Abstract
Highly differentiated CD8+CD28−CD27− T cells have short telomeres, defective telomerase activity, and reduced capacity for proliferation, indicating that they are close to replicative senescence. In addition, these cells express increased levels of the senescence-associated inhibitory receptor KLRG1 and have poor capacity for IL-2 synthesis and defective Akt (ser473) phosphorylation after activation. It is not known whether signaling via KLRG1 contributes to any of the attenuated differentiation-related functional changes in CD8+ T cells. To address this, we blocked KLRG1 signaling during T-cell receptor activation using antibodies against its major ligand, E-cadherin. This resulted in a significant enhancement of Akt (ser473) phosphorylation and T-cell receptor–induced proliferative activity of CD8+CD28−CD27− T cells. Furthermore, the increase of proliferation was directly linked to the Akt-mediated induction of cyclin D and E and reduction in the cyclin inhibitor p27 expression. In contrast, the reduced telomerase activity in highly differentiated CD8+CD28−CD27− T cells was not altered by KLRG1 blockade, indicating the involvement of other mechanisms. This is the first demonstration of a functional role for KLRG1 in primary human CD8+ T cells and highlights that certain functional defects that arise during progressive T-cell differentiation toward replicative senescence are maintained actively by inhibitory receptor signaling.
Introduction
The immune system undergoes dramatic restructuring with age. There is a marked decline in the number of naive T cells produced by the thymus that results from thymic atrophy.1,2 The reduced thymic T-cell production necessitates that the memory T-cell pool is maintained by continuous proliferation.3-5 The lifelong rechallenge, especially with persistent antigen, leads to the accumulation of highly differentiated T cells6-8 that are frequently expanded oligoclonally,5,6,8,9 and this is associated with a decline in immune responsiveness.10,11 These changes are collectively referred to as immune senescence, which is associated with an increase in the frequency and severity of infections12,13 and a higher incidence of malignancy in older adults.14
Human CD8+ T cells progressively lose expression of the costimulatory molecules CD28 and CD27 as they differentiate in vivo15 Relatively undifferentiated CD8+ T cells express a CD28+CD27+ phenotype, and after repeated activation, these cells differentiate via an intermediate CD28−CD27+ stage to a highly differentiated CD28−CD27− population.6,15,16 The CD28−CD27−CD8+T cells have the shortest telomeres, defective IL-2 production and proliferative activity, and lose the ability to phosphorylate the key signaling kinase Akt at the ser473 site.15 It was originally considered that the loss of CD28 was a major factor in the reduced activation and function of these highly differentiated cells17 ; however, we and others have demonstrated that alternative costimulatory receptors may be engaged to promote T-cell activation in some CD8+CD28− T-cell populations.15,18
Highly differentiated CD28−CD27− T cells in both the CD4+6,8,19,20 and CD8+6,7,15,16 T-cell compartments increase during aging.2,10,21 Because these cells have functional defects, this may explain the decreased efficiency of the immune system in older individuals.8 However, it is not clear how the decreased functionality is controlled in these highly differentiated T-cell populations. Because cellular senescence in fibroblasts has been shown to be reversible and maintained by active signaling,22 we hypothesized that the decreased functionality of highly differentiated primary human T cells may also be directly regulated by inhibitory signals.
The killer cell lectin–like receptor G1 (KLRG1) has been shown to identify senescent T cells in humans and rodents, as KLRG1+ T cells are unable to undergo clonal expansion after activation.23-25 KLRG1 contains an immunoreceptor tyrosine–based inhibitory motif (ITIM) in its cytoplasmic domain and on engagement with its ligands E-cadherin and N-cadherin26,27 mediates inhibitory effects through the recruitment of SHIP-1 and SHP-2.28 We, therefore, reason that signaling through KLRG1 may contribute in part to the dysfunctional profile of CD8+CD28−CD27− T cells. We demonstrate here that the defective Akt (ser473) phosphorylation and proliferation of highly differentiated CD8+CD28−CD27− T cells are actively regulated by KLRG1 signaling and that these defects can be reversed by blocking the interaction of this molecule with its ligand. Surprisingly, although our previous studies indicated a strong but indirect association between Akt (ser473) phosphorylation and telomerase activity in CD8+CD28−CD27− T cells, KLRG1 blockade did not reverse the telomerase defect in these cells after activation. This indicates that other Akt-independent mechanisms are involved in telomerase down-regulation in these cells. The manipulation of inhibitory signals mediated by KLRG1 and/or other inhibitory receptors on T cells29 may be potentially useful for increasing selective T-cell functions during immunotherapeutic regimes such as vaccination in older subjects.
