Abstract
Endothelial progenitor cells are critically involved in essential biologic processes, such as vascular homeostasis, regeneration, and tumor angiogenesis. Endothelial colony–forming cells (ECFCs) are endothelial progenitor cells with robust proliferative potential. Their profound vessel-forming capacity makes them a promising tool for innovative experimental, diagnostic, and therapeutic strategies. Efficient and safe methods for their isolation and expansion are presently lacking. Based on the previously established efficacy of animal serum–free large-scale clinical-grade propagation of mesenchymal stromal cells, we hypothesized that endothelial lineage cells may also be propagated efficiently following a comparable strategy. Here we demonstrate that human ECFCs can be recovered directly from unmanipulated whole blood. A novel large-scale animal protein-free humanized expansion strategy preserves the progenitor hierarchy with sustained proliferation potential of more than 30 population doublings. By applying large-scale propagated ECFCs in various test systems, we observed vascular networks in vitro and perfused vessels in vivo. After large-scale expansion and cryopreservation phenotype, function, proliferation, and genomic stability were maintained. For the first time, proliferative, functional, and storable ECFCs propagated under humanized conditions can be explored in terms of their therapeutic applicability and risk profile.
Introduction
Cellular therapy for organ vascularization and regeneration is a novel, conceptually challenging, but also highly controversial, issue.1,2 Endothelial progenitor cells (EPCs) are thought to play a central role in vascular homeostasis and repair, as well as contributing to the neovasculature of growing tumors.3,4 Circulating EPCs were first described in CD34+-enriched blood-borne cells, which contained a complex mixture of hematopoietic CD45+ as well as CD45− cell types contributing to vascular repair.5 The colony-like mixed cell clusters derived from these putative EPCs were later interpreted as colony-forming units of EPCs (CFU-Hill).6 The true nature of these in vitro–generated cell clusters was recently redefined by clonal analysis showing their hematopoietic origin.7-9 The composition of these colony-like structures was shown to result from a functional cross-talk between T cells and monocytes in vitro.10,11 One dominant function of these hematopoietic cells, collectively also termed circulating angiogenic cells (CACs), appears to be their proangiogenic activity.1,2,11-13 The contribution of bone marrow–derived CACs to vascular homeostasis and regeneration is a matter for debate.1,11,14-19
A major breakthrough toward achieving a better understanding of vascular progenitor cell biology was the identification of a novel hierarchy of endothelial colony–forming cells (ECFCs) in human blood that may originate from somatic EPCs within the vasculature.20,21 In contrast to CFU-Hill and other hematopoietic CACs, the ECFCs show robust proliferation in vitro and profound vessel formation in vivo making them attractive candidates for vascular regenerative therapy.8 However, applicability is limited by the low frequency of less than one ECFC per 1 million nucleated cells in steady-state peripheral blood (PB).20,22 Moderate mobilization of ECFCs into the bloodstream was seen in disease and after pharmacologic treatment.22 For both experimental and clinical studies, EPC mobilization may thus be an attractive strategy. However, despite rapid mobilization, EPCs cryopreserved after harvest lost functionality after thawing, and thus a method for large-scale EPC preparation is presently lacking.23 Another possibility for accessing larger numbers of EPCs is the ex vivo expansion of proliferating ECFCs. The currently established ECFC-enrichment methods start with density-gradient separation followed by subsequent adherence to matrix protein-coated tissue culture surfaces and depend on the presence of selected lots of fetal bovine serum (FBS). Current protocols for ECFC propagation are not yet suited to comprehensive testing or application of ECFCs as an investigational new drug.
In this study, we describe a novel strategy to efficiently recover ECFCs from unmanipulated human PB. We demonstrate that ECFCs can be propagated in an animal serum-free system to a true quantity of up to 150 million cells in large-scale animal protein-free cultures within a single passage after primary culture. Despite robust proliferation, the expanded ECFCs are genomically stable as evidenced by normal karyotype and balanced array-comparative genomic hybridization (array CGH) profiles. They display an endothelial phenotype and can be cryopreserved. After thawing, ECFCs showed preserved robust proliferation and maintained a progenitor hierarchy. Expanded ECFCs were successfully applied in test systems to form vascular networks in vitro and perfused human vessels in immune-deficient experimental animals in vivo.
