had their molar concentrations of antibody determined to be 94.6 plus or minus 0.08, 214.3 plus or minus 7.1, 2209.4 plus or minus 84.9, 2417.7 plus or minus 3.8 nM, respectively. Seven plasma samples designated as inhibitor-free were also analyzed and 2 of the 7, presented as samples GK1 and GK2, were measured to contain 112.9 plus or minus 1.9 nM and 71.1 plus or minus 8.5 nM anti-fVIII Ab.
Bethesda assay analysis of commercial plasmas with less than 1% fVIII activity
Two inhibitor-positive and 2 inhibitor-negative, as determined by the manufacturer, commercial plasma samples were reanalyzed in-house using the Bethesda assay kit. The 2 inhibitor-positive plasmas, 11 BU/mL and 106 BU/mL, were determined to contain 5 BU/mL and 101 BU/mL of anti-fVIII inhibitor, respectively. Two inhibitor-negative plasma samples were confirmed to be devoid of inhibitor activity, nonetheless, they were quantitated to have anti-fVIII antibody concentrations (GK1 = 112.1 ± 1.9 nM and GK2 = 71.1 ± 8.5 nM), comparable with that of 1 BU/mL inhibitor plasma (94.6 ± 0.8 nM) anti-fVIII antibody. These results validate the immunoassay for the quantitation of anti-fVIII antibodies not recognized by the standard Bethesda assay.
Analyses of inhibitor-free hemophilia A human plasma in FLI
Hemophilia A human plasma samples, reported as inhibitor-free, were quantitated for anti-fVIII Ab. Plasma samples with MFIU of the specific binding to rfVIII coupled beads greater than the MFIU of the ovalbumin nonspecific control were considered positive for the presence of anti-fVIII Ab. The net MFIU or the difference between the specific and nonspecific binding was correlated to the calibrator from which the molar concentration of the antibody was determined. Table 1 presents the classification of the 25 hemophilia A subjects with corresponding fVIII antigen concentrations, fVIII activities, and anti-fVIII Ab determinations. The subjects were classified as having severe, moderate, or mild hemophilia A according to the initial fVIII activity level analysis at diagnosis, using the standard determination or the APTT. Six of the 25 samples tested positive for anti-fVIII Ab. Subjects 1 through 11 received fVIII prophylaxis 0.25 to 4 days before phlebotomy as indicated (Table 1) and subject 19 received treatment 13 days before the second blood draw. Three subjects had a mean antibody concentration in the range of 1 to 10 nM: subject 2 had a mean concentration of 5.4 plus or minus 0.4 nM (2 draws, 4 determinations), subject 8 had a mean of 3.2 plus or minus 0.3 nM (1 draw, 2 determinations), and subject 20 had a mean of 1.2 plus or minus 0.2 nM (2 draws, 4 determinations). Samples from subjects 12 and 19 were determined as antibody-positive upon the second draw. Their antibody concentrations were 0.6 plus or minus 0.4 nM and 10.1 plus or minus 6.9 nM, respectively. In this group of hemophilia A subjects, the highest antibody concentration was found to be 20 plus or minus 4 nM in plasma from subject 18.
The antigen levels in a few subjects—particularly subjects 1, 10, 14, and 16—were undetectable by the immunoassay. Whether the lack of recognition is due to a mutation in the fVIII protein, and therefore the epitope was unrecognized by the monoclonal antibodies, or simply due to antigen levels below the detection limit of the assay, was not established. Also, the corresponding detectable activity of the samples did not exclude the possibility that the activity was due to activated fVIII, which is not recognized by the fVIII immunoassay (data not shown). Repeated analyses of the samples reproducibly showed undetectable antigen levels.
