Abstract
The kinase TAK1 is essential for T-cell receptor (TCR)–mediated nuclear factor κB (NF-κB) activation and T-cell development. However, the role of TAK1 in B-cell receptor (BCR)–mediated NF-κB activation and B-cell development is not clear. Here we show that B-cell–specific deletion of TAK1 impaired the transition from transitional type 2 to mature follicular (FO) B cells and caused a marked decrease of marginal zone (MZ) B cells. TAK1-deficient B cells exhibited an increase of BCR-induced apoptosis and impaired proliferation in response to BCR ligation. Importantly, TAK1-deficient B cells failed to activate NF-κB after BCR stimulation. Thus, TAK1 is critical for B-cell maturation and BCR-induced NF-κB activation.
Introduction
After successful rearrangement of heavy- and light-chain immunoglobulin genes, B-cell precursors express a surface immunoglobulin receptor (IgM) and are defined as immature B cells. Subsequently, immature B cells are subjected to further positive and negative selection and eventually give rise to mature B cells.1,2 On the basis of cell-surface markers, anatomical localization, and function, mature B cells are classified into 3 subsets.3 The recirculating follicular (FO) B cells localize to the B-lymphoid follicles of the spleen and lymph node.4 The usually nonrecirculating marginal zone (MZ) B cells reside primarily around the periphery of the splenic lymphoid nodules.3,5 The nonrecirculating B1 B cells are enriched in the peritoneal and pleural cavities.6 MZ and B1 B cells contribute substantially to the initial, rapid T cell–independent (TI) immunoglobulin M (IgM) antibody response and form a first line of defense against antigens,7,8 whereas FO B cells participate later in the T cell–dependent (TD) antibody responses.7 Signals from the B-cell receptor (BCR) are important for the development of FO, MZ, and B1 B cells.3,7,9
The family of NF-κB proteins, which consists of 5 members, nuclear factor (NF)–κB1 (p50), NF-κB2 (p52), c-Rel, RelB, and RelA (p65), is essential for B-cell development and function.10 NF-κB is normally associated with the IκB family of inhibitory proteins and thereby sequestered in the cytoplasm of quiescent B cells. Engagement of the BCR is able to induce phosphorylation and subsequent degradation of IκB, leading to NF-κB activation.11,12 Upon engagement, the BCR initiates sequential activation of members of 3 distinct families of cytoplasmic protein tyrosine kinases, including Lyn, Syk, and Btk.13-15 These kinases phosphorylate the important adaptor protein, B-cell linker protein (BLNK), and the transmembrane protein CD19, which facilitates activation of the lipid kinase, phosphatidylinositol 3-kinase (PI3K).15-18 An important outcome of these events is activation of phospholipase C (PLC)γ2, which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol trisphosphate (IP3), resulting in the activation of protein kinase C (PKC)β.19-22 PKCβ in turn triggers the formation of a 3-component complex composed of the CARD domain proteins CARMA1 and BCL10 and the paracaspase MALT1.23-30 The ternary complex leads to the activation of the IκB kinase (IKK) complex, consisting of 2 catalytic subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ, also known as NEMO). Activated IKK phosphorylates IκB molecules and triggers their ubiquitination and subsequent proteolysis by the proteasome complex.31,32 The degradation of IκB releases NF-κB, ultimately leading to the nuclear localization of NF-κB.33,34
The molecular mechanism by which the CARMA1-BCL10-MALT1 complex mediates BCR-induced IKK activation remains to be determined. However, the mechanism by which the ternary complex regulates T-cell receptor (TCR)–induced IKK activation is extensively characterized.23-30 During TCR signaling, the ternary complex functions to recruit the ubiquitin E3 ligase TRAF6 and the ubiquitin E2 enzyme Ubc13-Uev1A, resulting in a K63-linked ubiquitination of TRAF6 itself and IKKγ.35-38 In turn, TRAF6-mediated polyubiquitination activates a protein kinase complex consisting of the transforming growth factor-β–activated kinase 1 (TAK1) and its binding proteins TAB1, TAB2, and TAB3.38-40 Activated TAK1 phosphorylates IKKβ within the activation loop, leading to the activation of IKK.38 Activated TAK1 also phosphorylates and activates MAPK kinase 6 (MKK6) and MKK7, leading to the activation of p38 and JNK kinase pathways, respectively.38,39 The critical role of TAK1 in IKK activation by the TCR has been emphasized by studies of T cell–specific TAK1-deficient mice.41,42
On the basis of the high similarities between BCR and TCR signaling pathways, it is expected that TAK1 plays a critical role in BCR-mediated IKK and subsequent NF-κB activation. Surprisingly, previous studies of B cell–specific TAK1-deficient mice demonstrated that TAK1 is dispensable for BCR-induced IKK and NF-κB activation through the CARMA1-Bcl10-MALT1 complex and that TAK1 deficiency does not affect B-cell development.43 In this report, we independently generated B cell–specific TAK1-deficient mice and revealed that TAK1 is critical not only for BCR-mediated IKK and NF-κB activation but also the function and maturation of B cells. TAK1 deficiency rendered B cells unable to fully activate NF-κB or proliferate in response to BCR engagement. TAK1 deficiency resulted in an increase of BCR-induced apoptosis. In addition, the lack of TAK1 led to a marked reduction of FO, MZ, and B1 B cell numbers. Thus, TAK1 plays an essential role in BCR-induced NF-κB activation and subsequent B-cell maturation.
Methods
Flow cytometry
Bone marrow (BM), spleen, and lymph node cells obtained from control or mutant mice were treated with Gey solution (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, and 0.1 mmol/L Na2EDTA) to lyse red blood cells and then resuspended as single-cell suspensions in phosphate-buffered saline (PBS) with 2% fetal bovine serum. Peritoneal cells were obtained by lavage of the peritoneal cavity with PBS containing 2% fetal bovine serum. The cells were then stained with a mixture of fluorescence-conjugated monoclonal antibodies. Stained cells were analyzed on a BD LSR II with BD FACSDiva software. Phycoerythrin (PE)–conjugated anti-CD5 (340697), fluorescein isothiocyanate (FITC)–conjugated anti-CD21 (553818), PE-conjugated anti-CD43 (553271), and PE-conjugated anti-CD24 (553262) were purchased from BD Biosciences (San Jose, CA). Allophycocyanin (APC)–conjugated anti-IgM (17-5790), PE-conjugated anti-CD23 (12-0232), PE-Cy7–conjugated anti-B220 (25-0452), PE-conjugated anti-B220 (12-0452), FITC-conjugated anti-Thy1.2 (11-0902), APC-conjugated anti-B220 (17-0452), PE-Cy7-conjugated anti-CD23 (25-0232), and PE-Cy5.5-conjugated anti-B220 (35-0452) were purchased from eBioscience (San Diego, CA). FITC-conjugated anti-IgM (1140-02) was purchased from SouthernBiotech (Birmingham, AL). This study received approval for the use of mice from the institutional review board (IRB) of the Medical College of Wisconsin.