Methods
Blood sample collection and isolation
Heparinized peripheral blood samples were taken from healthy volunteers. Where the data are stratified by age, young is defined as individuals between 20 and 35 years (median age 30) and old as over 65 years (median age 78). All samples were obtained in accordance with and with the approval of the ethical committee of Royal Free and University College Medical School, and voluntary informed consent was obtained in accordance with the Declaration of Helsinki. Old donors did not have any comorbidity, were not on any immunosuppressive drugs, and retained physical mobility and lifestyle independence. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll Hypaque (GE Healthcare, Little Chalfont, United Kingdom) and either analyzed immediately or cryopreserved as described previously.30
Flow cytometric analysis and cell sorting
Five-color flow cytometric analysis was performed using the following antibodies: PE-conjugated or Alexa Fluor 488–conjugated KLRG1 (13F12),31 PD-1 (kind gift from Dr. G. Freeman, Dana-Farber Cancer Institute, Boston, MA), CD8 PerCP (SK1), CD27 FITC (M-T271), CD27 APC-H7 (M-T271) CD28 APC (CD28.2), CD45RA PE-Cy7 (L48), CD14 FITC (MjP9), CD19 PE (HD37), CD56 FITC (MY31), and CD209 APC (DCN), all from BD Biosciences (San Jose, CA). E- and N-cadherin were purchased from R&D Systems (Minneapolis, MN), CD303 FITC (AC144) from Miltenyi Biotec (Auburn, CA). For intracellular staining, the following antibodies were used: IL2 FITC (MQ1-17H12), interferon γ (IFN-γ) APC (B27), and Ki67 FITC (B56), all from BD Biosciences. Cells were stained for surface expression and then fixed and permeabilized (Fix and Perm Cell Permeabilization Kit; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For intranuclear staining, the following antibodies were used: pser473 Akt (193H12; Cell Signaling, Danvers, MA); cyclin D2 (DCS-5) and p27 (F-8), both from Santa Cruz Biotechnology (Santa Cruz, CA); and cyclin E (HE12; BD Biosciences) with secondary goat anti–rabbit Alexa 488 or rabbit anti–mouse Alexa 488 (Invitrogen). Cells were fixed in 4% paraformaldehyde for 10 minutes at 37°C, then permeabilized with 90% methanol on ice for 30 minutes. All samples were run using an LSR and analyzed using FlowJo software (TreeStar, Ashland, OR).
CD8+ T cells were purified by negative selection using the VARIOMACS system (Miltenyi Biotec) according to the manufacturer's instructions. Negatively selected CD8+ T cells were stained with CD28 biotin (CD28.2; BD Biosciences), washed, and then incubated with anti-biotin microbeads according to the manufacturer's instructions. Positively selected cells were CD8+28+27+. The CD8+CD28− negative fraction was further separated into CD8+28−27+ and CD8+28−27– using CD27 microbeads (Miltenyi Biotec).
Proliferation assays
CD8+ T cells were stimulated with anti-CD3 (purified OKT3, 0.5 μg/mL) and irradiated antigen-presenting cells (APCs), in a 1:1 ratio, for 3 days, and proliferation was assessed by [3H]thymidine incorporation or by staining against the nuclear antigen Ki67 as described previously.32 Irradiated APCs are used as a source of multiple costimulatory signals to activated T-cell subsets sorted on the presence or absence of CD28.15 Proliferation was expressed either as the mean [3H]thymidine incorporation (counts per minute [cpm]) of triplicate wells (± SD), or a proliferation index was calculated. Proliferation index was determined by calculating the ratio of cpm in cells proliferating in response to anti-CD3 stimulation versus the cpm in cells proliferating in response to E-cadherin blockade.
Assessment of CD8 telomere length
Telomere length of CD8+ T cells was measured using a modified version of the flow-fluorescence in situ hybridization (FISH) method that was previously described.30 In brief, peripheral blood mononuclear cells (PBMCs) were washed in phosphate-buffered saline (PBS) and fixed in BS3 (final concentration 1 mM; Perbio Science, Chester, United Kingdom) for 30 minutes at 4°C. The reaction was quenched using 1 mL of 50 mM Tris (pH 7.2) in PBS and incubated in the dark for 20 minutes at room temperature. After washing in PBS followed by hybridization buffer (70% formamide, 20 mM Tris, 150 mM NaCl, and 1% BSA), cells were incubated in 0.75 mg/mL of the nucleic acid telomeric probe (CCCTAA)3 conjugated to Cy5 (Applied Biosystems, Foster City, CA). Samples were then heated for 10 minutes at 82°C and left to hybridize in the dark at room temperature for 1 hour. Samples were washed in posthybridization buffer (70% formamide, 10 mM Tris, 150 mM NaCl, 0.1% BSA, and 0.1% Tween 20) followed by PBS and analyzed immediately by flow cytometry. Samples were analyzed with and without probe to control for differences in background fluorescence between samples. To ensure consistency of the results between experiments, cryopreserved PBMC samples with known telomere fluorescence and telomere lengths as determined by Southern blot analysis were used as standards. Results were obtained as median fluorescence intensity values, which could then be converted to telomere length in kilobases using a standard curve. The standard curve was constructed using 30 samples of varying telomere length analyzed both by flow-FISH and telomeric restriction fragment analysis.30
Measurement of telomerase activity
Purified CD8+ T-cell populations (2 × 105 cells) were snap-frozen after stimulation for 3 days with anti-CD3 (purified OKT3, 0.5 μg/mL) and irradiated APCs. Telomerase activity was determined using the TeloTAGGG telomerase ELISA kit from Roche according to the protocol provided by the manufacturer. The absolute numbers of CD8+ T cells were enumerated using trypan blue (Sigma-Aldrich, St Louis, MO) or Tru-count tubes (BD Biosciences) and Ki67 analysis. ELISAs was performed with samples adjusted to 500 Ki67+ T cells per reaction as described previously.32
Western blot analysis
Purified CD8+ T-cell populations were activated in the presence of anti-CD3 (purified OKT3, 0.5 μg/mL) and irradiated APCs for 3 days, after which time cell lysates were obtained by sonicating cells in 50 mM Tris-HCl (pH 7.5), 2 mM EGTA, 0.1% Triton X-100 buffer. Lysates from 2 × 106 cells were fractionated on SDS–polyacrylamide electrophoresis gels and analyzed by immunoblotting with pAkt1/2/3 (ser473), cyclin D2 (DCS-5), cyclin E (C-19), p27 (F-8), and HPRT (FL-218), all from Santa Cruz Biotechnology using the ECL Advanced Western Blotting Detection Kit (GE Healthcare), according to the protocol provided by the manufacturer.