Methods
ECFC recovery and humanized expansion
Blood samples (n = 20) were collected after written informed consent was obtained in accordance with the Declaration of Helsinki. The study protocols were approved by the Institutional Review Board of the Medical University of Graz (protocol numbers 19-252 ex 07/08 and 18-243 ex 06/07). Steady-state venous PB was obtained from healthy volunteers (maximum, 4 × 6 mL; 4 male, 2 female; age, 26-50 years) and patients with cardiovascular disease (CVD; 1 × 6 mL, 4 male, 3 female; age, 45-86 years). Umbilical cord blood (UCB; 20-45 mL; n = 7) was collected after full-term pregnancies. Endothelial growth medium (EGM) was prepared as described except that FBS was replaced by 10% pooled human platelet lysate (pHPL) to create a humanized culture system that may be translated into medically relevant applications.10,24 The pHPL was prepared from pooled platelet-rich plasma derived from a minimum of 40 whole blood donations as previously reported.24 The frequency of endothelial colonies was determined in primary unmanipulated blood as described except that cultures were supplemented with 10% pHPL replacing FBS in heparinized EGM.20 Heparinized blood was diluted without additional cell separation in EGM/10% pHPL and seeded directly into the culture vessels. Nonadherent cells were removed by washing with prewarmed phosphate-buffered saline after overnight culture. Cultures were maintained until the outgrowth of cobblestone-type colonies (for a maximum of 4 weeks). An extended methods section can be found in the online supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article).
ECFC large-scale expansion and clonogenicity
Primary culture–derived ECFCs were expanded each in EGM/10% pHPL in 2 4-layered cell factories (2528 cm2 CF-4; Nalge Nunc, Rochester, NY) for 11 to 25 days. Large-scale cultures were otherwise treated as previously established for clinical-grade mesenchymal stromal cell (MSC) production.25 Cells were cryopreserved as described.26 In preliminary experiments, serial dilutions were analyzed in triplicate to determine endothelial colony frequency in established ECFC populations. These pilot colony assays revealed optimum countable colonies starting from a seeding density of 10 ECFCs/cm2 (Figure S1A). Low proliferative potential (LPP) and high proliferative potential (HPP) colonies were defined, as published, as colonies comprising of 51 to 500 and more than 500 cells, respectively, at day 14 (Figure S2).20,21 Colonies were fixed, stained, and thereafter analyzed with ImageJ software (National Institutes of Health, Bethesda, MD).
Phenotyping, functional and genomic analyses in vitro and in vivo
Immune histochemistry was performed as published.8 Immune cytochemistry was done using a horseradish peroxidase polymer detection system (Thermo Fisher Scientific, Freemont, CA) following the manufacturer's instructions. ECFCs were analyzed for surface marker expression with a FACSCalibur instrument (BD Biosciences, San Jose, CA) as previously described for MSCs.27 In vitro vascular network formation was tested as previously described by seeding 7500 large-scale expanded ECFCs per 0.4-cm2 well.10 The vascular network formation was documented by acquiring video sequences at 30-minute intervals covering a time period of 24 hours with a cell observer (Carl Zeiss, Thornwood, NY).
