Abstract
Activin A is a dimeric protein, member of the transforming growth factor (TGF)–β family that plays a crucial role in wound repair and in fetal tolerance. Emerging evidence also proposes activin A as a key mediator in inflammation. This study reports that activin A induces the directional migration of immature myeloid dendritic cells (iDCs) through the activation of ALK4 and ActRIIA receptor chains. Conversely, activin A was not active on plasmacytoid dendritic cells (DCs) or mature myeloid DCs. iDC migration to activin A was phosphatidylinositol 3-kinase γ–dependent, Bordetella pertussis toxin– and cycloheximide-sensitive, and was inhibited by M3, a viral-encoded chemokine-binding protein. In a real-time video microscopy-based migration assay, activin A induced polarization of iDCs, but not migration. These characteristics clearly differentiated the chemotactic activities of activin A from TGF-β and classic chemokines. By the use of combined pharmacologic and low-density microarray analysis, it was possible to define that activin-A–induced migration depends on the selective and polarized release of 2 chemokines, namely CXC chemokine ligands 12 and 14. This study extends the proinflammatory role of activin A to DC recruitment and provides a cautionary message about the reliability of the in vitro chemotaxis assays in discriminating direct versus indirect chemotactic agonists.
Introduction
Activin A is a homodimeric protein that belongs to the transforming growth factor (TGF)-β family. Activin A signals through heterodimeric transmembrane serine/threonine kinase receptor complexes consisting of type I (ALK 2, 4, or 7) and type II receptors (ActRIIA and ActRIIB), which share the intracellular Smad signaling pathway with the TGF-β receptors. Activin A also binds follistatin, a protein that controls the biologic activity of Activin A acting as a decoy receptor.1,2
Activin A was initially identified for its ability to control the secretion of follicle-stimulating hormone,2 and then found to be a differentiating and trophic factor for embryonic tissues during ontogenesis and wound repair.3,4 However, emerging evidence proposes activin A is also a key mediator of inflammation. In vivo inoculation of lipopolysaccharide (LPS) induces a rapid increase of activin A circulating levels. This increase precedes and is independent of proinflammatory cytokine and prostaglandin production.4 In vitro, many cell types produce activin A in response to proinflammatory cytokines and/or LPS, and activin A by itself was reported to potently stimulate the production of inflammatory cytokines and nitric oxide.4
Dendritic cells (DCs) are highly specialized antigen-presenting cells that play a crucial role in the initiation of innate and adaptive immune responses5-7 and shape inflammatory responses through the production of proinflammatory cytokines and chemokines.5,8 DCs accumulate at the site of inflammation in response to the local production of inflammatory chemokines. In turn, the exposure of DCs to proinflammatory agonists induces their migration to regional lymph nodes, where they localize to the paracortical area by a C-C chemokine receptor 7–dependent mechanism.8,9
Recent data from us and others suggest a key role for activin A in regulating DC function. Stimulation of DCs in vitro with proinflammatory cytokines and bacterial agonists produces significant amounts of activin A.3,10 Furthermore, high levels of activin A were detected in vivo at sites of tissue repair such as hypertrophic scars, as well as in certain autoimmune diseases like oral lichen planus11 and inflammatory bowel disease.12
Activin A was also shown to regulate cytokine production by DCs and to attenuate the expansion of viral antigen-specific effector CD8+ T cells.10 Furthermore, activin A was reported to induce the differentiation of Langerhans cells from circulating and skin-resident precursor cells.11 Because in vivo the expression of activin A is associated with an increased tissue infiltration by DCs,11,13,14 the aim of this study was to investigate the possible role of activin A in DC migration. In this study, we show that activin A selectively promotes in vitro directional migration of immature myeloid DCs (iDCs) through a complex mechanism involving polarized chemokine production that differentiates the action of activin A from that of TGF-β and classical chemotactic factors.
Methods
Cytokines and reagents
Human granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 were from Prospec (Rehovot, Israel). C-C chemokine ligand (CCL)3/macrophage-inflammatory protein-1α was from PeproTech (Rocky Hill, NJ); CXC chemokine ligand (CXCL)12/stromal cell–derived factor-1 (SDF-1), CXCL14/breast- and kidney-expressed chemokine (BRAK), anti-CXCR4, anti-ALK4, and anti-ActRIIA monoclonal antibodies (mAbs) were purchased from R&D Systems (Minneapolis, MN). The activity of recombinant (R&D Systems) was similar in terms of dose response and potency to that of natural purified CXCL14 (provided by B. Moser, Cardiff University, Cardiff, United Kingdom). Activin A from PeproTech was routinely used; similar results were obtained with activin A from R&D Systems. Follistatin was from R&D Systems. All cytokines were endotoxin-free at working concentrations, as assessed by Limulus amebocyte assay (BioWhittaker, Walkersville, MD). Tumor necrosis factor (TNF)-α was a gift from P. Vandenabeele (Ghent University, Ghent, Belgium). Bordetella pertussis toxin was from Calbiochem (San Diego, CA); platelet-activating factor, cycloheximide, AMD 3100, SB431542, and ginkgolide B were from Sigma-Aldrich (St Louis, MO). Leukotriene B4 (LTB4), LY2552833, and U-75302 were from Cayman Chemical (Ann Arbor, MI). The murine gammaherpesvirus 68 chemokine-binding protein M3 (a gift from A. Alcami, Centro de Biologia Molecular Severo Ochoa, Madrid, Spain) fused to a C-terminal his-tag was expressed in the baculovirus system and purified by affinity chromatography.15 The ability of the purified M3 protein sample to bind chemokines and inhibit their activity was confirmed in vitro.
Purification and culture of DCs
Human DCs.
Peripheral blood mononuclear cells were isolated from buffy coats of healthy blood donors (through the courtesy of the Centro Trasfusionale, Spedali Civili di Brescia, Brescia, Italy) by Ficoll gradient (Amersham Biosciences, Uppsala, Sweden). Peripheral blood myeloid DCs and plasmacytoid DCs were magnetically sorted by blood antigen (BDCA)–1 and BDCA-4 cell isolation kits (Miltenyi Biotec, Auburn, CA), respectively. Monocyte-derived DCs were generated from CD14+ cells, as previously described.16
Murine DCs.
Eight- to 12-week-old 129sv wild-type and 129sv phosphatidylinositol 3-kinase (PI3K)γ−/− male mice were used.17 CD34+-derived myeloid DCs were generated and functionally characterized, as previously described.17 Mice were housed in the specific pathogen–free animal facility of the Istituto Clinico Humanitas (ICH) in individually ventilated cage systems. Procedures involving animals and their care were approved by the ICH ethical committee and were conformed with institutional guidelines in compliance with national (4D.L.N.116, G.U., supplement 40, 18-2-1992) and international (European Economic Community Council Directive 86/609, OJ L 358,1,12-12-1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals, US National Research Council 1996) laws and policies. All efforts were made to minimize the number of animals used and their suffering.