Phosphocytometry
CD8+ T cells were stimulated with anti-CD3 (purified OKT3, 0.5 μg/mL) and irradiated APCs for 3 days, after which the cells were starved of serum for 2 hours. CD8+ T cells were then restimulated, 1 μg/mL OKT3 was added, and the cells incubated on ice for 10 minutes followed by cross-linking with 20 μg/mL anti–mouse IgG (R&D Systems). Cells were transferred to 37°C, and stimulation was terminated after 10 minutes. The CD8+ T cells were fixed in 4% paraformaldehyde for 10 minutes at 37°C, then permeabilized with 90% methanol on ice for 30 minutes. Cells were sequentially incubated with pAkt(ser473) (193H12, Cell Signaling) and goat anti–rabbit Alexa 488 (Invitrogen) for 30 minutes at room temperature.
KLRG1 receptor blockade
Signaling through KLRG1 on purified total CD8+ and CD28/CD27 defined CD8+ subsets was blocked by adding 10 μg/mL E-cadherin antibody (67A4; Millipore Bioscience Research Reagents, Temecula, CA) or 10 μg/mL IgG1 isotype control (MOPC31c; Sigma-Aldrich) antibody at the start of culture during the 3-day stimulation period with of anti-CD3 (purified OKT3, 0.5 μg/mL) and irradiated APCs. E-cadherin is the main ligand for KLRG1 on human CD8+ T cells, and the addition of anti–E-cadherin antibody blocks the interaction of KLRG1 on the CD8+ T cells with E-cadherin on myeloid dendritic cells (DCs) in the irradiated APC (see below).
Inhibition of PI3K and Akt
Purified CD8+ CD28/27 T-cell populations were incubated with the PI3K inhibitor LY294002 at a final concentration 20 μM or the Akt inhibitor 1L-6-Hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate at a final concentration of 5 μM (both from Calbiochem, San Diego, CA). The concentrations of inhibitors chosen were optimized previously to result in minimal loss of cell viability.15 As before, CD8+ cells were activated for 3 days with anti-CD3 antibody (purified OKT3, 0.5 μg/mL) and irradiated APCs, with the inhibitors being added to the media at the start of the stimulation.
Statistical analysis
Statistical significance was evaluated using the Student t test. Differences were considered significant when P was less than .05.
Results
The accumulation of defective, highly differentiated CD8+CD28−CD27− T cells during aging
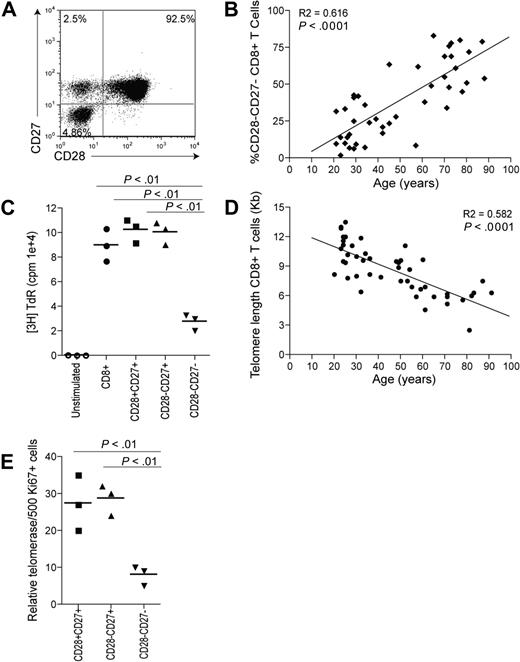
Extensive surface receptor changes occur on CD8+ T cells during progressive differentiation. Three main subsets of CD8+ T cells can be identified on the basis of CD28 and CD27 expression (Figure 1A): CD28+CD27+ (relatively undifferentiated), CD28−CD27+ (intermediate differentiation), and CD28–CD27− (highly differentiated), as described previously.6,15,16,19,33 Although human CD8+ T cells can be identified and isolated using combinations of different sets of surface markers such as CCR7 and CD45RA,33 the use of CD28/CD27 enabled the isolation of sufficient CD8+ cells from 3 discrete stages of differentiation for functional analysis. The percentage of highly differentiated CD28−CD27− T cells within the CD8+ T-cell pool increases significantly during aging (Figure 1B; P < .001). The CD28−CD27− T-cell population has reduced proliferative responses to stimulation with anti-CD3 and irradiated APC stimulation compared with the CD28+CD27+ T cells (P < .01) or CD28−CD27+ T cells (P < .01, Figure 1C), and this may be due in part to their decreased ability to synthesize IL-2.15 However, the CD28−CD27− T-cell population can synthesize high levels of IFNγ after stimulation and have high constitutive levels of perforin compared with the other subsets, indicating that they have undergone functional differentiation.15
Changes occurring to CD8+ T cells with age and differentiation. (A) Representative example of CD28/CD27 staining using purified PBMCs gated on CD8+ T cells. (B) Data showing the correlation between highly differentiated CD28−CD27− T cells, expressed as a percentage of CD8+ T cells, and age. (C) Graph showing the proliferation of CD28/CD27 subsets assessed by 3H thymidine incorporation on day 3 after stimulation with anti-CD3 and irradiated APCs. (D) Data showing the loss of telomere length with age within CD8+ T cells. (E) Graph showing telomerase activity in CD8+ CD28/CD27 subsets. Horizontal lines depict mean values. All P values were calculated using the Student t test.
