Abstract
The CXCR4–SDF-1 axis plays a central role in the trafficking and retention of normal and malignant stem cells in the bone marrow (BM) microenvironment. Here, we used a mouse model of acute promyelocytic leukemia (APL) and a small molecule competitive antagonist of CXCR4, AMD3100, to examine the interaction of mouse APL cells with the BM microenvironment. APL cells from a murine cathepsin G-PML-RARα knockin mouse were genetically modified with firefly luciferase (APLluc) to allow tracking by bioluminescence imaging. Coculture of APLluc cells with M2-10B4 stromal cells protected the leukemia cells from chemotherapy-induced apoptosis in vitro. Upon injection into syngeneic recipients, APLluc cells rapidly migrated to the BM followed by egress to the spleen then to the peripheral blood with death due to leukostasis by day 15. Administration of AMD3100 to leukemic mice induced a 1.6-fold increase in total leukocytes and a 9-fold increase of circulating APL blast counts, which peak at 3 hours and return to baseline by 12 hours. Treatment of leukemic mice with chemotherapy plus AMD3100 resulted in decreased tumor burden and improved overall survival compared with mice treated with chemotherapy alone. These studies provide a proof-of-principle for directing therapy to the critical tethers that promote AML-niche interactions.
Introduction
Hematopoietic stem cells (HSCs) reside in the bone marrow (BM) and interact with a highly organized microenvironment composed of a diverse population of stromal cells and an extracellular matrix rich in fibronectin, collagens, and various proteoglycans. The interaction between HSCs and the BM microenvironment is critical in regulating HSC processes such as trafficking, self-renewal, proliferation, and differentiation.
Egress (mobilization) of stem cells from the BM to the peripheral blood can be induced by cytokines (G-CSF and GM-CSF), chemokines (Gro-β and IL-8), or by small molecule inhibitors of both CXCR4 and VLA-4.1 Interaction between the chemokine, SDF-1, and its cognate receptor CXCR4 functions as a key regulator of stem cell mobilization and trafficking.2,3 Constitutive secretion of SDF-1 by marrow stromal cells creates a gradient by which HSCs expressing the receptor CXCR4 home to and interact with its marrow niche.4 In response to factors such as G-CSF, SDF-1 production is down-regulated by stromal cells that release HSCs into the peripheral circulation.5 AMD3100 is a bicyclam molecule that potently, selectively, and reversibly antagonizes the binding of SDF-1 to CXCR4.6 In multiple clinical studies, AMD3100 rapidly and effectively mobilizes HSCs into the peripheral circulation and is currently under development as a stem cell mobilization agent prior to high-dose chemotherapy for multiple myeloma, non-Hodgkin lymphoma, and other hematologic malignancies.7-9
In acute myeloid leukemia (AML), the bone marrow microenvironment provides the primary site of minimal residual disease after chemotherapy.10-12 Similar to normal HSCs, AML blasts express many of the same adhesion molecules as normal HSCs such as CXCR4, CD117, VLA-4, and CD44, which allow them to interact with the marrow stroma.13 These molecules have been shown to mediate antiapoptotic and proliferative effects in both normal CD34 stem cells and AML blasts.14 In leukemic blasts, these prosurvival processes contribute to chemotherapy resistance in a process termed cell adhesion–mediated drug resistance (CAM-DR).10 In addition, other cell-intrinsic genetic and epigenetic changes affecting DNA metabolism, nucleoside and nucleotide metabolism and transport, reactive oxygen species metabolism, and apoptosis have all been implicated in drug resistance.15-21
Because of the central role of the CXCR4/SDF-1 axis in mediating HSC-stromal interactions, we hypothesize that similar to normal HSCs, AMD3100 may disrupt the interaction of leukemic cells with the marrow microenvironment, thereby sensitizing these cells to genotoxic stresses. Using a genetically defined murine model of APL, we demonstrate that AMD3100 can rapidly and preferentially mobilize leukemic blasts into the peripheral circulation, which are then sensitized to the in vivo effects of cytotoxic chemotherapy.
Methods
Mice
C57BL/6J and 129Sv/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The mCGPR/+ strain has been previously described and was maintained on a C57BL/6 × 129/SvJ F1 background.22 Hybrid C57BL/6J × 129Sv/J F1 (B6129F1) mice at 8 to 14 weeks of age were used in all experiments. Animal care and humane killing protocols were approved by the Animal Studies Committee of Washington University School of Medicine.
Automated blood counts and flow cytometry
Peripheral blood from mice was obtained via the retro-orbital plexus under anesthesia and complete blood counts were obtained using an automated cell counter (Hemavet; CDC Technologies, Oxford, CT). Single-cell suspensions from blood, spleen, or BM were stained with allophycocyanin (APC)–conjugated antimurine CD34 and phycoerythrin (PE)–conjugated anti-Gr1 (BD Biosciences, San Diego, CA) monoclonal antibodies as previously described. Cells were analyzed with single-color staining using PE- or APC-conjugated isotype control antibodies to determine specific staining. For each sample, a minimum of 10 000 events were acquired on a Cytomics FC500 flow cytometer (Becton Dickinson, San Jose, CA) and data analyzed using FlowJo software (TreeStar, San Carlos, CA). APL cell death was assessed by flow cytometry using annexin V–PE and 7-amino-actinomycin D using the annexin V–PE apoptosis detection kit (BD Biosciences).
Mobilization protocol and CFC assays
AMD3100 (Genzyme, Cambridge, MA) was supplied as a sterile isotonic aqueous solution at 10 mg/mL and was administered at a dose of 5 mg/kg as a single subcutaneous injection. Colony-forming cell (CFC) assays were performed by plating blood in methocellulose-containing Iscove MDM supplemented with IL-3, IL-6, and stem cell factor (MethoCult 3534; Stem Cell Technologies, Vancouver, BC) according to standard techniques.23
Acute promyelocytic leukemia cells and transplantation
Acute promyelocytic leukemia (mCGPR/+ APL) cells from the spleens of mCG-PML-RARα knockin mice were harvested and cryopreserved.22 Cryopreserved cells were injected intravenously via the tail vein, or intraperitoneally into unconditioned genetically compatible B6129F1 recipients. To screen for development of leukemia, peripheral blood was obtained for complete blood counts and flow cytometry. Moribund animals were killed, and blood, spleen, and BM samples were analyzed for evidence of acute leukemia.
Retroviral vector construction and packaging
Firefly luciferase (Fluc) was amplified from pFR-luc (Stratagene, La Jolla, CA) by polymerase chain reaction (PCR) using the sense primer 5′-CAGCTTGGCAATCC-3′ and antisense primer 5′-TGCCATCGCTGAATACA-3′. The 1656-bp Fluc PCR product was then ligated into the pCR2.1 vector (Invitrogen, Carlsbad, CA) to give pCR2.1-Fluc. To construct the Fluc/IRES/EGFP vector, EcoRI fragments from pCR2.1-Fluc were inserted into an EcoRI site that is 5′ of the internal ribosome entry sequence in the murine stem cell virus (MSCV)–EGFP vector.24
Generation of APLluc cells
Cryopreserved mCGPR/+ APL cells were added to Fluc/IRES/EGFP retroviral supernatant containing 4 μg/mL polybrene at a multiplicity of infection of 4. Following 6-hour incubation, cells were washed and resuspended at 106 cells/mL in media. After 48 hours, EGFP-positive APL cells (APLluc) were collected with a MoFlo (Cytomation, Fort Collins, CO) high-speed fluorescence-activated cell-sorter scanner (FACS) and injected into genetically compatible B6129F1 mice. Leukemic splenocytes were harvested from moribund mice and cryopreserved in media containing 40% FBS and 10% DMSO.
