Abstract
Mast cells are key participants in allergic diseases via activation of high-affinity IgE receptors (FcϵRI) resulting in release of proinflammatory mediators. The biochemical pathways linking IgE activation to calcium influx and cytoskeletal changes required for intracellular granule release are incompletely understood. We demonstrate, genetically, that Pak1 is required for this process. In a passive cutaneous anaphylaxis experiment, Wsh/Wsh mast cell–deficient mice locally reconstituted with Pak1−/− bone marrow–derived mast cells (BMMCs) experienced strikingly decreased allergen-induced vascular permeability compared with controls. Consistent with the in vivo phenotype, Pak1−/− BMMCs exhibited a reduction in FcϵRI-induced degranulation. Further, Pak1−/− BMMCs demonstrated diminished calcium mobilization and altered depolymerization of cortical filamentous actin (F-actin) in response to FcϵRI stimulation. These data implicate Pak1 as an essential molecular target for modulating acute mast cell responses that contribute to allergic diseases.
Introduction
Mast cells are found in tissues where there is frequent contact between the host and the environment. These cells function as key sensors of foreign antigens and initiate inflammatory processes including anaphylaxis, allergy, and asthma.1 Antigenic challenge activates FcϵRI, the high-affinity IgE receptor, which triggers the immediate release of preformed mediators from cytoplasmic granules (degranulation) via secretory granule translocation followed by vesicle and plasma membrane fusion. Delayed responses to FcϵRI activation also include production and secretion of eicosanoids and inflammatory cytokines. Thus, mast cells play a central role in the initiation and propagation of a local inflammatory response, resulting in many of the symptoms of acute and chronic allergic diseases.
The tetrameric FcϵRI receptor binds IgE and transduces signals via a large macromolecular signaling complex at the plasma membrane. Subsequent activation of multiple kinases and phosphatases, as well as calcium and lipid signals must be coordinated to impact cytoskeletal changes resulting in mast cell degranulation. The importance of changes in intracellular calcium concentration in degranulation is well established.2 Allergen-induced mast cell degranulation involves a calcium-dependent process triggered by depletion of calcium from intracellular stores followed by calcium entry from the extracellular environment via store-operated calcium channels.3-6 The resultant sustained increase in cytoplasmic calcium facilitates granule movement, membrane fusion, and exocytosis via actin filament and microtubule reorganization.7,8 Speaking to the complexity of this molecular machinery, there is also evidence to suggest that cytoskeletal elements themselves participate in the regulation of calcium entry.9 Despite significant investigation and progress in understanding this complex process, much remains unknown about the downstream effectors that link early FcϵRI targets to cellular changes that promote degranulation.
Studies using pharmacological inhibitors and genetic models have established that, by interacting with multiple target proteins, PI3-K and small RhoGTPases regulate calcium mobilization and cytoskeletal changes that are integral to FcϵRI-mediated mast cell degranulation.10-13 One shared class of mediators that independently receives signals from PI3-K and RhoGTPases is the family of p21-activated kinases (Paks) (reviewed in Kumar et al14 and Hofmann et al15 ). Pak1 is a Pak family member whose expression is restricted principally to brain, muscle, and hematopoietic cells (reviewed in Bokoch16 and Sells et al17 ). In nonhematopoietic cells, Paks induce the temporal and spatial formation of cortical actin structures similar to those regulated by Rac and Cdc42, including membrane ruffles, lamellipodia, filopodia, and focal complexes.17-19 Given that degranulation involves complex cytoskeletal rearrangements and that Paks regulate filamentous actin (F-actin) dynamics, we investigated a potential role for Pak1 in allergen-stimulated mast cell de granulation.
Here we generated a Pak1−/− mouse to elucidate the role of Pak1 in allergen-induced mast cell function. We provide in vivo and in vitro evidence that Pak1 is necessary for appropriate mast cell degranulation, although loss of Pak1 does not impact the life span, growth, or fertility of the Pak1−/− mouse. We report a biochemical mechanism by which Pak1 impacts degranulation by regulation of F-actin, calcium signaling, and vesicle translocation. Our findings suggest that, although F-actin polymerization in response to IgE sensitization is independent of Pak1 function, Pak1 is critical for disassembly of cortical F-actin upon allergen stimulation, a process that provides mast cell granules access to the plasma membrane.13,20 These studies establish a vital role for Pak1 in regulating mast cell function and warrant further examination of Pak1 as a potential therapeutic target in allergen-mediated inflammatory processes.
Methods
Mice
Pak1+/− mice were backcrossed 10 generations onto a C57Bl/6J strain. Syngeneic WT and Pak1−/− mice progeny from this backcross were used for experimentation. Animal care and experimental procedures were conducted on a protocol approved by the Fox Chase Cancer Center Institutional Animal Care and Use Committee and the Indiana University Laboratory Animal Research Center.
Southern blot
Genomic DNA was isolated for Southern blot using a standard phenol chloroform extraction, followed by digestion with HindIII, separation of the DNA fragments via gel electrophoresis, transfer of the gel to a nitrocellulose membrane, and hybridization with a 32P-labeled 1-KB Xba1-BamHI genomic probe.
Genotyping by PCR
Purified (Puregene; Gentra, Minneapolis, MN) tail DNA was used for polymerase chain reaction (PCR) designed to amplify DNA fragments from the WT and targeted Pak1 alleles simultaneously. A common forward primer (5′-GCC CTT CAC AGG AGC TTA ATG A-3′) was used with a pak1-specific reverse primer (5′-GAA AGG ACT GAA TCT AAT AGC A-3′) to amplify a 240-bp product from the WT allele; and with a neo-specific reverse primer (5′-CAT TTG TCA CGT CCT GCA CGA-3′) to amplify a 360-bp product from the targeted allele.
Western blotting
Whole-cell lysates were prepared by addition of 1 × SDS sample buffer to 106 bone marrow–derived mast cells (BMMCs). Cells were sonicated, clarified by centrifugation, and quantified using a BCA reagent assay (Thermo Scientific, Waltham, MA). Samples were separated by SDS–polyacrylamide gel electrophoresis (PAGE) on a 4% to 12% gradient gel (Invitrogen, Frederick, MD) and transferred to PVDF membrane. Blots were probed with anti-Pak1 antibody (1:1000; Cell Signaling, Beverly, MA) then visualized with HRP-conjugated goat anti–rabbit IgG antibody (1:5000; R&D Systems, Minneapolis, MN). To prepare whole-cell lysates for the phospholipase Cγ1 blots, BMMCs (2 × 106) were stimulated (“Cell culture and activation”) and lysed with ProteoJET Mammalian Cell Lysis Reagent (Fermentas, Hanover, MD), clarified, and quantified using BCS reagent assay (Thermo Scientific). Proteins were separated on an 8% Tris-glycine polyacrylamide gel or 4% to 12% gradient Bis-Tris polyacrylamide precast gel (Invitrogen), transferred to PVDF, and probed with anti–phospho-phospholipase Cγ1 (1:1000; Cell Signaling) or anti–total phospholipase Cγ1 (1:1000; Cell Signaling). All blots used an HRP-conjugated antirabbit secondary antibody (Amersham, Arlington Heights, IL), and films were developed using the enhanced chemiluminescence (ECL) plus system (Amersham). Phosphorylated proteins were quantified by subjecting autoradiographs to densitometry (NIH Image software; National Institutes of Health [NIH], Bethesda, MD).
