Abstract
Extensive apoptosis of leukocytes during sepsis and endotoxic shock constitutes an important mechanism linked to the excessive mortality associated with these disorders. Caspase inhibitors confer protection from endotoxin-induced lymphocyte apoptosis and improve survival, but it is not clear which caspases mediate lipopolysaccharide (LPS)–induced lymphocyte apoptosis and mortality. We report here that the apoptotic executioner caspase-7 was activated in the splenocytes of LPS-injected mice, suggesting a role for caspase-7 in lymphocyte apoptosis. Indeed, caspase-7–deficient mice were resistant to LPS-induced lymphocyte apoptosis and were markedly protected from LPS-induced lethality independently of the excessive production of serum cytokines. These results reveal for the first time a nonredundant role for caspase-7 in vivo and identify caspase-7 inhibition as a component of the mechanism by which caspase inhibitors protect from endotoxin-induced mortality.
Introduction
Sepsis is the most common cause of mortality in patients treated in the intensive care setting, with more than 210 000 sepsis-related deaths occurring annually in the United States.1 Extensive apoptotic death of leukocytes is commonly observed in patients who died of sepsis2,3 and was suggested to contribute significantly to immune suppression and lethality.4-8 In this regard, synthetic caspase inhibitors and overexpression of the antiapoptotic protein Bcl-2 were shown to diminish lymphocyte apoptosis and improve survival in experimental sepsis models.8-12 However, it is currently incompletely understood which caspases promote lymphocyte apoptosis and contribute to lethality.
Together with caspase-3, the executioner caspase-7 performs central roles in the execution phase of apoptosis by cleaving a large set of substrates, ultimately resulting in the morphologic and biochemical hallmarks of apoptosis such as DNA fragmentation.13-16 Caspase-3/-7 double-deficient mice were recently shown to exhibit embryonic lethality, whereas mice singly deficient in either caspase are born at normal Mendelian ratios and display no gross abnormalities when maintained on a C57BL/6 genetic background.17 At this stage, the precise roles of caspase-7 in the adult animals remain to be elucidated.
In this study, we show that caspase-7 was activated in splenocytes of lipopolysaccharide (LPS)–treated mice and that caspase-7−/− mice were protected from LPS-induced splenocyte apoptosis. As a result, caspase-7 deficiency improved survival during endotoxemia without affecting cytokine levels.
Methods
Mice
Caspase-1−/−, caspase-3−/−, and caspase-7−/− mice were backcrossed to C57BL/6 background for 10 generations and have been described previously.17,18 Mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a pathogen-free facility. The animal studies were conducted under protocols approved by St Jude Children's Research Hospital Committee on Use and Care of Animals.
LPS-induced shock
Mice (6-8 weeks old) were injected intraperitoneally with either 20 or 30 mg kg−1 LPS from Escherichia coli (serotype 0111:B4; Sigma-Aldrich, St Louis, MO). The mice were monitored for signs of endotoxemia and lethality daily for 7 days. Differences in group survival were analyzed with the Kaplan-Meier test using Prism5 (GraphPad Software, La Jolla, CA). A value of P less than .05 was considered statistically significant.
Histology and apoptosis quantification
Caspase-7+/+ and caspase-7−/− mice were sham-operated or injected with LPS in the peritoneum. Spleens were collected 24 hours later, fixed overnight at 4°C in 10% buffered formalin, and embedded in paraffin. Apoptotic lymphocytes were quantified both by light microscopy read of hematoxylin and eosin (H&E)–stained sections and by counting terminal dUTP nick end labeling (TUNEL) staining. Image acquisition was performed at room temperature on an Olympus BX41 microscope fitted with Olympus UPlan 20×/0.5 numeric aperture (NA) and 40×/0.75 NA objectives (Olympus America, Center Valley, PA) and equipped with a Spot Insight 3.2 digital camera and corresponding acquisition software (Diagnostic Instruments, Sterling Heights, MI). Data were analyzed with the Student t test. A value of P less than .05 was considered statistically significant.
Western blotting
Mice (6-8 weeks old) were injected intraperitoneally with 20 mg kg−1 LPS from E coli (serotype 0111:B4; Sigma-Aldrich). Spleens were collected and splenocyte extracts were transferred to nitrocellulose membranes, immunoblotted with primary antibodies, and proteins were detected by enhanced chemiluminescence. Antibodies against active caspase-3, active caspase-7, and Grb2 were purchased from Cell Signaling Technology (Danvers, MA). The antibody against caspase-1 was kindly provided by Dr Peter Vandenabeele (Ghent University, Zwijnaarde, Gent, Belgium).