Bioluminescence imaging
APLluc cell trafficking and in vivo expansion in B6129F1 recipients was assessed noninvasively by bioluminescence imaging using an IVIS 100 CCD camera (Xenogen, Alameda, CA).25,26 Briefly, mice were shaved to reduce light attenuation and injected intraperitoneally with D-luciferin (150 μg/g in PBS). Images were acquired 10 minutes after D-luciferin injection (1- to 60-second exposure; binning: 8; f-stop: 1; field-of-view: 15 cm). Total photon flux (photons/second) was quantified on images using a rectangular region of interest encompassing the entire abdomen and thorax.
Treatment of APL cells in vitro
The murine stromal cell line M2-10B4 was obtained from ATCC (Manassas, VA). To assess APL cell death, APLluc cells (103 cells/well) were cultured in 96-well black-walled microtiter plates (Costar, Corning, NY) in the presence or absence of M2-10B4 cells (103 cells/well). After 48 hours, cells were treated with cytarabine (Ara-C), daunorubicin (DNR), or vehicle and cultured for an additional 48 hours. In some experiments, AMD3100 was added to a final concentration of 10 ng/mL. To image live cells, D-luciferin (150 μg/mL) was added to wells. Photon flux for each well was measured 8 to 10 minutes after addition of D-luciferin with the IVIS (60-second exposure; binning: 8; f-stop: 1; 1; field-of-view: 15 cm). To prepare conditioned medium, M2-10B4 cells were grown to confluence, washed with PBS, and incubated in supplemented RPMI 1640 (sRPMI) containing 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT), 100 units/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine at 37°C. After 72 hours, the medium was collected and centrifuged at 1000g for 10 minutes. Cell-free culture supernatant was obtained by passage through a 0.45-μm sterile filter.
Statistical analysis
Data are expressed as mean values plus or minus SD. Comparison between groups was performed using an unpaired Student t test (2 tailed), 95% confidence intervals, or analysis of variance (ANOVA). Survival curves were generated using the method of Kaplan and Meier and analyzed by log-rank test. Statistical analyses were performed using StatView software (Abacus Concepts, Berkeley, CA). Differences at a P value less than .05 were considered to be statistically significant.
Results
In vivo bioluminescence imaging of AML cell trafficking
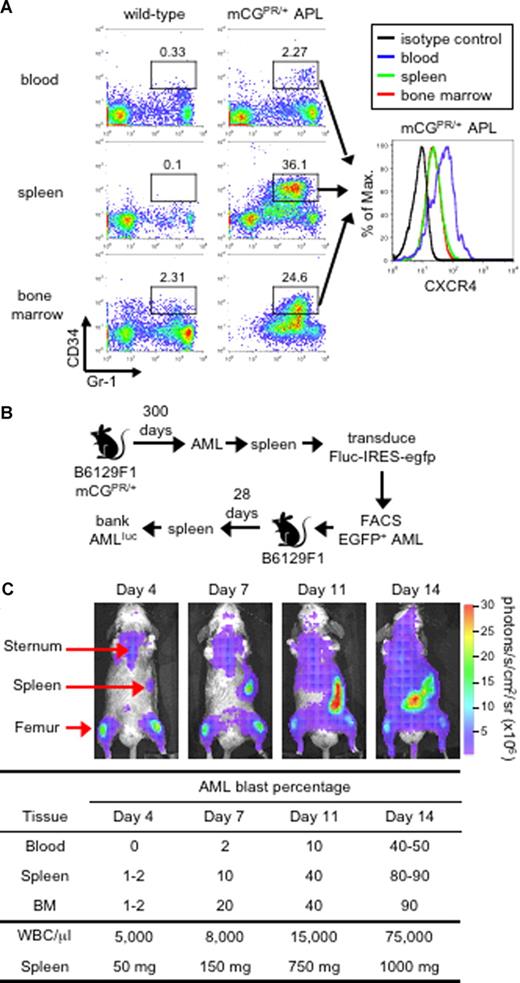
The mCGPR/+ murine model of APL was previously generated by knocking in the human PML-RARα cDNA found in acute promyelocytic leukemia into the 5′ regulatory sequence of the cathepsin G locus.22 These mice develop a fatal myeloid leukemia characterized by marked peripheral leukocytosis with infiltration of the spleen and bone marrow by myeloblasts with 90% to 100% penetrance and a latency of 150 to 400 days.22 We previously reported that leukemic blasts can be identified by the abnormal coexpression of the myeloid surface antigen Gr-1 and the early hematopoietic marker CD34 (Figure 1A).22,27,28 These Gr-1+CD34+ APL cells partially retain the ability to terminally differentiate toward mature granulocytes and can be adoptively transferred to secondary recipients, which develop an identical, rapidly fatal leukemia within 2 to 3 weeks (dose and tumor dependent) after tumor inoculation.22,27-29 Finally, as shown in Figure 1A, Gr-1+CD34+ blast cells express cell-surface CXCR4, with increased expression observed on cells obtained from the peripheral blood compared with the spleen and bone marrow.
Murine APL model. (A) Immunophenotype of APL cells. Peripheral blood, splenocytes, and bone marrow cells from a healthy wild-type B6129F1 mouse and a leukemic murine cathepsin G-PML-RARα knock-in (mCGPR/+) B6129F1 mouse with APL were stained for the myeloid surface antigen Gr-1 and the early hematopoietic progenitor marker CD34. The level of CXCR4 expression on Gr-1+/CD34+ in mCGPR/+ APL cells is shown in the histogram along with the isotype control (black line). The indicated gates were used to determine the percentage of cells positive for Gr-1 and CD34. (B) Generation of luciferase-labeled acute myeloid leukemia (APLluc) cells. APL cells obtained from the spleen of a mCGPR/+ knock-in mouse were transduced with a bicistronic retroviral vector containing firefly luciferase upstream of EGFP (Fluc-IRES-egfp). Following transduction, EGFP+ APL cells were purified by fluorescence-activated cell sorting (FACS) and passaged in genetically compatible B6129FI recipients. These secondary recipients developed a rapidly fatal acute leukemia characterized by pronounced leukocytosis, anemia, thrombocytopenia, and massive hepatosplenomegaly with leukemic cell infiltration. APLluc cells obtained from the spleens of secondary recipients were frozen and banked 28 days after injection. (C) Kinetics of APLluc engraftment and expansion. Genetically compatible B6129F1 recipients were injected with 106 APLluc cells. Tumor trafficking and growth were assessed at various time intervals by bioluminescence imaging. Images of a representative mouse are shown. Photon flux is indicated in the color scale bar. WBC counts were determined by automated counting and the percentage of leukemic blasts in the blood, spleen, and bone marrow by flow cytometry.