Reverse transcription–PCR
Total RNA was isolated with the RNeasy mini protocol kit (QIAGEN, Valencia, CA), using 2× excess DNAse I treatment (RNnase-Free DNase set; QIAGEN). Superscript First-Strand Synthesis System (Invitrogen) was used to synthesize cDNA from 5 μg RNA. cDNA was amplified with 2 mM MgCl2, 200 μM PCR nucleotide mix, 0.04 U Taq DNA polymerase, and 300 nM of the following primers: PAK1 sense, 5′-GGCAGCAAAGACACTGGAACCCTA-3′; PAK1 antisense, 5′-CTCATGTACTTCTTACTATTGGAGGTC-3′; GAPDH sense, 5′-CTGGTGCTGAGTATGTCGTG-3′; and GAPDH antisense, 5′-CAGTCTTCTGAGTGGCAGTG-3′. Amplification included initial denaturation at 95°C × 5 minutes, denaturation at 95°C × 1 minute, elongation at 58°C × 1 minute, and annealing at 72°C × 1 minute, for 34 cycles.
Cell culture and activation
BMMCs were selectively grown in IMDM medium supplemented with 10 U/mL penicillin, 0.1 mg/mL streptomycin, 1% l-glutamine, 10% FBS, and 5 ng/mL mIL-3 (Peprotech, Rocky Hill, NJ) for 5 to 8 weeks. BMMCs were cultured for 5 to 8 weeks, sensitized in media with 1.5 μg/mL anti-DNP IgE monoclonal antibody (clone SPE-7; Sigma-Aldrich, St Louis, MO) for 4 hours, and stimulated with 30 ng/mL dinitrophenyl conjugated to human serum albumin (DNP-HSA, 30-40 mol DNP/mol HSA; Sigma-Aldrich). IPA-3 is an isoform-selective, small molecule inhibitor that targets the autoregulatory mechanism of p21-activated kinases. For the IPA-3 treatment, cells were sensitized as described and then pretreated for 10 minutes with 30 μM IPA-3 prior to the addition of the DNP stimulation.21
Detection of c-kit/FcϵRI receptors
C-kit and FcϵRI expression were analyzed by fluorescence cytometry as described.11 Cells were blocked with unconjugated anti-FcγRII/III (BD Pharmingen, San Diego, CA) and stained with anti-DNP monoclonal antibody IgE clone SPE-7 (Sigma-Aldrich), anti–mouse CD 117 (c-kit) PE-conjugated antibody, and FITC-conjugated anti–mouse IgE (both BD Pharmingen) secondary antibody. Cells were washed and resuspended in 0.5% BSA PBS buffer. Aliquots of BMMCs were also stained with FITC- and PE-conjugated rat IgG2b, K; isotype antibodies as negative controls. Cells were analyzed on a fluorescence-activated cell sorting (FACS) Calibur (Becton Dickinson, San Jose, CA). Data were analyzed by unpaired, 2-tailed Student t test.
Pak1 kinase assay
BMMCs were sensitized and stimulated (see “Cell culture and activation”) at 37°C for 1 minute and the reaction was terminated by addition of 1 mM Na3VO4 in cold PBS. Whole-cell lysate (400 μg) was prepared as previously described,22 and a 10-μL aliquot of each sample was reserved for detection of β-actin to serve as a loading control. The remainder of the lysate was immunoprecipitated with 2 μg/mL α-Pak1 antibody (N20; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 18 hours before incubation with protein A/G plus beads (Santa Cruz Biotechnology) for 2 hours. Pak activity was assayed by incubating the immunobeads with 1 μg/reaction inactive Mek (Millipore, Billerica, MA) and 250 μM ATP (Sigma-Aldrich) in 30 μL kinase buffer.23 Samples were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti–Mek-phospho-serine 298 (1:1000; Biosource, Camarillo, CA). Phosphorylated Mek was quantified by subjecting autoradiographs to densitometry (NIH Image software).
Degranulation
BMMC degranulation was determined by β-hexosaminidase release as previously described24 with minor modification. IgE-primed (see “Cell culture and activation”) BMMCs were suspended at 2 × 106 cells/mL in Tyrode buffer (10 mM HEPES buffer, 130 mM NaCl, 5 mM Kcl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 0.05% BSA, pH = 7.4) then stimulated with 30 ng/mL DNP-HSA (Sigma-Aldrich) for 15 minutes at 37°C. For receptor-independent stimulation, unsensitized cells were incubated in Tyrode buffer and stimulated with 1 μM calcimycin for 15 minutes. The cell pellets were solubilized in Tyrode buffer, 0.5% Triton X-100. β-Hexosaminidase release was measured in both the supernatants and the cell pellets by incubating with 4-nitrophenyl N-acetyl-beta-d-glucosaminide (Sigma-Aldrich) in sodium citrate (pH 4.5) for one hour at 37°C. Sodium carbonate/sodium bicarbonate buffer (0.1 M, pH 10) was used to stop the reaction and absorbance was read at 405 λ. Degranulation was expressed as a percentage of β-hexosaminidase released = supernatant activity/total (supernatant plus pellet) activity ×100. Samples were assayed in triplicate. Data were graphed using Prism (GraphPad Software, San Diego, CA) and analyzed by unpaired, 2-tailed Student t test.
Calcium mobilization
IgE-primed BMMCs were suspended at 106 cells/mL in 0.1% BSA in RPMI 1640 containing 3 μM fura-2-AM (Molecular Probes, Eugene, OR) at 37°C for 1 hour. Cells were washed and resuspended at 106 cells/mL in calcium-containing HBSS without phenol red. Samples were warmed to 37°C and stimulated with either 1 μM calcimycin (A23187) or DNP-HSA (30 ng/mL). In some experiments, extracellular calcium was removed prior to stimulation by the addition of 10 mM EGTA. Fura-2 fluorescence was monitored using an F-2000 spectrophotometer (Hitachi, Tokyo, Japan) as previously described.25 Measurements were performed at 37°C with constant stirring. The excitation and emission wavelengths of fura-2 are 340λ and 380λ. Subsequent addition of 80 μg/mL digitonin then 10 mM EGTA allowed determination of maximum and minimum fura-2 fluorescence for calculation of [iCa]rest and [iCa]stim as described.26 Data were graphed using Prism (GraphPad Software) and analyzed by unpaired, 2-tailed Student t test.
Confocal microscopy
BMMCs were allowed to adhere to glass slides then fixed in 3.7% paraformaldehyde in phosphate-buffered saline (PBS: 1.76 mM KH2PO4, 10.14 mM Na2HPO4, 2.68 mM KCl, and 136.8 mM NaCl) for 15 minutes at room temperature. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 minutes, washed in PBS, then incubated with Alexa Fluor488 phalloidin (1 unit) or rhodamine-phalloidin (Invitrogen) for 30 minutes. After washing in PBS, fluorescence analysis was performed using the Zeiss LSM 510 confocal laser scanning system (Carl Zeiss, Heidelberg, Germany) using a 100× (oil) magnification. The intensity of F-actin staining was quantified using ImageJ software (NIH) and data were analyzed by unpaired, 2-tailed Student t test.