Measurement of cytokines
Serum cytokines and chemokines were measured with Multiplex assay (Bio-Rad, Hercules, CA). Data were analyzed with the Student t test. A value of P less than .05 was considered statistically significant.
Results and discussion
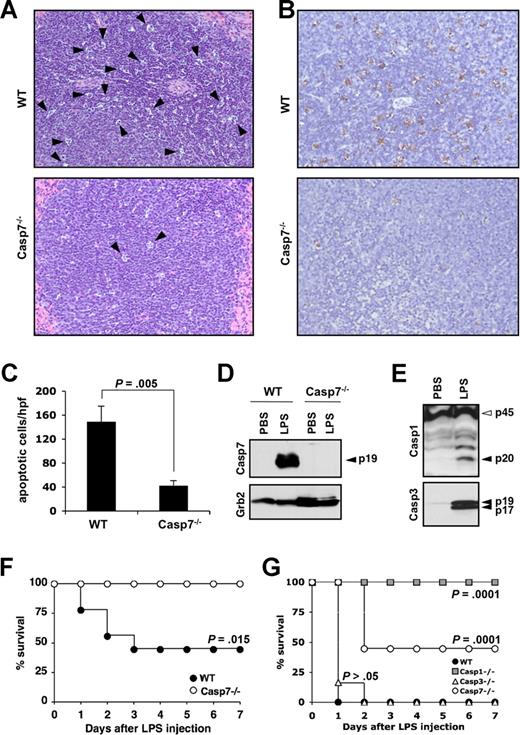
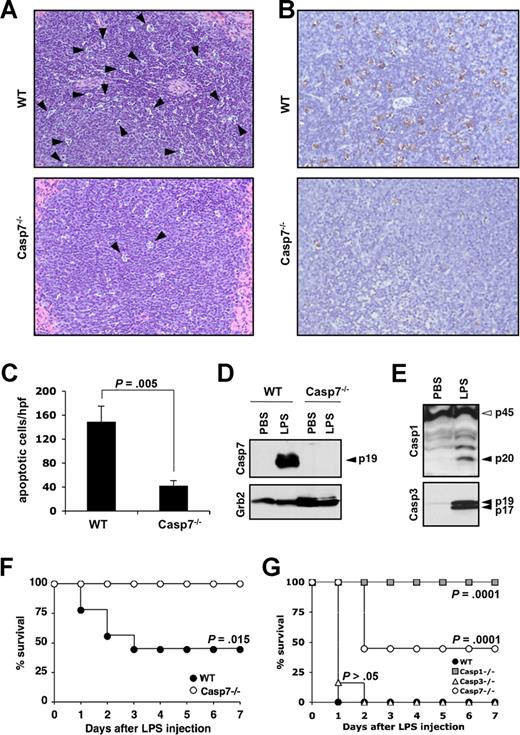
Extensive lymphocyte apoptosis is evident in the spleen, intestinal lamina propria, and in lymphoid organs of patients who died of sepsis2,19 and studies that used Bcl-2–overexpressing mice7,8,11 and synthetic caspase inhibitors9-12 demonstrated its importance for shock-induced lethality. Lethal endotoxemia is a widely used experimental model that mimics many features of septic shock, including elevated cytokine production and extensive leukocyte apoptosis.8,20 However, it is currently not well understood which caspases contribute to endotoxemia-associated lymphocyte apoptosis. To study whether caspase-7 was implicated in endotoxin-induced lymphocyte apoptosis, spleens of caspase-7+/+ and caspase-7−/− mice were collected 24 hours after intraperitoneal LPS injection (20 mg kg−1). Microscopic analysis of H&E-stained sections showed that the morphology of a significant number of lymphocytes in the splenic white pulp of caspase-7+/+ mice was consistent with apoptosis (Figure 1A top panel). Notably, this apoptotic phenotype was markedly reduced in the splenic white pulp of caspase-7−/− mice (Figure 1A bottom panel). To quantify these differences, apoptotic lymphocytes in spleens of wild-type and caspase-7−/− mice were stained with TUNEL. As expected, a significant number of lymphocytes stained positive for TUNEL in spleen of LPS-treated caspase-7+/+ mice (Figure 1B top panel). In contrast, splenic lymphocytes of caspase-7−/− were markedly protected from endotoxin-induced apoptosis (Figure 1B bottom panel). The number of TUNEL-positive lymphocytes in each genotype was quantified by counting the number of apoptotic cells in 5 randomly chosen high-power fields (×400) in the splenic white pulp of each mouse (n = 4). The number of apoptotic lymphocytes was significantly (P = .005) lower in caspase-7−/− mice compared with LPS-treated caspase-7+/+ mice (Figure 1C). These results demonstrate that caspase-7 is essential for endotoxin-induced lymphocyte apoptosis in vivo.