Murine APL model. (A) Immunophenotype of APL cells. Peripheral blood, splenocytes, and bone marrow cells from a healthy wild-type B6129F1 mouse and a leukemic murine cathepsin G-PML-RARα knock-in (mCGPR/+) B6129F1 mouse with APL were stained for the myeloid surface antigen Gr-1 and the early hematopoietic progenitor marker CD34. The level of CXCR4 expression on Gr-1+/CD34+ in mCGPR/+ APL cells is shown in the histogram along with the isotype control (black line). The indicated gates were used to determine the percentage of cells positive for Gr-1 and CD34. (B) Generation of luciferase-labeled acute myeloid leukemia (APLluc) cells. APL cells obtained from the spleen of a mCGPR/+ knock-in mouse were transduced with a bicistronic retroviral vector containing firefly luciferase upstream of EGFP (Fluc-IRES-egfp). Following transduction, EGFP+ APL cells were purified by fluorescence-activated cell sorting (FACS) and passaged in genetically compatible B6129FI recipients. These secondary recipients developed a rapidly fatal acute leukemia characterized by pronounced leukocytosis, anemia, thrombocytopenia, and massive hepatosplenomegaly with leukemic cell infiltration. APLluc cells obtained from the spleens of secondary recipients were frozen and banked 28 days after injection. (C) Kinetics of APLluc engraftment and expansion. Genetically compatible B6129F1 recipients were injected with 106 APLluc cells. Tumor trafficking and growth were assessed at various time intervals by bioluminescence imaging. Images of a representative mouse are shown. Photon flux is indicated in the color scale bar. WBC counts were determined by automated counting and the percentage of leukemic blasts in the blood, spleen, and bone marrow by flow cytometry.
To efficiently track mCGPR/+ APL cells, primary tumors were transduced with a bicistronic retroviral vector containing firefly luciferase, a bioluminescence imaging (BLI) optical reporter gene, upstream of EGFP (Fluc/IRES/EGFP). Large numbers of Fluc/EGFP+ mCGPR/+ APL cells (APLluc) were purified by high-speed cell sorting EGFP+ cells and passaged in genetically compatible secondary recipients (Figure 1B). Upon intravenous injection of 106 APLluc cells, the APLluc cells rapidly migrate to the BM with increased BLI signal in the femurs, spine, sternum, and skull as well as the spleen at 4 days after injection (Figure 1C and data not shown). Over the next 2 to 3 days, APLluc cells expand in the spleen followed rapidly by widespread dissemination and death due to leukemia by 14 to 16 days (Figure 1C). In this model, leukemia cells reside in the BM microenvironment in a manner that is similar to what is observed in human AML.10-12
Stromal cells protect mCGPR/+ APL cells from apoptosis in vitro
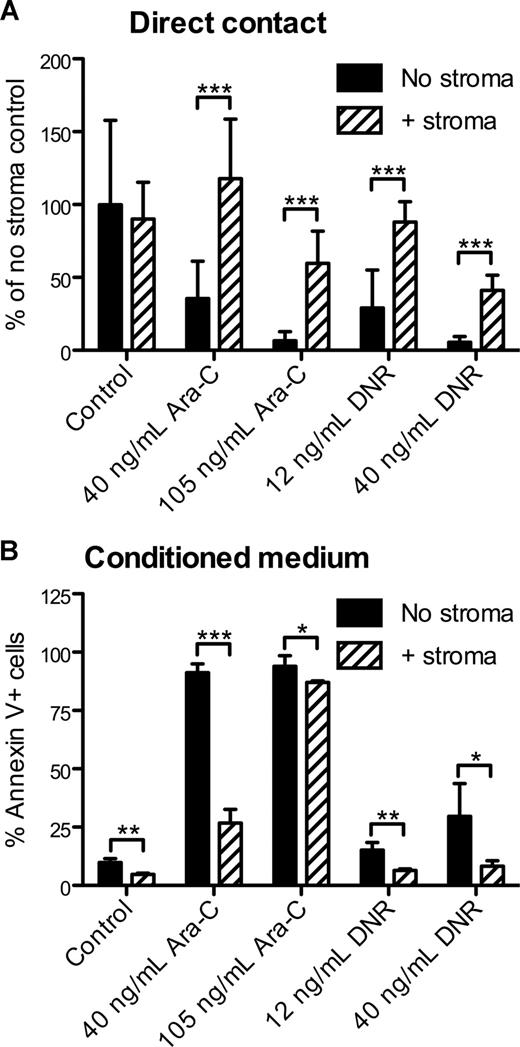
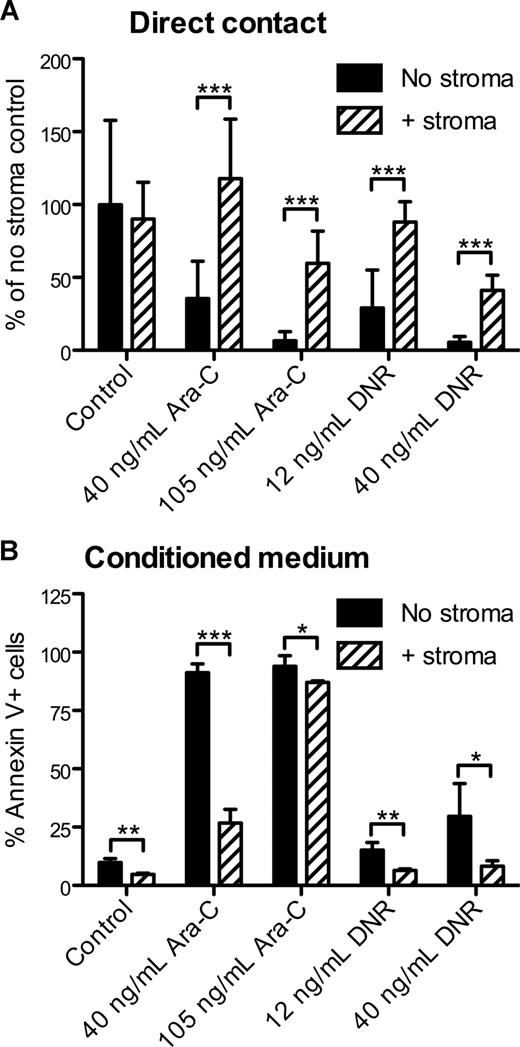
To model the effects of the stromal microenvironment on AML in vitro, APLluc cells were cultured in 96-well plates in the presence or absence of the M2-10B4 stromal cell line. After 48 hours, cells were treated with cytarabine (Ara-C) or daunorubicin (DNR) and cultured for an additional 48 hours. By BLI measurement, we observed no significant change in the survival of APLluc cells cocultured with stroma in the absence of chemotherapeutic agents (Figure 2A). Upon the addition of 40 ng/mL or 105 ng/mL Ara-C, the BLI signal intensity of APLluc cells cultured alone decreased to 36% and 7%, respectively, of control, whereas cells that were cocultured with stroma showed no change in BLI signal intensity at 40 ng/mL Ara-C and a decrease to only 60% of control in the presence of 105 ng/mL Ara-C. We observed similar trends in APLluc survival in the presence of 12 ng/mL or 40 ng/mL DNR (Figure 2A). These data suggest that M2-10B4 stromal cells protect mCGPR/+ APL cells against chemotherapy-induced apoptosis.
In vitro sensitivity of APL cells to chemotherapy. (A) Decreased apoptosis of APLluc cells following culture with stromal cells. APLluc cells (103 cells/well) were cultured in 96-well black-walled tissue culture plates in the presence or absence of the murine bone marrow stromal cell line M2-10B4. After 48 hours, APLluc cells were incubated for an additional 48 hours in medium containing 40 ng/mL cytarabine (Ara-C), 105 ng/mL Ara-C, 12 ng/mL daunorubicin (DNR), 40 ng/mL DNR, or vehicle alone (control). APLluc cell survival was assessed by bioluminescent imaging and results are presented as a percent of the no stroma control. Each bar represents the mean plus or minus SD of 2 separate experiments, for which each sample was assayed in quadruplicate. (B) Decreased apoptosis of APL cells following culture in M2-10B4–conditioned medium. Cell-free culture supernatant was obtained from 3-day cultures of M2-10B4 stromal cells. APL cells (103 cells/well) were incubated for 48 hours in unconditioned medium (no stroma) or M2-10B4–conditioned medium (+stroma) containing 40 ng/mL Ara-C, 105 ng/mL Ara-C, 12 ng/mL DNR, 40 ng/mL DNR, or vehicle alone (control). APL cell death was assessed by flow cytometry using annexin V–PE and 7-amino-actinomycin D. Each bar represents the mean ± SD of 2 separate experiments, for which each sample was assayed in triplicate. *P < .05; **P < .01; and ***P < .001.