Migration assay
Monocyte-derived DC migration was evaluated using a chemotaxis microchamber technique, as described previously.16 Twenty-seven microliters of chemoattractant solution or control medium (RPMI/1% fetal calf serum [FCS]) was added to the lower wells of a chemotaxis chamber (NeuroProbe, Pleasanton, CA). A polycarbonate filter (5-μm pore size; NeuroProbe) was layered onto the wells and covered with a silicon gasket and the top plate. Fifty microliters of cell suspension (0.7-1 × 106/mL) was seeded in the upper chamber. The chamber was incubated at 37°C for 90 minutes. At the end of the incubation, filters were removed and stained with Diff-Quik (Baxter, Rome, Italy), and 5 high-power oil-immersion fields (magnification 1000×) were counted. Each experiment was performed in triplicate. In some experiments, cells were preincubated with blocking antibodies (30 minutes at 4°C) or with chemical inhibitors. Purified recombinant M315 was added at the same concentration in both the lower and the upper well. Desensitization experiments were performed, pretreating cells with the selected chemotatic factor (30 minutes at 30°C, 5% CO2); subsequently, cells were washed and used in the chemotaxis assay.16 Changes in intracellular Ca2+ concentration ([Ca2+]i) were monitored using fura 2-acetoxymethyl ester, as previously described.16 Migration of blood-purified DCs was evaluated using 24-well Costar Transwell chambers (5 μm pore size; Corning, Life Science, Cambridge, MA). A quantity amounting to 100 μL of cell suspension (5 × 106 cells/mL) was seeded in the upper chambers, and 600 μL of chemoattractant or control medium was added to the lower wells. The chambers were incubated at 37°C for 4 hours. The number of migrated cells, recovered from the lower well, was expressed as the percentage of input cells. To evaluate the ex vivo migration of mouse DCs, ears were rinsed in 70% ethanol, air dried, and split in dorsal and ventral halves. Dorsal skin was placed in culture, and emigrated cells were collected and counted after 48 hours.17 Cell migration is expressed as numbers of migrated cells per milligram of tissue.
The amount of metalloproteases (MMPs) secreted in cell culture supernatant was estimated by zymography.18 Cells were incubated for 2 days without FCS in presence of activin A or CCL3. Subsequently, cells were mixed with sample buffer (126 mM Tris-HCl (pH 6.8), 20% (vol/vol) glycerol, 0.01% (wt/vol) bromphenol blue, 4% (wt/vol) sodium dodecyl sulfate), and proteins were separated by electrophoresis through the substrate gel (10% polyacrylamide containing 1 mg/mL gelatin; Sigma-Aldrich). The gel was renatured in 2.7% (vol/vol) Triton X-100 for 1 hour at room temperature and incubated overnight at 37°C in developing buffer (100 mM Tris-HCl (pH 7.46), 10 mM CaCl2, 5 μM ZnCl2, and 0.015% (vol/vol) Brij-35). The gel was stained with 0.25% (wt/vol) Coomassie brilliant blue (Sigma-Aldrich) and destained in 50% methanol and 10% acetic acid. Gels were scanned and analyzed by Scion Image program (a version of NIH Image; Scion, Bethesda, MD).
Cell bodies and pseudopodia were isolated, respectively, from the upper and lower side of the chemotactic filter, as previously described,19 with some modifications. Cells were placed in the upper compartment of a Transwell insert equipped with a 3-μm porous polycarbonate membrane, and activin A was added to the lower chamber. At the end of the incubation (30 minutes at 37°C), cells were fixed with 0.3% formaldehyde. Pseudopodia (lower side of the filter) and cell bodies (upper side of the filter) were scraped into lysis buffer (phosphate-buffered saline [PBS] without Ca2+/Mg2+, 10 mM Nonidet P-40, 5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM phenylmethylsulfonyl fluoride, protease, and phosphatase inhibitors mixture (50 μg/mL pepstatin, 50 μg/mL leupeptin, 10 μg/mL aprotinin, 1.0 mM Na3VO4; all from Sigma-Aldrich). Cell extracts were incubated at 4°C for 15 minutes and centrifuged, and the supernatants were tested for chemokine levels by enzyme-linked immunosorbent assay (ELISA).
Real-time video microscopy migration assays were carried out using bone marrow-derived immature murine DCs. DCs (after 10 days culturing) were grown on coverslip bottom (0.17 mm thickness) cell culture dishes (35 mm) for 16 hours before the experiment. The cells were washed with 1 × PBS, and the directional migration was determined on a heated microscope stage (37°C). The ligands (100 ng/mL activin A or 100 nM LTB4 in PBS) were loaded into micropipettes, and the tip of micropipette was placed in focus and at a desired distance from DCs using micromanipulators. Real-time images were captured using Nikon Inverted Microscope Eclipse TE300 with oil-immersion 60× objective lens and a Cool Snap HQ digital B/W CCD camera (Roper Scientific, Tuscon, AZ) at every 15-second time interval using Metamorph 6.1 software (Molecular Devices, Downingtown, PA). The movies were generated from these images using Metamorph 6.1 software (Universal Imaging, Downington, PA).
mRNA analysis
mRNA was extracted by TRIzol-LS reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized with GeneAmp RNA polymerase chain reaction kit, according to manufacturer's instructions; total reaction volume was 100 μL, containing 2 μg or total RNA. cDNA was analyzed with TaqMan low density array based on an Applied Biosystems MicroFluidic Card (Applied Biosystems, Carlsbad, CA) containing probe sets for the following chemotatic factors: CCL1, CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, CCL13, CCL14, CCL15, CCL16, CCL17, CCL18, CCL19, CCL20, CCL21, CCL22, CCL23, CCL24, CCL25, CCL26, CCL27, CCL28, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL12, CXCL14, XCL1, CX3CL1, and chemerin. A total of 8 ng of sample in 8-μL reaction/well was used. Low Density Array MicroFluidic Cards were analyzed with QR documents and RQ Manager Software for automated data analysis. Chemokine mRNA was normalized to the housekeeping gene 18S mRNA by subtracting the cycle threshold (Ct) value of 18S mRNA from the Ct value of the gene (ΔCt). Fold difference (RQ = 2−ΔΔCt) was calculated by comparing the ΔCt of the unknown samples with the ΔCt of unstimulated cells.
Cytokine evaluation by ELISA
The concentrations of human CXCL12, CXCL14, and chemerin were evaluated using colorimetric ELISA DuoSet (R&D Systems), according to the manufacturer's instructions.
Statistical analysis
Statistical significance between the experimental groups was determined using paired Student t test.