In vivo functionality was analyzed as published.28-31 In short, 3 × 105 bone marrow–derived MSCs were mixed with 1.2 × 106 ECFCs in Matrigel (Millipore, Billerica, MA) immediately before a subcutaneous injection of 0.2-mL aliquots into the right flank of immune-deficient nude mice. Both MSCs and ECFCs were derived from large-scale expansions representing approximately 20 population doublings since culture initiation. G-banding was performed according to standard laboratory procedures.32 Array CGH of ECFCs after large-scale expansion and in the course of long-term proliferation was performed and interpreted as described to determine the possible genomic alteration of cells expanded for 4 passages representing up to 37 population doublings and compared in selected samples to constitutional DNA.25,33 Telomere length analysis was done using a telomere peptide nucleic acid (PNA) kit (Dako Denmark, Glostrup, Denmark) according to the manufacturer's instructions with minor modifications as specified in the online supplemental data. Telomerase activity was measured using a real-time polymerase chain reaction-based telomeric repeat amplification protocol (TRAP) following exactly manufacturer's instructions (Allied Biotech, Ijamsville, MD). Animal experiments were approved by the Animal Care and Use Committee at the veterinary University of Vienna on behalf of the Austrian Ministry of Science and Research according to the criteria published in the Guide for the Care and Use of Laboratory Animals.34
Statistical analyses
Unless otherwise stated, data are shown as mean plus or minus SEM. SPSS 15.0 software (SPSS, Chicago, IL) was used for statistical analyses. Normal distribution was assessed by Kolmogorov-Smirnov analysis. Statistical differences were assessed using the analysis of variance test. Significance was set at P less than .05 and P less than .01.
Results
Recovery of ECFCs from PB and large-scale propagation in an animal protein–free humanized system
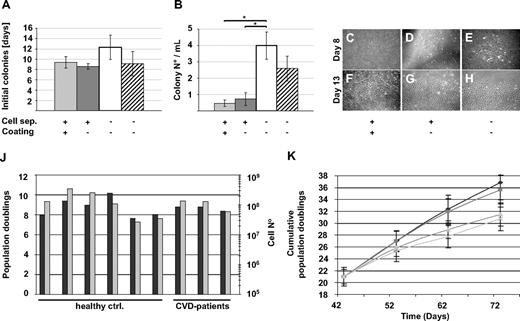
The abandonment of animal-derived substitutes is considered to be a critical prerequisite for standardized clinical scale propagation of human cells intended for transplantation purposes.35 Based on the previously demonstrated efficacy of animal serum-free large-scale clinical-grade propagation of MSCs from human bone marrow and UCB, we hypothesized that endothelial lineage cells may also be propagated efficiently without animal serum.24,25,27 In an initial set of experiments, we established that pHPL can replace FBS in standard cultures of established human umbilical vein endothelial cells and EPCs derived from human UCB, resulting in at least comparable colony outgrowth and proliferation (Figure S1B,C). Therefore, all subsequent experiments were performed in EGM supplemented with 10% pHPL (supplemental data). In addition, standard procedures for EPC propagation strictly depend on coating the culture surface with matrix protein mostly of animal origin, which may limit medical applicability.10,20,22,29,30 For that reason, we determined in a further step whether collagen coating is essential for the recovery of ECFCs. Mononuclear cells derived from the steady-state PB of 6 healthy volunteers were split and seeded onto either collagen-coated or uncoated tissue culture surfaces. The first colonies were detected after mean 9.4 plus or minus 1.1 days in collagen-coated compared with 8.6 plus or minus 0.5 days in uncoated cultures (Figure 1A). No significant difference was observed in the number of colonies recovered under either condition (Figure 1B). In a further series of experiments, we tested whether density gradient separation of mononuclear cells from PB was necessary to recover ECFCs. Seeding nonseparated whole blood, which had just been diluted in EGM/10% pHPL on uncoated culture surface, resulted in a delay in colony detection, but an almost 8-fold significant rise in recovered ECFC colonies compared with MNC separation. We recovered a mean of 4.0 plus or minus 0.8 colonies with typical cobblestone morphology per 1 mL of unseparated whole blood compared with less than one colony per milliliter after density gradient separation. Reproducibility of this procedure was confirmed by propagating ECFCs from the same 6 volunteers as technical replicates. Based on previous experience with UCB-derived EPCs propagated in conventional FBS-supplemented cultures,10 we also succeeded in generating ECFCs under animal protein-free conditions from unseparated UCB in 7 of 7 samples. Because CVD represents one interesting target for ECFC research and vascular regenerative therapy, unseparated PB from 7 patients with stable CVD was used for ECFC recovery experiments as a proof of principle. First colonies were detected after mean 9.1 plus or minus 5.6 days in uncoated cultures. The mean colony number recovered per 1 mL did not show significant differences compared with the healthy control volunteers (Figure 1A,B). Once established, colonies increased rapidly in size indicating a high proliferation rate (Figure 1C-H). As a result, in 6 independent primary cultures, a mean of 6.9 plus or minus 2.8 × 104 oligoclonal ECFCs could be harvested after 7 to 19 days of primary culture originating from 1 mL of normal unmanipulated PB. In comparison, a separate series of experiments was performed by seeding PB in 6-well plates with a 10-cm2 growth area to select for monoclonal colony growth by limited dilution. After primary culture, 3000 to 38 000 ECFCs were harvested from 4 single ECFC clones from 3 donors after 2 weeks. For reasons of practicability, the intended scale-up for large-scale cultures was performed with oligoclonally derived ECFCs available in higher quantity.