In vitro sensitivity of APL cells to chemotherapy. (A) Decreased apoptosis of APLluc cells following culture with stromal cells. APLluc cells (103 cells/well) were cultured in 96-well black-walled tissue culture plates in the presence or absence of the murine bone marrow stromal cell line M2-10B4. After 48 hours, APLluc cells were incubated for an additional 48 hours in medium containing 40 ng/mL cytarabine (Ara-C), 105 ng/mL Ara-C, 12 ng/mL daunorubicin (DNR), 40 ng/mL DNR, or vehicle alone (control). APLluc cell survival was assessed by bioluminescent imaging and results are presented as a percent of the no stroma control. Each bar represents the mean plus or minus SD of 2 separate experiments, for which each sample was assayed in quadruplicate. (B) Decreased apoptosis of APL cells following culture in M2-10B4–conditioned medium. Cell-free culture supernatant was obtained from 3-day cultures of M2-10B4 stromal cells. APL cells (103 cells/well) were incubated for 48 hours in unconditioned medium (no stroma) or M2-10B4–conditioned medium (+stroma) containing 40 ng/mL Ara-C, 105 ng/mL Ara-C, 12 ng/mL DNR, 40 ng/mL DNR, or vehicle alone (control). APL cell death was assessed by flow cytometry using annexin V–PE and 7-amino-actinomycin D. Each bar represents the mean ± SD of 2 separate experiments, for which each sample was assayed in triplicate. *P < .05; **P < .01; and ***P < .001.
We next evaluated whether the differences in apoptosis were contact dependent. APL cells were cultured for 48 hours with Ara-C or DNR in unconditioned medium (no stroma) or cell-free culture supernatant obtained from M2-10B4 cells. Using annexin V staining as a readout for cell survival, we observed that the survival benefit afforded by the stromal cells was largely maintained following separation of the APL cells from the M2-10B4 cells (Figure 2B). This observation suggests that the antiapoptotic stimulus provided by the M2-10B4 cells is partially mediated by soluble factors released from the stromal cells.
Circulating APLluc cells exhibit increased chemosensitivity
To study the effects of the hematopoietic microenvironment, we incubated unpurified or CD34-purified APLluc cells obtained from the blood, spleen, or BM with Ara-C. Interestingly, unpurified APLluc cells obtained from the peripheral blood were significantly more sensitive to chemotherapy than APLluc cells collected from the spleen (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, we found that APLluc cells purified from the BM and spleen exhibited the same ex vivo sensitivity to Ara-C as APLluc cells obtained from the peripheral blood (Figure S1B). This enhanced Ara-C chemosensitivity following APLluc purification suggests that a nonleukemic component(s) within the BM and spleen mediates chemoprotection. Therefore, we tested whether APL cell resistance to chemotherapy could be rescued by the addition of normal BM to purified APLluc cells in vitro. However, we were unable to rescue APL resistance to chemotherapy with the addition of normal BM to purified APLluc cells in vitro (data not shown). These in vitro studies further suggest that an interaction between leukemia cells and the hematopoietic microenvironment confers protection from chemotherapy.
Mobilization of APL blasts by AMD3100
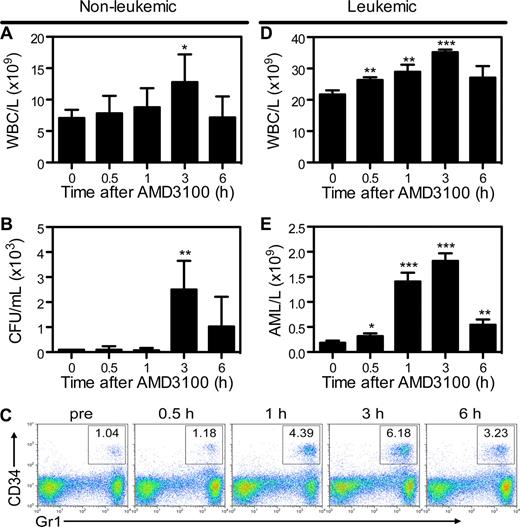
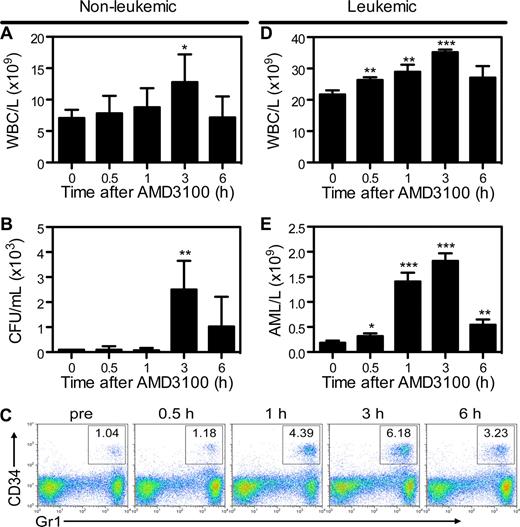
To determine whether mCGPR/+ APL cells can be mobilized into the peripheral circulation by AMD3100, B6129F1 recipients were injected with 106 mCGPR/+ APL cells or left untreated. A single dose of AMD3100 5 mg/kg was administered subcutaneously 12 or 25 days after tumor inoculation. Treatment of wild-type mice with AMD3100 results in rapid mobilization of white blood cells (WBCs) and hematopoietic progenitor cells (HPCs), with peak WBC and CFU levels achieved 3 hours after a single injection of AMD3100 (Figure 3A,B). Because mCGPR/+ APL cells aberrantly coexpress CD34 and Gr-1 on the cell surface,22,27,28 APL mobilization in leukemic mice can be tracked by flow cytometry (Figure 3C). Peak mobilization of Gr1+CD34+ mCGPR/+ APL cells also occurred 3 hours after the day-12 injection of AMD3100 and was characterized by a 1.6-fold increase in total WBC count (Figure 3D) and 9-fold increase in Gr1+CD34+ APL blast cells (Figure 3E) compared with baseline. Furthermore, similar to WBCs and HPCs in nonleukemic recipients, WBC and mCGPR/+ APL mobilization was transient, with cell counts returning to baseline levels within 12 hours (data not shown). However, we observed very different results if AMD3100 treatment of leukemic recipients was delayed until 25 days after APL inoculation. In this case, a total of 4 of 8 mice died of leukostasis as a result of the rapid and massive mobilization of blasts, with greater than 60% APL cells and 1 × 1011 WBC/L (100 000 WBC/μL) of blood 2 to 4 hours after the injection of AMD3100 (data not shown). Of note, since Gr-1+CD34+ APL cells partially retain the ability to terminally differentiate toward mature granulocytes, the majority of circulating baseline and mobilized cells in the leukemic mice shown in Figure 3C through E are mature granulocytes derived from the APL blast population.22,27,28
AMD3100 induces a rapid and transient mobilization of normal hematopoietic progenitor cells and APL blasts into the peripheral blood. Syngeneic B6129F1 recipient mice were left untreated (nonleukemic) or injected intravenously with APL cells (leukemic). Twelve days after APL injection, mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100. Peripheral blood samples were collected immediately before and 0.5, 1, 3, and 6 hours after AMD3100 administration. (A) Total white blood cell (WBC) counts per microliter of peripheral blood in nonleukemic mice were determined by automated counting. (B) Colony-forming units in the peripheral blood of nonleukemic mice. (C) Representative flow cytometry profiles showing mobilization of Gr1+CD34+ APL blast cells following treatment with AMD3100. (D) Total white blood cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting. (E) APL blast cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting and flow cytometry of Gr1+CD34+ APL blast cells. Each bar represents the mean ± SD of a single experiment, for which each sample was assayed in quadruplicate. Results are representative of 3 separate experiments. *P < .05; **P < .01; and ***P < .001.
