Abstract
We previously reported that RO+ expression correlated with increased mutation, activation, and selection among human germinal center (GC) B cells. Here, we subdivided human tonsillar B cells, including IgD−CD38+ GC B cells, into different fractions based on RB expression. Although each subset contained RB+ cells, when used as an intrasubset marker, differential RB expression effectively discriminated between phenotypically distinct cells. For example, RB+ GC B cells were enriched for activated cells with lower AID expression. RB inversely correlated with mutation frequency, demonstrating a key difference between RB- and RO-expressing GC B cells. Reduced RB expression during the transition from pre-GC (IgM+IgD+CD38+CD27−) to GCB cells was followed by a dramatic increase during the GC-to-plasmablast (IgD−CD38++CD27+) and memory (IgD−CD38−CD27+) transition. Interestingly, RB+ GC B cells showed increased signs of terminal differentiation toward CD27+ post-GC early plasmablast (increased CD38 and RO) or early memory (decreased CD38 and RO) B cells. We propose that as in T cells, differential RB expression directly correlates with development- and function-based transitions in tonsillar B cells. Application of this RB:RO system should advance our understanding of normal B-cell development and facilitate the isolation of more discrete B-cell populations with potentially different propensities in disease pathogenesis.
Introduction
Immune function is uncompromisingly governed by at least 3 interdependent principles including recognition, effector function, and transition. Early naive B and T cells that successfully bind foreign antigen become activated and undergo developmental transition toward more mature, faster responding memory subsets. In contrast, lymphocytes that bind self-antigen in the periphery are usually counterselected against and undergo a different type of transition, one toward apoptosis or anergy. Ineffectual negative selection against self-specific B cells has been associated with the development of autoimmune and neoplastic diseases. Unfortunately, potential factors responsible for the redirection or abrogation of transitions toward cell death and anergy have not been conclusively determined. Antibody sequence analyses revealed that B cells participating in autoreactive responses are often mutated and show signs of selection for self-antigen.1,2 This suggests that somatic hypermutation (SHM) during proliferation and activation-based transitions in the dynamic germinal center reaction may play a role (whether direct or indirect) in disease pathogenesis.
We therefore sought to develop an expedient approach that would allow for the reproducible identification of B-cell populations that exist at different transitional stages based on their states of activation, SHM-dependent diversification, and developmental progression toward adjacent downstream subsets. Delineating how surface marker profiles change as B cells transition between key stages (especially when immunoregulatory markers such as CD45 protein tyrosine phosphatase are included in such profiles) should increase our understanding of B-cell development and ultimately how to circumvent dysregulated processes that could lead to disease.
Over the past 3 decades, our research laboratory has investigated multiple aspects of human B-cell development and the molecular processes that collectively shape the peripheral BCR repertoire.3-6 We recently published that GC B cells that brightly expressed the CD45RO isoform (hereafter termed RO), compared with their RO− counterpart, had higher mutation frequencies, were more activated, showed increased signs of receptor-mediated selection and survival, and were enriched for CD77− centrocytes.7,8 These studies successfully showed that RO could distinguish between GC B cells that existed in different transitional stages of both SHM activity and selection. In this report, we look to improve upon the discriminatory power of the CD45-based system by investigating how SHM and selection correlate with a second major CD45 isoform, CD45RB (hereafter termed RB).
Previous studies in T cells demonstrated that surface RO expression delineated between resting naive (RO−) and activated effector/memory (RO+) subsets.9 However, high RO expression less effectively distinguishes between major B-cell subsets since multiple pools including GC, memory, and plasmablasts each contained RO+ cells (Jackson et al8 and in Figure 4). Nonetheless, the immunophenotype of RO+ GC B cells was different from their RO− counterpart, suggesting that RO, and perhaps other CD45 isoforms such as RB, may be more effective as distinctive intrasubset (within the same subset; ie, GC) markers than intersubset (between 2 or more subsets; ie, GC vs memory).
In T cells, differential RB (and RO) expression correlated with different stages of T-cell development, activation, cytokine production, and selection.10-12 In contrast to the relatively low percentage (≤ 2%) of RO+ tonsillar B cells,7,8 in this report we show that RB+ (or RBbright) B cells constitute a substantially larger (≈ 20%) fraction. Thus, potential RB-associated correlations with activation, diversification, and transitional development are likely to extend to a wider range of B cells in the secondary lymphoid organs. The pattern of RB expression in T cells varied with respect to subset, the type of activation-inducing stimuli administered, and the microenvironment in which stimulation was received.10,12-15 It is therefore uncertain whether RB-associated correlations observed among GC B cells will directly extrapolate to other B-cell subsets that developmentally precede (naive, pro-GC, pre-GC) or succeed (plasmablasts and memory cells) the GC B-cell stage (see Table 1 for B-cell subset surface marker profiles). In this report, we investigate the degree to which differential RB expression in B cells correlates with mutation frequency, AID expression, activation state, and developmental transition toward terminal effector memory and antibody secreting cells. We show that CD45RB, when used in conjunction with the standard panel of IgM, IgD, CD38, and CD27 markers, provides valuable information about cellular properties that would otherwise have required the simultaneous use of 5 or 6 additional markers (including CD69, CD40, CD25, Ki67, CD77, among others). Most correlations associated with RO+ GC B cells7,8 were also observed among RB+ fractions. Differential RO and RB surface expression both reproducibly subdivide the GC B-cell pool into fractions that differ in SHM activity and selection, but RB expression provides additional insights into developmental transition toward downstream B-cell subsets. As has been the case for almost all of the human B-cell subsets described in the last decade, it is likely that disease counterparts to these newly described subpopulations will be forthcoming.
Methods
Lymphocyte isolation and B-cell subset identification
Total lymphocytes were isolated from the tonsils of healthy children and prepared for cytometric analysis as previously described.3 All protocols were approved by the Institutional Review Board of Oklahoma Medical Research Foundation and informed consent was obtained in accordance with the Declaration of Helsinki. Antibodies for analyses, including multicolor antibody combinations used to distinguish between different B-cell subsets (and T cells), are listed in Table 1. After applying the original IgD versus CD38 gating strategy,5 4 independent, nonabutting gates (represented as Q1-4 in Figure 1A) were drawn to increase the purity of each subset. In addition, the upper limit of the CD38 gate used to identify GC B cells (CD19+IgD−CD38+) was conservatively drawn (toward the left) to exclude the CD38++ plasmablasts. Incubations with the appropriate concentrations of conjugated antibodies were for 20 minutes at 4°C in the dark following manufacturers' protocols. Stained cells were analyzed and sorted using the LSR II (BD Biosciences, San Diego, CA) or FACSAria (BD Biosciences) cytometers.