Evaluation of inhibitor-free severe hemophilia A plasma prior and subsequent to infusion with therapeutic fVIII
Seven inhibitor-negative hemophilia A subjects (Table 2, subjects 26-32) were tested for the presence of anti-fVIII Ab in FLI at baseline, before the infusions with therapeutic doses of 5 different fVIII products. These infusions were carried out under a pharmacokinetic study protocol with each PK study separated by a minimum of 30 days and extending as much as 3 months between infusions.1 Five of the 7 subjects (subjects 26-30) were found to be anti-fVIII Ab positive, as listed in Table 2. Four subjects (26, 27, 28, and 31) were followed through 5 cycles of therapy with 5 different fVIII concentrates (products A-E) accompanied by the determination of fVIII antigen concentration, fVIII activity, and anti-fVIII Ab. Three subjects (29, 30, and 32) were studied through 3 cycles of therapeutic intervention with the same product (B). Subject 26 showed a variable pattern of fVIII Ab through the 5 cycles, with molar concentrations between 1.1 plus or minus 0.1 nM and 17.6 plus or minus 0.4 nM. Subject 27 exhibited an increase in fVIII Ab presence after therapy cycle 2 (cycle 1 at 0.5 ± 0.3 nM and cycle 5 at 3.2 ± 2.0 nM), whereas subject 28 had a diminished concentration of fVIII Ab between cycle 1 (0.6 ± 0.2 nM) and cycle 5 (nondetectable antibody). The development of an anti-fVIII Ab was observed in subject 29, cycle 1 with nondetectable levels and cycle 3 with 2.5 plus or minus 2.1 nM. A constant concentration of the alloantibody was observed in subject 30 between cycle 1 with 4.6 plus or minus 0.2 nM and cycle 3 with 3.5 plus or minus 0.8 nM. All antibody concentrations were within the 1 to 10 nM range and below the concentration determined for 1 BU/mL plasma titer (94.6 ± 0.8 nM).
Discussion
The assay used for this study was calibrated with human anti-fVIII Ab and validated with inhibitor-positive plasma samples. Due to the limited amount of the human calibrator, we also validated a mouse anti-fVIII Ab as a suitable surrogate calibrator. Both assays are specific for anti-fVIII Abs in human plasma with a sensitivity of 40 pM. The human calibrator was validated using 2 different sources of fVIII, rfVIII, and IST fVIII, demonstrating similar affinities for each product. Due to the polyclonal nature of the anti-fVIII immunoglobulins generated by each subject, the affinities and the specific recognition sites of fVIII antibodies might differ. Four commercially available severe hemophilia A plasma samples with predetermined inhibitor activity (1, 11, 106, and 140 BU/mL inhibitor titer) were confirmed to be anti-fVIII Ab–positive in our assay with molar concentrations of 94, 214, 2209, and 2417 nM, respectively. Among the 150 normal controls, 4 (3%) were determined to be anti-fVIII Ab–positive with concentrations of 0.6 to 6.2 nM, 1 to 2 orders of magnitude below that for a 1 BU/mL (94 nM) hemophilia A plasma. The incidence of autoantibodies in the healthy population examined by our assay is at the low end of reported frequency (0%-36%) for healthy individuals.41,,,,–46 The high incidence of antibodies in healthy donors reported by others might arise due to the lack of nonspecific binding controls in the detection assays used to analyze the samples. Inclusion of a binding control in our assay largely eliminates from analyses signals from the nonspecific binding of plasma proteins. In some cases, the self-directed immune response in normal controls has been reported to be temporary, with detectable levels disappearing over time.45,47,,–50
Thirteen of 39 (33%) inhibitor-negative hemophilia A subjects were positive for anti-fVIII Abs. The samples from these 39 subjects can be divided into 3 groups. One consisted of 25 samples among which some were subjected to fVIII therapeutic intervention before the time of collection (Table 1). Measured fVIII antigen concentration and activity levels were reflective of hemophilia A, that is, these levels were less than or equal to 40% of those observed for normal donor plasma (normal 0.7 nM). Subjects were grouped as having severe, moderate, and mild hemophilia A according to reported classifications based on percent fVIII activity relative to normal.2 Three of the 25 individuals had blood drawn on 2 separate occasions, both times with consistent antibody concentrations. Two subjects were antibody-positive only at the second blood draw. Interestingly, one of these subjects had received fVIII therapy just before the phlebotomy.
The second group, consisting of 7 inhibitor-free severe hemophilia A subjects enrolled in a study evaluating the fVIII PKs, was analyzed for the development of alloantibodies before and during treatment with the fVIII products. Each subject's plasma collected before each PK cycle was tested for fVIII antigen concentration, fVIII activity, and the presence of anti-fVIII Abs (Table 2). Five of the 7 subjects (71%) demonstrated the presence of alloantibodies in the range of 0.5 to 17 nM. The concentrations of antibodies in 2 of the subjects increased significantly over the course of the treatments. The direct cause of this augmentation in the immune response is not clear. One of the 7 subjects showed a stable antibody level, whereas another subject demonstrated a decline or even the disappearance of detectable antibody despite continued therapy.