Immunofluorescent histologic analysis
Spleen tissue was embedded in OCT compound (Sakura Tissue-Tek, Torrance, CA) and flash-frozen in liquid nitrogen. Sections (5-μm) were fixed in cold-acetone, air-dried, and incubated in PBS containing 1% bovine serum albumin, 10% normal rat serum, and 10% normal goat serum for 1 hour at room temperature. Subsequently, the tissue sections were stained with TRITC-conjugated goat anti–mouse IgM (SouthernBiotech) and FITC-conjugated rat anti–mouse metallophilic macrophages, MOMA-1 (AbD Serotec, Raleigh, NC) at 4°C overnight. After staining, sections were washed and mounted with Vectashield (Vector Laboratories, Burlingame, CA) and examined on a fluorescence microscope (Zeiss Axioskop, Carl Zeiss, Jena, Germany) equipped with a mercury lamp and FITC and Rhodamine filters. A 10× objective lens (numerical aperture 0.3) and a charge-coupled device (CCD) camera (Sensys; Photometrics, Tucson, AZ) were used. Data were collected and analyzed with the use of MetaMorph version 6.1 software (Molecular Devices, Downingtown, PA).
Proliferation and cell-cycle analysis
Splenic B cells were isolated by negative selection with the use of anti-CD4, anti-CD8, and anti-CD11b MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), and then stained with biotin-conjugated anti-AA4.1 (eBioscience). Immature B cells (AA4.1+) were isolated with the use of streptavidin-conjugated microbeads with the flow through constituting the mature B cells (AA4.1−). Purified B cells (5 × 104) were stimulated for 48 hours with lipopolysaccharide (LPS; 10 μg/mL), anti-IgM (10 μg/mL) or anti-IgM (10 μg/mL) plus interleukin-4 (IL-4;10 ng/mL) in a 96-well plate. Subsequently, the cells were stained with propidium iodide for cell-cycle analysis or pulsed for 16 hours with 3H-thymidine (1 μCi/well). Thymidine-pulsed samples were collected with the use of a MACH III harvester (TOMEC, Hamden, CT), and the incorporation of 3H-thymidine was determined with a Wallac MicroBeta TriLux scintillation system (PerkinElmer, Waltham, MA).
Western blot analysis
The splenic mature B cells (AA4.1−) from mutant and control mice were purified by microbeads as described previously. Cells (2 × 106) were stimulated with anti-IgM (10 μg/mL) or LPS (10 μg/mL) at 37°C for the indicated times and cell lysates were subjected to Western blot analysis with the indicated antibodies. Rabbit polyclonal anti-TAK1 (sc-7162), anti-p38 (sc-535), and anti-JNK (sc-572) and mouse monoclonal anti–phospho-ERK (pThr202/pTyr204 sc-7384) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-phospho–IκBα (Ser32/36, no. 9246), anti-phospho-JNK (pThr183/pTyr185 no. 9255), and anti–phospho-p38 (pThr180/pTyr182 no. 9216) and rabbit polyclonal anti-IκBα (no. 9242), rabbit polyclonal anti–phospho-IKKα(Ser180)/IKKβ(Ser181) (no. 2681), and rabbit monoclonal anti-IKKβ (2C8, no. 2370) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal anti-Myc (no. 06-340) was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal anti–Bcl-XL (B22630) was purchased from Transduction Laboratories (Lexington, KY).
NF-κB gel mobility shift assay
The splenic mature B cells (AA4.1−) from mutant and control mice were purified by microbeads as described previously. After stimulation with LPS (10 μg/mL) for 3 hours or anti-IgM (10 μg/mL) for 1, 2, or 6 hours, the cells (106) were lysed in the lysis buffer (20 mmol/L HEPES pH 7.9, 350 mmol/L NaCl, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, 0.5 mol/L DTT, 20% glycerol, 1% NP-40). Cell lysates were incubated with 32P-labeled NF-κB probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Santa Cruz Biotechnology) for 20 minutes at room temperature and then resolved on a 4% polyacrylamide gel at 4°C.
Results
Generation of B cell–specific TAK1-deficient mice
Mice with floxed Tak1 gene were generated as previously described.42 The Tak1fl/+ mice were crossed with CD19Cre transgenic mice, which express the Cre recombinase gene under the control of the B cell–specific CD19 promoter, which begins to be active at the early pro-B stage.44 Deletion of the Tak1 gene was examined by semiquantitative polymerase chain reaction (PCR) analysis of FACS-sorted B cells at different developmental stages. CD19Cre-mediated deletion of the floxed Tak1 gene initiated at the pro/pre–B-cell stage and continued throughout B-cell maturation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). At the immature and mature B-cell stages, the deletion of Tak1 was comparable (Figure S1). Moreover, the deletion of TAK1 in splenic mature B cells derived from CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice was confirmed by Western blot analysis (Figure 1A). Therefore, CD19 promoter–driven expression of Cre mediates specific deletion of the floxed Tak1 in B cells.
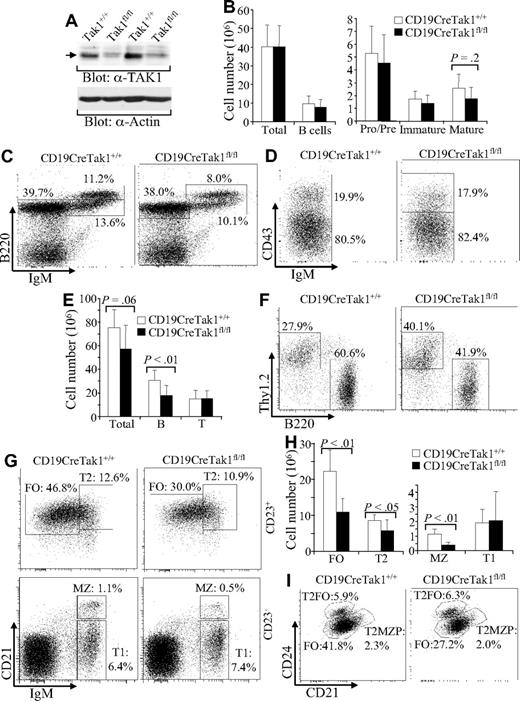
B-cell development in TAK1-deficient mice. (A) Western blot analysis of TAK1 deletion in mature B cells. Splenic mature B cells were isolated from 2 pairs of CD19CreTak1+/+ and CD19CreTak1fl/fl mice, and total cell lysates were subjected to Western blot analysis with anti-TAK1 antibody. Anti-actin immunoblotting was used as a protein loading control. (B) The numbers of total B cells and B-cell subpopulations in BM of TAK1-deficient mice. Histograms show the numbers of total BM cells, total B cells, pro/pre-, and immature and mature B cells in BM of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (C) B-cell subpopulations in BM of TAK1-deficient mice. BM cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-IgM antibodies. Percentages indicate cells in the gated lymphoid population. (D) Pro- and pre-B populations in BM of Tak1-deficient mice. BM cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220, anti-CD43, and anti-IgM antibodies. Percentages indicate cells in the gated B220+IgM− population. (E) Reduction of total splenocytes and total B cells in the spleens of TAK1-deficient mice. Histograms show the numbers of total splenocytes, B220+ B cells and Thy1.2+ T cells in the spleens of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (F) Reduction of B-cell populations in the spleen of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-Thy1.2 antibodies. Percentages indicate cells in the gated lymphoid population. (G) Reduction of FO and MZ B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to IgM, CD21, and CD23. In CD23+-gated cells, T2 B cells (CD21hiIgMhi) and FO B cells (CD21intIgMlo) are shown. In CD23−-gated cells, MZ B cells (CD21hiIgMhi) and T1 B cells (CD21loIgMhi) are shown. The percentages of cells in the gated lymphoid populations are indicated. (H) Reduction of T2, FO, and MZ B cell numbers in the spleens of TAK1-deficient mice. Histograms show the numbers of T1, T2, FO, and MZ B cells in the spleens of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (I) Reduction of FO B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to B220, CD21, CD23, and CD24. In B220+CD23+-gated cells, FO (CD21intCD24int), T2FO (CD21intCD24hi), and T2MZP (CD21hiCD24hi) B cells are shown. Percentages indicate cells in the gated lymphoid population. Data shown are obtained from or representative of 7 (B-D), 8 (E-H), or 5 (I) 8- to 12-week-old mice of each genotype.