TB45 gating strategy to demark CD45 isoform expression levels
We recently defined a “TB45” gating strategy designed to increase consistency in gating for different CD45 isoform expression levels (ie, CD45−, CD45+/−, CD45+).7,8 This approach was used to define RB gating limits. The B-cell RB+ gate lower limit was set between the T-cell RB+RO− and RB+/−RO+ fractions. Unfortunately, the boundary between RB− and RB+/− fractions was less evident in T cells. Therefore, the upper limit for the B-cell RB− gate was determined using an isotype control antibody.
RNA isolation and RT-PCR
Total RNA was immediately isolated from cells collected during cell sorting using the RNAqueous kit (Ambion, Austin, TX) according to the manufacturer's protocol. VH4 IgM RNA transcripts were amplified using the One Step reverse-transcription–polymerase chain reaction (RT-PCR) kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Amplification parameters were performed as previously described.3 PCR products were gel purified using Qiaquick (QIAGEN), ligated into the pDrive vector (QIAGEN PCR Cloning Kit; QIAGEN), and used to transform Escherichia coli bacteria grown on agar plates containing kanamycin. Plasmid DNA was isolated and purified using the QIAGEN QIAprep Spin Miniprep Kit and submitted for DNA sequencing (ABI 3730 DNA sequencer; Applied Biosystems, Foster City, CA).
DNA sequence and statistical analysis
Four hundred fifty-six IgVH4 family IgM transcripts were amplified and sequenced from GC, pre-GC, Pblast, naive, and memory B cells (n = 229, 64, 58, 52, and 53, respectively) from 6 individual tonsils (GC = T81, T95, T100, and T101; pre-GC = T100, T101; Pblast = T85, T86; naive and memory = T95). Determination of VH4 family gene use among sequences was analyzed using the National Center for Biotechnology Information IgBlast (http://www.ncbi.nlm.nih.gov/igblast)16 and IMGT/V-QUEST (http://imgt.cines.fr/)17 databases. Sequence comparisons and alignments were performed using VectorNTI (Invitrogen, Carlsbad, CA). To ascertain statistical significance we performed 2-tailed heteroscedastic t tests using Microsoft Excel for Windows (Redmond, WA).
Results
The percentage of RB+ cells varies among human tonsillar B-cell subsets
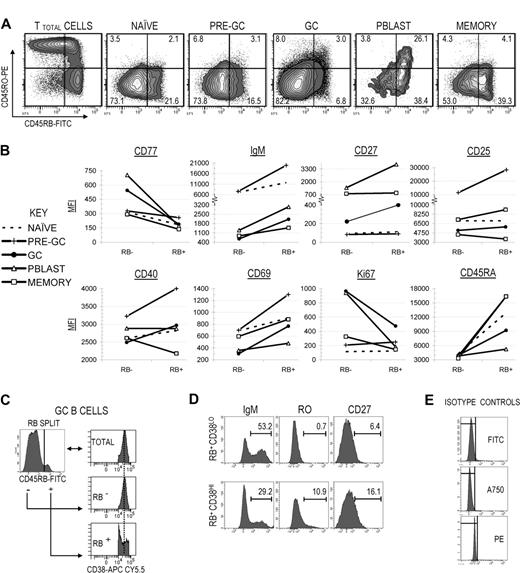
Tonsillar naive, pre-GC, GC, Pblast, and memory B-cell populations (representing early, middle, and late stages of human peripheral B-cell development) were cytometrically identified in 6 different tonsils using surface marker profiles (Table 1) and immunofluorescent gating strategies that have been previously described by our laboratory5,8 (illustrated in Figure 1A,B). The percentage of RB+ (or RBbright) cells was determined for each B-cell subset. Illustrated in Figure 1C, approximately 20% of tonsillar naive and pre-GC B cells were RB+. More than half of post-GC Pblast (56.7% ± 5.6%) and memory (51.2% ± 9.1%) B cells were RB+ (RBbright), suggesting that RB increases during peripheral B-cell development in tonsils. However, the RB increase was not linear since the percentage of RB+ GC B cells (6.3%) was significantly lower by 3- and 9-fold compared with pre-GC (P ≤ .01) and Pblast (P ≤ .001) B cells, respectively. These data suggest that the transition from GC to terminal effector B cells is marked by a significant increase in RB expression such that 1 of 2 tonsillar Pblast and memory B cells were RB+.
Cytometric identification of tonsillar GC B cells and quantification of surface CD45RB expression. (A) Human tonsillar B cells were cytometrically identified using procedures previously described.7 Gates Q1 (CD38+IgD−), Q2 (CD38+IgD+), Q3(CD38−IgD+), and Q4(CD38−IgD−) were determined for total tonsillar B cells based on distinct staining patterns using antibodies against CD38 and IgD. (B) GC B cells were identified from Q1 as CD38+IgD− and excluded CD38++ cells. An anti-CD27 antibody was used to further identify the Pblast pool from Q1 as IgD−CD38++CD27+. Antibodies against IgM and/or CD27 were used to identify the pre-GC (from Q2 as IgM+IgD+CD38+CD27−), naive (from Q3 as IgM+IgD+CD38−CD27−), and memory (from Q4 as IgD−CD38−CD27+) B-cell pools. (C) Tonsillar B cells were additionally stained with anti-CD45RB. The percentage of RB+ cells within each B-cell subset is indicated. Values represent the average of 6 tonsils. Standard deviations are included as error bars. Intersubset differences in the percentage of RB+ cells were statistically significant (P ≤ .01, denoted by a double asterisk [**]) between GC and all other subsets.
Cytometric identification of tonsillar GC B cells and quantification of surface CD45RB expression. (A) Human tonsillar B cells were cytometrically identified using procedures previously described.7 Gates Q1 (CD38+IgD−), Q2 (CD38+IgD+), Q3(CD38−IgD+), and Q4(CD38−IgD−) were determined for total tonsillar B cells based on distinct staining patterns using antibodies against CD38 and IgD. (B) GC B cells were identified from Q1 as CD38+IgD− and excluded CD38++ cells. An anti-CD27 antibody was used to further identify the Pblast pool from Q1 as IgD−CD38++CD27+. Antibodies against IgM and/or CD27 were used to identify the pre-GC (from Q2 as IgM+IgD+CD38+CD27−), naive (from Q3 as IgM+IgD+CD38−CD27−), and memory (from Q4 as IgD−CD38−CD27+) B-cell pools. (C) Tonsillar B cells were additionally stained with anti-CD45RB. The percentage of RB+ cells within each B-cell subset is indicated. Values represent the average of 6 tonsils. Standard deviations are included as error bars. Intersubset differences in the percentage of RB+ cells were statistically significant (P ≤ .01, denoted by a double asterisk [**]) between GC and all other subsets.