AMD3100 induces a rapid and transient mobilization of normal hematopoietic progenitor cells and APL blasts into the peripheral blood. Syngeneic B6129F1 recipient mice were left untreated (nonleukemic) or injected intravenously with APL cells (leukemic). Twelve days after APL injection, mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100. Peripheral blood samples were collected immediately before and 0.5, 1, 3, and 6 hours after AMD3100 administration. (A) Total white blood cell (WBC) counts per microliter of peripheral blood in nonleukemic mice were determined by automated counting. (B) Colony-forming units in the peripheral blood of nonleukemic mice. (C) Representative flow cytometry profiles showing mobilization of Gr1+CD34+ APL blast cells following treatment with AMD3100. (D) Total white blood cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting. (E) APL blast cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting and flow cytometry of Gr1+CD34+ APL blast cells. Each bar represents the mean ± SD of a single experiment, for which each sample was assayed in quadruplicate. Results are representative of 3 separate experiments. *P < .05; **P < .01; and ***P < .001.
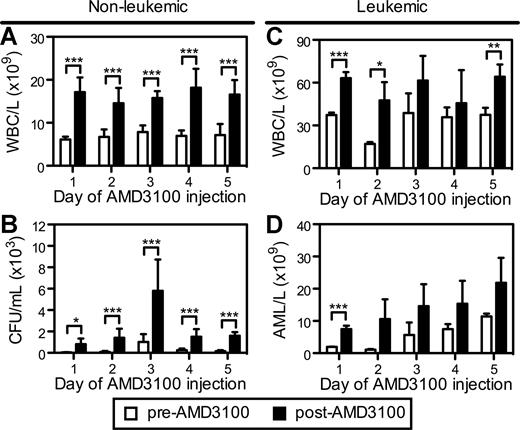
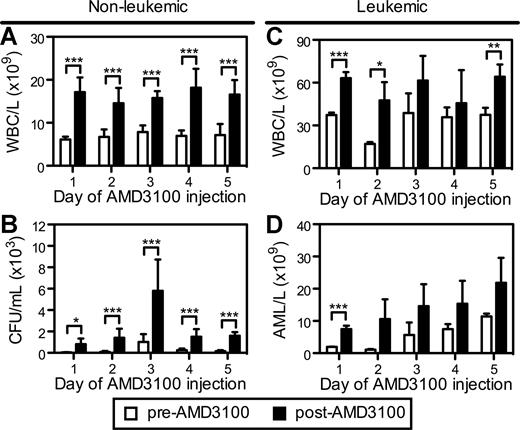
We next evaluated whether repetitive daily injections of AMD3100 could remobilize normal hematopoietic progenitors and APL cells into the circulation. In these studies, B6129F1 mice were injected with 106 mCGPR/+ APL cells and administered a single dose of AMD3100 for 5 consecutive days 12 days after mCGPR/+ APL injection. Repetitive subcutaneous injection of AMD3100 to healthy (nonleukemic) mice every 24 hours resulted in a similar 2.0- to 2.8-fold mobilization of total WBCs after each injection (Figure 4A). Although the total number of CFUs/mL of blood was increased on the third day of AMD3100 administration (Figure 4B), the fold change in CFUs after each dose of AMD3100 was similar. These data demonstrate that AMD3100 can be given daily, resulting in similar kinetics and magnitude of progenitor mobilization with no obvious tachyphylaxis.
Repetitive mobilization of normal hematopoietic stem cells and APL blasts into the peripheral blood. Syngeneic B6129F1 recipient mice were left untreated (nonleukemic) or injected intravenously with 106 APL cells (leukemic). Twelve days after APL injection, mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100 for 5 consecutive days. Peripheral blood samples were collected immediately before (pre-AMD3100) and 3 hours after (post-AMD3100) each daily dose of AMD3100. (A,C) Total white blood cell counts per microliter of peripheral blood in nonleukemic (A) and leukemic (C) mice. (C) Colony-forming units in the peripheral blood of nonleukemic mice. (D) Blast cell counts per microliter of peripheral blood in leukemic mice. APL blast cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting and flow cytometry of Gr1+CD34+ APL blast cells. Each bar represents the mean ± SD of a single experiment, for which each sample was assayed in triplicate. *P < .05; **P < .01; and ***P < .001.
Repetitive mobilization of normal hematopoietic stem cells and APL blasts into the peripheral blood. Syngeneic B6129F1 recipient mice were left untreated (nonleukemic) or injected intravenously with 106 APL cells (leukemic). Twelve days after APL injection, mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100 for 5 consecutive days. Peripheral blood samples were collected immediately before (pre-AMD3100) and 3 hours after (post-AMD3100) each daily dose of AMD3100. (A,C) Total white blood cell counts per microliter of peripheral blood in nonleukemic (A) and leukemic (C) mice. (C) Colony-forming units in the peripheral blood of nonleukemic mice. (D) Blast cell counts per microliter of peripheral blood in leukemic mice. APL blast cell counts per microliter of peripheral blood in leukemic mice were determined by automated counting and flow cytometry of Gr1+CD34+ APL blast cells. Each bar represents the mean ± SD of a single experiment, for which each sample was assayed in triplicate. *P < .05; **P < .01; and ***P < .001.
A similar, repetitive mobilization pattern of mCGPR/+ APL cells by AMD3100 was observed in leukemic mice (Figure 4C,D). As expected, the total WBC levels in the circulation were increased in the leukemic mice (Figure 4C). Furthermore, the baseline (pre-AMD3100) number of mCGPR/+ APL blast cells in the peripheral blood before each daily dose of AMD3100 increased from 2 × 103 to 11 × 103 per microliter of blood during the course of the 5 days of ADM3100 administration (Figure 4D). This increase in circulating blast cell numbers is due to disease progression in this model. Following AMD3100 administration, the number of mobilized mCGPR/+ APL cells increased from 1.3- to 10.8-fold, with the greatest fold change in circulating APL blast cell numbers occurring during the first 2 days of drug treatment.