Results
Activin A is chemotactic for human and murine DCs
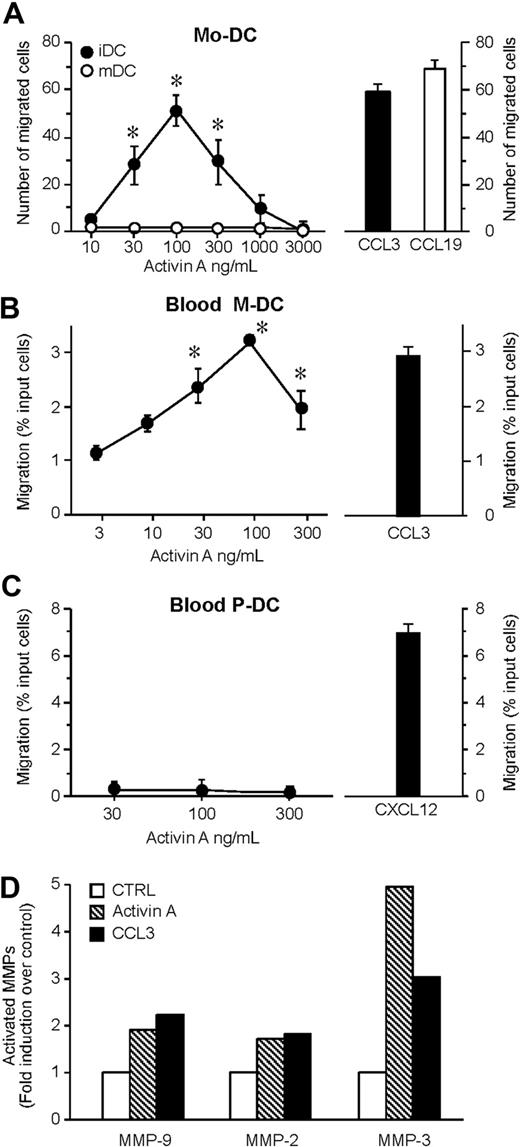
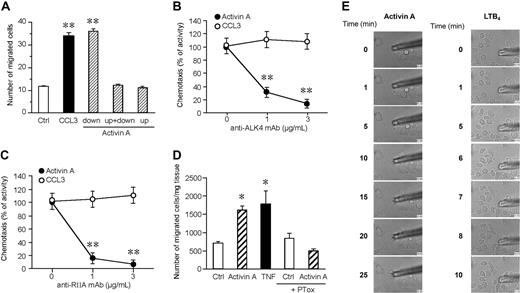
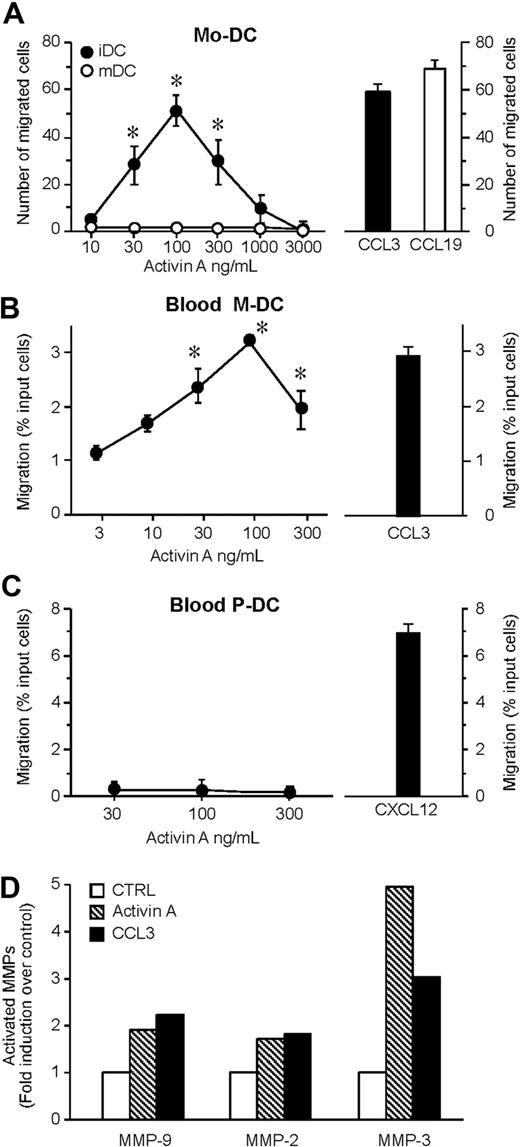
The ability of activin A to induce the migration of monocyte-derived DCs was examined using a 48-well microchemotaxis chamber assay. Activin A, tested over a wide range of concentrations (10-3000 ng/mL), started to induce a statistical significant migration of iDCs at 30 ng/mL and reached a peak at 100 ng/mL, and the activity started to decline at 300 ng/mL. At the peak concentration, the number of migrated cells was similar to that induced by an optimal concentration of CCL3, used as a prototypic iDC chemotactic factor (Figure 1A). Similarly, activin A induced the migration of circulating iDCs (Figure 1B). Conversely, no migration was observed when plasmacytoid DCs were tested (Figure 1C). Because activin A also induced the migration of Langerhans cells (data not shown), these results indicate that, at least in vitro, activin A is a selective chemotactic factor for iDCs. Indeed, no migration was observed when mature DCs were tested, suggesting that activin A may play a relevant role in inducing DC recruitment during inflammation (Figure 1A). Of note, mature DCs retained the expression of the activin A receptor chains10 (data not shown), and activin A was able to induce Akt phosphorylation in mature DCs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These data suggest that mature DCs express functional activin A receptors that, however, are uncoupled from the cell migration machinery. An important event crucial for DC migration is the release of activated MMPs.18,20 Consistently, activin A induced the production and secretion of the active forms of MMP-2, MMP-3, and MMP-9 (Figure 1D).
Activin A induces DC migration and MMP-2, -3, and MMP-9 activation. (A) Dose-dependent migration of monocyte-derived DCs (Mo-DC) was evaluated using modified Boyden chambers. The right panel shows the migration in response to an optimal concentration of CCL3 (100 ng/mL; iDC = immature DC) or CCL19 (100 ng/mL; mDC = mature DC). Maturation of DC was achieved after incubation with 100 g/mL LPS for 24 hours. (B,C) Blood myeloid (M-DC) and plasmacytoid (P-DC) DC subsets were isolated and tested for their migration in response to activin A or to an optimal concentration (100 ng/mL) of CCL3 (M-DC) or CXCL12 (P-DC). Values represent the mean plus or minus SD of 7 (A) or 3 (B,C) different experiments performed with independent donors. (D) DCs were stimulated with 100 ng/mL activin A for 48 hours. MMP-2, MMP-3, and MMP-9 activity was evaluated by gelatin zymography. Band intensity of the bands was analyzed by Scion Image program. Results represent the average values of 2 independent experiments. *P < .05 by paired Student t test versus control group.
Activin A induces DC migration and MMP-2, -3, and MMP-9 activation. (A) Dose-dependent migration of monocyte-derived DCs (Mo-DC) was evaluated using modified Boyden chambers. The right panel shows the migration in response to an optimal concentration of CCL3 (100 ng/mL; iDC = immature DC) or CCL19 (100 ng/mL; mDC = mature DC). Maturation of DC was achieved after incubation with 100 g/mL LPS for 24 hours. (B,C) Blood myeloid (M-DC) and plasmacytoid (P-DC) DC subsets were isolated and tested for their migration in response to activin A or to an optimal concentration (100 ng/mL) of CCL3 (M-DC) or CXCL12 (P-DC). Values represent the mean plus or minus SD of 7 (A) or 3 (B,C) different experiments performed with independent donors. (D) DCs were stimulated with 100 ng/mL activin A for 48 hours. MMP-2, MMP-3, and MMP-9 activity was evaluated by gelatin zymography. Band intensity of the bands was analyzed by Scion Image program. Results represent the average values of 2 independent experiments. *P < .05 by paired Student t test versus control group.