ECFC recovery from human steady-state PB in an animal serum-free humanized system. (A,B) Peripheral blood (PB) from healthy volunteers was density gradient-separated (+) to enrich for mononuclear cells ( ) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated (
) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated ( ) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in Figure S1D.) Images were captured with a DS-Fi1 camera on a Nikon (Lijnden, Netherlands) Diaphot 300 inverted microscope (original magnification 4×/0.13 NA objective) with the NIS-Elements D3.0 image acquisition software (Nikon). (J) Population doublings (
) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in Figure S1D.) Images were captured with a DS-Fi1 camera on a Nikon (Lijnden, Netherlands) Diaphot 300 inverted microscope (original magnification 4×/0.13 NA objective) with the NIS-Elements D3.0 image acquisition software (Nikon). (J) Population doublings ( ) and expanded cell number (
) and expanded cell number ( ) determined after large-scale expansion of ECFCs from 6 healthy volunteers (healthy controls) and 3 CVD patients are shown. (K) Cumulative population doublings (mean ± SD) as obtained during large-scale expansion of ECFCs from 6 healthy volunteers after large-scale expansion are shown. Large-scale expansion-derived cells bear a history of mean 21 population doublings before initiating long-term culture at cell seeding densities of 10 (◆), 100 (■), 1000 (▲), and 10 000 cells/cm2 (x). Cells were reseeded during long-term culture at indicated time points according to their initial seeding density.
) determined after large-scale expansion of ECFCs from 6 healthy volunteers (healthy controls) and 3 CVD patients are shown. (K) Cumulative population doublings (mean ± SD) as obtained during large-scale expansion of ECFCs from 6 healthy volunteers after large-scale expansion are shown. Large-scale expansion-derived cells bear a history of mean 21 population doublings before initiating long-term culture at cell seeding densities of 10 (◆), 100 (■), 1000 (▲), and 10 000 cells/cm2 (x). Cells were reseeded during long-term culture at indicated time points according to their initial seeding density.
ECFC recovery from human steady-state PB in an animal serum-free humanized system. (A,B) Peripheral blood (PB) from healthy volunteers was density gradient-separated (+) to enrich for mononuclear cells ( ) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated (
) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated ( ) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in Figure S1D.) Images were captured with a DS-Fi1 camera on a Nikon (Lijnden, Netherlands) Diaphot 300 inverted microscope (original magnification 4×/0.13 NA objective) with the NIS-Elements D3.0 image acquisition software (Nikon). (J) Population doublings (
) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in Figure S1D.) Images were captured with a DS-Fi1 camera on a Nikon (Lijnden, Netherlands) Diaphot 300 inverted microscope (original magnification 4×/0.13 NA objective) with the NIS-Elements D3.0 image acquisition software (Nikon). (J) Population doublings ( ) and expanded cell number (
) and expanded cell number ( ) determined after large-scale expansion of ECFCs from 6 healthy volunteers (healthy controls) and 3 CVD patients are shown. (K) Cumulative population doublings (mean ± SD) as obtained during large-scale expansion of ECFCs from 6 healthy volunteers after large-scale expansion are shown. Large-scale expansion-derived cells bear a history of mean 21 population doublings before initiating long-term culture at cell seeding densities of 10 (◆), 100 (■), 1000 (▲), and 10 000 cells/cm2 (x). Cells were reseeded during long-term culture at indicated time points according to their initial seeding density.