Changes occurring to CD8+ T cells with age and differentiation. (A) Representative example of CD28/CD27 staining using purified PBMCs gated on CD8+ T cells. (B) Data showing the correlation between highly differentiated CD28−CD27− T cells, expressed as a percentage of CD8+ T cells, and age. (C) Graph showing the proliferation of CD28/CD27 subsets assessed by 3H thymidine incorporation on day 3 after stimulation with anti-CD3 and irradiated APCs. (D) Data showing the loss of telomere length with age within CD8+ T cells. (E) Graph showing telomerase activity in CD8+ CD28/CD27 subsets. Horizontal lines depict mean values. All P values were calculated using the Student t test.
We have previously shown that these highly differentiated T cells have shortest telomeres15 and the accumulation of these differentiated cells during aging (Figure 1B) explains the strong correlation between the loss of telomere length and age (Figure 1D; P < .001). The short telomeres in the CD8+CD28−CD27− T cells results from their significantly reduced ability to up-regulate telomerase after activation compared with the less differentiated subsets (Figure 1E; P < .01). The CD8+CD28−CD27− T cells that accumulate during aging therefore have characteristics of cells that are approaching end-stage differentiation or replicative senescence. However, it is not known how the functional defects in these cells, which include the inability to phosphorylate the key signaling kinase Akt, are regulated.15
The percentage of CD8+ T cells expressing of the senescence-associated receptor KLRG1 significantly increases on CD8+ T cells during aging (Figure 2A, P < .001, Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, in both young (< 35 years) and old (> 65 years) subjects, KLRG1 expression is greatest on the CD8+ CD28−CD27− T-cell subset (Figure 2B, Figure S1B), and there is also a significant positive correlation between KLRG1 expression and the percentage of CD28−CD27− T cells present in the total CD8+ pool (P < .001; data not shown). In contrast to KLRG1, the expression of another inhibitory receptor PD-1 that has been shown to be important in regulating CD8+ T-cell function34,35 does not increase significantly with age (Figure 2C). Although KLRG1 expression on NK cells and T cells has been shown to correlate with reduced cytotoxic activity in both mice and humans,25-27,36,37 a role for this molecule in the regulation of T-cell proliferation has only been demonstrated in the mouse.27 The ability of KLRG1 to regulate human T-cell proliferation is a crucial consideration for the regulation of replicative senescence in these cells.
Expression of inhibitory receptors on CD8+ T cells. (A) Data showing the correlation between KLRG1 expression on CD8+ T cells and age. (B) Graph showing the percentage of CD8+ T cells expressing KLRG1 in young and old donors on CD28/CD27 subsets. Horizontal lines depict mean values. (C) Graph showing the correlation between PD1 expression on CD8+ T cells and age. All P values were calculated using the Student t test.
Expression of inhibitory receptors on CD8+ T cells. (A) Data showing the correlation between KLRG1 expression on CD8+ T cells and age. (B) Graph showing the percentage of CD8+ T cells expressing KLRG1 in young and old donors on CD28/CD27 subsets. Horizontal lines depict mean values. (C) Graph showing the correlation between PD1 expression on CD8+ T cells and age. All P values were calculated using the Student t test.
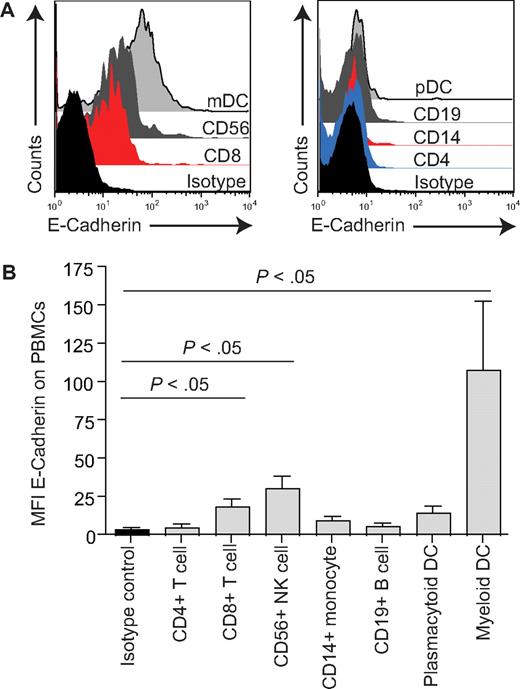
Although blocking antibodies to KLRG1 are available, their use in our system has the potential to cross-link KLRG1 and provide a negative signal. We therefore used antibodies to E- and N-cadherin, the main ligands for KLRG1, to block interactions with this molecule.25-27 Little is known about the expression of these cadherins on human PBMCs. In our experimental system, to stimulate CD8+ T-cell subsets that have lost CD28 expression, we use anti-CD3 antibodies and irradiated autologous PBMCs (APCs) as a source of alternative costimulatory signals as described in detail previously.15 We first examined if E- and N-cadherin were expressed by cells within the irradiated PBMC populations. We show by flow cytometry that whereas E-cadherin is expressed on multiple leukocyte subsets, the highest levels of E-cadherin are expressed by myeloid dendritic cells defined by the presence of DC-SIGN (Figure 3). N-cadherin was not expressed by leukocytes (data not shown). We therefore examined whether blocking E-cadherin on the irradiated APCs during anti-CD3 antibody stimulation revealed a role for KLRG1 signaling in our experimental system.
Expression of E-cadherin on PBMCs. (A) Representative examples of E-cadherin staining using purified PBMCs gated on the cells types indicated. (B) Graph showing MFI of E-cadherin on various cell types. Graph shows the mean ± SE for 3 donors. P values were determined using the Student t test.