AMD3100-induced APL mobilization occurs from hematopoietic tissues
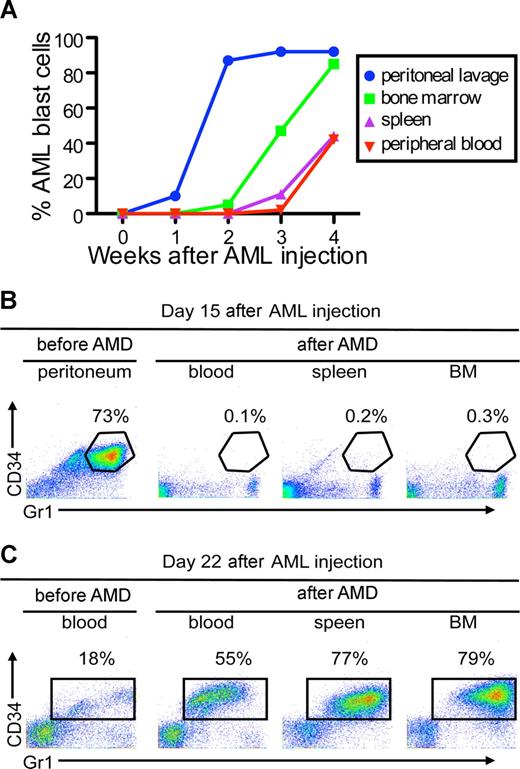
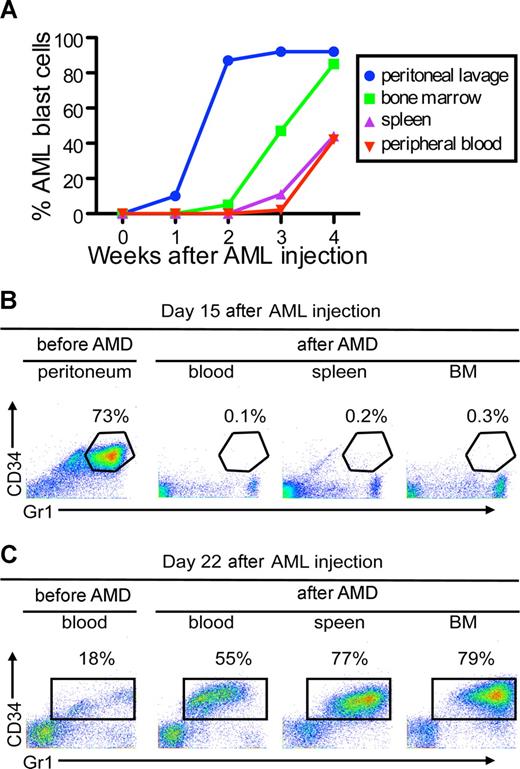
To examine whether CXCR4/SDF1 specifically regulates leukemia cell interaction with a hematopoietic microenvironment, we developed a protocol to allow mCGPR/+ APL cell expansion in hematopoietic and nonhematopoietic compartments. After intraperitoneal injection of 106 APL cells into syngeneic B6129F1 recipients, mCGPR/+ APL cells expanded into the peritoneal cavity in the first 2 weeks, followed by engraftment in the BM and spleen during the third and fourth week, respectively (Figure 5A). A single subcutaneous injection of AMD3100 (5 mg/kg) was capable of mobilizing mCGPR/+ APL cells to the blood by the fourth week after injection, but not from the peritoneal cavity during the second week after injection (Figure 5B,C). During the fourth week after mCGPR/+ APL injection, when the APL cells had engrafted and expanded into the BM, we observed a significant increase in blood mCGPR/+ APL cells from 18% to 55% one hour after AMD3100 injection (Figure 5C). In contrast, during the period of exclusive intraperitoneal expansion of the leukemic cells (second week), we were unable to detect Gr1+CD34+ mCGPR/+ APL cells in blood after AMD3100 administration (Figure 5B).
AMD3100 does not mobilize APL cells from the peritoneal cavity. (A) Kinetics of APL progression following intraperitoneal administration of APL cells. Syngeneic B6129F1 recipient mice (n = 10) were injected intraperitoneally with 106 APL cells and the percentage of Gr-1+CD34+ APL cells in the peritoneum, peripheral blood, spleen, and BM was determined weekly by flow cytometry. APL cells expanded into the peritoneal cavity during the first 2 weeks followed by engraftment in the BM and spleen during the third and fourth weeks. (B,C) Fifteen (B) or 22 (C) days after APL injection, leukemic mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100 and killed 3 hours later. Baseline peritoneum and peripheral blood samples were collected immediately before (pre-AMD) administration of AMD3100. AMD3100-mobilized APL cells into the peripheral blood (B) during the fourth week, but not from the peritoneal cavity (A) during the second week.
AMD3100 does not mobilize APL cells from the peritoneal cavity. (A) Kinetics of APL progression following intraperitoneal administration of APL cells. Syngeneic B6129F1 recipient mice (n = 10) were injected intraperitoneally with 106 APL cells and the percentage of Gr-1+CD34+ APL cells in the peritoneum, peripheral blood, spleen, and BM was determined weekly by flow cytometry. APL cells expanded into the peritoneal cavity during the first 2 weeks followed by engraftment in the BM and spleen during the third and fourth weeks. (B,C) Fifteen (B) or 22 (C) days after APL injection, leukemic mice were treated with a single subcutaneous dose of 5 mg/kg AMD3100 and killed 3 hours later. Baseline peritoneum and peripheral blood samples were collected immediately before (pre-AMD) administration of AMD3100. AMD3100-mobilized APL cells into the peripheral blood (B) during the fourth week, but not from the peritoneal cavity (A) during the second week.
AMD3100 sensitizes APL to chemotherapy in vivo
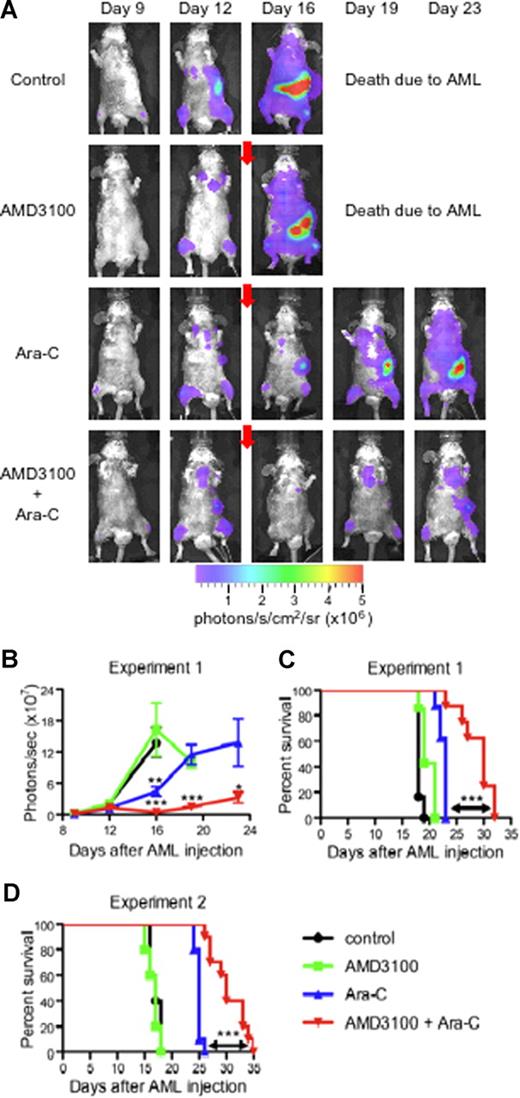
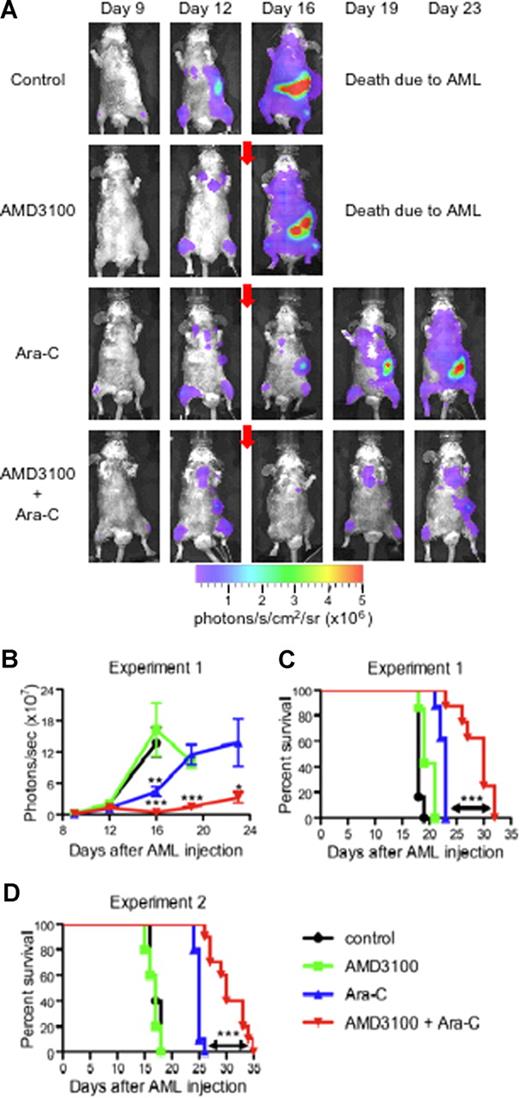
In our final set of experiments, we studied the effect of AMD3100 on the mobilization of APLluc cells into the peripheral blood and their sensitivity to chemotherapeutic agents in vivo. For these studies, we injected a total of 29 healthy B6129F1 mice with 106 APLluc cells and evaluated APLluc engraftment and trafficking by flow cytometry and BLI. At 12 days after APLluc injection, all mice had comparable numbers of circulating white blood cells (WBCs; Figure S2A) and APLluc blast cells (Figure S2B), with similar BLI signal patterns in their femurs, spine, sternum, skull, and spleen (Figure 6A,B). Immediately following the day-12 BLI evaluations, cohorts of leukemic mice were treated with AMD3100, Ara-C, or both. Eight mice received subcutaneous injections of Ara-C only (500 mg/kg) on days 12 and 13, and another group of 8 mice received subcutaneous injections of Ara-C and AMD3100 (5 mg/kg) 1 hour before and 3 hours after each Ara-C injection. Six mice received only AMD3100 and an additional 6 mice were left as untreated controls. As shown in Figure 6, treatment with Ara-C alone had a significant cytoreductive effect, as evidenced by the decreased day-16 whole-body BLI signal (Figure 6A,B; P = .004 between Ara-C alone and untreated control) and prolonged survival compared with the untreated control (Figure 6C; P < .001 between untreated vs Ara-C alone, Figure 6D; P < .001 between untreated vs Ara-C alone). More importantly, we found that AMD3100 treatment of the leukemic recipients significantly enhanced the efficacy of the Ara-C chemotherapy. The number of white blood cells (Figure S2A) and APLluc blast cells (Figure S2B) per microliter of blood as well as total body BLI