Higher mutation frequencies inversely correlate with surface RB expression on tonsillar GC B cells
In similar fashion to our RO studies,7,8 we next investigated whether RB+ and RB−/lo GC B cells represented antipodean fractions regarding SHM frequency. More than 200 IgM+ IgVH4 RT-PCR amplified transcripts were cloned and sequenced from RB−/lo (n = 118; RB− and RB+/− inclusive) and RB+ (n = 111) GC B cells that were isolated from the tonsils of 4 different subjects (T81, T95, T100, and T101). Somatic mutation frequencies (average number of mutations per ∼ 300-base pair IgVH variable region gene segment; Figure 2A) and the spectrum of mutations (percentage of clones within a given mutation range; Figure 2B) were quantified and compared for each RB fraction.
Somatic mutation is inversely correlated with CD45RB expression among GC B cells. IgVH4 IgM transcripts were sequenced from different tonsillar B-cell subsets that were sorted into separate RB−/lo and RB+ fractions. (A) Mutation frequencies (average number of mutations per transcript in the region spanning FW1 through FW3) were calculated for GC B cells from 4 independent tonsils. Combined data (T81 + T95 + T100 + T101) are also presented (T-TOTAL). (B) Mutation distributions among RB−/lo and RB+ GC B cells were calculated as the percentage of sequences with mutation counts that fall within the indicated range. Centrally encircled numbers indicate sample sizes (n values) for each RB fraction. (C) IgVH4 mutation analyses were performed for pre-GC and Pblast B cells to verify that sorted populations used in subsequent AID expression experiments (Figure 3) were phenotypically consistent with conventionally accepted mutation patterns for these cell types (ie, mutation frequency pre-GC < GC {9i} Pblast). In each case, 2 independent tonsils were used as the source of pre-GC (T100 and T101) or Pblast (T85 and T86) B cells. Pre-GC and Pblast pools were independently compared against values for GC B cells that were isolated from the same respective tonsils (ie, [T100 + T101] pre-GC vs [T100 + T101] GC; [T85 + T86] Pblast vs [T85 + T86] GC). (D) Mutation distributions were calculated for pre-GC and Pblast B cells as in panel B. (E) Mutation frequencies were determined for RB-subdivided naive, GC, and memory B cells that were all isolated from the same tonsil (T95). Note that the correlative relationship between RB expression and SHM is opposite for the naive and memory B-cell pools which, respectively, represent early and late stages of peripheral B-cell development. (F) Mutation distributions were calculated for T95 (IgM+IgD+CD38−CD27−) naive and GC B cells as in panel B. Where appropriate, standard deviations are included as error bars. Statistically significant differences between compared populations (RB−/lo vs RB+ in panels A and E; or GC vs pre-GC/Pblast in panel C) are indicated by 1, 2, or 3 asterisks (P ≤ .05, P ≤ .01, or P ≤ .001, respectively).
Somatic mutation is inversely correlated with CD45RB expression among GC B cells. IgVH4 IgM transcripts were sequenced from different tonsillar B-cell subsets that were sorted into separate RB−/lo and RB+ fractions. (A) Mutation frequencies (average number of mutations per transcript in the region spanning FW1 through FW3) were calculated for GC B cells from 4 independent tonsils. Combined data (T81 + T95 + T100 + T101) are also presented (T-TOTAL). (B) Mutation distributions among RB−/lo and RB+ GC B cells were calculated as the percentage of sequences with mutation counts that fall within the indicated range. Centrally encircled numbers indicate sample sizes (n values) for each RB fraction. (C) IgVH4 mutation analyses were performed for pre-GC and Pblast B cells to verify that sorted populations used in subsequent AID expression experiments (Figure 3) were phenotypically consistent with conventionally accepted mutation patterns for these cell types (ie, mutation frequency pre-GC < GC {9i} Pblast). In each case, 2 independent tonsils were used as the source of pre-GC (T100 and T101) or Pblast (T85 and T86) B cells. Pre-GC and Pblast pools were independently compared against values for GC B cells that were isolated from the same respective tonsils (ie, [T100 + T101] pre-GC vs [T100 + T101] GC; [T85 + T86] Pblast vs [T85 + T86] GC). (D) Mutation distributions were calculated for pre-GC and Pblast B cells as in panel B. (E) Mutation frequencies were determined for RB-subdivided naive, GC, and memory B cells that were all isolated from the same tonsil (T95). Note that the correlative relationship between RB expression and SHM is opposite for the naive and memory B-cell pools which, respectively, represent early and late stages of peripheral B-cell development. (F) Mutation distributions were calculated for T95 (IgM+IgD+CD38−CD27−) naive and GC B cells as in panel B. Where appropriate, standard deviations are included as error bars. Statistically significant differences between compared populations (RB−/lo vs RB+ in panels A and E; or GC vs pre-GC/Pblast in panel C) are indicated by 1, 2, or 3 asterisks (P ≤ .05, P ≤ .01, or P ≤ .001, respectively).
Unlike RO+ GC B cells that had the highest mutation frequency, RB+ GC B cells consistently averaged fewer mutations per sequence (by a factor of 1.6) compared with cells in their respective RB−/lo fractions (Figure 2A). Statistical significance was achieved for T81 (P ≤ .001), T100 (P ≤ .005), and in T total (combined tonsils; P ≤ .001). GC B cells with 8 or more mutations were prevalent in the RB− fraction (ie, TTotal RB− vs RB+ = 44% vs 29%). Unmutated sequences were equivalently represented between fractions (ie, TTotal RB− vs RB+ = 17% vs 19%; Figure 2B). Therefore, lower RB+ mutation frequencies appeared to be influenced more by a diminution of B cells with highly mutated IgVH sequences, which is in sharp contrast to the enrichment of highly mutated sequences among RO+ GC B cells.7,8 These data suggest that RB+ and RO+ GC B-cell fractions may not enrich for identical subpopulations and that RO may serve as a stronger discriminatory marker for mutation frequency than RB.
AID expression is inversely correlated with surface RB expression during early, mid, and late stages of the GC reaction
We previously reported that RO+ GC B cells, despite having higher mutation frequencies, had reduced AID expression (at the mRNA level).8 These, among other data, suggested that these cells are unlikely to be in the active stage of incorporating somatic mutations. We next investigated whether higher mutation averages among RB− GC B cells were potentially due to ongoing SHM, of which elevated AID expression would be partially indicative. AID transcripts were semiquantitatively (use of beta actin) RT-PCR amplified from fluorescence-activated cell sorting (FACS)–sorted RB+ and RB−/lo GC B cells (Figure 3A). AID was positively detected in both RB fractions, but expression was more than 2.5-fold higher among RB−/lo cells (Figure 3B). Therefore, AID and surface RB expression are inversely proportional in GC B cells, similar to RO studies.8
AID expression is reduced among RB+ GC and pre-GC B cells. (A) AID transcripts from RB−/lo and RB+ fractions of GC, pre-GC, and Pblast B-cell subsets were semiquantitatively amplified via RT-PCR. RNA concentrations were normalized using beta actin. The 500–base pair molecular weight marker (MW) is indicated to the right. In each case, AID and RB expression were inversely related. (B) The AID expression ratio between RB−/lo versus RB+ fractions (RB−/loAID EXPRESSION/RB+AID EXPRESSION) was determined by comparing digitally quantified band intensities. Each AID band was first normalized to its respective beta actin band (AID intensity/beta actin intensity = AIDNORM). RB−/lo versus RB+ AID expression ratios were next calculated as (RB−/lo AIDNORM/RB+ AIDNORM). In the case of GC B cells, AID expression is 2.4-fold greater among the RB−/lo fraction (relative to the RB+ counterpart).