) determined after large-scale expansion of ECFCs from 6 healthy volunteers (healthy controls) and 3 CVD patients are shown. (K) Cumulative population doublings (mean ± SD) as obtained during large-scale expansion of ECFCs from 6 healthy volunteers after large-scale expansion are shown. Large-scale expansion-derived cells bear a history of mean 21 population doublings before initiating long-term culture at cell seeding densities of 10 (◆), 100 (■), 1000 (▲), and 10 000 cells/cm2 (x). Cells were reseeded during long-term culture at indicated time points according to their initial seeding density.
Based on previous experience with optimizing large-scale MSC expansion,24-27 we seeded oligoclonal ECFCs derived from 13 to 30 colonies from healthy volunteers after primary culture in a low density of 18 to 100 cells/cm2 into 2 cell factories (covering an area of 2 × 2528 cm3) per culture sample (Video S1). In 4 of 6 cases, we obtained more than 108 ECFCs after 11 to 25 days of large-scale culture representing 7.6 to 10.2 population doublings. Two other cultures that were initiated from a starting cell density of 27 cells/cm2 generated 2.7 × 107 and 3.4 × 107 after 25 and 20 days, respectively. Large-scale expansions of primary ECFCs (16-33 colonies) from 3 CVD patients did not show significant differences in final cell number compared with healthy volunteers (Figure 1J).
Preserved ECFC phenotype, proliferation, progenitor hierarchy, and genomic stability after large-scale expansion and cryopreservation
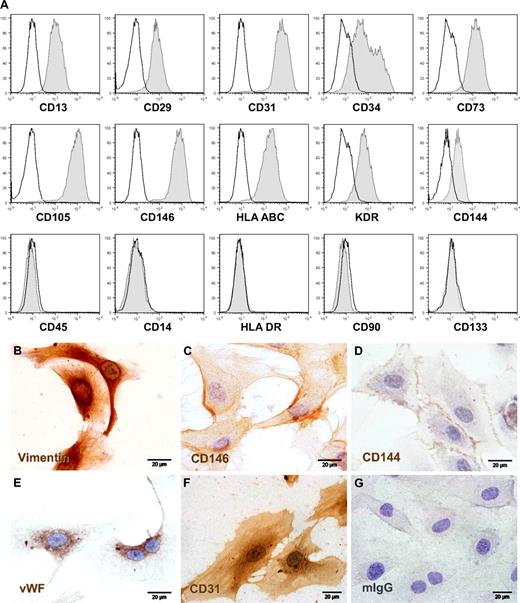
Proliferating ECFCs showed the typical cobblestone morphology of endothelial lineage cells (ECs) in vitro (Figures 1C-H, S1D). Immune profiling by flow cytometry revealed the homogeneous reactivity of consistently more than 95% of the generated cells with monoclonal antibodies directed against human EC lineage marker molecules after large-scale expansion and before cryopreservation. Cultures contained less than 0.1% contamination with CD45+ hematopoietic cells after large-scale propagation (Figure 2A). Immune phenotype was unchanged after freezing, thawing, and additional culture of cryopreserved ECFCs and was comparable with UCB-derived EPCs, human umbilical vein endothelial cells, and human microvascular ECs (Figure S3). Immune cytochemistry confirmed CD144 (vascular endothelial cadherin) and CD146 (melanoma and endothelial cell adhesion molecule) surface expression and demonstrated endothelial Weibel-Palade body–specific anti–von Willebrand factor and mesoderm-specific antivimentin antibody reactivities in appropriate subcellular localization as well as typical diffuse CD31 (platelet-endothelial cell adhesion molecule-1) staining pattern (Figure 2B-F).