Caspase-7 deficiency protects against LPS-induced splenocyte apoptosis and lethality. (A,B) Wild-type (WT) and caspase-7−/− mice (Casp7−/−; both n = 4) were injected intraperitoneally with 20 mg kg−1 LPS for 24 hours before spleens were collected and sections were stained with H&E (A) or TUNEL (B). (C) The number of TUNEL-positive cells in 5 high-power fields (hpf, ×400) from the white pulp of the spleen of each animal was quantified. Results represent mean plus or minus SD for each genotype. Data were analyzed by the Student t test. (D) Wild-type and caspase-7−/− mice (n = 2) were either sham-operated or injected intraperitoneally with 20 mg kg−1 LPS for 24 hours before spleens were collected and extracts were probed with an antibody against active caspase-7 (listed as Casp7; p19 denotes the large catalytic subunit) and Grb2. (E) Spleen extracts of wild-type mice that were either sham-operated or injected intraperitoneally with 20 mg kg−1 LPS for 24 hours were probed with an antibody against caspase-1 (p45, procaspase-1; p20, large catalytic subunit) and active caspase-3 (p19 and p17 denote the large catalytic subunit). (F) Caspase-7−/− (Casp7−/−; n = 9) and wild-type mice (WT; n = 8) were injected intraperitoneally with 20 mg kg−1 LPS, and their survival was monitored. Data were analyzed with the Kaplan-Meier test. Results are representative of 5 independent experiments. (G) Wild-type (WT; n = 8), caspase-1−/− (Casp1−/−; n = 8), caspase-3−/− (Casp3−/−; n = 6), and caspase-7−/− (Casp7−/−; n = 9) mice were injected intraperitoneally with 30 mg kg−1 LPS and their survival was monitored. Data were compared with wild-type mice and analyzed with the Kaplan-Meier test. Results are representative of 2 independent experiments.
Caspase-7 deficiency protects against LPS-induced splenocyte apoptosis and lethality. (A,B) Wild-type (WT) and caspase-7−/− mice (Casp7−/−; both n = 4) were injected intraperitoneally with 20 mg kg−1 LPS for 24 hours before spleens were collected and sections were stained with H&E (A) or TUNEL (B). (C) The number of TUNEL-positive cells in 5 high-power fields (hpf, ×400) from the white pulp of the spleen of each animal was quantified. Results represent mean plus or minus SD for each genotype. Data were analyzed by the Student t test. (D) Wild-type and caspase-7−/− mice (n = 2) were either sham-operated or injected intraperitoneally with 20 mg kg−1 LPS for 24 hours before spleens were collected and extracts were probed with an antibody against active caspase-7 (listed as Casp7; p19 denotes the large catalytic subunit) and Grb2. (E) Spleen extracts of wild-type mice that were either sham-operated or injected intraperitoneally with 20 mg kg−1 LPS for 24 hours were probed with an antibody against caspase-1 (p45, procaspase-1; p20, large catalytic subunit) and active caspase-3 (p19 and p17 denote the large catalytic subunit). (F) Caspase-7−/− (Casp7−/−; n = 9) and wild-type mice (WT; n = 8) were injected intraperitoneally with 20 mg kg−1 LPS, and their survival was monitored. Data were analyzed with the Kaplan-Meier test. Results are representative of 5 independent experiments. (G) Wild-type (WT; n = 8), caspase-1−/− (Casp1−/−; n = 8), caspase-3−/− (Casp3−/−; n = 6), and caspase-7−/− (Casp7−/−; n = 9) mice were injected intraperitoneally with 30 mg kg−1 LPS and their survival was monitored. Data were compared with wild-type mice and analyzed with the Kaplan-Meier test. Results are representative of 2 independent experiments.
Binding of LPS to receptors on leukocytes triggers the production of potent proinflammatory cytokines such as interleukin-1β (IL-1β), IL-18, tumor necrosis factor-α (TNF-α), and IL-6 as well as chemokines such as CXCL1/KC and CXCL2/MIP-2α.21,22 These cytokines and chemokines are believed to be important mediators of organ injury in endotoxic shock.20,21,23 Therefore, we addressed whether caspase-7 mediates lymphocyte apoptosis indirectly by regulating the secretion of proinflammatory cytokines and chemokines. After endotoxin challenge, we observed that amounts of all cytokines and chemokines measured in the serum of caspase-7−/− mice were not significantly different from those found in the serum of wild-type mice (Table 1). These results indicate that caspase-7 is not required for the secretion of proinflammatory cytokines and chemokines during endotoxemia and suggest that caspase-7 contributes to splenocyte apoptosis independent of systemic cytokine release. Indeed, an antibody directed against active caspase-7 confirmed the potent activation of caspase-7 in splenocytes of LPS-injected mice (Figure 1D). Caspase-7 activation was not observed in splenic extracts of phosphate-buffered saline (PBS)–injected mice, indicating that caspase-7 activation in splenocytes was associated with endotoxemia. The absence of immunoreactive bands in splenic extracts of LPS-treated caspase-7−/− mice confirmed that the antibody was specific for caspase-7 and did not cross-react with other caspases. In addition to caspase-7, caspase-3 and the inflammatory caspase-1 also were activated in splenic extracts of LPS-challenged mice (Figure 1E). Caspase-1 and caspase-3 activation were associated with endotoxemia because processing was not observed in splenic extracts of sham-operated mice.