AID expression is reduced among RB+ GC and pre-GC B cells. (A) AID transcripts from RB−/lo and RB+ fractions of GC, pre-GC, and Pblast B-cell subsets were semiquantitatively amplified via RT-PCR. RNA concentrations were normalized using beta actin. The 500–base pair molecular weight marker (MW) is indicated to the right. In each case, AID and RB expression were inversely related. (B) The AID expression ratio between RB−/lo versus RB+ fractions (RB−/loAID EXPRESSION/RB+AID EXPRESSION) was determined by comparing digitally quantified band intensities. Each AID band was first normalized to its respective beta actin band (AID intensity/beta actin intensity = AIDNORM). RB−/lo versus RB+ AID expression ratios were next calculated as (RB−/lo AIDNORM/RB+ AIDNORM). In the case of GC B cells, AID expression is 2.4-fold greater among the RB−/lo fraction (relative to the RB+ counterpart).
To determine whether the inverse AID and RB correlation is maintained throughout the GC response, we performed similar semiquantitative AID RT-PCR amplifications on RB-subdivided pre-GC and Pblast B cells. Respectively, these 2 GC-proximal B-cell subsets are examples of early (founder) and later (recent emigrant) stages of the GC reaction. As in GC B cells, AID expression was highest among RB−/lo pre-GC and Pblast B cells compared with their counterpart RB+ fractions (Figure 3A). Digital quantification and normalization (beta actin) of AID band intensities show an approximately 1.5-fold increase among RB−/lo pre-GC and Pblast B cells (Figure 3B). Although the magnitude of difference is less for pre-GC and Pblast B cells compared with GC B cells, the inverse relationship between AID and surface RB expression appears to be maintained throughout the GC reaction. Therefore, cells within the RB−/lo fraction are more likely to be incorporating mutations, whereas those in the RB+ fraction may be terminating (AID down-regulation) or preparing to reinitiate (AID up-regulation from a naught level) SHM.
Mutation analysis of FACS-sorted pre-GC and Pblast IgVH4 sequences indicates patterns that are consistent with early- and late-stage germinal center reaction B cells
To establish the accuracy with which sorted IgM+IgD+CD38+CD27− pre-GC and IgD−CD38++CD27+ Pblast B cells represented early and later stages of the GC reaction, we performed similar mutation analyses on IgM+ IgVH4 sequences from each subset. It is generally accepted that pre-GC18 and Pblast B cells, respectively, represent early (germline; modestly mutated) and late (extensively mutated) stages of the GC reaction. A total of 64 pre-GC sequences (from 2 tonsils: T100 and T101) and 58 Pblast sequences (also from 2 tonsils: T85 and T86) were analyzed.
As would be expected, 62% to 84% of pre-GC sequences had no or few (≤ 4) mutations and their average mutation frequencies were significantly lower (P ≤ .01) than frequencies among GC B cells from the same tonsils (3.9 ± 1.8 vs 5.9 ± 3.5 mutations, respectively; Figure 2C, D). In contrast to pre-GC B cells, only 5% of Pblast IgVH4 IgM sequences were unmutated, approximately 50% had 8 or more mutations, and average mutation frequencies were virtually identical to those among same-tonsil GC B cells (9.8 ± 3.6 vs 9.6 ± 3.5 mutations, respectively; Figure 2C,D). These data suggest that pre-GC and Pblast B cells, respectively, represent earlier and later developmental stages of the GC reaction.
Predominant RB expression level predictably varies among germinal center–proximal B-cell subsets
We previously reported that differential surface RO expression among GC B cells directly correlated with reproducible changes in various immunophenotypes associated with somatic mutation frequency, receptor-mediated selection, developmental stage, and activation state.7,8 Having demonstrated that both mutation frequency and AID expression are inversely correlated with surface RB expression on GC B cells, we next investigated whether properties that are partially indicative of activation-dependent transitional development also vary with respect to surface CD45RB expression. Primary focus was given to GC and GC-proximal subsets, though naive (earliest) and memory (among the latest) B cells were also included to bookend the tonsillar B-cell developmental stages. Illustrated in Figure 4A, the “TB45BO” gating system was applied to establish gate limits between RB−/lo (combined RB− and RB+/−) and RB+, and between RO− and RO+ B-cell fractions. Due to the very low percentage of RO+ B cells, all cells above the RO− gate limit were placed into a single RO-expressing fraction (combined RO+/− and RO+ fractions).
RB expression correlates with increased signs of activation and selection and is highest among late-stage and/or terminally differentiated peripheral B-cell subsets. (A) RB versus RO dot plots were generated for naive, pre-GC, GC, Pblast, and memory B-cell subsets that collectively represent the spectrum of peripheral B-cell development, ranging from early (naive) to late (memory). The “TB45BO” system was used to assign RB−/lo, RB+, RO−, and RO+ (combined RO+/− and RO+) gate limits. The percentage of cells in each fraction is indicated. (B) Mean fluorescence intensities (MFIs) for several additional markers were quantified among RB−/lo and RB+ fractions from each B-cell subset. Markers were partially indicative of selection (CD77, IgM), late-stage development or terminal differentiation (CD27), potential interactions with accessory cells (CD25, CD40), and activation/proliferation (CD69, Ki67). (C) The total GC (IgD−CD38+) pool was subdivided into RB−/lo and RB+ fractions. Each RB fraction was then applied to a CD38 histogram to characterize the distribution of cells across the CD38 spectrum. RB−/lo GC B cells exhibited a bell-curve distribution across the CD38 spectrum, showing a strong central tendency (indicated by the bisecting dotted line). In contrast, RB+ GC B cells produced a “V-shaped” distribution across the CD38 spectrum and were skewed toward either the CD38− or CD38++ gate limits. (D) RB+ GC B cells were further split into CD38LO and CD38HI fractions in accordance with the dividing gate depicted in panel C. In separate analyses, the percentage of IgM+, RO+, and CD27+ cells were calculated for each fraction. RB+CD38HI GC B cells were relatively more similar to Pblast B cells than memory cells (↑RO, ↑CD27). Conversely, the RB+CD38LO fraction was more consistent with memory B cells than Pblast. (E) Isotype control antibodies for FITC (RB), Alexa Fluor 750 (A750; CD27), and PE analyses (RO).