B-cell development in TAK1-deficient mice. (A) Western blot analysis of TAK1 deletion in mature B cells. Splenic mature B cells were isolated from 2 pairs of CD19CreTak1+/+ and CD19CreTak1fl/fl mice, and total cell lysates were subjected to Western blot analysis with anti-TAK1 antibody. Anti-actin immunoblotting was used as a protein loading control. (B) The numbers of total B cells and B-cell subpopulations in BM of TAK1-deficient mice. Histograms show the numbers of total BM cells, total B cells, pro/pre-, and immature and mature B cells in BM of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (C) B-cell subpopulations in BM of TAK1-deficient mice. BM cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-IgM antibodies. Percentages indicate cells in the gated lymphoid population. (D) Pro- and pre-B populations in BM of Tak1-deficient mice. BM cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220, anti-CD43, and anti-IgM antibodies. Percentages indicate cells in the gated B220+IgM− population. (E) Reduction of total splenocytes and total B cells in the spleens of TAK1-deficient mice. Histograms show the numbers of total splenocytes, B220+ B cells and Thy1.2+ T cells in the spleens of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (F) Reduction of B-cell populations in the spleen of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-Thy1.2 antibodies. Percentages indicate cells in the gated lymphoid population. (G) Reduction of FO and MZ B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to IgM, CD21, and CD23. In CD23+-gated cells, T2 B cells (CD21hiIgMhi) and FO B cells (CD21intIgMlo) are shown. In CD23−-gated cells, MZ B cells (CD21hiIgMhi) and T1 B cells (CD21loIgMhi) are shown. The percentages of cells in the gated lymphoid populations are indicated. (H) Reduction of T2, FO, and MZ B cell numbers in the spleens of TAK1-deficient mice. Histograms show the numbers of T1, T2, FO, and MZ B cells in the spleens of CD19CreTak1+/+ and CD19CreTak1fl/fl mice. (I) Reduction of FO B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to B220, CD21, CD23, and CD24. In B220+CD23+-gated cells, FO (CD21intCD24int), T2FO (CD21intCD24hi), and T2MZP (CD21hiCD24hi) B cells are shown. Percentages indicate cells in the gated lymphoid population. Data shown are obtained from or representative of 7 (B-D), 8 (E-H), or 5 (I) 8- to 12-week-old mice of each genotype.
TAK1 deficiency impairs B-cell maturation
To determine the role of TAK1 in B-cell biology, we first examined the development of pro-, pre-, immature, and mature B cells in BM derived from CD19CreTak1fl/fl mice. The numbers of total BM and B cells (B220+) were comparable between CD19CreTak1fl/fl and CD19CreTak1+/+ mice (Figure 1B). In addition, the numbers and percentages of pro/pre- (B220+IgM−) and immature (B220+IgM+) B cells in BM were comparable between CD19CreTak1fl/fl and CD19CreTak1+/+ mice (Figure 1B,C). However, the population of mature (B220hiIgM+) B cells was slightly, but not significantly (P = .2), reduced in BM from CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 1B,C). Within B220+IgM− pro/pre-B cells, the populations of pre- (B220+CD43−IgM−) and pro- (B220+CD43+IgM−) B cells were comparable between CD19CreTak1fl/fl and CD19CreTak1+/+ mice (Figure 1D). Of note, thymic T-cell development was normal in CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice, based on the presence of comparable populations of CD4 and CD8 double- and single-positive lymphocytes in the thymus (data not shown). Therefore, B cell–specific deletion of TAK1 does not affect B-cell development in the BM.
We next examined the effect of TAK1 deletion on B-cell maturation in peripheral lymphoid organs. The number of total splenocytes was slightly reduced in CD19Cre Tak1fl/fl relative to CD19CreTak1+/+ mice (P = .06; Figure 1E). Importantly, the number of total B220+ B cells was markedly decreased (P < .01) in CD19CreTak1fl/fl mice compared with that of CD19CreTak1+/+ mice (Figure 1E). In addition, the percentage of B220+ B cells was markedly decreased in CD19CreTak1fl/fl relative to CD19Cre Tak1+/+ mice (Figure 1F). Because of the reduction of B cells, the percentage of Thy1.2+ T cells was increased in the spleen derived from CD19CreTak1fl/fl relative to CD19Cre Tak1+/+ mice (Figure 1F). In contrast, the numbers of total, CD4+, and CD8+ T cells in the spleen were comparable between CD19CreTak1fl/fl and CD19CreTak1+/+ mice (Figure 1E and data not shown). On the basis of the expression of IgM, CD21, and CD23, splenic B cells can be separated into CD23+ and CD23− populations to define the different subsets of splenic B cells.45 Among the populations of CD23+-gated cells, FO (CD23+CD21intIgMlo) B cells were markedly (P < .01) and T2 (CD23+CD21hiIgMhi) B cells were slightly (P < .05) reduced in CD19CreTak1fl/fl compared with CD19CreTak1+/+ mice (Figure 1G,H). In some experiments, splenic B cells from PLCγ2-deficient mice that have a marked reduction of FO B cells but a relatively normal T2 population were used as gating control (Figure S2).46 In addition, CD23+ B cells can be defined as CD21intCD24int FO, CD21intCD24hi T2-FO precursor (T2FO), and CD21hiCD24hi T2-MZ precursor (T2MZP) B cells.47,48 In CD23+-gated cells, FO B cells (CD21intCD24int) were markedly decreased in CD19CreTak1fl/fl mice (Figure 1I). In CD23−-gated cells, the population of T1 B cells (CD23−CD21loIgMhi) was comparable between the mutant and control mice whereas the population of MZ B cells (CD23−CD21hiIgMhi) was markedly reduced (P < .01) in CD19CreTak1fl/fl compared with CD19CreTak1+/+ mice (Figure 1G,H).