To examine the survival of caspase-7−/− mice when challenged with a lethal dose of LPS, wild-type and caspase-7−/− mice were injected with 20 mg kg−1 of LPS intraperitoneally. By 72 hours, all caspase-7−/− mice survived, whereas approximately 60% of the wild-type mice succumbed to LPS administration (Figure 1F). Caspase-7 activation in LPS-activated macrophages was recently demonstrated to require the caspase-1 inflammasome.24 To compare the resistance of caspase-1−/− and caspase-7−/− mice, a cohort of age-matched caspase-1−/− mice was challenged with 20 mg kg−1 of LPS. In line with the reported resistance of caspase-1−/− mice,18,25 all caspase-1−/− mice survived the insult (data not shown). We repeated the experiment with an increased dose of 30 mg kg−1 of LPS and included a cohort of caspase-3−/− mice to allow comparison across the different genotypes. The complete caspase-1−/− group survived the increased LPS dose, whereas the survival rate was approximately 50% for the cohort of caspase-7−/− mice (Figure 1G). In contrast to caspase-7−/− mice, all caspase-3–deficient mice succumbed at a rate comparable with that observed with wild-type mice (Figure 1G). These results demonstrate that the absence of caspase-7 confers significant protection against LPS-induced mortality and support the notion that caspase-3 and caspase-7 are functionally distinct in the adult animal.16,24 In addition, caspase-1−/− mice were significantly (P = .01) more resistant to endotoxemia compared with caspase-7−/− mice. One explanation is that in addition to the reduced lymphocyte apoptosis shared with caspase-7−/− mice,10 proinflammatory cytokine levels are significantly attenuated in caspase-1−/− mice18,25 but unaffected in caspase-7−/− mice (Table 1). Caspase-1 may therefore function upstream of caspase-7 during endotoxemia, as recently observed in LPS-activated macrophages.24 Alternatively, a caspase-1–independent mechanism may account for the activation of caspase-7 in LPS-challenged mice.
Regardless, we showed here that caspase-7−/− mice display a marked protection against LPS-induced lymphocyte apoptosis and endotoxemia-associated mortality despite elevated cytokine levels. These results demonstrate for the first time a nonredundant in vivo role for caspase-7 and identify caspase-7 inhibition as a potential mechanism by which caspase inhibitors protect from endotoxin-induced lymphocyte apoptosis and lethality. Indeed, studies that use Bcl-2–overexpressing mice and synthetic caspase inhibitors identified leukocyte apoptosis as a major contributor of sepsis- and endotoxemia-induced lethality.7-12 Thus, therapeutics targeting apoptosis of lymphoid tissues constitute promising new approaches for the treatment of sepsis and endotoxemia. The results presented here suggest caspase-7 inhibition as a reasonable approach that warrants further study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jennifer Rogers from the Cellular Immunology Core Facility of St Jude Children's Research Hospital for technical support and Dr Peter Vandenabeele (Ghent University) for providing caspase-1 antibody.
This work was supported in part by grants from the National Institutes of Health (Bethesda, MD; AR056296) and the American Lebanese Syrian Association Charities (Memphis, TN) to T.-D.K.
National Institutes of Health
Authorship
Contribution: M.L. and T.-D.K. designed the research, performed experiments, analyzed data and wrote the paper; L.O.M, P.M., and D.C.J.S. performed experiments; histologic evaluation was performed by an experienced veterinary pathologist (K.L.B); and P.J.M and D.R.G. analyzed data and helped in editing successive drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thirumala-Devi Kanneganti, Department of Immunology, St Jude Children's Research Hospital, MS #351, 570 St Jude Pl, Suite E7004, Memphis, TN 38105-2794; e-mail: Thirumala-Devi.Kanneganti@StJude.org; or Mohamed Lamkanfi, Department of Physiological Chemistry, Genentech, 1 DNA Way, South San Francisco, CA 94080; e-mail: Lamkanfi.Mohamed@gene.com.