Passive cutaneous anaphylaxis
Adoptive transfer studies were conducted as previously described27 using mast cell–deficient Kit Wsh/Wsh mice purchased from Jackson Laboratories (Bar Harbor, ME). BMMCs (106) in 40 μL IMDM were injected intradermally into each ear of 6- to 8-week-old female Kit Wsh/Wsh mice. Twelve weeks after intradermal injection, each mouse was primed to express an IgE-dependent passive cutaneous anaphylaxis (PCA) reaction. Mice were anesthetized with avertin and received an intradermal injection to the right ear of 20 μL of 1:44 dilution of monoclonal anti-DNP IgE in PBS (clone SPE-7; Sigma-Aldrich). The left ear received an intradermal injection of 20 μL PBS alone. Twenty hours after injection, the mice received 300 μL of a 10-mg/mL DNP-human serum albumin (HSA) (Sigma-Aldrich) and 1% Evans blue (Sigma-Aldrich) solution intravenously. Thirty minutes later, the mice were killed, imaged using an Epson perfection 4990 photo scanner. Tissue samples were acquired by 5-mm punch biopsy at the sensitization site. Dye was extracted with 1N KOH overnight at 37°C. The next day 900 μL extraction buffer (85% H3PO4, acetone, and H2O) was added to digested ear and followed by sample agitation and centrifugation. Samples were read at 620 nm with a spectrophotometer. Data were analyzed by unpaired, 2-tailed Student t test.
Generation of recombinant Pak1 construct and supernatants
The BsmB1-adapted pEGFP-PAK1 cDNA was ligated into the Age1/BsmB1 site of the pCL1 lentiviral backbone provided by Dr Helmut Hanenberg. LV-containing supernatant was generated via transfection of 293T cells with a recombinant foamy virus envelope plasmid and the LV helper plasmid pCD/NL-BH using 45 μg polyethylenimine (PEI; Sigma-Aldrich). Packaging cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 10 U/mL penicillin, and 0.1 mg/mL streptomycin, 1% l-glutamine, and 2% NaHCO3 as previously described.23
Generation of recombinant CD63-EGFP supernatants
The pMX-CD63-EGFP retroviral plasmid was kindly provided by Dr Keigo Nishida at Riken Research Center for Allergy and Immunology (Kanagawa, Japan). RV-containing supernatant was generated via transfection in an ecotropic packaging cell line using a calcium phosphate transfection kit (Invitrogen) to generate recombinant retrovirus.
Transduction of mast cell progenitors
Transduction of mast cells was accomplished using previously published methods from our laboratory.23 Mice were injected with 5-fluorouracil (150 mg/kg; American Pharmaceutical Partners, Schaumburg, IL). Bone marrow cells were harvested 48 hours after injection. Mononuclear cells obtained after density gradient centrifugation were cultured for 2 days in the presence of stem cell factor 100 ng/mL, G-GSF 100 ng/mL, and IL-6 200 U/mL (all from Peprotech). The cells were infected with either pCL1EGFP or pCL1EGFPPAK 1 virus supernatant on FN CH-296–coated plates overnight. On day 3 after infection, the green fluorescent protein–positive cells were isolated by fluorescence-activated cell sorter (FACStar Plus; Becton Dickinson) under sterile conditions and expanded in BMMC conditions for 6 to 8 weeks.
Results
Genetic disruption of murine Pak1
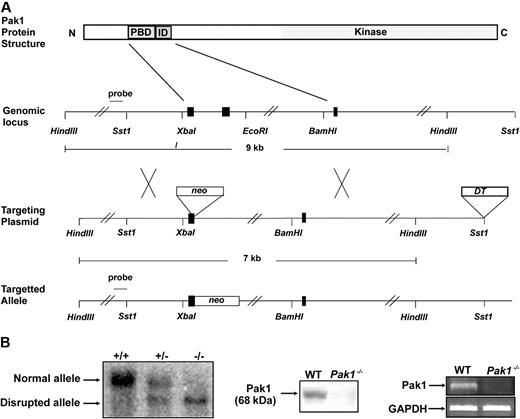
A Pak1−/− mouse was generated by targeted disruption of the Pak1 allele in embryonic stem (ES) cells. The resultant allele contains a neomycin cassette and is lacking 2 kb genomic DNA encoding the p21-binding domain (Figure 1A). Southern blot analysis of tail DNA from Pak1+/+, Pak1+/−, and Pak1−/− reveals the presence of the expected genotypes (Figure 1B). Pak1−/− mice are observed at the predicted Mendelian frequency and are viable and fertile. Pak1−/− mice have a normal life span and normal peripheral blood cell indices (Table 1). To verify that Pak1−/− mice produce no Pak1 protein, cell lysates isolated from WT and Pak1−/− BMMCs were subjected to Western blot using a Pak1-specific antibody (Figure 1B). Western blots of whole brain lysates and murine embryonic fibroblasts were also performed (data not shown). No Pak1 protein was observed in any cells from the Pak1−/− mice. To further document the absence of Pak1 protein being expressed at levels below detection of Western blot, reverse-transcription (RT)–PCR was conducted to look for expression of the Pak1 mRNA transcript. As expected, Pak1 mRNA was detected in WT BMMCs, but no Pak1 mRNA was detected in Pak1−/− BMMCs (Figure 1B).
Targeted disruption of the Pak1 allele. (A) Partial restriction map of the native Pak1 gene (genomic locus), the targeting vector replacing the coding sequence of a portion of the N-terminus, including the p21-binding domain (PBD) and the inhibitory domain (ID) with the Neo-resistance gene in the antisense orientation (targeting vector), and the organization of the targeted Pak1 allele (targeted allele). The 1-kb genomic probe used for screening is indicated along with the expected sizes of the wild-type (WT) and targeted HindIII fragments. (B) Genomic Southern blot analysis (left panel). The nontargeted Pak1 allele (10 kb) is visualized in the WT (+/+) mice and the targeted allele (8 kb) is visualized in the Pak1−/− (−/−) mice. Both bands can be appreciated in the heterozygous (+/−) mice. Western blot analysis (center panel). WT and Pak1−/− bone marrow–derived mast cell (BMMC) lysates were subjected to immunoblotting with anti-Pak1. The 68-kDa Pak1 protein is present in WT BMMCs and absent in the Pak1−/− cells. RT-PCR analysis (right panel). Pak1 cDNA was amplified by PCR from BMMCs to generate a 352–base pair fragment (corresponding to base pairs 306-658) in the WT cells, which is absent in the Pak1−/− cells. GAPDH mRNA in WT and Pak1−/− BMMCs is also shown.
Targeted disruption of the Pak1 allele. (A) Partial restriction map of the native Pak1 gene (genomic locus), the targeting vector replacing the coding sequence of a portion of the N-terminus, including the p21-binding domain (PBD) and the inhibitory domain (ID) with the Neo-resistance gene in the antisense orientation (targeting vector), and the organization of the targeted Pak1 allele (targeted allele). The 1-kb genomic probe used for screening is indicated along with the expected sizes of the wild-type (WT) and targeted HindIII fragments. (B) Genomic Southern blot analysis (left panel). The nontargeted Pak1 allele (10 kb) is visualized in the WT (+/+) mice and the targeted allele (8 kb) is visualized in the Pak1−/− (−/−) mice. Both bands can be appreciated in the heterozygous (+/−) mice. Western blot analysis (center panel). WT and Pak1−/− bone marrow–derived mast cell (BMMC) lysates were subjected to immunoblotting with anti-Pak1. The 68-kDa Pak1 protein is present in WT BMMCs and absent in the Pak1−/− cells. RT-PCR analysis (right panel). Pak1 cDNA was amplified by PCR from BMMCs to generate a 352–base pair fragment (corresponding to base pairs 306-658) in the WT cells, which is absent in the Pak1−/− cells. GAPDH mRNA in WT and Pak1−/− BMMCs is also shown.