RB expression correlates with increased signs of activation and selection and is highest among late-stage and/or terminally differentiated peripheral B-cell subsets. (A) RB versus RO dot plots were generated for naive, pre-GC, GC, Pblast, and memory B-cell subsets that collectively represent the spectrum of peripheral B-cell development, ranging from early (naive) to late (memory). The “TB45BO” system was used to assign RB−/lo, RB+, RO−, and RO+ (combined RO+/− and RO+) gate limits. The percentage of cells in each fraction is indicated. (B) Mean fluorescence intensities (MFIs) for several additional markers were quantified among RB−/lo and RB+ fractions from each B-cell subset. Markers were partially indicative of selection (CD77, IgM), late-stage development or terminal differentiation (CD27), potential interactions with accessory cells (CD25, CD40), and activation/proliferation (CD69, Ki67). (C) The total GC (IgD−CD38+) pool was subdivided into RB−/lo and RB+ fractions. Each RB fraction was then applied to a CD38 histogram to characterize the distribution of cells across the CD38 spectrum. RB−/lo GC B cells exhibited a bell-curve distribution across the CD38 spectrum, showing a strong central tendency (indicated by the bisecting dotted line). In contrast, RB+ GC B cells produced a “V-shaped” distribution across the CD38 spectrum and were skewed toward either the CD38− or CD38++ gate limits. (D) RB+ GC B cells were further split into CD38LO and CD38HI fractions in accordance with the dividing gate depicted in panel C. In separate analyses, the percentage of IgM+, RO+, and CD27+ cells were calculated for each fraction. RB+CD38HI GC B cells were relatively more similar to Pblast B cells than memory cells (↑RO, ↑CD27). Conversely, the RB+CD38LO fraction was more consistent with memory B cells than Pblast. (E) Isotype control antibodies for FITC (RB), Alexa Fluor 750 (A750; CD27), and PE analyses (RO).
The percentage of cells in each fraction is graphed in Figure 4A; results are from a single representative tonsil. Data were consistent among 6 different cytometrically analyzed tonsils. Approximately 10% of GC B cells were RB+, which was significantly lower than values for antecedent and post-GC populations (Figure 1C). Interestingly, only 31% of RB+ GC B cells also expressed surface RO. This suggested that RO and RB can be independently expressed and that RO+ and RB+ fractions do not obligatorily enrich for the same subsets of GC B cells. A similar RB−/loRO+ predominance was observed in antecedent GC populations (naive and pre-GC). RO+ Pblast B cells were almost exclusively RB+ (87.9%), but this trend was not maintained among all post-GC subsets since RO+ memory B cells were equivalently divided between RB−/lo and RB+ fractions (4.3% and 4.1%, respectively). Therefore, not only do RO and RB expression change during peripheral B-cell development and diversification in tonsils, but the surface ratio between both CD45 isoforms changes in a consistent pattern.
Surface marker profile of RB+ germinal center B cells is consistent with activation and potential receptor-mediated selection
We next investigated whether differential RB expression reproducibly correlated with physiologically relevant changes in tonsillar B-cell immunophenotype. Multicolor FACS analyses were performed focusing on several markers that are indicative of activation/proliferation (CD69, Ki67), potential communication with accessory cells (CD40, CD25), transitional development (CD77, surface IgM), and terminal differentiation (CD27). Each B-cell subset in Figure 4A (naive through memory) was first subdivided into separate RB−/lo and RB+ fractions. Next, mean fluorescence intensities (MFI) of each marker were calculated for each RB fraction (Figure 4B). Presented data are from a single representative tonsil, though observed trends were consistent with findings in the 6 different tonsils surveyed.
Ki67 MFI for the RB+ GC B-cell fraction was relatively lower than for RB− (RB+ vs RB−: 474 vs 965 MFI), suggesting constituent cells are generally less proliferative, or that fewer cells within the RB+ fraction are currently dividing. This, combined with lower AID expression, suggests RB+ GC B cells are also less likely to be in the active process of incorporating somatic mutations. CD69 MFI was 2.5-fold greater among RB+ GC B cells indicating increased activation. In addition, CD25 and CD40 MFI values were modestly higher (RB+ vs RB−: CD25 = 5865 vs 5208; CD40 = 2968 vs 2489). These data suggest that similar to RO+ GC B cells, the RB+ GC B-cell fraction is enriched for activated cells.
RB+ germinal center B cells show increased signs of terminal differentiation toward CD27+ Pblast and memory B cells
CD27 MFI for memory B cells did not vary with respect to RB expression (Figure 4B). In contrast, CD27 MFI increased concordantly with surface RB for Pblast and GC B cells. For GC B cells, maximal CD27 MFI was observed in the RB+ fraction (RB+ vs RB−: 400 vs 222), but was substantially lower than minimum values for mature CD27+ Pblast (1400 MFI) and memory cells (794 MFI). We next investigated whether elevated CD27 expression in the RB+ GC B-cell fraction correlated with cells beginning to developmentally transition toward early CD27+CD38++ Pblast and/or CD27+CD38− memory B cells, as indicated by expected changes in CD27, CD38, surface Ig, and RO surface marker profiles. Figure 4C depicts a CD38 histogram of RB−/lo and RB+ GC B cells. Unlike RB−/lo GC B cells, those in the RB+ fraction were predominantly polarized toward either the CD38hi or CD38lo gate limits, resulting in a “V-shaped” distribution pattern.
We next subdivided RB+ GC B cells based on CD38hi or CD38lo expression (RB+CD38hi and CD38+CD38lo; Figure 4D). In Figure 4B, we observed that CD27 and RO MFI values were higher among Pblast than memory B cells and that surface IgM was lower. Data in Figure 4D suggest that RB+ CD38-subdivided GC B cells showed the potential for transitioning toward terminal subsets. The RB+CD38hi fraction was higher in CD27 and RO, and lower in IgM. The converse was observed for the RB+CD38lo fraction. This suggests that a subpopulation of RB+CD38lo GC B cells is developmentally similar to early Pblasts, whereas a subpopulation in the RB+CD38lo fraction is developmentally closer to early memory cells.