signal (Figure 6A,B) at days 19 and 23 after APLluc injection were all significantly decreased in the group that received Ara-C and AMD3100 compared with the Ara-C–only control. Importantly, the decrease in leukemic burden translated into a significant survival advantage (Figure 6C). The median survival times for the untreated control, AMD3100 alone, Ara-C alone, and Ara-C + AMD3100 cohorts were 18, 19, 23, and 30 days, respectively (P < .001 between Ara-C vs Ara-C + AMD3100 cohorts; Figure 6C). In addition, it is important to note that administration of AMD3100 with chemotherapy did not increase general hematologic toxicity, as hemoglobin (Figure S2C) and platelet counts (Figure S2D) after chemotherapy were similar between the Ara-C–only and Ara-C + AMD3100 groups. Similar decreases in leukemic burden with improved overall survival were observed with a different nontransduced mCGPR/+ APL tumor (Figure 6D) and with doxorubicin in AMD3100-treated recipients (data not shown).
AMD3100 sensitization of APL to Ara-C. (A-C) Syngeneic B6129F1 recipient mice (n = 29) were intravenously injected with 106 APLluc cells. Twelve days after APL injection, mice were left untreated (control; n = 6) or treated with AMD3100 alone (n = 7), Ara-C alone (n = 8), or the combination of AMD3100 and Ara-C (n = 8). Mice treated with chemotherapy received a single subcutaneous injection of Ara-C (500 mg/kg) on days 12 and 13 after APL injection. Mice treated with AMD3100 received subcutaneous injections of AMD3100 (5 mg/kg) 1 hour before and 3 hours after each Ara-C injection. (A) In vivo bioluminescent imaging of APLluc cells. One representative animal for each group is shown over time. Photon flux is indicated in the color scale bar. Red arrow indicates initiation of treatment with AMD3100 and/or Ara-C. (B) Expansion of APLluc cells was quantified in emitted photons over total body area (ventral view). BLI signal intensity at days 15, 19, and 23 after APL injection was significantly reduced in mice receiving the combination of AMD3100 and Ara-C compared with mice receiving Ara-C alone. Each bar represents the mean ± SD of a single experiment with the number of mice in each group exactly as described earlier in the legend. (C) Kaplan-Meier plot of overall survival of mice. Overall survival of leukemic mice is significantly prolonged when mice are treated with the combination of AMD3100 and Ara-C (P < .001 between Ara-C versus Ara-C + AMD3100 cohorts). (D) Syngeneic B6129F1 recipient mice (n = 30) were intravenously injected with 106 nontransduced APL cells. Twelve days after APL injection, mice were left untreated (control; n = 5) or treated with AMD3100 alone (n = 5), Ara-C alone (n = 10), or the combination of AMD3100 and Ara-C (n = 10) exactly as described earlier in the legend. Overall survival of leukemic mice is significantly prolonged when mice are treated with the combination of AMD3100 and Ara-C (P < .001 between Ara-C vs Ara-C + AMD3100 cohorts). *P < .05; **P < .01; and ***P < .001.
AMD3100 sensitization of APL to Ara-C. (A-C) Syngeneic B6129F1 recipient mice (n = 29) were intravenously injected with 106 APLluc cells. Twelve days after APL injection, mice were left untreated (control; n = 6) or treated with AMD3100 alone (n = 7), Ara-C alone (n = 8), or the combination of AMD3100 and Ara-C (n = 8). Mice treated with chemotherapy received a single subcutaneous injection of Ara-C (500 mg/kg) on days 12 and 13 after APL injection. Mice treated with AMD3100 received subcutaneous injections of AMD3100 (5 mg/kg) 1 hour before and 3 hours after each Ara-C injection. (A) In vivo bioluminescent imaging of APLluc cells. One representative animal for each group is shown over time. Photon flux is indicated in the color scale bar. Red arrow indicates initiation of treatment with AMD3100 and/or Ara-C. (B) Expansion of APLluc cells was quantified in emitted photons over total body area (ventral view). BLI signal intensity at days 15, 19, and 23 after APL injection was significantly reduced in mice receiving the combination of AMD3100 and Ara-C compared with mice receiving Ara-C alone. Each bar represents the mean ± SD of a single experiment with the number of mice in each group exactly as described earlier in the legend. (C) Kaplan-Meier plot of overall survival of mice. Overall survival of leukemic mice is significantly prolonged when mice are treated with the combination of AMD3100 and Ara-C (P < .001 between Ara-C versus Ara-C + AMD3100 cohorts). (D) Syngeneic B6129F1 recipient mice (n = 30) were intravenously injected with 106 nontransduced APL cells. Twelve days after APL injection, mice were left untreated (control; n = 5) or treated with AMD3100 alone (n = 5), Ara-C alone (n = 10), or the combination of AMD3100 and Ara-C (n = 10) exactly as described earlier in the legend. Overall survival of leukemic mice is significantly prolonged when mice are treated with the combination of AMD3100 and Ara-C (P < .001 between Ara-C vs Ara-C + AMD3100 cohorts). *P < .05; **P < .01; and ***P < .001.