Mast cell development is not affected by genetic disruption of Pak1
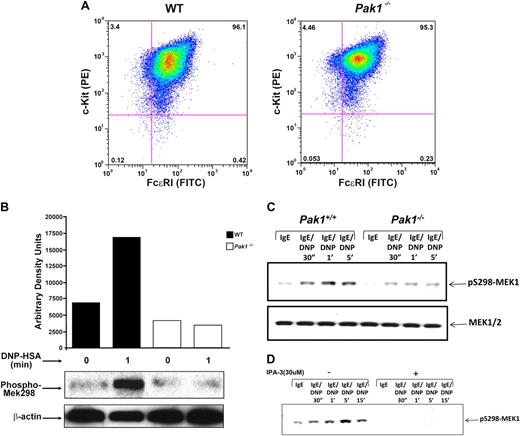
Mast cells were cultured from murine bone marrow in the presence of serum and interleukin 3 (IL-3), and expression of cell surface receptors typical of mature mast cells, including c-kit receptor and FcϵRI receptor, was examined. Fluorescence cytometric analysis of WT and Pak1−/− mast cells demonstrated equivalent expression of both c-kit and FcϵRI receptors (mean 96.1 ± 2.3 SEM % vs 95.3 ± 1.7 SEM % double-positive cells, respectively; n = 6). A representative mast cell population of each genotype is shown in Figure 2A. Further, Pak1−/− cells had typical cellular morphology and staining of cytoplasmic granules upon Alcian blue/saffranin-O staining (data not shown). The frequency of mast cells in the peritoneum and in the dermis of the ear is equivalent in WT and Pak1−/− mice (Table 1). These results indicate that genetic disruption of Pak1 did not influence mast cell differentiation in vitro or in vivo.
Characterization of Pak1−/− bone marrow–derived mast cells. (A) Bone marrow–derived mast cell (BMMC) receptor expression. BMMCs were maintained in culture for 5 weeks, and expression of c-kit and FcϵRI was measured by incubation with antimouse CD 117 (c-kit) PE-conjugated antibody, and anti-DNP monoclonal antibody IgE clone SPE-7 followed by incubation with FITC-conjugated anti–mouse IgE secondary antibody. Double-positive cells (top right quadrant) are mature mast cells, expressing both c-kit and FcϵRI. Data shown are representative of 6 independent lines from each genotype. (Mean WT = 96.1 + 2.3 SEM % vs Pak1−/− = 95.3 + 1.7 SEM % double-positive cells, n = 6.) (B) IgE-mediated Pak1 activation in BMMCs (representative of 3 independent experiments). IgE-primed BMMCs were stimulated with antigen (DNP) for the indicated times, lysates were precipitated with anti-Pak1 antibody, and Pak1 activity was assayed. (C) Pak1 activation of pS298-MEK1 in Wt and Pak1−/− BMMCs. IgE-primed BMMCs were stimulated with antigen (DNP) for the indicated times. Cell lysates (“Western blotting”) were subjected to immunoblotting with anti–phospho-S298 MEK1 (top blot) or anti–total MEK1/2 (bottom blot). (D) Effect of IPA-3 treatment in Wt BMMCs. The length of activation and the addition of inhibitor are indicated.
Characterization of Pak1−/− bone marrow–derived mast cells. (A) Bone marrow–derived mast cell (BMMC) receptor expression. BMMCs were maintained in culture for 5 weeks, and expression of c-kit and FcϵRI was measured by incubation with antimouse CD 117 (c-kit) PE-conjugated antibody, and anti-DNP monoclonal antibody IgE clone SPE-7 followed by incubation with FITC-conjugated anti–mouse IgE secondary antibody. Double-positive cells (top right quadrant) are mature mast cells, expressing both c-kit and FcϵRI. Data shown are representative of 6 independent lines from each genotype. (Mean WT = 96.1 + 2.3 SEM % vs Pak1−/− = 95.3 + 1.7 SEM % double-positive cells, n = 6.) (B) IgE-mediated Pak1 activation in BMMCs (representative of 3 independent experiments). IgE-primed BMMCs were stimulated with antigen (DNP) for the indicated times, lysates were precipitated with anti-Pak1 antibody, and Pak1 activity was assayed. (C) Pak1 activation of pS298-MEK1 in Wt and Pak1−/− BMMCs. IgE-primed BMMCs were stimulated with antigen (DNP) for the indicated times. Cell lysates (“Western blotting”) were subjected to immunoblotting with anti–phospho-S298 MEK1 (top blot) or anti–total MEK1/2 (bottom blot). (D) Effect of IPA-3 treatment in Wt BMMCs. The length of activation and the addition of inhibitor are indicated.
Pak1 is activated upon antigen cross-linking of FcϵRI
We sought to determine the role of Pak1 in signal transduction pathways activated by FcϵRI cross-linking. Lysates from IgE-stimulated mast cells of both genotypes were subjected to an in vitro kinase assay to determine whether Pak1 is normally activated as a consequence of IgE stimulation. Recombinant Mek1 protein was used as a phosphorylation substrate following immunoprecipitation of Pak1, since Pak1 and Pak2 have been established to specifically phosphorylate Mek1 at serine 298 (Mek1 S298).28 There is minimal background phosphorylation of the Mek1 substrate at baseline and upon stimulation of Pak1−/− BMMCs. In contrast, a 2.5-fold increase in Mek1 S298 phosphorylation was observed in WT mast cells (Figure 2B), demonstrating that FcϵRI cross-linking results in Pak1 activation. In an independent experiment, Mek1 S298 phosphorylation was measured by Western blot at baseline and following FcϵRI activation. Consistent with the kinase assay, a significant reduction in Mek 1 S298 phosphorylation was observed in Pak1−/− cells at both 1 and 5 minutes following activation (Figure 2C). Finally, in a replicate experiment, cells were incubated with an established inhibitor of Pak1 and Pak2 prior to FcϵRI cross-linking. Complete inhibition of Mek1 S298 phosphorylation was observed at multiple time points (Figure 2D). Collectively these genetic, biochemical, and pharmacologic data indicate that Pak1 is activated following FcϵRI activation.
Pak1 is required for normal mast cell function in vitro
To determine the impact of loss of Pak1 on mast cell degranulation, we compared FcϵRI-mediated degranulation in WT and Pak1−/− mast cells. Purified populations of IgE-sensitized WT and Pak1−/− BMMCs were stimulated with antigen to achieve FcϵRI cross-linking. The concentration of β-hexosaminidase, an enzyme present in preformed mast cell granules, was measured in the supernatant and in the cell pellet. Total β-hexosaminidase content in BMMCs of the 2 genotypes was equivalent, indicating that Pak1 is not required for synthesis of this enzyme (data not shown). However, Pak1−/− BMMCs had a 3-fold reduction in release of β-hexosaminidase upon FcϵRI stimulation (10.7% ± 1.1%) compared with cells from syngeneic, WT age- and sex-matched controls (32.1% ± 4.2%) (n = 4, Figure 3A). Stimulation of mast cells in a non–receptor-dependent fashion by calcimycin (A23187) resulted in comparable levels of degranulation between the 2 genotypes (Figure 3A). These data implicate a specific role for Pak1 in mediating FcϵRI-stimulated degranulation.