Expression of E-cadherin on PBMCs. (A) Representative examples of E-cadherin staining using purified PBMCs gated on the cells types indicated. (B) Graph showing MFI of E-cadherin on various cell types. Graph shows the mean ± SE for 3 donors. P values were determined using the Student t test.
KLRG1 blockade reverses defective Akt (ser473) phosphorylation in highly differentiated CD8+ T cells
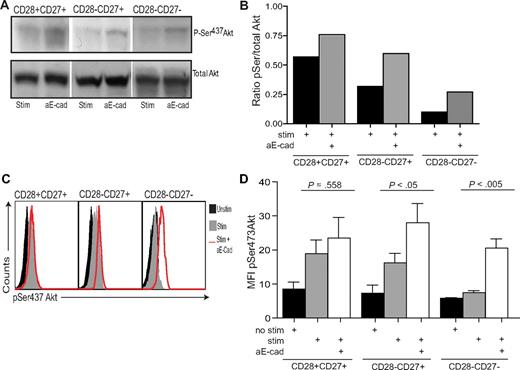
Akt activation is controlled by phosphorylation at 2 different sites, Thr308 and Ser473.38,39 We have recently reported that highly differentiated CD8+CD28−CD27− T cells are unable to phosphorylate Akt at the Ser473 phosphorylation site.15 We showed by Western blot analysis, that in the presence of E-cadherin–blocking antibody, there is an enhancement of Akt(ser473) phosphorylation in CD8+CD28−CD27− T cells after activation for 3 days with anti-CD3 and irradiated PBMC (Figure 4A,B). The addition of E-cadherin antibody in the absence of anti-CD3 stimulation did not have any effect on CD8+ T-cell responses (data not shown). Limitations in the availability of sufficient cells for the analysis of changes in cell signaling in primary human CD8+ T cells prompted us to develop the technology for the study of these processes by flow cytometry. We therefore investigated the change in Akt phosphorylation by flow cytometry. The staining intensity of Akt (ser473) was very similar to that observed in previous studies40 and confirmed the data obtained using Western blot analysis that highly differentiated CD8+CD28−CD27− T cells had a defect in Akt (ser473) phosphorylation compared with the less differentiated subsets (Figure 4C,D).15 Furthermore, we also showed that the detection of pAkt (ser473) by flow cytometry in these experiments was specific, as the staining was abrogated when the experiments were performed in the presence of a PI3K inhibitor (data not shown). The blockade of KLRG1 signaling significantly enhanced the phosphorylation of Akt (ser473) in the CD28−CD27+ cells and CD28−CD27− cells (P < .05 and P < .005, respectively; Figure 4D). Moreover, the phosphorylation defect in the CD28−CD27− cells after activation was completely reversed (Figure 4D).
KLRG1 blockade increases ser473 Akt phosphorylation. (A) Representative immunoblots of pser473 and total Akt for CD8+ CD28/CD27 subsets after E-cadherin blockade. (B) Densitometric analysis of one Western blot showing the ratio between pSer Akt/total Akt after E-cadherin blockade in CD28/CD27 subsets. (C) Representative histograms of pser473 Akt flow cytometry in the CD8+ CD27/28 populations with or without E-cadherin. (D) Pooled data showing the change in pser473 Akt phosphorylation levels in donors after E-cadherin blockade. Graphs show the mean ± SE for 4 donors.
KLRG1 blockade increases ser473 Akt phosphorylation. (A) Representative immunoblots of pser473 and total Akt for CD8+ CD28/CD27 subsets after E-cadherin blockade. (B) Densitometric analysis of one Western blot showing the ratio between pSer Akt/total Akt after E-cadherin blockade in CD28/CD27 subsets. (C) Representative histograms of pser473 Akt flow cytometry in the CD8+ CD27/28 populations with or without E-cadherin. (D) Pooled data showing the change in pser473 Akt phosphorylation levels in donors after E-cadherin blockade. Graphs show the mean ± SE for 4 donors.
KLRG1 blockade enhances CD8+ T-cell proliferation but not telomerase activity
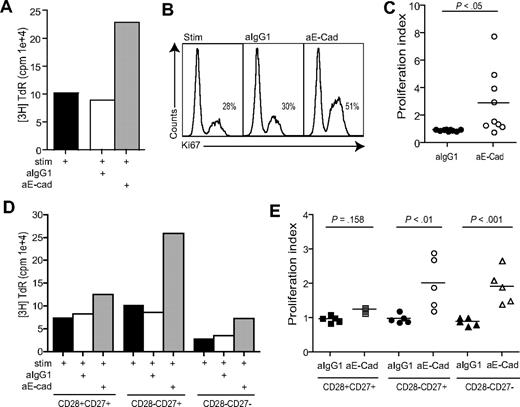
We determined whether other defective functions of highly differentiated CD8+CD28−CD27− T cells could be reversed by blocking KLRG1 signaling. The PI3K/Akt signaling pathway has been shown to play a key role in regulating cell proliferation.41 We found that blockade of the KLRG1 signaling pathway by addition of E-cadherin antibody increased the proliferative capacity of unfractionated CD8+ T cells measured by [3H] thymidine incorporation(Figure 5A) or expression of Ki67 a marker for cellular proliferation (Figure 5B) on CD8+KLRG1+ gated T cells. The cumulative data using [3H] thymidine incorporation as a readout indicate that the inhibition of the KLRG1 signaling pathway significantly enhances the proliferative capacity of unfractionated CD8+ T cells (Figure 5C; P < .05).