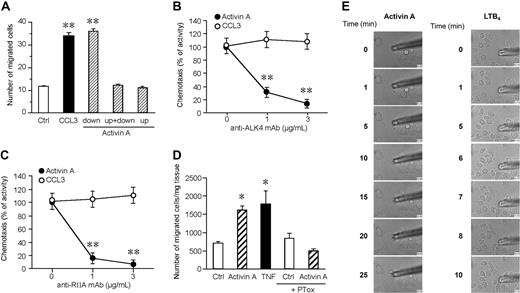
To evaluate whether activin A promoted true chemotaxis (ie, directional migration) rather than chemokinesis (ie, activated random migration), checkerboard analysis was performed. Figure 2A shows that activin A is active only in the presence of a positive gradient (ie, when it is added to the lower compartment of the chemotaxis chamber) with no activity in the presence of a negative gradient (activin A in the upper well) or in absence of a chemotactic gradient (activin A in both the lower and upper wells). The use of blocking mAbs demonstrated that iDC migration to activin A is mediated by the engagement of ALK4 and ActRIIA receptor chains, the 2 main receptors expressed by DCs10 (Figure 2B,C). Finally, the chemotactic activity of activin A was tested on mouse-resident cutaneous DCs ex vivo. When segments of dorsal ear skin were placed in culture, the number of DCs emigrating into the culture medium was strongly increased in the presence of an optimal concentration of activin A, with efficacy comparable with the prototypic agonist TNF-α17 (Figure 2D).
Activin A induces directional migration of DCs through the activation of ALK4 and ActRIIA receptors. (A) Checkerboard analysis was performed using 100 ng/mL activin A in the lower, upper, or both lower and upper wells. Results show that DCs migrated only in the presence of an activin A–positive (in the lower chamber) gradient. (B,C) DC migration in response to activin A was assessed in the presence of blocking mAbs for ALK4 or ActRIIA. Migration of DCs in response to 100 ng/mL CCL3 was used as positive control. Values represent the mean plus or minus SD of 3 different experiments. (D) Egression of mouse skin DCs in response to 100 ng/mL activin A. DC egression into the medium was evaluated after 48-hour culture; TNF-α (20 ng/mL) was used as a positive control. The results are the mean plus or minus SEM of 3 to 10 independent experiments. PTox: B pertussis toxin. *P < .05 and **P < .005 by paired Student t test versus control group. (E) Real-time DC migration in response to activin A (100 ng/mL) and LTB4 (100 nM). The micropipettes loaded with activin A or LTB4 were positioned in culture dishes with DCs, and bright-field images were collected using a Nikon TE-300 microscope, as indicated in “Migration assay.”
Activin A induces directional migration of DCs through the activation of ALK4 and ActRIIA receptors. (A) Checkerboard analysis was performed using 100 ng/mL activin A in the lower, upper, or both lower and upper wells. Results show that DCs migrated only in the presence of an activin A–positive (in the lower chamber) gradient. (B,C) DC migration in response to activin A was assessed in the presence of blocking mAbs for ALK4 or ActRIIA. Migration of DCs in response to 100 ng/mL CCL3 was used as positive control. Values represent the mean plus or minus SD of 3 different experiments. (D) Egression of mouse skin DCs in response to 100 ng/mL activin A. DC egression into the medium was evaluated after 48-hour culture; TNF-α (20 ng/mL) was used as a positive control. The results are the mean plus or minus SEM of 3 to 10 independent experiments. PTox: B pertussis toxin. *P < .05 and **P < .005 by paired Student t test versus control group. (E) Real-time DC migration in response to activin A (100 ng/mL) and LTB4 (100 nM). The micropipettes loaded with activin A or LTB4 were positioned in culture dishes with DCs, and bright-field images were collected using a Nikon TE-300 microscope, as indicated in “Migration assay.”
To determine whether activin A can directly induce DC migration, we performed a real-time migration assay. In this assay, chemoattractants, like LTB4, induce rapid migration of DCs (Figure 2E right panel, and Video S1). In contrast, activin A caused cell polarization, but not appreciable migration of DCs (Figure 2E left panel, and Video S2). Because both LTB4 and activin A caused similar levels of migration in classical Boyden chamber assays (data not shown), these results suggest that activin A might be inducing DC migration through a secondary mechanism.
Activin-A–induced iDC chemotaxis is protein synthesis and Giα protein dependent
The results reported above prompted a more detailed characterization of the mechanisms used by activin A to induce DC chemotaxis. Figures 2D and 3A show that B pertussis toxin inhibits the migration to activin A of both mouse and human DCs. As expected based on previous reports, the toxin also inhibited DC migration to CCL3,8 but it was inactive on the response to TGF-β21 and phorbol myristate acetate (PMA), a nonreceptor chemotactic factor.22 Similarly to TGF-β,21 activin A chemotactic activity was dependent on protein synthesis, as documented by the use of cycloheximide. As expected, under the same experimental conditions, the effect of CCL3 and PMA was not affected by the drug (Figure 3A). B pertussis toxin and cycloheximide pretreatments were not toxic and did not affect basal DC migration (data not shown). Furthermore, exposure of DC to activin A (Figure 3B), similarly to TGF-β,21 did not result in changes of cytosolic [Ca2+]i, a classic feature of chemokine signaling.16 Finally, migration of DCs in response to activin A and CCL3, but not to TGF-β, was inhibited by the PI3K inhibitor wortmannin (Figure 3C). Consistently, DCs generated from PI3Kγ−/− mice did not migrate in response to CCL3, as expected on the basis of previous observations,17 and to activin A, but exhibited a normal response to TGF-β (Figure 3D). Furthermore, in light of the known ability of activin A to induce phospholipid metabolism,23 it was important to investigate the possible involvement of chemotactic lipids in activin A–induced DC migration. However, the involvement of main lipid chemotactic agonists was ruled out by the use of selective receptor antagonists for LTB4 (3-30 μM LY2552833 and U-75302) and platelet-activating factor (5-50 μM ginkgolide B) (data not shown). Finally, DC migration to activin A was completely inhibited by M3, a viral chemokine-binding protein with a large spectrum of specificity15 (Figure 3E). Collectively, these data suggested that activin A promotes DC migration through the secondary induction of chemokines.
Activin A–induced chemotaxis is protein synthesis, PI3Kγ, and G-protein dependent. (A) Effect of B pertussis toxin (PTox; 3 μg/mL) or cycloheximide (CHX; 1 μg/mL) on DC migration in response to activin A, TGF-β, CCL3, and PMA. Results represent average values ( ± SD) of 3 independent experiments. (B) [Ca2+]i in DC stimulated with activin A. One experiment representative of 3 independent determinations is shown. (C) Effect of 10 ng/mL wortmannin on DC migration. (D) Migration to activin A of DCs generated from wild-type (WT) or PI3Kγ−/− mice. (E) Effect of the viral chemokine-binding protein M3 (100 nM) on DC migration. CCL3, activin A, PMA, and TGF-β were tested at the respective concentrations of 100 ng/mL, 100 ng/mL, 10 nM, and 10 pg/mL. **P < .005 by paired Student t test versus respective control groups.
Activin A–induced chemotaxis is protein synthesis, PI3Kγ, and G-protein dependent. (A) Effect of B pertussis toxin (PTox; 3 μg/mL) or cycloheximide (CHX; 1 μg/mL) on DC migration in response to activin A, TGF-β, CCL3, and PMA. Results represent average values ( ± SD) of 3 independent experiments. (B) [Ca2+]i in DC stimulated with activin A. One experiment representative of 3 independent determinations is shown. (C) Effect of 10 ng/mL wortmannin on DC migration. (D) Migration to activin A of DCs generated from wild-type (WT) or PI3Kγ−/− mice. (E) Effect of the viral chemokine-binding protein M3 (100 nM) on DC migration. CCL3, activin A, PMA, and TGF-β were tested at the respective concentrations of 100 ng/mL, 100 ng/mL, 10 nM, and 10 pg/mL. **P < .005 by paired Student t test versus respective control groups.