Pak1 is a critical mediator of mast cell degranulation in vitro. Mast cell degranulation was assessed by measuring the release of β-hexosaminidase. (A) IgE-primed WT and Pak1−/− BMMCs were stimulated with DNP-HSA for 15 minutes. To determine FcϵRI-independent degranulation, cells were alternatively stimulated with calcimycin (A23187). In all conditions, β-hexosaminidase activity was measured in the supernatant and the extent of degranulation is reported as a percentage of total cellular β-hexosaminidase activity. Data are means plus or minus SEM from triplicate samples in 4 independent experiments. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (B) Confocal laser scanning microscopy images of CD63-EGFP in BMMCs. CD63-EGFP fusion protein was introduced into WT and Pak1−/− progenitors by retroviral transduction as described in “Methods.” Incorporation of the expressed CD63-EGFP into secretory vesicle membranes allows visualization of vesicle location. Fluorescence images of CD63-EGFP (left) and DAPI (right) are shown. Images are representative of 3 independent experiments. (C) Wild-type Pak1 was reintroduced into Pak1−/− BMMCs by lentiviral transduction. Expression of Pak1 in transduced Wt and Pak1−/− BMMCs. The recombinant and endogenous Pak1 proteins are indicated. (D) Release of β-hexosaminidase was measured after sensitization and antigen stimulation as in panel A. Data are expressed as a percentage of WT degranulation. Proof of phenotypic rescue by reintroduction of Pak1 is shown for a single transduced mast cell line assayed in triplicate.
Pak1 is a critical mediator of mast cell degranulation in vitro. Mast cell degranulation was assessed by measuring the release of β-hexosaminidase. (A) IgE-primed WT and Pak1−/− BMMCs were stimulated with DNP-HSA for 15 minutes. To determine FcϵRI-independent degranulation, cells were alternatively stimulated with calcimycin (A23187). In all conditions, β-hexosaminidase activity was measured in the supernatant and the extent of degranulation is reported as a percentage of total cellular β-hexosaminidase activity. Data are means plus or minus SEM from triplicate samples in 4 independent experiments. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (B) Confocal laser scanning microscopy images of CD63-EGFP in BMMCs. CD63-EGFP fusion protein was introduced into WT and Pak1−/− progenitors by retroviral transduction as described in “Methods.” Incorporation of the expressed CD63-EGFP into secretory vesicle membranes allows visualization of vesicle location. Fluorescence images of CD63-EGFP (left) and DAPI (right) are shown. Images are representative of 3 independent experiments. (C) Wild-type Pak1 was reintroduced into Pak1−/− BMMCs by lentiviral transduction. Expression of Pak1 in transduced Wt and Pak1−/− BMMCs. The recombinant and endogenous Pak1 proteins are indicated. (D) Release of β-hexosaminidase was measured after sensitization and antigen stimulation as in panel A. Data are expressed as a percentage of WT degranulation. Proof of phenotypic rescue by reintroduction of Pak1 is shown for a single transduced mast cell line assayed in triplicate.
CD63 is located on granule and plasma membranes in resting basophils and mast cells. We transduced WT and Pak1−/− mast cells with a retrovirus encoding a CD63-EGFP fusion protein to visualize degranulation.29,30 Cells were also stained with DAPI to allow nuclear detection. Following FACS sorting of the transduced cells, BMMCs of both genotypes were imaged at baseline, after IgE sensitization, and after FcϵRI cross-linking. Significantly more cells containing CD63-EGFP–positive vesicles remained after stimulation in the Pak1−/− BMMCs compared with WT mast cells (Figure 3B) consistent with the genotypic differences in β-hexosaminidase release.
To verify that the defect in degranulation is due to disruption of the Pak1 gene, as a proof of principle, we transduced Pak1−/− hematopoietic progenitors with a lentivirus encoding an EGFP-Pak1 fusion protein (or “vector alone,” encoding EGFP only in the case of controls), sorted for transduced cells, and differentiated these cells into BMMCs under standard culture conditions. The recombinant Pak1 transgene is expressed at levels that are close to that of endogenous Pak1 (Figure 3C). Pak1−/− BMMCs transduced with vector alone showed decreased degranulation compared with WT controls (Figure 3D), consistent with previous experiments. Importantly, Pak1−/− BMMCs reconstituted with WT Pak1, were able to degranulate in response to FcϵRI cross-linking to the same degree as WT control BMMCs. This finding underscores the importance and specificity of Pak1 in allergen-induced mast cell degranulation in vitro.
FcϵRI-dependent calcium mobilization is altered in Pak1−/− BMMCs
Stimulation of mast cells by calcimycin in a non–receptor-dependent fashion caused equivalent increases in intracellular calcium concentration ([Ca2+]; data not shown) and comparable levels of degranulation between the 2 genotypes (Figure 3A). This observation together with the profound decrease in degranulation of Pak1−/− BMMCs following FcϵRI-dependent degranulation led us to question the involvement of Pak1 in allergen-induced calcium mobilization. IgE-sensitized BMMCs of both genotypes were loaded with fura-2, suspended in calcium-containing HBSS, and stimulated with DNP-HSA. Spectrophotofluorimetric analysis measuring calcium-bound versus unbound fura-2 fluorescence was used to measure baseline and stimulated intracellular calcium concentration. Baseline levels of intracellular [Ca2+] were similar in WT and Pak1−/− BMMCs and, in both genotypes, maximum emission from calcium-bound fura-2 occurred within seconds of stimulus addition. Compared with controls, the change in intracellular [Ca2+] was significantly diminished in Pak1−/− BMMCs after antigen stimulation (Figure 4A).
Calcium responses are altered in Pak1−/− BMMCs. IgE-primed WT and Pak1−/− BMMCs were loaded with Ca2+-sensitive dye (fura2) and suspended in Ca2+-containing medium (A). To detect release from intracellular stores, cells were suspended in Ca2+-free media and treated with EGTA prior to stimulation (B). FcϵRI was activated by addition of DNP-HSA and changes in intracellular calcium concentration (i[Ca2+]) were measured by spectrophotofluorimetry. (A) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating decreased Ca2+-bound fura-2 in the Pak1−/− cells after antigen stimulation (left panel). Changes in i[Ca] after addition of digitonin followed by EGTA are also shown. Average change in i[Ca2+] (right panel) expressed as a percentage of WT change in i[Ca2+] from 4 independent experiments, *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (B) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating similar responses upon antigen stimulation after depletion of extracellular calcium (left panel). Average percentage increase in i[Ca2+] (right panel) from baseline in WT and Pak1−/− BMMCs from 3 independent experiments, P < .0.57, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Western blot and densitometry for activated PLCγ1. NS indicates cells at baseline (nonprimed, nonstimulated). IgE-primed WT and Pak1−/− BMMCs were stimulated with DNP for 30 seconds or 1 minute. Cell lysates (“Western blotting”) were subjected to immunoblotting with anti–phospho-PLCγ1 (top blot) or anti–total PLCγ1 (bottom blot) and densitometry was performed. Intensity of phosphorylated PLCγ1 bands is represented as the ratio of phospho/total PLCγ1 for 1 of n = 4 experiments (P = .79 and P = .47 at 30 seconds and 60 seconds, respectively).