The third group analyzed consisted of 7 commercially available inhibitor-free plasma samples with less than 1% fVIII activity. The plasma samples (different lots) revealed the presence of anti-fVIII Abs in 2 samples. The concentration of the antibody in both samples was significant, with molar quantities comparable with the concentrations found in 1 BU/mL inhibitor-positive plasma. The overall prevalence of anti-fVIII Abs in hemophilia A subjects evaluated by our immunoassay was approximately 33%, relative to the healthy donor population in which 3% were determined as positive (≥ 40 pM) for fVIII Ab. The 33% frequency of antibodies in hemophilia A patients as quantitated by our assay is within the reported range (ie, 15%-50%) of inhibitory antibodies in hemophilia A subjects as determined by the Nijmegen method.6,–8 The reported frequency of noninhibitory alloantibodies in qualitative studies of hemophilia A patients is 8% to 60%.33,49,51 Our quantitative study, with a sensitivity of greater than or equal to 40 pM, indicates that such alloantibodies occur in 33% of inhibitor-negative (as determined by the Bethesda assay) hemophilia A population with concentrations ranging between 0.5 and 200 nM. Levels of fVIII antigen, fVIII activity, and anti-fVIII Ab in the hemophilia A population were compared with one another to determine correlations between the 3 parameters. No correlations were observed between the fVIII antigen and anti-fVIII Ab or the fVIII activity and anti-fVIII Ab. The most common sites of recognition by the noninhibitory antibodies in healthy subjects have been found in the nonfunctional domains of fVIII or the A1, A3, and B domains. These 3 domains are also the most frequently recognized by noninhibitory alloantibodies in hemophilia A patients, whereas the A2, A3, and C2 domains are the most commonly recognized by antibodies in hemophilia A patients with inhibitors.12,41,42,52
The present report describes the development of a novel fluorescence based immunoassay that uses a human anti-fVIII Ab calibrator as a standard for the absolute quantitation of anti-fVIII alloantibodies in human plasma. Inhibitory alloantibodies, quantitated in terms of their capacity to inactivate plasma fVIII expressed in Bethesda Units, have been shown to neutralize the activity of exogenous and residual endogenous fVIII either by inhibiting binding of the antigen to phospholipid membranes and VWF or by blocking the activation of fVIII by thrombin and factor Xa.52,53 The relevance and natural history of noninhibitory antibodies, however, has been largely ignored, mainly due to the inability of the Bethesda assay to determine their identification and the failure of binding assays to quantify concentration in absolute (molar) terms. The clinical importance of antibodies, unrecognized by the standard Bethesda assay, has not been systematically studied and the mechanism of action and the effect on fVIII therapy remains to be elucidated.
This work was presented at the 50th Annual American Society of Hematology Meeting and Exposition, December 6-9, 2008, San Francisco, CA and presented in part at the XXth Congress of the International Society on Thrombosis and Haemostasis, August 6-12, 2005, Sydney, Australia (abstract number P0650) and at the XXVIIIth International Congress of the World Federation of Hemophilia, June 1-5, 2008, Istanbul, Turkey (abstracts 03 PO 37 and 04 FP 24).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Lundblad for providing us with rfVIII and Dr Fass for providing anti–fVIII-68 mAb. We also thank Drs Undas and Tracy for kindly supplying the healthy donor plasma samples. We thank Matthew Gissel and Matthew Whelihan for their technical assistance.
This study was supported by grant P01HL46703 from the National Institutes of Health (K.G.M.); a research grant from Baxter Healthcare (E.D.G. and K.G.M.); a Career Development Award from the National Hemophilia Foundation (K.B.Z.); and a research grant from Bayer Canada (G.E.R.).
National Institutes of Health
Authorship
Contribution: J.A.-K. performed the research, analyzed data, and wrote the paper; B.P.-S. designed and performed the fVIII antigen and antibody assays, and analyzed data; S.B. contributed to data analysis and directed fVIII activity assays; K.B.-Z. contributed to data analysis and writing the paper; E.D.G. provided inhibitor-negative hemophilia A plasma and contributed to the analysis; G.E.R. provided inhibitor-negative hemophilia A plasma and plasma from a hemophilia A patient used for isolation of the human anti-fVIII antibody calibrator; and K.G.M. designed the research protocol, analyzed data, and contributed to writing the paper.
Conflict-of-interest disclosure: K.G.M. is a consultant to Baxter Healthcare and Chairman of the Board of Haematologic Technologies. E.D.G. is a consultant to Baxter Healthcare. The remaining authors declare no competing financial interests.
Correspondence: Kenneth G. Mann, Department of Biochemistry, University of Vermont, 208 South Park Drive, Suite 2, Room T227, Colchester, VT 05446; e-mail: kenneth.mann@uvm.edu.