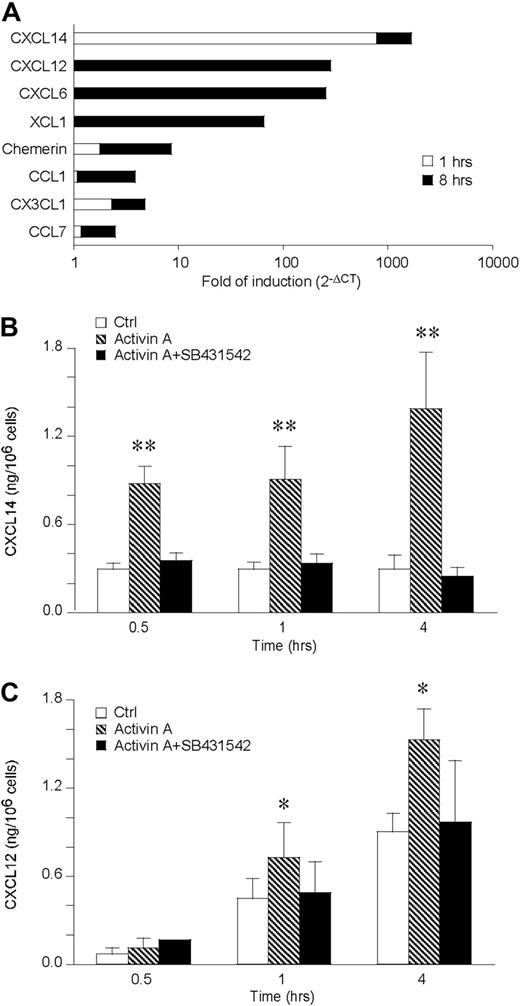
Activin A induces a rapid release of CXCL12 and CXCL14 by DCs
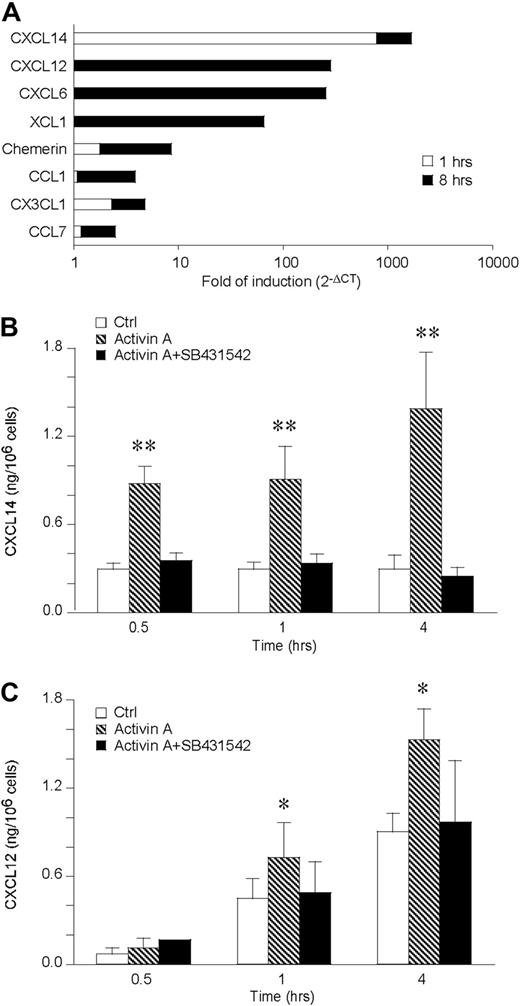
To investigate the ability of activin A to induce chemokine production, chemokine transcripts induced in DCs stimulated for 1 and 8 hours with activin A were tested by the use of a low-density microarray. Among the 34 genes investigated, only 8 were induced by activin A at the time points tested. CXCL14, CX3CL1, and chemerin resulted already induced after 1-hour stimulation and further increased at 8 hours, whereas CXCL12, CXCL6, XCL1, CCL1, and CCL7 mRNA were up-regulated only at the longest time of stimulation (Figure 4A). Among the 8 induced genes, we focused our interest on those known to be chemotactic for iDCs, namely CXCL14, CXCL12, and chemerin. CXCL14 was up-regulated 800- and 900-fold at 1 and 8 hours, respectively. CXCL12 was induced 284-fold at 8 hours, whereas chemerin was induced 2- and 7-fold at 1 and 8 hours, respectively. The induction of these chemotactic agonists was then tested at the protein level by ELISA, using supernatants of activin A–stimulated DCs (Figure 4B,C). Consistent with the increased mRNA levels, CXCL14 was released at low concentrations under basal conditions and induced by activin A along all the kinetics investigated. CXCL12 was already produced at conspicuous levels under basal conditions (eg, at 1 and 4 hours), and activin A further increased its levels. Both CXCL12 and CXCL14 production was completely blocked in the presence of SB431542, an inhibitor of the ALK4 signaling transducer chain (Figure 4B,C). In contrast, chemerin was not detected under basal conditions and was not induced by activin A up to 12 hours stimulation (data not shown).
Activin A up-regulates CXCL14 and CXCL12 in DCs. (A) Low-density microarray of DC mRNA extracted after 1 and 8 hours of activin A (100 ng/mL) stimulation. The data are expressed as fold of induction versus control DC. Only regulated chemokines are shown. (B,C) Kinetics of CXCL14 and CXCL12 release by 100 ng/mL activin-A–stimulated DC. SB431542, an ALK4 inhibitor, was used at the concentration of 5 μM. Data are the mean plus or minus SD of 4 independent experiments. *P < .05, **P < .005 by paired Student t test versus control groups.
Activin A up-regulates CXCL14 and CXCL12 in DCs. (A) Low-density microarray of DC mRNA extracted after 1 and 8 hours of activin A (100 ng/mL) stimulation. The data are expressed as fold of induction versus control DC. Only regulated chemokines are shown. (B,C) Kinetics of CXCL14 and CXCL12 release by 100 ng/mL activin-A–stimulated DC. SB431542, an ALK4 inhibitor, was used at the concentration of 5 μM. Data are the mean plus or minus SD of 4 independent experiments. *P < .05, **P < .005 by paired Student t test versus control groups.