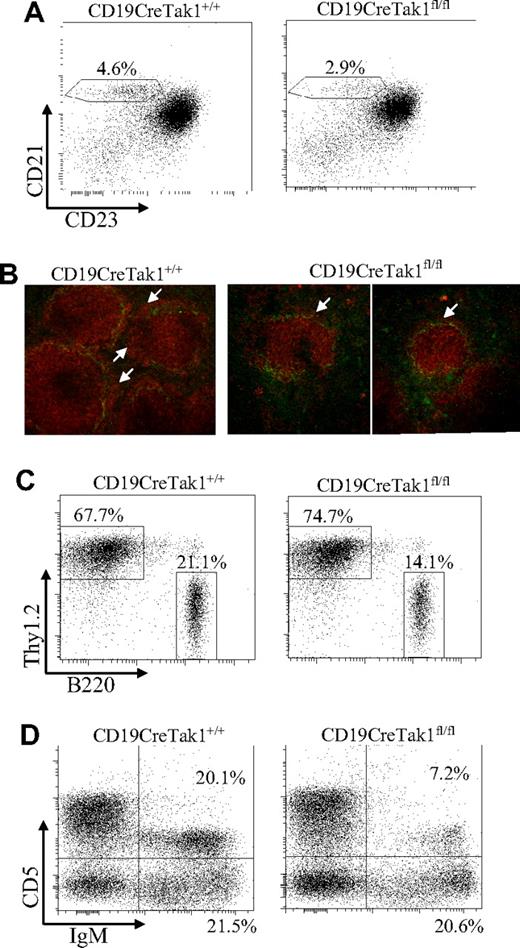
In addition, the MZ B cells can be recognized as B220+CD21hiCD23lo cells based on B220, CD21, and CD23 expression. In B220+-gated cells, MZ B cells (CD21hiCD23lo) were decreased in CD19CreTak1fl/fl mice (Figure 2A). The severe reduction of MZ B cells was further confirmed by immunofluorescent staining. Frozen spleen sections from CD19CreTak1fl/fl compared with CD19CreTak1+/+ mice were stained with tetramethyl rhodamine isothiocyanate (TRITC)–conjugated goat anti–mouse IgM and FITC-conjugated rat anti–mouse MOMA-1, a specific marker for metallophilic macrophages. The ring of metallophilic macrophages permits visualization of the border between the follicular and marginal zones. In agreement with the flow cytometric results, the width of MZ B-cell area was barely detectable in spleens derived from CD19CreTak1fl/fl mice (Figure 2B). These results confirm the physical disappearance of MZ B cells in CD19CreTak1fl/fl mice despite a normal follicular architecture. Consistent with the observation in the spleen, the population of B220+ B cells was markedly decreased in the lymph nodes of CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 2C). Because of the decrease in the B-cell population, the percentage of Thy1.2+ T cells was increased in the lymph nodes of CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 2C).
Impaired development of MZ and reduced lymph node and B1 B cells in TAK1-deficient mice. (A) Reduction of MZ B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to B220, CD21, and CD23. Percentages indicate MZ B cells (CD21hiCD23lo) in the gated B220+ population. (B) Impaired MZ development in the spleens of TAK1-deficient mice. Frozen splenic sections derived from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-MOMA-1 (green) and anti-IgM (red). The IgM+ MZ B-cell layers external to the ring of metallophilic macrophages are indicated with arrows. (C) Reduction of lymph node B cells in TAK1-deficient mice. Lymphocytes from the lymph nodes of CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-Thy1.2 antibodies. The percentages of cells in the gated lymphoid populations are indicated. (D) Reduction of B1 B cells in the peritoneal cavities of TAK1-deficient mice. Cells from the peritoneal cavities of CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to IgM and CD5. The percentages of cells in the gated lymphoid populations are indicated. Data shown are representative of 9 (A), 3 (B,C), or 6 (D) independent experiments.
Impaired development of MZ and reduced lymph node and B1 B cells in TAK1-deficient mice. (A) Reduction of MZ B cells in the spleens of TAK1-deficient mice. Splenocytes from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to B220, CD21, and CD23. Percentages indicate MZ B cells (CD21hiCD23lo) in the gated B220+ population. (B) Impaired MZ development in the spleens of TAK1-deficient mice. Frozen splenic sections derived from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-MOMA-1 (green) and anti-IgM (red). The IgM+ MZ B-cell layers external to the ring of metallophilic macrophages are indicated with arrows. (C) Reduction of lymph node B cells in TAK1-deficient mice. Lymphocytes from the lymph nodes of CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with anti-B220 and anti-Thy1.2 antibodies. The percentages of cells in the gated lymphoid populations are indicated. (D) Reduction of B1 B cells in the peritoneal cavities of TAK1-deficient mice. Cells from the peritoneal cavities of CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stained with antibodies to IgM and CD5. The percentages of cells in the gated lymphoid populations are indicated. Data shown are representative of 9 (A), 3 (B,C), or 6 (D) independent experiments.
The self-renewing mature B1 B cells reside primarily in the peritoneal and pleural cavities. We examined the effect of TAK1 deficiency on the development of B1 cells. In peritoneal lymphocytes, the populations of B1 B cells (IgM+CD5+) were markedly reduced in CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 2D), consistent with the previous report.43 However, the population of B2 cells (IgM+CD5−) was comparable between CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 2D). Taken together, these data demonstrate that B cell–specific deletion of TAK1 impairs B-cell maturation, resulting in a marked reduction of T2, FO, MZ, and B1 B cells.
Impaired proliferation and increased apoptosis of TAK1-deficient B cells
To further study the effect of TAK1 deletion on B cells, we examined the proliferation and apoptosis of TAK1-deficient B cells. First, the proliferation of TAK1-deficient B cells to BCR engagement was examined by in vitro 3H-thymidine incorporation assay. Purified immature (AA4.1+) or mature (AA4.1−) B cells from the spleens of CD19CreTak1fl/fl and CD19CreTak1+/+ mice were stimulated with anti-IgM in the absence or presence of IL-4. The 3H-thymidine incorporation rate of the purified TAK1-deficient immature or mature B cells in response to stimulation of anti-IgM or anti-IgM plus IL-4 was markedly reduced compared with that of the corresponding wild-type cells (Figure 3A left). Similarly, the LPS-induced 3H-thymidine incorporation rate was dramatically decreased in TAK1-deficient immature or mature B cells relative to corresponding wild-type cells (Figure 3A right).