Calcium responses are altered in Pak1−/− BMMCs. IgE-primed WT and Pak1−/− BMMCs were loaded with Ca2+-sensitive dye (fura2) and suspended in Ca2+-containing medium (A). To detect release from intracellular stores, cells were suspended in Ca2+-free media and treated with EGTA prior to stimulation (B). FcϵRI was activated by addition of DNP-HSA and changes in intracellular calcium concentration (i[Ca2+]) were measured by spectrophotofluorimetry. (A) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating decreased Ca2+-bound fura-2 in the Pak1−/− cells after antigen stimulation (left panel). Changes in i[Ca] after addition of digitonin followed by EGTA are also shown. Average change in i[Ca2+] (right panel) expressed as a percentage of WT change in i[Ca2+] from 4 independent experiments, *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (B) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating similar responses upon antigen stimulation after depletion of extracellular calcium (left panel). Average percentage increase in i[Ca2+] (right panel) from baseline in WT and Pak1−/− BMMCs from 3 independent experiments, P < .0.57, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Western blot and densitometry for activated PLCγ1. NS indicates cells at baseline (nonprimed, nonstimulated). IgE-primed WT and Pak1−/− BMMCs were stimulated with DNP for 30 seconds or 1 minute. Cell lysates (“Western blotting”) were subjected to immunoblotting with anti–phospho-PLCγ1 (top blot) or anti–total PLCγ1 (bottom blot) and densitometry was performed. Intensity of phosphorylated PLCγ1 bands is represented as the ratio of phospho/total PLCγ1 for 1 of n = 4 experiments (P = .79 and P = .47 at 30 seconds and 60 seconds, respectively).
Since calcium release from intracellular stores is a discrete event preceding calcium influx from the extracellular environment, we differentiated the 2 processes by also performing experiments in the absence of extracellular calcium. In calcium-free media plus EGTA, the peak increase in calcium concentration after antigen stimulation is similar between the 2 genotypes (Figure 4B), indicating normal calcium release from intracellular stores. Consistent with this data, allergen-stimulated PLCγ1 activation, which leads to IP3-dependent release of calcium from the endoplasmic reticulum, is similar in WT and Pak1−/− mast cells (Figure 4C). We also confirmed equivalent Rac activation after FcϵRI cross-linking in WT and Pak1−/− BMMCs (data not shown). Thus, the role of Pak1 in regulating calcium mobilization appears to be distinct from that of Rac proteins, which exert their effect on calcium mobilization through regulation of PLCγ1/2 and altered release from intracellular stores.10,31 Our results suggest that, in BMMCs, Pak1 is involved in receptor-mediated calcium influx from the extracellular environment.
Pak1−/− BMMCs have altered actin cytoskeletal dynamics
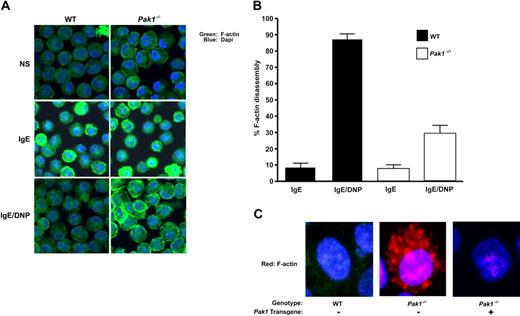
An increase in intracellular calcium is essential for FcϵRI-mediated F-actin ring disassembly and degranulation.30 Given our finding of an attenuated increase in intracellular calcium in Pak1−/− BMMCs, as well as published observations that overexpression of a kinase dead K299R Pak1 mutant stabilizes F-actin in breast cancer cells,32 we hypothesized that Pak1 may affect degranulation through alteration of actin cytoskeletal dynamics. To test this hypothesis, we used confocal microscopy to visualize changes in cortical F-actin in WT and Pak1−/− BMMCs. IgE sensitization of a WT mast cell leads to enhancement of the cortical F-actin ring and subsequent FcϵRI cross-linking induces disassembly of this structure in a majority of cells (Figure 5A). Sensitization of Pak1−/− BMMCs also induces organization of cortical F-actin that appears exaggerated in some cells. Importantly, in Pak1−/− BMMCs, cortical F-actin persists after allergen stimulation (Figure 5A). In 4 experiments conducted from independent primary mast cell lines, the percentage of cells displaying fragmentation of the cortical F-actin ring upon FcϵRI stimulation was significantly greater in the WT BMMCs (87.5% ± 3.6%) compared with the Pak1−/− BMMCs (29.0% ± 5.3%; n = 400 cells, Figure 5B). A similar reduction in total F-actin was observed using fluorescence cytometry. Thirty seconds after cross-linking of the FcϵRI receptor, there is a 35% reduction in F-actin compared with only a 5% to 7% reduction in F-actin in Pak1−/− mast cells. The genotypic differences in F-actin are maintained in serial measurements over 5 minutes (data not shown). Addition of latrunculin, a compound that promotes F-actin depolymerization, enhanced F-actin depolymerization in both WT and Pak1−/− BMMCs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Analogous to the correction of FcϵRI-stimulated β-hexosaminidase release in Pak1−/− BMMCs by lentiviral reintroduction of WT Pak1 (Figure 3B), expression of the Pak1 transgene in Pak1−/− BMMCs restored F-actin disassembly to WT levels, whereas gene transfer with only a reporter gene did not (Figure 5C). Overall, this microscopy data argue for a central role for Pak1 in mediating F-actin disassembly.
Pak1 is required for normal allergen-induced cytoskeletal changes and vesicle trafficking. In all panels, cells were imaged at baseline (NS), after IgE-priming (4 hours), or following IgE-priming and DNP-HSA stimulation (5 minutes) as indicated. (A) WT and Pak1−/− BMMCs were fixed and stained with Alexa-488 phalloidin (to detect F-actin intensity) and DAPI nuclear stain. The images are representative of 5 experiments. (B) The number of cells showing F-actin disassembly was estimated under indicated conditions using a quantitative intensity analysis of Image J. Data are expressed as a percentage of cells with fragmented rings (= number of cells with fragmented ring/total number of cells imaged × 100) for 4 independent experiments (100 cells counted per experiment); *P < .001, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Confocal laser scanning microscopy images of WT or Pak1−/− BMMCs transduced with pCL1EGFP (control, “−”) or pCL1EGFP-PAK1 (Pak1 transgene, “+”) as indicated. Imaged cells have been treated with IgE and DNP.
Pak1 is required for normal allergen-induced cytoskeletal changes and vesicle trafficking. In all panels, cells were imaged at baseline (NS), after IgE-priming (4 hours), or following IgE-priming and DNP-HSA stimulation (5 minutes) as indicated. (A) WT and Pak1−/− BMMCs were fixed and stained with Alexa-488 phalloidin (to detect F-actin intensity) and DAPI nuclear stain. The images are representative of 5 experiments. (B) The number of cells showing F-actin disassembly was estimated under indicated conditions using a quantitative intensity analysis of Image J. Data are expressed as a percentage of cells with fragmented rings (= number of cells with fragmented ring/total number of cells imaged × 100) for 4 independent experiments (100 cells counted per experiment); *P < .001, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Confocal laser scanning microscopy images of WT or Pak1−/− BMMCs transduced with pCL1EGFP (control, “−”) or pCL1EGFP-PAK1 (Pak1 transgene, “+”) as indicated. Imaged cells have been treated with IgE and DNP.