Discussion
The tumor microenvironment is increasingly being recognized as a critical factor in mediating drug resistance. Resistance may occur both through the soluble release of growth factors and by contact-dependent mechanisms via cell-cell and cell–extracellular matrix interactions. The term, cell adhesion–mediated drug resistance (CAM-DR) has been used to describe the phenotype in which interaction of malignant cells with stroma confers resistance to chemotherapy. CAM-DR has been described in vitro for both primary tumors and cell lines derived from several hematologic malignancies when cultured in fibronectin-coated plates or with stromal cell lines.10,30-32 Engagement of fibronectin and vascular cell adhesion molecule 1 (VCAM1) to α4β1 and α5β1 integrins and of CD44 to hyaluronin have both been implicated as important mediators of CAM-DR.33,34 Although the mechanisms by which CAM-DR mediates drug resistance are largely unknown, several different signaling pathways have been implicated including p27kip, NF-κB, and PI3K/Akt.14,35,36
In this paper, we describe a murine APL model that exhibits the characteristics of microenvironment-mediated drug resistance in vitro through both contact-dependent and -independent mechanisms. Furthermore, we show that these interactions can be blocked in vivo by AMD3100, a small molecule inhibitor of CXCR4 that mobilizes leukemic blasts from hematopoietic niches into the peripheral blood. Finally, disruption of the CXCR4/SDF-1 axis by AMD3100 improves overall survival of APLluc mice when treated with cytotoxic chemotherapy. Similar results have been described in which the combination of blocking antibodies against VLA-4 and cytarabine in immunodeficient mice that received a transplant of either U937 cell lines or primary AML tumors increased survival compared with cytarabine alone.14 High expression of both CXCR4 and VLA-4 has recently been found to be associated with an unfavorable prognosis in AML associated with a higher risk of disease relapse.14,37,38 These data suggest a critical and pivotal role of the CXCR4/SDF-1 axis in AML survival and the persistence of minimal residual disease through interaction with the marrow microenvironment.12
The concept of “priming” leukemic cells to increase in vivo sensitivity to chemotherapy has also been extensively studied in AML. Preclinical studies have suggested that priming with GM-CSF and G-CSF prior to chemotherapy induces the proliferation of AML cells, thus rendering them more sensitive to cell cycle–specific agents such as cytarabine.39-41 Alternatively, G-CSF–induced down-regulation of SDF-1 in BM stroma and osteoblasts may also disrupt the AML-stromal interaction, rendering these cells more susceptible to chemotherapy.5,42 The largest randomized trial of G-CSF priming for the treatment of newly diagnosed AML was performed by the Dutch-Belgian Hemato-Oncology Cooperative Group and the Swiss Group for Clinical Cancer Research.43 Although patients with AML who were randomized to receive induction chemotherapy plus G-CSF had superior disease-free and overall survivals compared with those patients randomized to standard induction chemotherapy without G-CSF, the rates of achieving a complete response (CR) after induction were the same in both groups. Although the reasons for these results remain unclear, one explanation is that the addition of G-CSF to the induction chemotherapy may have a significant impact on improving the quality and duration of remission in those patients who achieve CR after induction chemotherapy by limiting or reducing the amount of residual AML prior to consolidation chemotherapy. In this study, G-CSF was given on the same day that the induction chemotherapy was given. Based on what is known relating to the effect of G-CSF on the kinetics of BM stroma SDF-1 expression and on normal stem cell mobilization, delaying induction chemotherapy for 3 to 5 days after the initiation of G-CSF priming may actually provide for even greater clinical efficacy. However, this approach may be clinically undesirable because G-CSF is a potent growth and survival factor for AML cells.39-41 In contrast, AMD3100 rapidly and effectively mobilizes AML cells without inducing their proliferation. Although these features make AMD3100 more attractive as a potential chemosensitization agent, only appropriately designed clinical trials in AML patients using AMD3100 and G-CSF will answer this question.
Several cytokines and adhesion molecules have been shown to play similar roles in protecting other malignant cell types such as myeloma and lymphoma against various genotoxic stresses. These include IL-6, IL-7, VEGF, CXCR4, RANTES, MIP-1, JAGGED, and IGF-1.44-49 Recent studies suggest stroma-lymphoma cross talk via stromally produced sonic hedgehog (Shh) results in antiapoptotic signaling and is mediated by direct contact.50 Hedgehog proteins activate transmembrane receptor Patched (PTC) on lymphoma cells resulting in PTC-mediated suppression of SMO, a 7-transmembrane protein, resulting in activation of multiple downstream signaling pathways, and culminating in increased survival and diminished sensitivity to chemotherapy and other genotoxic stresses. Pharmacologic inhibition of this pathway using a synthetic broad-based inhibitor of Shh, cyclopromine, results in increased spontaneous apoptosis and increased sensitivity of lymphoma cells (in the presence of stromal elements) to chemotherapy and an increased accumulation of lymphoma cells in G1. Accumulation of malignant cells in G1 via either direct contact to stroma or via soluble factors (Shh) released in close proximity to stromal elements may, in a similar fashion to SDF-1 interacting to its receptor, CXCR4, also contribute to the relative resistance of lymphoma cells and murine APL cells in this study to chemotherapy. Thus, attempts to target stroma-malignant cell interactions using multiple and cell-specific targets may enhance the clinical efficacy of chemotherapy.
These studies presented in this report provide a compelling preclinical rationale for priming patients with AMD3100 and/or G-CSF prior to administering cytotoxic chemotherapy in AML. In fact, if the mechanism for AMD3100-induced chemosensitization that we have observed in our in vivo murine APL model is simply due to physical interruption of the APL-BM stroma interaction, then the addition of additional mobilizing agents that are known to synergize with AMD3100, such as G-CSF, may further enhance AML killing in response to chemotherapy. We have recently initiated a clinical study of AMD3100 in combination with cytotoxic chemotherapy in relapsed or refractory AML to test the safety and feasibility of this approach. We believe that this approach may have broad implications for the treatment of other CXCR4+ hematologic malignancies including acute lymphoblastic leukemia, multiple myeloma, and chronic lymphocytic leukemia.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in abstract form at the 48th Annual Meeting of the American Society of Hematology, Orlando, FL, December 10, 2006.51
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants RO1 CA83845 (J.F.D.), R21 CA110489 (J.F.D.), P01 CA0101937 (T.J.L.), and P50 CA94056 (D.P.-W.). M.P.R. is an American Society of Hematology Fellow Scholar in Basic Science Research. G.L.U. is an American Society of Hematology Fellow Scholar in Clinical/Translational Research.
National Institutes of Health
Authorship
Contribution: B.N., P.R., M.P.R., and J.F.D. designed and performed research, analyzed data, and wrote the paper; M.S.H., J.K.R., and J.L.P. performed research and analyzed data; G.L.U. analyzed data and wrote the paper; and D.P.-W., T.J.L., and G.B. edited the paper and provided vital reagents.
Conflict-of-interest disclosure: J.F.D. received honoraria from Genzyme Corp. G.B. is an employee of Genzyme. The remaining authors declare no competing financial interests.
Correspondence: John F. Dipersio, Siteman Cancer Center, Washington University School of Medicine, 660 South Euclid, Box 8007, St Louis, MO 63110: e-mail: jdipersi@im.wustl.edu.
References
Author notes
*B.N. and P.R. contributed equally to this study.