Reduced proliferation and survival of TAK1-deficient B cells. (A) Impaired thymidine incorporation of TAK1-deficient B cells in response to anti-IgM or LPS. Splenic immature (AA4.1+) and mature (AA4.1−) B cells were purified from CD19CreTak1+/+ and CD19CreTak1fl/fl mice and then stimulated with anti-IgM, anti-IgM + IL-4, or LPS. Proliferative responses were determined by [3H]thymidine incorporation. (B) Cell-cycle profile of TAK1-deficient immature B cells in response to anti-IgM or LPS. After stimulation, AA4.1+ B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1 and S + G2/M population in live cells are indicated. (C) Cell-cycle profile of TAK1-deficient mature B cells in response to anti-IgM or LPS. After stimulation, AA4.1− B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1, and S + G2/M population in live cells are indicated. (D) Reduced survival of TAK1-deficient B cells in response to anti-IgM or LPS. AA4.1+ and AA4.1− B cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stimulated with anti-IgM + IL-4 or LPS. At the indicated time points, cell survival rates were determined by PI staining. Data are representative of 5 (A) or 3 (B-D) independent experiments.
Reduced proliferation and survival of TAK1-deficient B cells. (A) Impaired thymidine incorporation of TAK1-deficient B cells in response to anti-IgM or LPS. Splenic immature (AA4.1+) and mature (AA4.1−) B cells were purified from CD19CreTak1+/+ and CD19CreTak1fl/fl mice and then stimulated with anti-IgM, anti-IgM + IL-4, or LPS. Proliferative responses were determined by [3H]thymidine incorporation. (B) Cell-cycle profile of TAK1-deficient immature B cells in response to anti-IgM or LPS. After stimulation, AA4.1+ B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1 and S + G2/M population in live cells are indicated. (C) Cell-cycle profile of TAK1-deficient mature B cells in response to anti-IgM or LPS. After stimulation, AA4.1− B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1, and S + G2/M population in live cells are indicated. (D) Reduced survival of TAK1-deficient B cells in response to anti-IgM or LPS. AA4.1+ and AA4.1− B cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stimulated with anti-IgM + IL-4 or LPS. At the indicated time points, cell survival rates were determined by PI staining. Data are representative of 5 (A) or 3 (B-D) independent experiments.
The reduction of BCR-induced 3H-thymidine incorporation in TAK1-deficient B cells could be attributable to a decrease of cell proliferation and/or an increase of cell apoptosis. Thus, propidium iodide (PI) staining in combination with FACS analysis was used to detect the stimulation-induced cell proliferation and apoptosis of TAK1-deficient B cells. Immature or mature B cells were purified from splenocytes derived from CD19Cre Tak1fl/fl and CD19CreTak1+/+ mice and then stimulated with anti-IgM plus IL-4 or LPS. Subsequently, the cells were collected, stained with PI, and FACS analyzed. Upon anti-IgM plus IL-4 stimulation, the entry into S and G2/M phase was markedly reduced in TAK1-deficient immature (AA4.1+) and slightly reduced in TAK1-deficient mature (AA4.1−) B cells compared with that of corresponding wild-type cells (Figure 3B,C). Of note, LPS-induced entry into S and G2/M phase was markedly reduced in both TAK1-deficient immature or mature B cells (Figure 3B,C).
Activation of the BCR by anti-IgM in the presence of IL-4 also induces immature and mature B cells to undergo apoptosis. After anti-IgM plus IL-4 stimulation, the population of apoptotic cells (subG0) was slightly greater in TAK1-deficient immature B cells and markedly greater in TAK1-deficient mature B cells compared with that of corresponding wild-type cells (Figure 3B,C). Of note, upon LPS stimulation, TAK1-deficient immature and mature B cells underwent apoptosis markedly greater than corresponding wild-type B cells (Figure 3B,C).
In addition, immature or mature B cells were purified from splenocytes derived from CD19CreTak1fl/fl and CD19CreTak1+/+ mice and then stimulated with anti-IgM plus IL-4 or LPS. At the indicated time points, the cells were collected and their survival rate was determined by PI staining. Upon anti-IgM plus IL-4 stimulation, the survival rate was slightly reduced in TAK1-deficient immature (AA4.1+) and markedly reduced in TAK1-deficient mature (AA4.1−) B cells compared with that of corresponding wild-type cells (Figure 3D). Of note, upon LPS stimulation, TAK1-deficient immature and mature B cells displayed much lower survival rates than corresponding wild-type B cells (Figure 3D). Taken together, these data demonstrate that the B cell–specific deletion of TAK1 severely impairs BCR- and LPS-induced B-cell proliferation and survival.
Immune response in TAK1-deficient mice
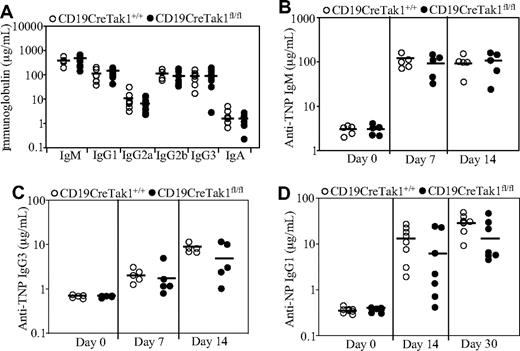
Nonmanipulated CD19CreTak1fl/fl mice displayed similar levels of IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 in their sera as CD19CreTak1+/+ mice (Figure 4A). Further, the humoral immune responses to TI and TD antigen challenge were examined in the mutant mice. CD19CreTak1fl/fl or CD19CreTak1+/+ mice were immunized with the TI antigen TNP-Ficoll or the TD antigen NP-CGG. After the immunization, serum titers of antigen-specific antibodies were examined. In response to TNP-Ficoll, CD19CreTak1fl/fl and CD19CreTak1+/+ mice produced comparable levels of antigen-specific IgM and IgG3 (Figure 4B,C). Of note, CD19CreTak1fl/fl mice produced slightly, but not significantly (P = .3 at day 14 and P = .1 at day 30), less NP-specific IgG1 in response to NP-CGG than CD19CreTak1+/+ mice (Figure 4D). Therefore, TAK1-deficient mice have a normal TI immune response and a slightly, but not significantly, impaired TD immune response.
Basal serum immunoglobulin levels and immune responses in TAK1-deficient mice. (A) Basal serum immunoglobulin levels in TAK1-deficient mice. Basal serum levels of IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 in nonmanipulated CD19CreTak1+/+ (n = 7) and CD19CreTak1fl/fl (n = 9) mice were determined by ELISA. ―indicates mean values. (B,C) Immune response to TI-specific antigens in TAK1-deficient mice. CD19CreTak1+/+ (n = 5) and CD19CreTak1fl/fl (n = 5) mice were immunized intraperitoneally with the TI antigen, TNP-Ficoll. At 7 or 14 days after immunization, the titers of TNP-specific IgM (B) and IgG3 (C) in sera were determined by enzyme-linked immunosorbent assay (ELISA). ―indicates mean values. (D) Immune response to TD-specific antigens in TAK1-deficient mice. CD19CreTak1+/+ (n = 8) and CD19CreTak1fl/fl (n = 7) mice were immunized intraperitoneally with TD antigen, NP-CGG. Fourteen or 30 days after immunization, the titers of NP-specific IgG1 in sera were determined by ELISA. ―indicates mean values.