Blockade of KLRG1 causes an increased proliferation in CD8+ T cells. (A) Representative example of KLRG1 blockade measured after 3 days by 3H thymidine incorporation. (B) Representative example of KLRG1 blockade measured after 3 days by Ki67 staining. Plots shown are gated on CD8+ KLRG1+ T cells showing the percentage Ki67 staining within each group. (C) Pooled data showing proliferation of CD8+ T cells after E-cadherin antibody block. (D) Representative example of KLRG1 blockade in CD28/CD27 subsets measured after 3 days by 3H thymidine incorporation. (E) Pooled data showing the effect of E-cadherin antibody block on proliferative capacity in CD8+ CD28/CD27 T-cell subsets. Proliferation index was calculated by determining the ratio between cells proliferating in the presence of anti-CD3 stimulation versus E-cadherin blockade. All P values were calculated using the Student t test.
Blockade of KLRG1 causes an increased proliferation in CD8+ T cells. (A) Representative example of KLRG1 blockade measured after 3 days by 3H thymidine incorporation. (B) Representative example of KLRG1 blockade measured after 3 days by Ki67 staining. Plots shown are gated on CD8+ KLRG1+ T cells showing the percentage Ki67 staining within each group. (C) Pooled data showing proliferation of CD8+ T cells after E-cadherin antibody block. (D) Representative example of KLRG1 blockade in CD28/CD27 subsets measured after 3 days by 3H thymidine incorporation. (E) Pooled data showing the effect of E-cadherin antibody block on proliferative capacity in CD8+ CD28/CD27 T-cell subsets. Proliferation index was calculated by determining the ratio between cells proliferating in the presence of anti-CD3 stimulation versus E-cadherin blockade. All P values were calculated using the Student t test.
We next investigated the effect of E-cadherin block on CD28/CD27 defined CD8+ T-cell populations (Figure 5D,E). We found that blocking KLRG1 signaling significantly increases proliferative activity in the intermediate and highly differentiated CD28−CD27+ and CD28−CD27− populations (P < .01, P < .001, respectively). Furthermore, the proliferation rate observed after KLRG1 blocking in the CD28−CD27− population was similar to that in the stimulated (no blocking antibody added) CD28+CD27+ and CD28−CD27+ populations (Figure 5D).
We previously showed that defective Akt (ser473) phosphorylation and defective telomerase activity were both found in CD8+CD28−CD27− T cells and postulated from this indirect evidence that both phenomena may be related.15 However, we now show that the defective Akt in these cells can be reversed by KLRG1 blockade and that telomerase activity is not enhanced (Figure S1C). This suggests that although telomerase activity is dependent on the activation of Akt,15 the down-regulation of telomerase function in CD8+CD28−CD27− T cells is not related to Akt (ser473) phosphorylation. In addition, we investigated whether the blockade of KLRG1 signaling could enhance interleukin 2 (IL-2) production. We found that even though we could slightly increase IL-2 production in 4 of 5 experiments performed, this increase was not significant (Figure S1D).
KLRG1 blockade enhances CD8+ T-cell proliferation via the up-regulation of cyclin D2 and E and decrease of p27 expression
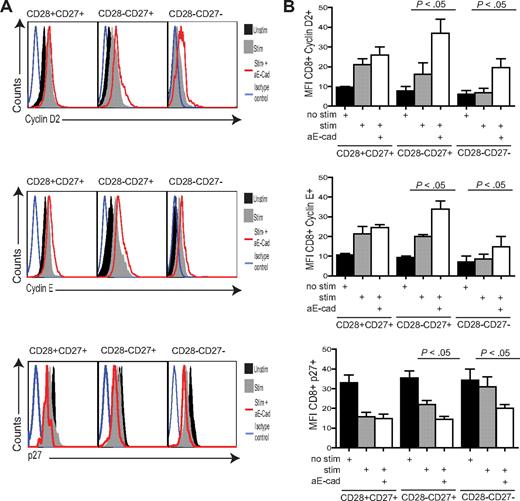
It was not clear whether the increase in CD8+ T-cell proliferation after KLRG1 blockade was linked to up-regulation of cell cyclins, down-regulation of cyclin inhibitors, or both. We therefore investigated the change in expression of cyclin D2, cyclin E, and p27 inhibitor protein by flow cytometry analysis (Figure 6). After activation, both cyclin D2 and cyclin E increase significantly in the CD28+CD27+ (P < .009) and CD28−CD27+ (P < .05) but not CD28−CD27− subset (Figure 6A,B). In contrast, although the expression of the cyclin inhibitor p27 is high in all 3 subsets before activation, it is only down-regulated significantly in the CD28+CD27+ and CD28+CD27− populations after activation (Figure 6A,B), and these results were also confirmed by Western blot analysis (not shown). When we added anti–E-cadherin antibody to block KLRG1 signaling during activation of the different subsets, there was an enhancement in cyclin D2 and cyclin E in intermediate and highly differentiated CD8+ T cells relative to the stimulus alone. There was a concomitant significant decrease in p27 expression by these cells during KLRG1 blockade relative to the stimulated cultures that were not blocked (Figure 6A,B). The expression of cyclins and p27 was not affected by KLRG1 blockade of the relatively undifferentiated CD28+CD27+ cells, which was probably due to their low expression of KLRG1 relative to the other subsets (Figure 2). These data indicate that the enhancement of proliferation after KLRG1 blockade in CD8+CD28−CD27− T cells is due to an effect on both cyclin up-regulation and cyclin inhibitor down-regulation. However, it is not clear if these effects are directly linked to changes in Akt activation after KLRG1 blockade.