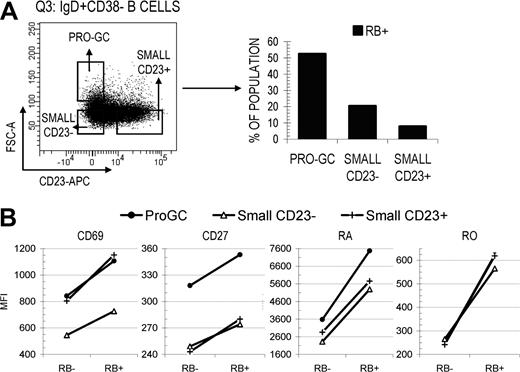
Surface RB expression is increased on the earliest naive-like founder GC subset, pro-GC B cells
Since RB expression appears to increase on some B cells exiting the GC reaction (or those passing through certain selection-based checkpoints), we next investigated whether early naive B cells entering the GC reaction showed a similar increase in surface RB. In a previous study from our laboratory, Kolar et al19 described that pro-GC B cells (IgD+CD38−CD23−FSChi) have an activated naive-like surface marker profile, and proposed they are the earliest candidate founder GC population. This conclusion was based on their localization within germinal centers and positive AID expression. Illustrated in Figure 5A, B cells within the naive-enriched IgD+CD38− quadrant were separated into 3 pools including pro-GC, small CD23−, and small CD23+ and subsequently subdivided into RB−/lo and RB+ fractions. Pro-GC B cells had a 2.6- to 6.6-fold increase in RB+ cells compared with small CD23− and CD23+ naive B cells (52.5 vs 20.4 and 7.9%, respectively). CD27 MFI was highest in pro-GC B cells and was maximal in the RB+ fraction (Figure 5B). True for all 3 subsets (and all B-cell subsets analyzed in this study), CD69 MFI increased in direct proportion to RB surface density. These data suggest that both the transition to enter the GC reaction as an early founder cell and the transition to exit as a terminally differentiated cell may preferentially/predominantly occur among cells that express elevated levels of surface RB (and perhaps CD2720 ).
Pro-GC B cells that are intermediate between naive and GC B cells are predominantly RB+ and express higher levels of CD27. (A) Pro-GC B cells (IgD+CD38− CD23−FSCHI) were cytometrically identified from within the Q3 IgD+CD38− quadrant and are considered to be developmentally intermediate between small resting naive B cells and early GC B cells.20 Differential RB expression (%RB−/lo and RB+) was determined for pro-GC B cells and small naive B cells that were either CD23− or CD23+. (B) CD69 and CD27 MFI values were calculated for each fraction. The majority of small naive B cells were RB− independent of CD23 expression. Pro-GC B cells were predominantly RB+ and expressed the highest level of CD27.
Pro-GC B cells that are intermediate between naive and GC B cells are predominantly RB+ and express higher levels of CD27. (A) Pro-GC B cells (IgD+CD38− CD23−FSCHI) were cytometrically identified from within the Q3 IgD+CD38− quadrant and are considered to be developmentally intermediate between small resting naive B cells and early GC B cells.20 Differential RB expression (%RB−/lo and RB+) was determined for pro-GC B cells and small naive B cells that were either CD23− or CD23+. (B) CD69 and CD27 MFI values were calculated for each fraction. The majority of small naive B cells were RB− independent of CD23 expression. Pro-GC B cells were predominantly RB+ and expressed the highest level of CD27.
RB expression successfully distinguishes between unmutated and mutated B cells within the naive-enriched IgD+CD38− pool
Multiple B-cell subsets can be identified by the IgD+CD38− surface marker profile including naive, pro-GC, and IgD+ memory cells,5,21 each differing in their average mutation frequency (respectively, ranging from unmutated to < 7 mutations per IgVH transcript). We investigated whether the transition toward CD27+ expression among RB+IgM+IgD+CD38−CD27− cells correlated with higher mutation frequencies as was previously shown for IgM+IgD+CD27+ memory cells. Sequence mutation analyses showed that RB+IgM+IgD+CD38−CD27− B cells averaged 5.3 ± 2.2 mutations and more closely resembled pre-GC (3.9 ± 1.8) or IgM+IgD+CD27+ memory B cells (3.7-6.6 mutation average range; from Klein et al21 ; Figure 2E). In contrast, RB−IgM+IgD+CD38−CD27− cells were essentially unmutated (1.4 ± 0.7 mutations) and were more consistent with antigen-inexperienced bona fide naive B cells.5,19 Therefore, RB enriches for a subset of mutated IgM+IgD+38− cells that expresses low levels of CD27 that are intermediate between CD27− naive cells and CD27bright memory cells. Thus, in addition to the population of IgM+IgD+CD27+ memory B cells described by Weller22 and Weill23 to have incorporated mutations during development of the pre-immune Ig repertoire, there may also exist a unique RB+ population within the IgM+IgD+CD38− pool that introduces mutations before reaching “maximal” CD27 expression characteristic of IgM+IgD+CD27+ B cells. Collectively, these data show that by including RB in the current panel of lymphocyte-delineating markers (ie, IgM, IgD, CD38, and CD27) one gains additional discriminatory power particularly in distinguishing between developmentally divergent populations (naive vs IgD+ memory) occupying the same IgD versus CD38 pool.
Discussion
In this report, we show that RO and RB are independently expressed on not only GC B cells, but also all known major tonsillar B-cell subsets. Our primary focus was to determine whether differential gating on surface RB levels could enrich for highly mutated and selected GC B cells, or whether this attribute was unique to variable RO expression. Our data show that like RO,7,8 variable RB expression reproducibly correlates with differences in SHM frequency, though their directional correlations are opposite (ie, inverse for RB, direct for RO). This may be attributable to the observation that most (73%) RO+ GC B cells were also RB−/lo (Figure 4A), supporting the hypothesis that highly mutated cells would be enriched in the RB−/lo fraction.
Data also revealed that RB+ inversely correlated with AID expression (Figure 3) and cell proliferation (Figure 4B), suggesting that RB+ GC B cells are less likely to be in the process of incorporating mutations than their RB−/lo counterparts. Lower AID expression among RB+ and RO+ GC-proximal subsets may be due to at least 2 factors: an anti-CD45 induced down-regulation of AID (which Zhou et al24 demonstrated occurs in a concentration-dependent manner), or a relatively lower percentage of AID+ cells in RB+ and RO+ fractions resulting in a lower AID+/AID− ratio. Several studies showed that surface expression of smaller CD45 isoforms, including RB increases after cell stimulation, but could thereafter decrease due to unstable expression.25 It is therefore plausible that GC B cells may have up-regulated surface RB expression in response to antigenic/accessory stimuli,25,26 after which potential interactions between CD45 and its putative ligand(s) may have down-regulated AID expression. Isoform-specific inhibition of AID has yet to be demonstrated so the possibility of inhibitory anti-RB remains uncertain. Collectively, these data show that RB−/lo (AIDhi, Ki67hi, SHM++) and RB+ (AIDlo, Ki67lo, SHM+) GC B cells generally exist at different stages of the SHM process. RB-related differences may prove useful in identifying those cases of B-cell chronic lymphocytic leukemia (B-CLL) where CD38 and ZAP-70 levels were prognostic in patient survival, yet neither consistently predicted the mutational status of the B-cell receptor.27
IgVH sequence analysis of RB-subdivided GC and IgD+CD38− B cells suggests that the directional correlation between SHM and RB expression is subset dependent (ie, inverse for GC, and direct for IgD+CD38−). The RB− fraction of IgM+IgD+CD38−CD27− cells was essentially unmutated (1.4 ± 0.7 mutations) and was more consistent with antigen-inexperienced bona fide naive B cells. In contrast, the RB+ fraction from the same IgM+IgD+CD38−CD27− pool averaged 5.3 ± 2.2 mutations and was CD27−, thus resembling pre-GC B cells18 more so than the modestly mutated IgM+IgD+CD27+ memory B-cell subset21 (Figure 2E).