Basal serum immunoglobulin levels and immune responses in TAK1-deficient mice. (A) Basal serum immunoglobulin levels in TAK1-deficient mice. Basal serum levels of IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 in nonmanipulated CD19CreTak1+/+ (n = 7) and CD19CreTak1fl/fl (n = 9) mice were determined by ELISA. ―indicates mean values. (B,C) Immune response to TI-specific antigens in TAK1-deficient mice. CD19CreTak1+/+ (n = 5) and CD19CreTak1fl/fl (n = 5) mice were immunized intraperitoneally with the TI antigen, TNP-Ficoll. At 7 or 14 days after immunization, the titers of TNP-specific IgM (B) and IgG3 (C) in sera were determined by enzyme-linked immunosorbent assay (ELISA). ―indicates mean values. (D) Immune response to TD-specific antigens in TAK1-deficient mice. CD19CreTak1+/+ (n = 8) and CD19CreTak1fl/fl (n = 7) mice were immunized intraperitoneally with TD antigen, NP-CGG. Fourteen or 30 days after immunization, the titers of NP-specific IgG1 in sera were determined by ELISA. ―indicates mean values.
TAK1 deficiency impairs BCR-induced JNK and NF-κB activation
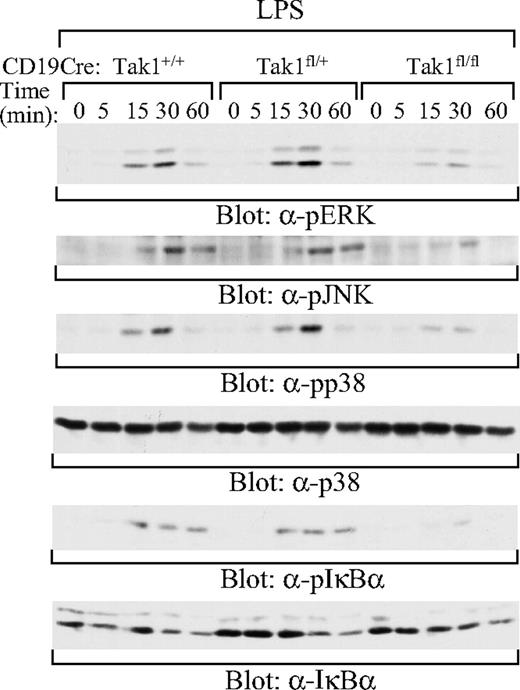
To elucidate the molecular basis of the impairment of BCR- and LPS-induced B-cell proliferation and survival in TAK1-deficient B cells, we examined the effect of TAK1 deficiency on LPS and BCR signaling. Mature (AA4.1−) B cells were purified from the spleens of CD19CreTak1+/+, CD19CreTak1fl/+, and CD19CreTak1fl/fl mice. Activation of ERK1 and ERK2 was evaluated by immunoblotting with antibodies that detect the phosphorylation of pThr202//pTyr204 within ERK1 and pThr185//pTyr187 within ERK2.49,50 Activation of JNK was evaluated by immunoblotting with antibodies that detect the phosphorylation of pThr183/pTyr185 within JNK,51 whereas p38 activation was evaluated by immunoblotting with antibodies that detect the phosphorylation of pThr180/pTyr182 within p38.52 After LPS stimulation, the activation of ERK, JNK, and p38 was impaired in mature B cells derived from CD19CreTak1fl/fl, relative to CD19CreTak1fl/+ or CD19CreTak1+/+ mice (Figure 5). In addition, LPS-induced phosphorylation of IκBα was impaired in mature B cells derived from CD19CreTak1fl/fl mice (Figure 5). Therefore, TAK1 is essential for LPS-induced activation of ERK, JNK, p38, and NF-κB.
Impaired activation of MAPK family members and NF-κB by LPS in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+, CD19CreTak1fl/+, and CD19CreTak1fl/fl mice. Cells were stimulated with LPS for the indicated times. Whole-cell lysates were subjected to direct Western blot analysis with the indicated antibodies. Data are representative of 2 independent experiments.
Impaired activation of MAPK family members and NF-κB by LPS in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+, CD19CreTak1fl/+, and CD19CreTak1fl/fl mice. Cells were stimulated with LPS for the indicated times. Whole-cell lysates were subjected to direct Western blot analysis with the indicated antibodies. Data are representative of 2 independent experiments.
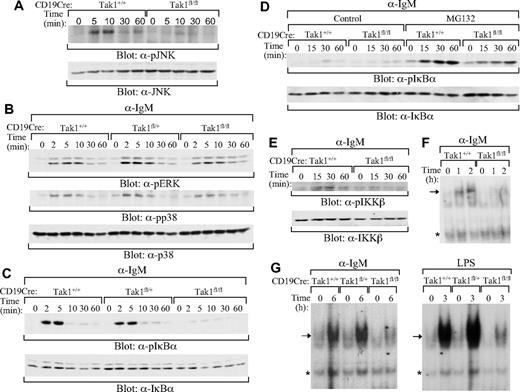
Moreover, mature splenic B cells from CD19CreTak1+/+, CD19CreTak1fl/+, and CD19CreTak1fl/fl mice were stimulated with anti-IgM. The activation of JNK was impaired in mature B cells derived from CD19CreTak1fl/fl, relative to CD19CreTak1+/+, mice (Figure 6A). In contrast, the activation of ERK and p38 were comparable in mature B cells derived from CD19CreTak1fl/fl, CD19CreTak1fl/+, and CD19CreTak1+/+ mice upon BCR engagement (Figure 6B). Importantly, BCR-induced phosphorylation of IκBα was impaired in mature B cells derived from CD19CreTak1fl/fl, relative to CD19CreTak1fl/+ or CD19CreTak1+/+, mice (Figure 6C). Because of proteasome-mediated degradation, phosphorylated IκBα in wild-type and mutant mature B cells could barely be detected at late time points in the absence of proteasome inhibitor (Figure 6D). However, in the presence of the proteasome inhibitor, MG132, the level of phosphorylated IκBα was reduced in mature B cells derived from CD19CreTak1fl/fl, relative to CD19CreTak1+/+, mice (Figure 6D). In addition, BCR-induced phosphorylation of IKKβ was impaired in mature B cells derived from CD19CreTak1fl/fl, relative to CD19CreTak1+/+, mice (Figure 6E). The impairment of BCR-induced activation of NF-κB in TAK1-deficient mature B cells was further confirmed by gel mobility shift assays. BCR engagement-induced formation of the NF-κB complex at early (1 and 2 hours) and late (6 hours) time points was severely impaired in mature B cell isolated from CD19CreTak1fl/fl, relative to CD19CreTak1fl/+ or CD19Cre Tak1+/+, mice (Figure 6F,G). Thus, TAK1 is not required for BCR-induced activation of ERK and p38 but is essential for BCR-mediated activation of JNK and NF-κB.