KLRG1 blockade causes a change in the expression of cell-cycle molecules. (A) Representative histograms of cyclin D2, cyclin E, and p27 flow cytometry in the CD8+ CD27/28 populations with or without the E-cadherin–blocking antibody. (B) Graphs showing the change in cyclin D2, cyclin E, and p27 phosphorylation levels in donors after E-cadherin blockade. Graphs show the mean ± SE for 3 donors. All P values were calculated using the Student t test.
KLRG1 blockade causes a change in the expression of cell-cycle molecules. (A) Representative histograms of cyclin D2, cyclin E, and p27 flow cytometry in the CD8+ CD27/28 populations with or without the E-cadherin–blocking antibody. (B) Graphs showing the change in cyclin D2, cyclin E, and p27 phosphorylation levels in donors after E-cadherin blockade. Graphs show the mean ± SE for 3 donors. All P values were calculated using the Student t test.
The change in cell-cycle molecules after KLRG1 blockade is prevented by PI3K and Akt inhibitors
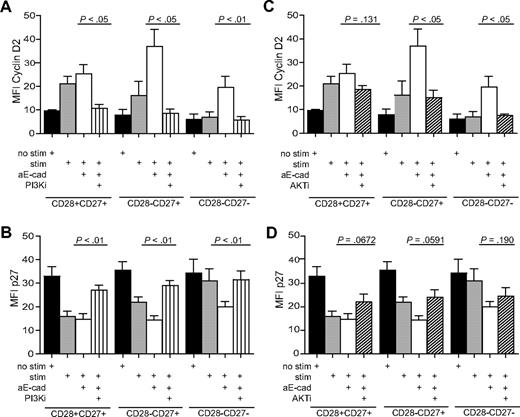
To clarify whether the KLRG1 changes of cyclin/cyclin inhibitor expression after KLRG1 blockade occurred via a direct effect on Akt activation, we added cell-permeable inhibitors specific for PI3K (LY294002, 20 μM) or Akt (1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate, 5 μM) to CD8+ subsets that were activated with anti-CD3 and irradiated PBMC. As shown previously, the blockade of KLRG1 signaling by the addition of E-cadherin antibody induced a significant increase in cyclin D2 and E (Figure 7A,C; results for only cyclin D2 are shown) and a down-regulation of p27 in CD28−CD27+ and CD28−CD27− T cells relative to stimulated cultures without E-cadherin (Figure 7B,D). The addition of the PI3K inhibitor together with the E-cadherin–blocking antibody reduced the expression of the cyclin D2 (Figure 7A) and cyclin E (not shown), and enhanced the expression of p27 (Figure 7B) to levels found in the unstimulated control cultures. This indicates that KLRG1 mediates its effects via the PI3K pathway.
The change in cell-cycle molecules after KLRG1 blockade is prevented by PI3K and Akt inhibitors. Graphs showing the change in expression of cyclin D2 (A,C) and p27 (B,D) after blockade with E-cadherin with or without PI3K inhibitor (A,B) or Akt inhibitor (C,D). All P values were calculated using the Student t test.
The change in cell-cycle molecules after KLRG1 blockade is prevented by PI3K and Akt inhibitors. Graphs showing the change in expression of cyclin D2 (A,C) and p27 (B,D) after blockade with E-cadherin with or without PI3K inhibitor (A,B) or Akt inhibitor (C,D). All P values were calculated using the Student t test.
When the Akt inhibitor was added to KLRG1-blocked CD8+ T-cell subsets, the expression of cyclin D2 (Figure 7C) and cyclin E (not shown) were reduced to the levels found in stimulated cultures without the addition of inhibitors. The addition of the Akt inhibitor therefore only blocked the increase in cyclin expression of stimulated T cells, which was induced by blocking KLRG1 signalling. In addition, the Akt inhibitor partially reversed the KLRG1 mediated down-regulation of p27 (Figure 7D). This indicates that KLRG1 signaling via a direct effect on Akt and induces changes in both cyclin and cyclin-inhibitor expression in CD8+ T cells. However, because cyclins were still induced above the unstimulated controls even in the presence of the Akt inhibitor, other PI3K signals that are independent of Akt are involved in the regulation of the cyclins D2 and E and the inhibitor p27 in human CD8+ T cells.
Discussion
The CD28 costimulatory pathway was initially considered to be essential for optimal activation of T cells.17,42 However, subsequent studies have suggested a greater plasticity in regard to costimulatory receptor usage among T cells. For example, costimulation through ICOS, a CD28 family member and CD137 and CD134, both members of the tumor necrosis factor family, have all been shown to enhance proliferation15,18,43,44 and telomerase activity in CD8+CD28− T cells.15 This redundancy in costimulatory receptor usage suggests that changes in addition to the loss of costimulatory receptors are involved in T-cell dysfunction during aging. KLRG1 is a coinhibitory molecule that has been suggested to be a marker of replicative senescence,23 and we and others have found it to increase on both highly differentiated primary human CD8+CD28−CD27− T cells and with age.23,45 We also find increased KLRG1 expression on undifferentiated CD8+CD28+CD27+ T cells with age. We postulate that this rise in expression occurs as a result of increased turnover of existing naive T cells replenishing the T-cell compartment as a result of thymic involution. We investigated whether certain aspects of the dysfunction that is observed in highly differentiated CD8+ T cells were maintained actively by KLRG1 receptor signaling.