Phenotypic characterization of large-scale expanded ECFCs. (A) Representative flow cytometry histograms of ECFCs after humanized large-scale expansion showing reactivity with EC-expressed marker molecules (right-shifted filled gray curves compared with black lined open curves of the appropriate isotype controls) and lack of reactivity with hematopoietic (CD14 and CD45) and stem cell-associated (CD90 and CD133) or activation (human leukocyte antigen class II type DR, HLA-DR) markers. (B-G) Representative cellular localization of (B) mesodermal cytoskeleton component vimentin, (C) CD146/melanoma and endothelial cell adhesion molecule detected by prototype antibody P1H12, (D) CD144/vascular endothelial-cadherin, (E) endothelial von Willebrand factor (VWF), (F) hematopoietic stem cell and vascular EC-specific CD31/platelet-endothelial cell adhesion molecule-1, compared with (G) one representative isotype control example. ECFCs were cryopreserved after large-scale expansion and thawed before seeding in chamber slides to obtain adherent cells for cytochemistry. Staining was done as specified in supplemental data. The brown color results from precipitation of the chromogen diaminobenzidine mediated by antibody binding to the target molecule. Images were captured with an Olympus (Hamburg, Germany) DP71 camera on an Olympus BX51 microscope (original magnification 100×/1.35 NA oil objective) with the Olympus cell D image acquisition software.
Phenotypic characterization of large-scale expanded ECFCs. (A) Representative flow cytometry histograms of ECFCs after humanized large-scale expansion showing reactivity with EC-expressed marker molecules (right-shifted filled gray curves compared with black lined open curves of the appropriate isotype controls) and lack of reactivity with hematopoietic (CD14 and CD45) and stem cell-associated (CD90 and CD133) or activation (human leukocyte antigen class II type DR, HLA-DR) markers. (B-G) Representative cellular localization of (B) mesodermal cytoskeleton component vimentin, (C) CD146/melanoma and endothelial cell adhesion molecule detected by prototype antibody P1H12, (D) CD144/vascular endothelial-cadherin, (E) endothelial von Willebrand factor (VWF), (F) hematopoietic stem cell and vascular EC-specific CD31/platelet-endothelial cell adhesion molecule-1, compared with (G) one representative isotype control example. ECFCs were cryopreserved after large-scale expansion and thawed before seeding in chamber slides to obtain adherent cells for cytochemistry. Staining was done as specified in supplemental data. The brown color results from precipitation of the chromogen diaminobenzidine mediated by antibody binding to the target molecule. Images were captured with an Olympus (Hamburg, Germany) DP71 camera on an Olympus BX51 microscope (original magnification 100×/1.35 NA oil objective) with the Olympus cell D image acquisition software.