Impaired activation of JNK and NF-κB by BCR in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+, CD19CreTak1fl/+, or CD19CreTak1fl/fl mice. Cells were stimulated with anti-IgM for the indicated times and then lysed. (A) Impaired activation of JNK by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-JNK or anti-JNK antibodies. (B) Normal activation of ERK and p38 by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-ERK, anti–phospho-p38 or anti-p38 antibodies. (C,D) Impaired phosphorylation of IκBα by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-IκBα or anti- IκBα antibodies. (D) Cells were stimulated with anti-IgM for the indicated times in the absence (control) or presence of MG132 before Western blot analysis. (E) Impaired phosphorylation of IKKβ by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-IKKα/β or anti-IKKβ antibodies. (F,G) Impaired activation of NF-κB by BCR in TAK1-deficient B cells. Cell lysates were subjected to NF-κB gel mobility shift analysis. LPS-stimulated mature B cells were used as controls. *A nonspecific band that serves as a loading control. Data are representative of 3 individual experiments.
Impaired activation of JNK and NF-κB by BCR in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+, CD19CreTak1fl/+, or CD19CreTak1fl/fl mice. Cells were stimulated with anti-IgM for the indicated times and then lysed. (A) Impaired activation of JNK by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-JNK or anti-JNK antibodies. (B) Normal activation of ERK and p38 by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-ERK, anti–phospho-p38 or anti-p38 antibodies. (C,D) Impaired phosphorylation of IκBα by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-IκBα or anti- IκBα antibodies. (D) Cells were stimulated with anti-IgM for the indicated times in the absence (control) or presence of MG132 before Western blot analysis. (E) Impaired phosphorylation of IKKβ by BCR in TAK1-deficient B cells. Cell lysates were subjected to direct Western blot analysis with anti–phospho-IKKα/β or anti-IKKβ antibodies. (F,G) Impaired activation of NF-κB by BCR in TAK1-deficient B cells. Cell lysates were subjected to NF-κB gel mobility shift analysis. LPS-stimulated mature B cells were used as controls. *A nonspecific band that serves as a loading control. Data are representative of 3 individual experiments.
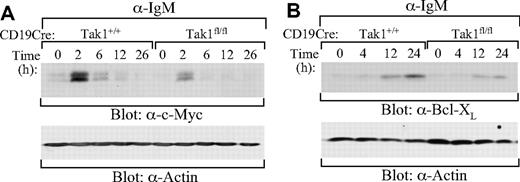
Further, BCR-induced expression of NF-κB–dependent genes were examined in TAK1-deficient B cells. BCR-induced expression of c-Myc and Bcl-XL, 2 downstream targets of NF-κB,53-55 was impaired in splenic mature B cells derived from CD19CreTak1fl/fl relative to CD19CreTak1+/+ mice (Figure 7A,B). Thus, TAK1 is important for BCR-induced expression of NF-κB–dependent genes, c-Myc and Bcl-XL.
Impaired BCR-induced expression of c-Myc and Bcl-XL in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+ and CD19CreTak1fl/fl mice. Cells were stimulated with anti-IgM for the indicated times. Cell lysates were subjected to direct Western blot analysis with anti-c-Myc (A), anti-Bcl-XL (B), or anti-actin antibodies. Data are representative of 2 individual experiments.
Impaired BCR-induced expression of c-Myc and Bcl-XL in TAK1-deficient B cells. Splenic mature B cells (AA4.1−) were isolated from CD19CreTak1+/+ and CD19CreTak1fl/fl mice. Cells were stimulated with anti-IgM for the indicated times. Cell lysates were subjected to direct Western blot analysis with anti-c-Myc (A), anti-Bcl-XL (B), or anti-actin antibodies. Data are representative of 2 individual experiments.
Discussion
In this study, we demonstrated that TAK1 is critical for the normal maturation of B cells. B cell–specific deletion of TAK1 resulted in the reduction of all mature B-cell subsets (ie, FO, MZ, and B1 B cells). Importantly, TAK1 deficiency disrupted BCR-induced NF-κB activation. However, our current findings stand in obvious contrast to the previous study of TAK1-deficient mice.43 The reasons for the discrepancy in these findings are not clear, but they could be the result of mouse strain variations and/or differences in the technical design of the knockouts. The previous study used E14.1 ES cells (129/Ola) to produce chimeric animals, which were then backcrossed to strain C57BL/6 mice.43 In our study, AB2.2 ES cells (129/SvEv) were used to produce chimeric animals, which were then backcrossed to strain C57BL/6 mice.42 Subsequently, the Tak1fl/+ mice were crossed with the CD19Cre transgenic mice to generate B cell–specific-null offspring in both studies. Notably, the Tak1-targeting strategy used in the previous study deleted exon 2 of the Tak1 gene, producing mice that had an altered TAK1 protein with deletion of 37 amino acids from the kinase domain.43 In contrast, the knockout mice used in the current study were generated by deleting the transcriptional initiation site containing exon 1 of Tak1 and lacked any detectable TAK1 protein.42 Although some slight mouse strain variations may exist between the TAK1-deficient mice involved in the 2 studies, the complete absence of TAK1 protein may more likely account for the findings that TAK1 deficiency results in abnormalities of all 3 mature B-cell populations and an impairment of BCR-induced NF-κB activation. Of note, a recent study has demonstrated that the complete lack of TAK1 in TAK1-deficient chicken DT40 cell lines abolishes BCR-induced activation of IKK and NF-κB.56
TAK1 is known to play a critical role in TCR-mediated NF-κB and JNK activation.41,42 Upon TCR engagement, the CARMA1-BCL10-MALT1 complex forms and recruits TRAF6, resulting in K63 ubiquitination of TRAF6 itself and the subsequent activation of TAK1.35-40 Activated TAK1 phosphorylates and activates IKK and MKK7, leading to the activation of NF-κB and JNK, respectively.38,39 Studies also have demonstrated that the CARMA1-BCL10-MALT1 complex is essential for BCR-induced activation of NF-κB and possibly JNK.31,57 Deficiency of CARMA1, Bcl10, or MALT1 clearly impairs BCR-mediated activation of NF-κB,23-29 although one study has reported that MALT1-deficient B cells have relatively normal BCR-mediated NF-κB activation.30 Deficiency of CARMA1 impairs BCR-mediated JNK activation23,24 whereas a lack of MALT1 seems to have normal BCR-mediated JNK activation.30 Importantly, deficiency of CARMA1, Bcl10, or MALT1 hardly has any effect on early B-cell development but impairs B-cell maturation, resulting in a dramatic decrease of FO, MZ, and/or B1 B cells.23-26,28-30 Here, we have found that TAK1 deficiency blocks BCR-induced activation of NF-κB and JNK, and impairs B-cell maturation. These findings clearly demonstrate that TAK1 is the missing link connecting the CARMA1-BCL10-MALT1 complex with NF-κB activation during BCR signaling in B cells, similar to its role during TCR signaling in T cells.