KLRG1 has been shown to be a cadherin receptor recognizing E-, N-, and R-cadherin.25-27 The cadherins comprise a family of transmembrane glycoproteins that mediate Ca2+-dependent cell-cell adhesion.46 Classically, E-cadherin is expressed on epithelial cells and Langerhans cells, whereas N- and R-cadherin are expressed by the nervous system. We find in this study no expression of N-cadherin on PBMCs, but we do find E-cadherin to be expressed on peripheral blood cells, notably on myeloid DCs. Our work demonstrates, therefore, that E-cadherin is not only found in epithelium but on a wide range of antigen presenting cells, suggesting a broader range of scenarios for immune control by KLRG1/cadherin interactions. E-cadherin can also interact with the alpha E integrin CD10347 ; however, we found that CD103 was only expressed on 5% on CD8+ T cells (data not shown), confirming previous reports.48 Although we cannot rule out the possibility that some of the effects we observe when using anti–E-cadherin antibody are due to the disruption of its interaction with CD103, the interaction of CD8+ T cells with KLRG1 predominates, as it is constitutively expressed on more than 80% of highly differen-tiated cells.
A recent report has shown that both KLRG1 and CD3/T-cell receptor signals have to be provided in a spatially restricted manner to inhibit T-cell activation.37 The authors suggest that KLRG1 inhibits T-cell function only when MHC/antigen and KLRG1 ligands are expressed on the same target cells, strongly indicating that KLRG1-mediated effects conform to the general rule that inhibition by ITIM-containing receptors requires coengagement with activating receptors.49
KLRG1 contains one ITIM motif in its cytoplasmic domain mediating its effects through the recruitment of SHIP-1 and SHP-2.28 These effectors degrade PIP3 to PIP2 regulating the function of PI3K.28 PI3K plays a crucial role in a broad range of cellular functions in response to extracellular signals. A key downstream effector of PI3K is the serine-threonine kinase Akt, which in response to PI3K activation, phosphorylates and regulates the activity of several targets, including kinases, transcription factors, and other regulatory molecules.50 The activation of Akt requires the binding of its pleckstrin homology domain to the phosphoinositide products of PI3K, resulting in its recruitment to the plasma membrane. Once there, Akt activation is controlled by phosphorylation at 2 different sites, Thr308 and Ser473. Highly differentiated CD8+CD28−CD27− T cells are unable to phosphorylate Akt (ser473), with the Thr308 phosphorylation site being unaffected.15 Using an E-cadherin–blocking antibody, we found that the defective Akt phosphorylation in highly differentiated CD28−CD27− T cells is restored to the levels that are found after activation in the less differentiated CD8+ subsets. This indicates that the defect in Akt phosphorylation is not a passive consequence of antigenic driven differentiation of CD8+ T cells but is instead actively maintained by KLRG1 signaling.
Previous studies have shown that hTERT, the catalytic component of telomerase is phosphorylated by Akt for optimal activity.51,52 Furthermore, the direct inhibition of Akt also resulted in the inhibition of telomerase activity in CD8+ T cells at all stages of differentiation.15 We previously identified a strong but indirect association between decreased Akt (ser473) phosphorylation and decreased hTERT phosphorylation in CD8+CD28−CD27− T cells.15 However, we now show that Akt (ser473) phosphorylation alone is not sufficient to up-regulate telomerase, as KLRG1 blockade significantly increases Akt (ser473) phosphorylation but not the low telomerase activity of the CD8+CD28−CD27− subset. This suggests that additional changes occur in these cells that result in the defective telomerase activity. Apart form Akt, other key signaling molecules such as ERK and NF-kB are activated after PI3K activation.41 A recent report suggests that telomerase activity may be increased in human T cells using compounds that activate the ERK pathway,53 and it would be interesting to find whether they can up-regulate telomerase activity in the CD8+CD28−CD27− subset. It is possible that other inhibitory receptors on T cells may regulate telomerase activity. Although the blockade of PD-1 or CTLA-4 signaling in T cells from patients with HIV54-56 or hepatitis C57 can boost virus-specific proliferative responses, we found that the low telomerase activity in CD8+CD28−CD27− T cells is unaffected by blocking both these receptors (data not shown). Ongoing studies in our laboratory are directed at understanding further the mechanism for telomerase down-regulation in highly differentiated human T cells.
It is well recognized that older humans have decreased responses to vaccination,14,58,59 and it is possible that modulating certain inhibitory receptors like KLRG1 that are preferentially expressed in highly differentiated T cells and during aging may boost these responses. Although we observed a significant reversal of the defective Akt (ser473) phosphorylation in CD8+ T cells isolated from old individuals after KLRG1 blockade (data not shown), this did not translate into increased proliferation; the enhancement was of a lower magnitude compared with young individuals (1.5- to 2.1-fold increase compared with 1.5- to 8-fold increase, respectively). This suggests that other age-related defects are contributing to the poor proliferative responses of CD8+ T cells from old donors and these remain to be clarified. The current challenge is to identify the extent to which other inhibitory pathways may be involved in the reduction of certain functions in highly differentiated T cells29 and whether they can be manipulated to improve immune responsiveness during aging.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Gordon Freeman for his kind gift of anti-PD1 antibody.
This work was supported by a Research into Ageing Fellowship to S.M.H. and funding from the British Biotechnology and Biological Sciences Research Council (United Kingdom) to A.N.A.
Authorship
Contribution: S.M.H. wrote the paper, designed and performed the experiments, and analyzed the data; O.F., R.M., V.L., R.I.A, S.K.-A., F.J.P., J.E.M., S.J., and S.J.G., performed experiments; H.-P.P. provided reagents; and M.V.D.S. and A.N.A. designed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sian Henson or Arne N. Akbar, Department of Immunology, University College London, 46 Cleveland St, London, W1T 4JF, United Kingdom; e-mail: s.henson@ucl.ac.uk or a.akbar@ucl.ac.uk.