CXCL12 and CXCL14 are responsible for activin A–induced migration of DCs
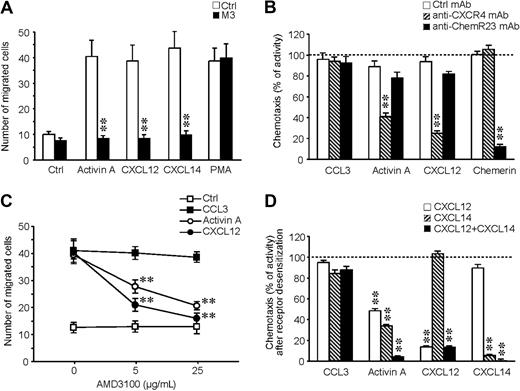
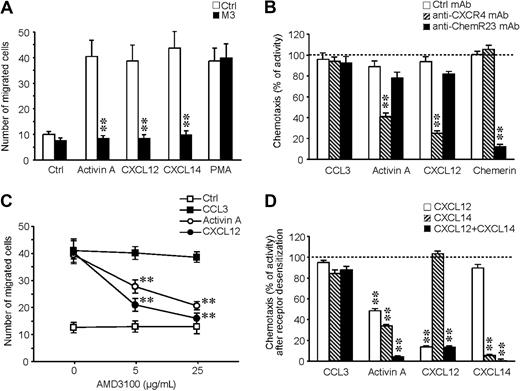
To investigate the role of CXCL12 and CXCL14 in activin A–induced migration, it was first confirmed that M3 can inhibit iDC migration to CXCL12 and CXCL14. Figure 5A shows that the action of both chemokines, as well as that of activin A, was completely blocked by the viral protein. The role of CXCL12 in activin A–induced DC migration was further addressed by the use of a specific blocking mAb and a CXCR4 receptor antagonist (AMD 3100). Figure 5B shows that the anti-CXCR4 mAb selectively caused a partial inhibition of activin A–induced chemotaxis, whereas, as expected, migration to CXCL12 was strongly inhibited; conversely, a blocking anti-ChemR23 mAb did not affect activin A–induced migration. The effect was specific because neither of the 2 antibodies affected iDC migration to CCL3. Similarly, AMD 3100 suppressed activin A– and CXCL12-induced migration in a dose-dependent manner (Figure 5C), with no effects on CCL3 chemotaxis. Because CXCL14 receptor is still unknown, the role of this chemokine in activin A–induced DC migration was evaluated, taking advantage of the fact that chemotactic factors induce specific homologous desensitization of their receptors.24 As shown in Figure 5D, a partial reduction of the migration to activin A was obtained by the pretreatment of iDCs with either CXCL12 or CXCL14 (51.6% ± 2.2% and 66.6% ± 1.7%, respectively; n = 3), whereas a complete inhibition (95.9% ± 1.0%; n = 3) was obtained after the simultaneous pretreatment of the cells with both chemokines. Specificity of the pretreatment was documented by the lack of effect on CCL3-induced chemotaxis. As expected, pretreatment of iDCs with chemerin did not affect activin A response (data not shown). Finally, the addition of both CXCL12 and CXCL14 in the upper well of the chemotactic chamber completely inhibited iDC migration to activin A, but did not affect the migration to CCL3 (data not shown). Taken together, these results demonstrate that activin A induces iDC migration through the secondary production of CXCL12 and CXCL14.
The endogenous production of CXCL12 and CXCL14 is responsible for activin A–induced DC migration. (A) M3 (100 nM) inhibits DC migration in response to activin A, CXCL12, and CXCL14. (B) Effect of anti-CXCR4 (7.5 μg/mL)- and anti-ChemR23 (3 μg/mL)–blocking antibodies on DC migration. (C) Effect of AMD3100, a CXCL12 receptor antagonist, on DC migration. Cells were preincubated for 30 minutes with different concentrations of the inhibitor/mAb and then tested in chemotaxis assays. (D) Cross-desensitization experiments using 100 ng/mL CXCL12, 100 ng/mL CXCL14, 100 ng/mL CCL3, and 3 nM chemerin. Cells were preincubated with the different chemotactic agonists at 37°C for 30 minutes, washed, and tested in the chemotaxis assay. Migration was performed using 100 ng/mL activin A, 100 ng/mL CCL3, 100 ng/mL CXCL12, 100 ng/mL CXCL14, 3 nM chemerin, and 10 nM PMA. **P < .005 by paired Student t test versus respective control groups.
The endogenous production of CXCL12 and CXCL14 is responsible for activin A–induced DC migration. (A) M3 (100 nM) inhibits DC migration in response to activin A, CXCL12, and CXCL14. (B) Effect of anti-CXCR4 (7.5 μg/mL)- and anti-ChemR23 (3 μg/mL)–blocking antibodies on DC migration. (C) Effect of AMD3100, a CXCL12 receptor antagonist, on DC migration. Cells were preincubated for 30 minutes with different concentrations of the inhibitor/mAb and then tested in chemotaxis assays. (D) Cross-desensitization experiments using 100 ng/mL CXCL12, 100 ng/mL CXCL14, 100 ng/mL CCL3, and 3 nM chemerin. Cells were preincubated with the different chemotactic agonists at 37°C for 30 minutes, washed, and tested in the chemotaxis assay. Migration was performed using 100 ng/mL activin A, 100 ng/mL CCL3, 100 ng/mL CXCL12, 100 ng/mL CXCL14, 3 nM chemerin, and 10 nM PMA. **P < .005 by paired Student t test versus respective control groups.
Activin A induces polarized release of CXCL12 by migrating DCs
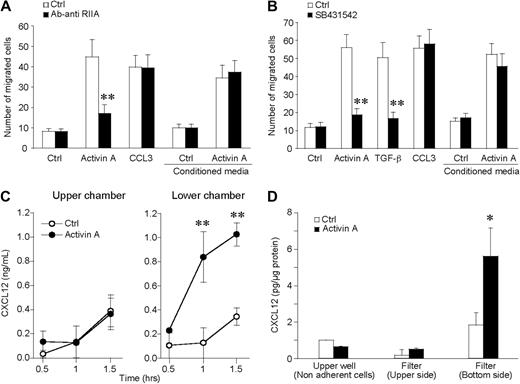
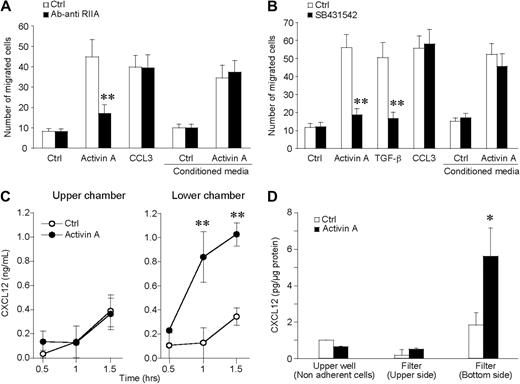
To confirm the importance of CXCL14 and CXCL12 production by iDCs during activin A–induced migration, conditioned medium-obtained incubating iDCs with activin A for 90 minutes was used, in the chemotactic assay, as a source of chemoattractant. Figure 6A shows that activin A–conditioned medium induced DC migration in an activin A receptor-independent manner. Indeed, the anti-ActRIIA mAb inhibited DC migration in response to activin A, but did not affect the migration in response to the conditioned (chemokine-containing) medium. Furthermore, SB431542 inhibited activin A–induced CXCL12 and CXCL14 release (Figure 4B,C) and blocked iDC migration to activin A with no effect on the migration to activin A–conditioned medium (Figure 6B).
Activin A induces the polarized production of CXCL12 by migrating DCs. (A) Conditioned medium of activin A–stimulated DCs (100 ng/mL for 90 minutes) was used as source of chemotactic factor for DCs. The anti-ActRIIA-blocking antibody (3 μg/mL; anti-RIIA) was used to neutralize the effect of activin A present in the supernatant. (B) SB431542 (5 μM), the inhibitor of signaling transducer chains ALK4, blocked the migration to activin A as well as to TGF-β, but not to CCL3. The inhibitor did not alter the migration to conditioned media. (C) At the end of the migration test, CXCL12 is present in higher concentrations in the lower well of the chemotactic chamber. (D) Evaluation of cell-associated CXCL12 in nonadherent cells and in cells adherent to the upper side or to the lower side (pseudopods + cells) of the filter. This experiment reveals that CXCL12 is selectively released in a polarized manner. *P < .05, **P < .005 by paired Student t test versus respective control groups.