Therefore RB not only distinguishes between some major macro-B-cell populations such as IgD+ naive and IgD+ memory B cells (intersubset delineation), but its differential expression profile is consistently specific enough to separate otherwise “quasihomogenous” populations (ie, IgM+IgD+CD38−CD27−) into immunophenotypically distinct micropopulations (intrasubset delineation). CD27 can also be used to distinguish between resting naive and memory B cells. However, it is now appreciated that the CD27+ B-cell pool is more heterogeneous than was previously appreciated, containing activated recently naive-like or centroblast founder GC B cells,20 B cells that mutated very early during ontogeny,22,23 and GC B cells transitioning into early Pblast and memory B cells (this report). The greatest discriminatory power may be achieved using RB in conjunction with CD27 (and other lymphocyte-delineating markers).
We show that RB (and RO) expression levels change over the course of peripheral B-cell development in a subset-dependent manner. GC B cells had a significantly lower percentage of RB+ cells compared with populations that were entering (pre-GC and pro-GC) or had completed (Pblast and memory) the GC reaction. It is uncertain whether the decreased number of RB+ GC B cells is due to down-regulation of all isoforms that use the RB exon, or due to activation-induced changes in how isoforms are glycosylated.28,29
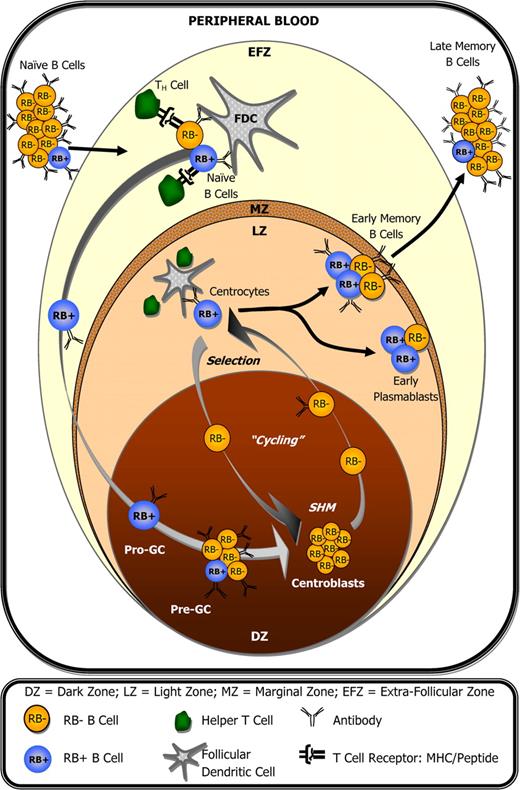
In T cells, it has been demonstrated that RB expression is cyclic,30,31 correlates with both positive and negative selection,12 and that homing to and/or retention within lymph nodes is reduced following antibody-mediated ligation of RB (evidenced by reduced CD62L expression).32,33 It is therefore plausible that CD45 isoform expression in GC B cells is also cyclic, and that RB cycling correlates with GC B cells that themselves are cycling between active/inactive SHM, selection, and/or between lymph node retention/exodus. Here we show that RB expression changes in accordance with cellular immunophenotype within a given subset. For example, RB+ GC B cells show increased potential for undergoing receptor-mediated selection based on their enrichment for CD77− centrocytes, and relatively higher MFI values for surface Ig, CD25, CD40, and CD69 (Figure 4B). Accordingly, RB+ GC B cells could be the most likely candidate cells to leave the GC microenvironment as early CD27+ Pblast and memory B cells.34-36 Interestingly, the pro-GC B-cell population described by Kolar et al19 as the earliest potential founder GC B-cell exhibited a 2- to 7-fold increase in surface RB expression compared with other tonsillar naive B cells. These data suggest that both the transition to enter the GC reaction as an early founder cell and the transition to exit the GC as a terminally differentiated B cell may preferentially/predominantly occur among cells that express elevated levels of surface RB. These observations are summarized in Figure 6, which illustrates how surface RB expression changes with respect to peripheral B-cell development during the germinal center reaction, and in correlation with B-cell activation following interactions with accessory T cells and follicular dendritic cells. Interestingly, the circulating memory B-cell pool in peripheral blood appears to have fewer RBbright cells (S.M.J., personal observation, June 12, 2007; data not shown) compared with the memory pool within tonsils, a finding that may correlate with important phenotypical differences that will hopefully come to be revealed in future studies.
CD45RB expression during the germinal center reaction and peripheral B-cell development. Within lymph nodes (tonsils), small resting naive B cells transition into activated pro-GC B cells after encountering antigen and accessory help. This transition coincides with elevated surface RB expression by a fraction of cells. However, RB expression can decrease as early as the pre-GC B-cell stage and remains low among centroblasts during phases of GC-associated somatic hypermutation and proliferation. Elevated surface RB is again observed among a fraction of centrocytes, presumably during B-cell receptor–mediated selection, and remains high in approximately 1 of every 2 plasmablast and memory B cells within lymph nodes. Compared with their tonsillar counterparts, a significantly lower percentage of circulating peripheral blood memory B cells are RBbright.
CD45RB expression during the germinal center reaction and peripheral B-cell development. Within lymph nodes (tonsils), small resting naive B cells transition into activated pro-GC B cells after encountering antigen and accessory help. This transition coincides with elevated surface RB expression by a fraction of cells. However, RB expression can decrease as early as the pre-GC B-cell stage and remains low among centroblasts during phases of GC-associated somatic hypermutation and proliferation. Elevated surface RB is again observed among a fraction of centrocytes, presumably during B-cell receptor–mediated selection, and remains high in approximately 1 of every 2 plasmablast and memory B cells within lymph nodes. Compared with their tonsillar counterparts, a significantly lower percentage of circulating peripheral blood memory B cells are RBbright.