Pak1 is required for normal mast cell degranulation in vivo
Passive cutaneous anaphylaxis (PCA) is a profound localized allergic reaction triggered by allergen-induced cross-linking of FcϵRI by the binding of an allergen-specific IgE placed beneath the skin. We used a PCA model to examine whether Pak1 is important in mast cell degranulation in vivo. The ears of WT and Pak1−/− mice were first sensitized by intradermal injection of monoclonal anti-DNP IgE. Twenty hours after cutaneous sensitization, IgE cross-linking was induced by systemic injection of the antigen, DNP-HSA, with Evans blue dye. The Evans blue dye extravasation was quantified 20 minutes after antigen challenge. This extravasation process is reflective of increased local vascular permeability, a process dependent on mast cell release of histamine and serotonin upon degranulation. The amount of dye in the ear was both qualitatively (Figure 6A) and quantitatively (Figure 6B) reduced in the Pak1−/− mice, indicating an important role for Pak1 in the mast cell–mediated immediate-phase PCA reaction.
Genetic disruption of Pak1 diminishes PCA in vivo. Evans blue extravasation after antigen challenge. (A,B) Pak1−/− or WT mice (n = 5 genotype) were sensitized by intradermal injection of anti-DNP IgE (1:44 dilution, 1 μg/mL) into the right ear and with PBS into the left ear. After 20 hours, mice were challenged by intravenous injection of antigen (DNP-HSA) in PBS/Evans blue. IgE-primed (right) ears 30 minutes after antigen challenge are shown qualitatively (bottom). From each ear, Evans blue was extracted and the intensity of the dye was measured by absorption at 620 nm. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Mast cell–deficient Wsh/Wsh mice (n = 5) were reconstituted locally in the ears by intradermal injection of WT or Pak1−/− mast cells. The mice were then sensitized by intradermal injection of anti-DNP IgE into the right ear and with PBS into the left ear as in panel A. IgE-primed (right) ears from mice reconstituted with WT or Pak1−/− mast cells 30 minutes after antigen challenge are shown qualitatively (bottom). From each ear, Evans blue was extracted and the intensity of the dye was measured by absorption at 620 nm. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test.
Genetic disruption of Pak1 diminishes PCA in vivo. Evans blue extravasation after antigen challenge. (A,B) Pak1−/− or WT mice (n = 5 genotype) were sensitized by intradermal injection of anti-DNP IgE (1:44 dilution, 1 μg/mL) into the right ear and with PBS into the left ear. After 20 hours, mice were challenged by intravenous injection of antigen (DNP-HSA) in PBS/Evans blue. IgE-primed (right) ears 30 minutes after antigen challenge are shown qualitatively (bottom). From each ear, Evans blue was extracted and the intensity of the dye was measured by absorption at 620 nm. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Mast cell–deficient Wsh/Wsh mice (n = 5) were reconstituted locally in the ears by intradermal injection of WT or Pak1−/− mast cells. The mice were then sensitized by intradermal injection of anti-DNP IgE into the right ear and with PBS into the left ear as in panel A. IgE-primed (right) ears from mice reconstituted with WT or Pak1−/− mast cells 30 minutes after antigen challenge are shown qualitatively (bottom). From each ear, Evans blue was extracted and the intensity of the dye was measured by absorption at 620 nm. *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test.
To validate that the genotypic reduction in extravasation is indicative of a cell-autonomous genotypic effect on mast cells, WT or Pak1−/− mast cell progenitors were transplanted into the subcutaneous tissue of the ears of mast cell–deficient (Wsh/Wsh) mice as previously described.27 The cells were allowed to differentiate in this microenvironment for 12 weeks. Thus, in this model, only the mast cells varied in genotype (WT or Pak1−/−) with all other cell lineages (other inflammatory cells, endothelial cells, fibroblasts, etc) expressing endogenous Pak1. Mice that underwent transplantation were sensitized by intradermal injection of monoclonal anti-DNP IgE in one ear (and PBS in the other as a control), followed by systemic injection of the antigen, DNP-HSA, with Evans blue dye the following day as in previous studies, and Evans blue dye extravasation was quantified after antigen challenge as in previous studies. The amount of dye in the ear was dramatically reduced in the mice locally reconstituted with Pak1−/− BMMCs compared with mice reconstituted with WT BMMCs (Figure 6C), indicating an important role for Pak1 in the mast cell–mediated immediate-phase PCA reaction. The profound reduction in PCA in the Pak1−/− mice is consistent with the observed reduction in β-hexosaminidase release in vitro. Collectively, these cellular and in vivo data indicate that Pak1 is critical in IgE-mediated mast cell degranulation.
Discussion
Local or systemic IgE-mediated hypersensitivity affects greater than 25% of populations in industrialized countries and clinically manifests as allergic asthma, allergic skin inflammation, and anaphylaxis, among others.33 Mast cells function as key effector cells that contribute to immediate- and late-phase IgE-mediated inflammatory responses in each of these conditions. Mast cells contribute to chronic inflammation in IgE-associated allergic responses via the release of preformed granules that include histamine, prostaglandin, and leukotrienes. The expulsion of these products has immediate consequences that result in local tissue damage and acute changes in local blood flow. The molecular regulation of the degranulation process involves multiple biochemical pathways and cytoskeletal changes resulting from the aggregation and activation of high-affinity IgE receptors (FcϵRI). Drugs targeting critical intracellular signaling molecules could provide alternative or adjunctive therapy to medications currently available, many of which are nonspecific.
The demonstration that Pak1 is activated following cross-linking of the IgE receptor together with a profound reduction in β-hexosaminidase release in Pak1−/− mast cells provided the first evidence for Pak1 in influencing immediate mast cell responses in allergen-mediated degranulation. This functional decrease in degranulation was linked to the inability of Pak1−/− cells to undergo cortical F-actin disassembly, a process central to degranulation.30,34 The specificity of these observations was verified by correction of the degranulation phenotype in Pak1−/− BMMCs transduced with the Pak1 transgene. The significance of these in vitro phenotypes was then validated using adoptive transfer of WT and Pak1−/− mast cells into Wsh/Wsh mice followed by passive cutaneous anaphylaxis. Since Wsh/Wsh mice lack endogenous mast cells, but are phenotypically normal with regard to other lineages implicated in PCA, the approximate 3-fold reduction in vascular leak observed in mice reconstituted with Pak1−/− BMMCs indicates a vital role for Pak1 in mast cells, specifically in allergen-mediated mast cell degranulation in vivo.
Our findings establish that loss of Pak1 results in a defect in calcium mobilization and impaired degranulation in vitro. However, when calcium entry is equalized via the non–receptor-dependent stimulus, calcimycin, degranulation is corrected to WT levels. Rac proteins, upstream effectors of Paks, regulate changes in intracellular calcium in response to FcϵRI activation in RBL cells via effects on PLCγ-1/2 and release of calcium from intracellular stores.10,31 However, activation of PLCγ-1 and release of calcium from intracellular stores are normal in Pak1−/− BMMCs. Precisely how Pak1 is involved in calcium mobilization is unclear, but our data indicate that Pak1 is required for events occurring between the release of calcium from intracellular stores and the influx of extracellular calcium.