Activin A induces the polarized production of CXCL12 by migrating DCs. (A) Conditioned medium of activin A–stimulated DCs (100 ng/mL for 90 minutes) was used as source of chemotactic factor for DCs. The anti-ActRIIA-blocking antibody (3 μg/mL; anti-RIIA) was used to neutralize the effect of activin A present in the supernatant. (B) SB431542 (5 μM), the inhibitor of signaling transducer chains ALK4, blocked the migration to activin A as well as to TGF-β, but not to CCL3. The inhibitor did not alter the migration to conditioned media. (C) At the end of the migration test, CXCL12 is present in higher concentrations in the lower well of the chemotactic chamber. (D) Evaluation of cell-associated CXCL12 in nonadherent cells and in cells adherent to the upper side or to the lower side (pseudopods + cells) of the filter. This experiment reveals that CXCL12 is selectively released in a polarized manner. *P < .05, **P < .005 by paired Student t test versus respective control groups.
Results reported in Figure 2A clearly indicate that activin A promotes directional migration of iDCs. This finding implies that activin A induces the formation of a chemotactic gradient between the lower and upper wells of the chemotaxis chamber. To better document this possibility, the concentration of CXCL12 was evaluated in the 2 compartments of the chemotactic chamber. Figure 6C shows that at the end of the 90-minute assay CXCL12 was present in the lower well at a concentration that was 2.8-fold higher than that found in the upper well. The levels of CXCL12 production in the lower well fell within the active chemotactic concentrations (Figure S2a), and the kinetics of CXCL12 production precedes activin A–induced iDC migration (Figure S3). Figure S2b,c also shows that CXCL12 and CXCL14 have an additive chemotactic action, suggesting that the 2 chemokines may act in a coordinated manner in the induction of iDC migration. To investigate the possibility that migrating DC may release CXCL12 in a polarized manner, CXCL12 concentration was evaluated in 3 different compartments, namely nonadherent cells recovered from the upper well of the chemotactic chamber; cells adherent to the upper side of filter and undergoing the process of transmigration of the filter; and finally, DC pseudopodia and early transmigrated cells adherent to the lower side of the filter. Results reported in Figure 6D show that activin A induces the selective expression of CXCL12 at the lower side of the filter; this finding provides the mechanism responsible for the formation of activin A–induced chemotactic gradient during the 90-minute assay.
Discussion
This study reports that activin A, a member of the TGF-β family, is able to induce the directional migration of myeloid iDCs in vitro and ex vivo. Activin A showed a classic bell-shaped dose-response curve, and had an efficacy (number of migrated cells) similar to CCL3, a prototypic chemotactic factor for iDCs.16 The peak chemotactic concentrations for both activin A and CCL3 were 100 ng/mL, which corresponds to 3.9 nM and 12 nM, respectively. Therefore, activin A, compared with CCL3, is characterized by a potency (peak chemotactic concentration) approximately 4-fold higher and by a similar efficacy. Checkerboard experiments revealed that activin A promoted iDC directional migration, therefore acting as a true chemotactic factor. Activin A was also chemotactic for blood myeloid DC; conversely, it did not induce the migration of plasmacytoid DC. Finally, activin A was only active on immature DC, with no chemotactic effect on LPS-activated cells. Similarly to many other chemotactic factors,18,20 activin A induced the activation of MMP-2, MMP-3, and MMP-9, which are enzymes known to be required for DC migration across extracellular matrix components.8 This latter result is consistent with previous reports showing that activin A induces the activation of MMP activity in other cell types, including trophoblasts and decidual cells.25-27 As demonstrated by the use of specific mAbs, the action of activin A was dependent on the activation of the 2 main receptor chains expressed by iDCs,10 namely ALK4 and ActRIIA. DC migration to activin A was sensitive to the action of cycloheximide, but differently from TGF-β, was also sensitive to the effect of B pertussis toxin and M3, a 44-kDa chemokine-binding protein encoded by the murine gammaherpesvirus 68.15 These results confirm previous observations obtained with TGF-β in other cell types21,28 and provide evidence that in spite of the similar signaling pathways of their receptors, activin A and TGF-β induce cell migration through different activation mechanisms.
The inhibitory effect of B pertussis toxin, cycloheximide, and M3 strongly suggested that the chemotactic action of activin A was dependent on the secondary production of chemokines. This hypothesis was tested by the use of a low-density microarray and of different complementary experimental approaches based on the use of receptor-blocking antibodies, receptor antagonists, and cross-desensitization experiments. These experiments allowed us to unequivocally define that the chemotactic activity of activin A completely relies on the production of 2 chemokines, namely CXCL12 and CXCL14. Both chemokines are produced in a constitutive manner in many tissues, including epithelial cells in the skin and gut, where they are believed to play a crucial role in the recruitment of lymphoid and myeloid cells and their production is further induced under pathologic conditions.29 The results reported in this study further extend these findings, showing that also iDCs spontaneously produce CXCL12 and CXCL14, in vitro, and that this production is further increased by activin A, although with different kinetics, CXCL14 being more rapidly induced than CXCL12. These results further distinguish the action of activin A from TGF-β, which was reported to down-regulate CXCL12 production in bone marrow stromal cells.30
The fact that a proinflammatory cytokine stimulates the production of chemotactic proteins is not uncommon. Many primary proinflammatory stimuli, such as LPS, TNF-α, and IL-1, are known to be strong inducers of chemokines.8,31 Similarly, certain cytokines such as basic fibroblast growth factor, GM-CSF, macrophage colony-stimulating factor, and TGF-β1 were reported to induce chemokine production.32-34 Therefore, activin A can be added to the list of cytokines able to regulate leukocyte recruitment through the secondary induction of chemokines. However, what makes the action of activin A unique is as follows: first, the ability to selectively induce a very limited pattern of chemokines among the 34 chemotactic factors investigated; second, to promote the directional migration of iDCs in an in vitro assay. Indeed, checkerboard assays clearly demonstrated that activin A is a pure chemotactic agonist for iDCs. This evidence is further sustained by the finding that during the chemotaxis assay, a gradient is formed between the lower and the upper wells, with the highest concentrations of the chemokine being present in the lower well of the chemotactic chamber. To investigate in a more direct way the mechanism(s) sustaining the formation of this chemotactic gradient, we performed real-time migration assays and took advantage of a fractionation scheme recently described.19 By the use of these experimental approaches, it was possible to demonstrate that activin A induced cell polarization, but not migration, in real-time microscopy assays and, during the migration to activin A, CXCL12 is selectively enriched in pseudopodes, cell protrusions, and early migrating cells. Previous data have reported that neutrophils release adenosine triphosphate at leading edge of migrating cells.35 This polarized release is likely to be functional to the amplification of the chemotactic gradient and contributes to cell orientation.35,36 Our study extends this observation, providing evidence that also the release of chemokines may happen in a polarized fashion. The mechanism reported in this study in vitro is likely to be relevant also in vivo for the recruitment of leukocytes both in steady state and under inflammatory conditions. During maturation, DCs produce activin A.3,10 Previous work has shown that the inhibition of endogenous activin A production, by the use of follistatin, increases CD40L-induced cytokine release.10 However, this was not the case for chemokines, because follistatin inhibited CXCL12 and CXCL14 production in CD40L- and LPS-stimulated DCs (data not shown).