The B-cell defects in B cell–specific TAK1-deficient mice are caused, least in part, by the impaired ability of these cells to activate NF-κB. The NF-κB family of proteins is involved in both the development and functions of FO, MZ, and B1 B cells.10 The lack of both NF-κB1 and NF-κB2 or both c-Rel and RelA impedes the development of FO B cells.10,58 In addition, the absence of NF-κB1 impairs MZ B-cell development.59 Therefore, the failure of normal BCR-mediated activation of NF-κB in TAK1-deficient B cells, as we report here, likely contributes to the defective maturation of the 3 subsets of B cells. Other signals (for example, derived from chemokines60 ) also participate in the maturation of peripheral B cells.
Nonetheless, although not formally examined in this study, it is possible that TAK1 could participate in the activation of NF-κB by as-yet-undefined chemokines to contribute to B-cell maturation. Despite the defective B-cell maturation, our TAK1-deficient mice have normal serum immunoglobulin levels, a normal TI immune response, and a slightly (but not significantly) impaired TD immune response. The previous study also has demonstrated that TAK1-deficient mice display largely normal serum immunoglobulin levels and immune responses, except for a markedly defective IgG1 response to the TD antigen NP-CGG.43 Of note, deficiency of CARMA1, Bcl10, or MALT1 impairs immune response.23-30 It is highly possible that CD19 promoter-driven expression of Cre does not delete the floxed Tak1 in all B cells and the remaining mature B cells with an intact Tak1 gene in CD19CreTak1fl/fl mice may be enough to initiate immune response upon antigen challenge.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported in part by National Institutes of Health (Bethesda, MD) grants R01 AI52327 (R.W.), RO1 AI60919 (Z.J.C.), R01 AI079087 (D.W.), and R01 HL073284 (D.W.) and by a Scholar Award from the Leukemia & Lymphoma Society (D.W.).
National Institutes of Health
Authorship
Contribution: J.S. and Y.C. designed and performed experiments, analyzed and interpreted data, and drafted the manuscript; A.P., M.Y., and H.-H.L. performed some experiments and analyzed data; R.W. analyzed data and critically reviewed the manuscript; Z.J.C. contributed vital new mutant mice and critically reviewed the manuscript; and D.W. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Demin Wang, Blood Research Institute, BloodCenter of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: demin.wang@bcw.edu.
References
Author notes
*J.S. and Y.C. contributed equally to this work.


![Figure 3. Reduced proliferation and survival of TAK1-deficient B cells. (A) Impaired thymidine incorporation of TAK1-deficient B cells in response to anti-IgM or LPS. Splenic immature (AA4.1+) and mature (AA4.1−) B cells were purified from CD19CreTak1+/+ and CD19CreTak1fl/fl mice and then stimulated with anti-IgM, anti-IgM + IL-4, or LPS. Proliferative responses were determined by [3H]thymidine incorporation. (B) Cell-cycle profile of TAK1-deficient immature B cells in response to anti-IgM or LPS. After stimulation, AA4.1+ B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1 and S + G2/M population in live cells are indicated. (C) Cell-cycle profile of TAK1-deficient mature B cells in response to anti-IgM or LPS. After stimulation, AA4.1− B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1, and S + G2/M population in live cells are indicated. (D) Reduced survival of TAK1-deficient B cells in response to anti-IgM or LPS. AA4.1+ and AA4.1− B cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stimulated with anti-IgM + IL-4 or LPS. At the indicated time points, cell survival rates were determined by PI staining. Data are representative of 5 (A) or 3 (B-D) independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-08-176057/7/m_zh80170934270003.jpeg?Expires=1769260477&Signature=yEjTIOpYjumKFVHWHFxzIf4HD~llgPf3uOopkhmDSmc5lGipN-LSab~LmA6QZJGWPsmc~wp-u8okPWIvFNdtG99MWgil9fgZGDlrWADTyvlWeiqeku4VbyM9ZpsVMRyr4hLJinLQpuep0JEc~2XNKczjM7WTgV4KzCHP0xSVHsHUnyTjlYalsTlwNyFBfDPd2MfLxnA1WVcNV~YFJK4xLmgNzFz0dSiIW40mjZb3VBlFt7rlOMvmoV0HM0AYDvniQ-2O82bZZ5JQVfQcideRXthI4JnIAQ2RNUggtlpMD8QBZ-nDMc2EwSA0GWN8ProBwM9Lc1rJxIi5bAgvPr73Gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 3. Reduced proliferation and survival of TAK1-deficient B cells. (A) Impaired thymidine incorporation of TAK1-deficient B cells in response to anti-IgM or LPS. Splenic immature (AA4.1+) and mature (AA4.1−) B cells were purified from CD19CreTak1+/+ and CD19CreTak1fl/fl mice and then stimulated with anti-IgM, anti-IgM + IL-4, or LPS. Proliferative responses were determined by [3H]thymidine incorporation. (B) Cell-cycle profile of TAK1-deficient immature B cells in response to anti-IgM or LPS. After stimulation, AA4.1+ B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1 and S + G2/M population in live cells are indicated. (C) Cell-cycle profile of TAK1-deficient mature B cells in response to anti-IgM or LPS. After stimulation, AA4.1− B cells were collected, stained with PI, and analyzed for cell-cycle profile by FACS. The percentages of subG0 populations in total cells and percentages of G0/G1, and S + G2/M population in live cells are indicated. (D) Reduced survival of TAK1-deficient B cells in response to anti-IgM or LPS. AA4.1+ and AA4.1− B cells from CD19CreTak1+/+ and CD19CreTak1fl/fl mice were stimulated with anti-IgM + IL-4 or LPS. At the indicated time points, cell survival rates were determined by PI staining. Data are representative of 5 (A) or 3 (B-D) independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-08-176057/7/m_zh80170934270003.jpeg?Expires=1769260478&Signature=v6dap5sb0A2P8TcCaNdK40FddzUVKXENaBbA5vDASLrXB5msxUnE7Fx9WfHsHmD5TG2HkcF3SbJ~nJ5fS61mH-TLIfe8nIhWs9ZE-4H8AfXoF048QzbwAa3P75E8VUTQY4G1lKJf4J-LxINI1cFH-PGYloaTeQ2A-Wyz2DKnDqjkO5OT0c2Oov9jAkmR4d15AMSY5CvD8nrPu0yHdmJWe9QYUZmjZDMQ3~97alOB3KgzQm95D26OUV2KKw8DaXUAHfdGHK6PWBZk3iWzXntbUk1-maCRQsMvkxuk8vfAwRbyeL2taiNsDy4BDoNcB~tLuZseuoA7Qe1fDHWnUEUtOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)