Tracking B cells during the different stages of SHM and selection is critical to better understand when and where imbalances between these 2 processes have the greatest potential for promoting disease. In addition, a systematic analysis of B lymphoid malignancies for their RO/RB phenotype is warranted as a potential way of further subdividing these malignancies. Understanding the molecular mechanisms behind RB-associated correlations (and RO) would better facilitate the design of CD45-based therapeutic approaches against disease. Antibodies against RB have been shown to have both an immunosuppressive37-39 and stimulatory40,41 effect on lymphoid cell behavior depending on various environmental factors, including differential associations with multiple surface molecules.42,43 Anti-RB treatment is useful as an immunotherapeutic agent and induces transplant tolerance.39,44-47 It must be stressed that B cells play a critical role in these processes.48 Therefore additional studies into the impact of CD45RB ligation on the surface of B cells are needed. From our findings, future therapies targeting RB are likely to influence peripheral B-cell developmental, receptor-mediated selection, and SHM induction/termination. This would prove useful in counteracting the endogenous and SHM-driven generation of autospecific effector B cells, including antibody-secreting Pblast and plasma B cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Richard A. Goldsby and Dr Patrick L. Williamson (Amherst College, MA), Dr Diana Hamilton and Jacob Bass (Paul Kincade Laboratory and Flow Cytometry laboratories, OMRF), Dr J. Jireh (Parker Ford, PA, personal ongoing oral communication, 2008), and members of the Building Young Scientists (BYS) Science Program for their timeless dedication, support, and advice administered during this project.
This work was supported by grants AI 12127 and RR15577 from the National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: S.M.J. wrote the paper, designed the research project, performed the research, and analyzed the data; research was also performed by N.H., D.P., J.W., E.D.S., and U.K.D.; and J.A.J. and J.D.C. supervised the research and reviewed the data and final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Donald Capra, Molecular Immunogenetics, Oklahoma Medical Research Foundation, 825 Northeast 13th St, Oklahoma City, OK 73104; e-mail: jdonald-capra@omrf.org.

![Figure 1. Cytometric identification of tonsillar GC B cells and quantification of surface CD45RB expression. (A) Human tonsillar B cells were cytometrically identified using procedures previously described.7 Gates Q1 (CD38+IgD−), Q2 (CD38+IgD+), Q3(CD38−IgD+), and Q4(CD38−IgD−) were determined for total tonsillar B cells based on distinct staining patterns using antibodies against CD38 and IgD. (B) GC B cells were identified from Q1 as CD38+IgD− and excluded CD38++ cells. An anti-CD27 antibody was used to further identify the Pblast pool from Q1 as IgD−CD38++CD27+. Antibodies against IgM and/or CD27 were used to identify the pre-GC (from Q2 as IgM+IgD+CD38+CD27−), naive (from Q3 as IgM+IgD+CD38−CD27−), and memory (from Q4 as IgD−CD38−CD27+) B-cell pools. (C) Tonsillar B cells were additionally stained with anti-CD45RB. The percentage of RB+ cells within each B-cell subset is indicated. Values represent the average of 6 tonsils. Standard deviations are included as error bars. Intersubset differences in the percentage of RB+ cells were statistically significant (P ≤ .01, denoted by a double asterisk [**]) between GC and all other subsets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-03-145979/4/m_zh80080931430001.jpeg?Expires=1769372310&Signature=PueBkAdHx-Pi9AKOMOhCn2Q8KwRFJ5dqgqbwJKEaqlarzVlyrY5rOF7a-VGZ9rSLw6BpNcoh0raZuxICmZgcakEtltkoi6ztdoufEix6tqshUeBsxJmJIBsxxBCGZfu0Dntxgmp8nR58iWjcfnrV3LYnyuK945yjp~Cq0PCFjf23r7knoX9LWtHsMHYosZkKx6pFUwCwvpqopZVL3DySXZiir6CX0Y-bjkqU29wXMZTjIDEy7Snwvm-fo8GX-4IrFa65zB2hsjvx7zA8mGdTxGewAslPQK-FG-Ks6l24plEexdIWFJ1WQHQWQNQxcny8hU~FDRJLZefeFNJ3OiI8UA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Somatic mutation is inversely correlated with CD45RB expression among GC B cells. IgVH4 IgM transcripts were sequenced from different tonsillar B-cell subsets that were sorted into separate RB−/lo and RB+ fractions. (A) Mutation frequencies (average number of mutations per transcript in the region spanning FW1 through FW3) were calculated for GC B cells from 4 independent tonsils. Combined data (T81 + T95 + T100 + T101) are also presented (T-TOTAL). (B) Mutation distributions among RB−/lo and RB+ GC B cells were calculated as the percentage of sequences with mutation counts that fall within the indicated range. Centrally encircled numbers indicate sample sizes (n values) for each RB fraction. (C) IgVH4 mutation analyses were performed for pre-GC and Pblast B cells to verify that sorted populations used in subsequent AID expression experiments (Figure 3) were phenotypically consistent with conventionally accepted mutation patterns for these cell types (ie, mutation frequency pre-GC < GC {9i} Pblast). In each case, 2 independent tonsils were used as the source of pre-GC (T100 and T101) or Pblast (T85 and T86) B cells. Pre-GC and Pblast pools were independently compared against values for GC B cells that were isolated from the same respective tonsils (ie, [T100 + T101] pre-GC vs [T100 + T101] GC; [T85 + T86] Pblast vs [T85 + T86] GC). (D) Mutation distributions were calculated for pre-GC and Pblast B cells as in panel B. (E) Mutation frequencies were determined for RB-subdivided naive, GC, and memory B cells that were all isolated from the same tonsil (T95). Note that the correlative relationship between RB expression and SHM is opposite for the naive and memory B-cell pools which, respectively, represent early and late stages of peripheral B-cell development. (F) Mutation distributions were calculated for T95 (IgM+IgD+CD38−CD27−) naive and GC B cells as in panel B. Where appropriate, standard deviations are included as error bars. Statistically significant differences between compared populations (RB−/lo vs RB+ in panels A and E; or GC vs pre-GC/Pblast in panel C) are indicated by 1, 2, or 3 asterisks (P ≤ .05, P ≤ .01, or P ≤ .001, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-03-145979/4/m_zh80080931430002.jpeg?Expires=1769372310&Signature=v1bQiftydlvREQD2qG3YwiFPhvIz7ZdAfECUP4OQBxhrNIBsOZK7kKXYAiiJj4~-QQfcy-JshLgKep0NoQ0BOxZZ65D6ue6BjM6z4Br8m-VYCeig4N2sesxxnFizsatZFi4D4pJ4EDtpfXuwZPnmZboT78Nb2OgdgbgEvtQYqPOdV0SWkxkINPWtE9hgfMiTs7nbXchaCXNYQdMDLyWvoZDdO9M25Z5FEKquaRdFvXBDsKk7bTlx~meU4T6AQuoK2J6spDnC4h~uFEAavfQGN17rK-yaU~T9K-ukyn10WPaN18Ppla7YaGi-Jgqmi6FCbvJmTweGHP8ketZLbyyrKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)