Circulating DCs are rapidly recruited from the blood compartment to the sites of inflammation and tissue injury in response to locally produced chemotactic factors.8 Activin A is expressed at low levels in normal skin,13 but is abundantly up-regulated during wound healing and hypertrophic scar formation13 and during certain inflammatory/autoimmune diseases, like liken planus11 and fibrohistiocytoma (W. Vermi, F. Facchetti, and S.S., unpublished observations, July 2008). In diseased skin, activin A is mostly produced by dermal fibroblasts,13 DC3 and by vascular endothelial cells.37 Therefore, activin A may contribute to the local accumulation of myeloid DCs and, as recently shown by our group, may also shape the local immune response through the induction of Langerhans cell differentiation from local and recruited precursor cells.11 Furthermore, activin A is a well-known cytokine produced by placenta and involved in human reproduction, where it is thought to function as a paracrine/endocrine regulator of immune fetal-maternal interactions.38,39 Several lines of evidence have recently implicated activin A in tumor cell biology. In breast and prostate carcinoma, the circulating levels of activin A correlate with tumor grade and the number of bone metastases.25,40 Finally, activin A may also promote tumor dissemination through the release of metalloproteases by tumor cells25,26 and DCs. Furthermore, CXCL14 expression was directly correlated with DC recruitment in tumors,41 whereas CXCL12 production was associated to tumor dissemination.42,43 Recent data suggest that activin A may skew DCs to a tolerogenic phenotype.10 Therefore, activin A may actively contribute to the formation of a microenvironment permissive to tumor progression characterized by a state of chronic inflammation, the production of metalloproteases and proangiogenic factors, and the recruitment of tolerogenic DC.
In conclusion, the results reported in this study not only describe the peculiar ability of activin A to induce the migration of myeloid DCs through the induction of the polarized secretion of CXCL12 and CXCL14, but also provide new information to better understand the enigmatic role of a cytokine apparently regulated in many different physiologic and pathologic conditions, ranging from embryonic development, inflammatory/autoimmune conditions, tumor growth, bone remodeling, and central nervous system protection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Antal Rot for fruitful discussion, Bernard Moser for providing purified natural CXCL14, Emilio Hirsch for providing PI3Kγ−/− mice, and Antonio Alcami for providing the M3 protein. We are indebted to Karel Otero and Annalisa Del Prete for their assistance in intracellular calcium evaluation and mouse ex vivo DC migration.
This work was supported by Associazione Italiana per la Ricerca sul Cancro; Istituto Superiore di Sanità; Ministero dell'Istruzione dell'Università e della Ricerca; and Fondazione Cariplo's Operational Network for Biomedicine par Excellence in Lombardy (NOBEL) Project, Ministero della Salute, FP-7 EU Projects “MOODINFLAME” and “INNOCHEM.”
Authorship
Contribution: L.S. performed research, analyzed data, and wrote the paper; D.B. performed research and analyzed data; M.M. and V.R.J. performed research and analyzed data; M.L. analyzed data; B.H. analyzed and discussed some of the data; T.M. performed research; and S.S. planned the experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Silvano Sozzani, Section of General Pathology and Immunology, University of Brescia, viale Europa 11, 25123 Brescia, Italy; e-mail: sozzani@med.unibs.it.

![Figure 3. Activin A–induced chemotaxis is protein synthesis, PI3Kγ, and G-protein dependent. (A) Effect of B pertussis toxin (PTox; 3 μg/mL) or cycloheximide (CHX; 1 μg/mL) on DC migration in response to activin A, TGF-β, CCL3, and PMA. Results represent average values ( ± SD) of 3 independent experiments. (B) [Ca2+]i in DC stimulated with activin A. One experiment representative of 3 independent determinations is shown. (C) Effect of 10 ng/mL wortmannin on DC migration. (D) Migration to activin A of DCs generated from wild-type (WT) or PI3Kγ−/− mice. (E) Effect of the viral chemokine-binding protein M3 (100 nM) on DC migration. CCL3, activin A, PMA, and TGF-β were tested at the respective concentrations of 100 ng/mL, 100 ng/mL, 10 nM, and 10 pg/mL. **P < .005 by paired Student t test versus respective control groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/23/10.1182_blood-2008-12-194597/4/m_zh89990936690003.jpeg?Expires=1769281238&Signature=KWnXHHE8R2TJTW5Lgf~u889VnvrnG0tW1wusbdAS7L~geNcN5817RwmYx9tZZJCBwKt7O1H8~Jm3WQ~t6gxxXwr2vwFj0vIAjnTPdJJGiEzkdpkipxm4QCcRJjDrO0Fpn6zJLks-zWqDEZjYX83UU2QJG~PsDgG0oRvdaLLeaWj~lorFkY55~aPSasAFuKQhohe7S0OFyWNqLQFj6M16rQq4yDc4Hj9~Wam94YzX0rgBw2NkK1KXGo5efDWHDADTbFXKvvlBBjQGDirqr26OftMrEmmADH0A9zg9XWr3sQ41Om-m5~vxJ0ZGSUUlUq-F4q4l4H1k9qdk5ekKj1Y5HQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Activin A–induced chemotaxis is protein synthesis, PI3Kγ, and G-protein dependent. (A) Effect of B pertussis toxin (PTox; 3 μg/mL) or cycloheximide (CHX; 1 μg/mL) on DC migration in response to activin A, TGF-β, CCL3, and PMA. Results represent average values ( ± SD) of 3 independent experiments. (B) [Ca2+]i in DC stimulated with activin A. One experiment representative of 3 independent determinations is shown. (C) Effect of 10 ng/mL wortmannin on DC migration. (D) Migration to activin A of DCs generated from wild-type (WT) or PI3Kγ−/− mice. (E) Effect of the viral chemokine-binding protein M3 (100 nM) on DC migration. CCL3, activin A, PMA, and TGF-β were tested at the respective concentrations of 100 ng/mL, 100 ng/mL, 10 nM, and 10 pg/mL. **P < .005 by paired Student t test versus respective control groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/23/10.1182_blood-2008-12-194597/4/m_zh89990936690003.jpeg?Expires=1769950570&Signature=Q3bwopndJqTHTD~FE6e~hyF~KVGJ1YU8hMNAz87QqJJcLI0DmQXWmbPDahtzFuAvb-fkOkAH~ohhc9Y35oI0~mMCUl2mlfvKKsEEWr-~cWRg-IXRxheTuamnjE5l-arRcJ-2Zi6ZzNntDrMm5Arfd3YT3wWnxPCIwU9W5BoLB7q-DcKWtN4pO2ehLmUpUi6ohVCxGXu1q4uae2C28h-xxLbbDqhE9JJibBELMPZx6vwG3UpZIGN~Iinu4knMYF~6~kJYuXKjbColgYhj-fsRoQoJAzpvuGC5wcPa9d4MYRv-hmlQHVRI8oTF2DyKcHewU9I4DYE7ODBOsZxsgNX7hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)