The manner by which intracellular stores of calcium communicate to regulatory proteins and calcium channels in the plasma membrane is of broad interest and is an area of ongoing investigation applicable to many pathophysiologic conditions (reviewed in Lewis6 and Parekh et al35 ). Our studies in mast cells unambiguously position Pak1 as important in this process. The precise mechanism by which Pak1 influences these events is incompletely resolved and will be an area of future investigation. One possibility is that loss of Pak1 leads to disruptions in cytoskeletal elements that change cell morphology and organelle location thereby altering signaling from intracellular stores to plasma membrane events that result in calcium entry. Studies reported here demonstrate alterations in F-actin dynamics. It is plausible that Pak1 influences calcium influx by modulating other cytoskeletal elements as well. For instance, one recent report observed that the microtubule cytoskeleton is important to maintain allergen-induced calcium influx in mast cell degranulation.9 The lack of secretory vesicle movement to the periphery of the allergen-stimulated Pak1−/− cell is analogous to confocal images of mast cells treated with nocodazole, a microtubule depolymerizing agent (Figure S1). The data reported here, together with the previous link between calcium influx to microtubules,9 as well as literature supporting a role for Pak1 in microtubule regulation,36-38 provide a potential link between Pak1 and calcium mobilization. Alternatively, or in addition to a mechanism involving cytoskeletal dynamics, Pak1 may mediate calcium mobilization via interaction with signaling molecules directly involved in calcium influx. Ke et al provide evidence in cardiac myocytes that Pak1 impacts calcium entry via regulation of PP2A,39 a phosphatase also involved in vesicular fusion in mast cells40 (reviewed in Blank and Rivera8 ). Further studies will be required to explore the underpinnings of the altered calcium influx in Pak1−/− mast cells.
The interaction of Pak1 with signaling molecules, including PP2A by physical interaction and/or phosphorylation, may have a central role in governing actin dynamics in mast cell degranulation as well. Recent studies support the long-standing hypothesis that cortical actin filaments function as a barrier to prevent granules from reaching the plasma membrane for exocytosis and that disassembly of cortical F-actin is required for vesicle-membrane fusion (reviewed in Blank and Rivera8 ). In our studies, after IgE sensitization of Pak1−/− BMMCs, the cortical F-actin ring forms as expected, but subsequent fragmentation of that cortical ring occurs to a much lesser extent than in a normal mast cell. This finding is consistent with studies by Adam et al who demonstrated that overexpression of a kinase-dead Pak1 mutant in breast cancer cells led to stabilization of F-actin,32 suggesting a possible role for Pak1 in F-actin depolymerization. Given the importance of cortical F-actin disassembly in mast cell degranulation30 (reviewed in Blank and Rivera8 ), it is plausible that perturbation of normal cortical actin dissolution by genetic disruption of Pak1 would result in a significant reduction in degranulation in Pak1−/− mast cells, as established here. Pak1 participates in the regulation of several proteins important in F-actin turnover including the LIMK/cofilin pathway and filamin A (reviewed in Bokoch16 ). Altered phosphorylation of these substrates in the absence of Pak1 represents one potential mechanism to explain our findings. In addition, the recently reported interaction between Pak1 and PP2A39 may be of particular relevance to our work since PP1 and PP2 are involved in plasma membrane events during late steps of exocytosis.40 We have generated preliminary data that warrant further investigation of regulation of these phosphatases by Pak1. Clearly, genetic disruption of Pak1 disturbs the complex machinery responsible for the breakdown of cortical F-actin, which normally allows release of secretory granules in response to mast cell allergen challenge.
In summary, despite minimal in vivo consequence to the unchallenged mouse, as evidenced by grossly normal growth, development, life span, and many normal hematopoietic parameters, genetic disruption of Pak1 significantly impairs allergen-stimulated mast cell function in vitro and in vivo. This designates Pak1 as a prospective therapeutic target for reduction of allergen-mediated responses. Small molecular Pak inhibitors are under development and will allow further study of potential therapeutic uses in IgE-mediated allergic disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mark Marshall for valuable discussions, Helmut Hanenberg for help with lentiviral construct design, and Paula Riggen, Janice Walls, and Susan Stanley for paper preparation.
This work was supported by the following grants from the National Institutes of Health: Pediatric Scientist Development Program AO4738, NIH P01HL069974-05, NIH R01CA074177-10, and NIH R01CA117884-01; and by the following grants from the Department of Defense: W81XWH-06-1-0213 and SPAR 08-0049.
National Institutes of Health
Authorship
Contribution: J.D.A., S.-J.P., S.B., S.C., E.D.-Y., E.G.M., A.M., W.K.B., J.B.T., and S.J.A. collected data; Z.M.J., C.H., and M.A.S. generated the KO mouse and contributed important reagents; J.D.A. wrote the paper; and D.A.I., J.C., and D.W.C. edited and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: D. Wade Clapp, Indiana University School of Medicine, Wells Center for Pediatric Research, Cancer Research Institute, 1044 W Walnut St, R4 402A, Indianapolis, IN 46202; e-mail: dclapp@iupui.edu; or Jonathan Chernoff, Fox Chase Cancer Center, Room W451, 333 Cottman Ave, Philadelphia, PA 19111-2497; e-mail: j_chernoff@fccc.edu.
References
Author notes
*J.D.A. and Z.M.J. contributed equally to this work and are co–first authors.
†J.C. and D.W.C. contributed equally to this work.


![Figure 4. Calcium responses are altered in Pak1−/− BMMCs. IgE-primed WT and Pak1−/− BMMCs were loaded with Ca2+-sensitive dye (fura2) and suspended in Ca2+-containing medium (A). To detect release from intracellular stores, cells were suspended in Ca2+-free media and treated with EGTA prior to stimulation (B). FcϵRI was activated by addition of DNP-HSA and changes in intracellular calcium concentration (i[Ca2+]) were measured by spectrophotofluorimetry. (A) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating decreased Ca2+-bound fura-2 in the Pak1−/− cells after antigen stimulation (left panel). Changes in i[Ca] after addition of digitonin followed by EGTA are also shown. Average change in i[Ca2+] (right panel) expressed as a percentage of WT change in i[Ca2+] from 4 independent experiments, *P < .05, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (B) Representative experiment of a WT (black) versus Pak1−/− (red) BMMC sample demonstrating similar responses upon antigen stimulation after depletion of extracellular calcium (left panel). Average percentage increase in i[Ca2+] (right panel) from baseline in WT and Pak1−/− BMMCs from 3 independent experiments, P < .0.57, WT versus Pak1−/−, unpaired, 2-tailed, Student t test. (C) Western blot and densitometry for activated PLCγ1. NS indicates cells at baseline (nonprimed, nonstimulated). IgE-primed WT and Pak1−/− BMMCs were stimulated with DNP for 30 seconds or 1 minute. Cell lysates (“Western blotting”) were subjected to immunoblotting with anti–phospho-PLCγ1 (top blot) or anti–total PLCγ1 (bottom blot) and densitometry was performed. Intensity of phosphorylated PLCγ1 bands is represented as the ratio of phospho/total PLCγ1 for 1 of n = 4 experiments (P = .79 and P = .47 at 30 seconds and 60 seconds, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/12/10.1182_blood-2008-06-160861/4/m_zh80130932510004.jpeg?Expires=1765188015&Signature=2IGXl6-p5BchC~gsy9tICKYugHNj~G21duBwDv4emLltM77~t5RYwlfU~GjVrNkRGEO5PywAj8b8YSGNbP1Kpn1joP-QxETvTB4I-~ffVZPLncfpQ6ohncNbu4XVInOsI0XyIrL5omqyoAm6vR~~Al08dw-4a9xFqm9F0nf8MAz9fIGQQ9BKc9DvkIMC9067GKtASgwYuVd6Oh5uGcJeTy5zpY6fzUtzNY5bznD6wX-DsnJ3Gbfk9cOdgSFHCmpnQLY85eAv7lqQTq-0HMsRhiNjOxh7fjFXZt4M~joubsSs-p3V129FexksKpfA2zBHc4MxPcfjMDOIpOSw2l0fHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)