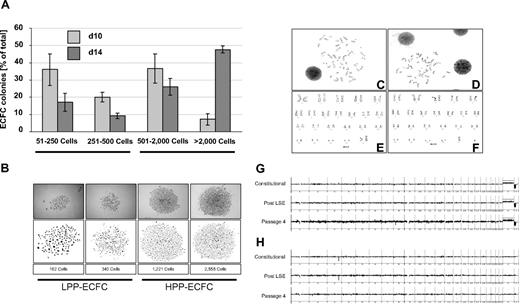
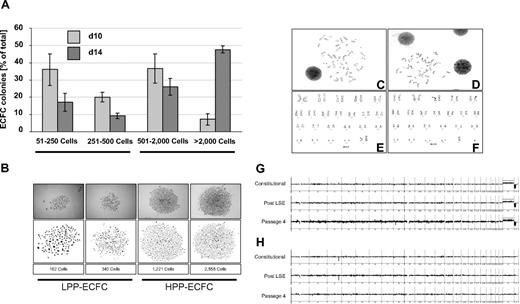
One hallmark of ECFC biology is the presence of a hierarchy of progenitor cells in any given ECFC population, resulting in the appearance of colonies of different size and cell number under specific culture conditions in vitro.8,11,20,21 In initial experiments, we noted that colony number and total cell recovery were higher than expected under our optimized culture conditions with pHPL replacing FBS compared with published data.20,22 To test for long-term colony formation, culture products after subsequent passages were diluted in 10-fold serial dilution steps starting from 10 000 down to 10 ECFCs/cm2. Results revealed countable colony frequencies only in assays initiated with 10 ECFCs/cm2 (Figure S1A). Indeed, the highest long-term proliferation resulting in 36.8 plus or minus 2.9 and 35.65 plus or minus 2.4 cumulative population doublings was seen after long-term culture initiated with ECFC seeding densities of 10 and 100 cells/cm2, respectively. Reduced proliferation was observed in long-term cultures initiated from more conventional seeding densities of 1000 and 10 000 cells/cm2 resulting in 31.4 plus or minus 1.9 and 30.7 plus or minus 2.2 cumulative population doublings, respectively (Figure 1K). This indicates that the influence of cell seeding density on ECFC proliferation was preserved during long-term culture as previously observed for MSCs from human bone marrow and UCB.24,27 To determine clonogenicity, endothelial colonies derived from the cryopreserved cells of 6 donors after large-scale propagation were subjected to a precise colony analysis after large-scale expansion using ImageJ software. We found that the entire hierarchy of LPP and HPP20 progenitors was maintained in all samples. As expected, large HPP colonies matured during the final culture phase at the expense of smaller ones (Figures 3A,B and S2).
ECFC clonogenicity and genomic stability. ECFCs after large-scale expansion representing more than or equal to 20 population doublings of the colony-initiating cells were subjected to an endothelial colony assay in triplicate in a seeding density of 10 ECFCs/cm2 in EGM/10% pHPL. (A) Colony assays were performed with ECFCs from 3 different donors each for 10 ( ) or 14 days (
) or 14 days ( ). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies20 are depicted (Figure S2). Representative chromosome G-banding derived from ECFC nuclei after large-scale expansion of (C) female and (D) male ECFCs and corresponding sorted (E) female and (F) male karyograms are shown. Representative array CGH depiction of constitutional initial white blood cell–derived DNA compared with ECFC-derived DNA post large-scale expansion and after passage 4 of the same (G) female and (H) male volunteers as shown in panels C and E and D and F, respectively (further examples in Figure S4).
). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies20 are depicted (Figure S2). Representative chromosome G-banding derived from ECFC nuclei after large-scale expansion of (C) female and (D) male ECFCs and corresponding sorted (E) female and (F) male karyograms are shown. Representative array CGH depiction of constitutional initial white blood cell–derived DNA compared with ECFC-derived DNA post large-scale expansion and after passage 4 of the same (G) female and (H) male volunteers as shown in panels C and E and D and F, respectively (further examples in Figure S4).
ECFC clonogenicity and genomic stability. ECFCs after large-scale expansion representing more than or equal to 20 population doublings of the colony-initiating cells were subjected to an endothelial colony assay in triplicate in a seeding density of 10 ECFCs/cm2 in EGM/10% pHPL. (A) Colony assays were performed with ECFCs from 3 different donors each for 10 ( ) or 14 days (
) or 14 days ( ). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies20 are depicted (Figure S2). Representative chromosome G-banding derived from ECFC nuclei after large-scale expansion of (C) female and (D) male ECFCs and corresponding sorted (E) female and (F) male karyograms are shown. Representative array CGH depiction of constitutional initial white blood cell–derived DNA compared with ECFC-derived DNA post large-scale expansion and after passage 4 of the same (G) female and (H) male volunteers as shown in panels C and E and D and F, respectively (further examples in Figure S4).
). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies20 are depicted (Figure S2). Representative chromosome G-banding derived from ECFC nuclei after large-scale expansion of (C) female and (D) male ECFCs and corresponding sorted (E) female and (F) male karyograms are shown. Representative array CGH depiction of constitutional initial white blood cell–derived DNA compared with ECFC-derived DNA post large-scale expansion and after passage 4 of the same (G) female and (H) male volunteers as shown in panels C and E and D and F, respectively (further examples in Figure S4).
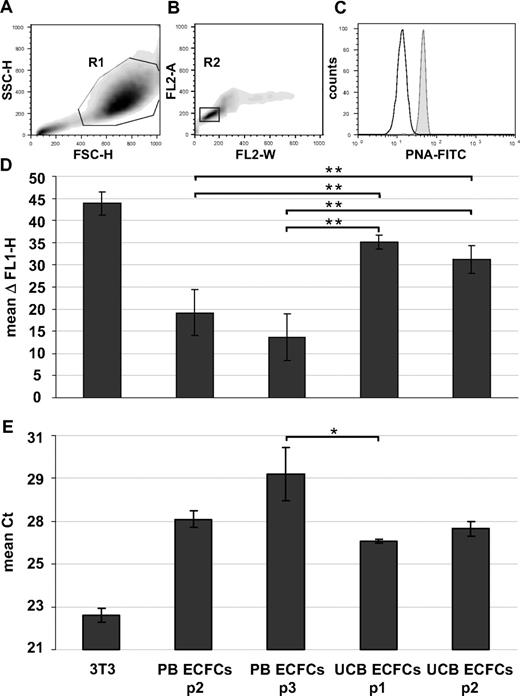
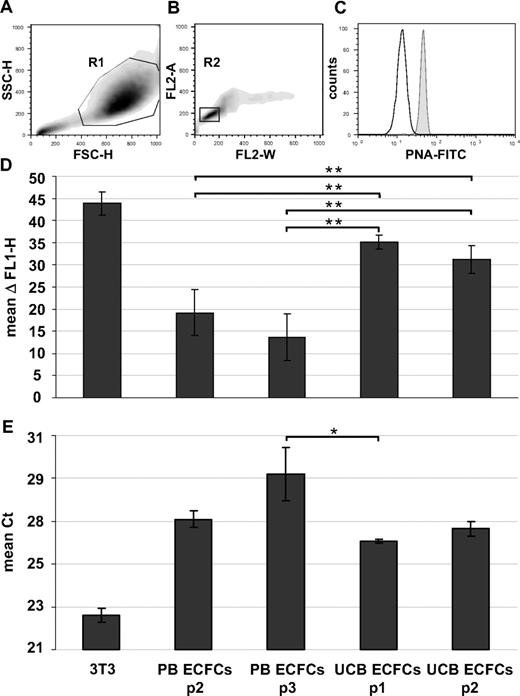
Karyotyping of early cultures showed normal chromosome content of ECFC progeny recovered from female and male volunteers (Figures 3C-F and S4A,B). Array CGH confirmed preservation of the constitutional CGH profile during culture and thus genomic stability (Figures 3G,H and S4C-G). Flow fluorescence in situ hybridization analysis showed shorter telomere length in PB- and UCB-derived ECFCs compared with mouse 3T3 cells, which were used as a positive control. PB ECFCs displayed significantly shorter telomeres than UCB ECFCs. Furthermore, we observed a nonsignificant but measurable decrease in telomere length through passaging compatible with an aging process of progenitor cells (Figure 4A-D). Quantitative telomerase activity measurement using TRAP supported these observations (Figure 4E). TRAP also indicated a higher telomerase activity (mean Ct ± SD) in cells derived from HPP (Ct = 26.8 ± 0.3 cycles) compared with LPP (Ct = 29.9 ± 0.2 cycles) colonies.
Telomere length and telomerase activity. Human ECFCs derived from PB (PB ECFCs; n = 4) and umbilical cord blood (UCB ECFC; n = 4) after different culture passages (p1-p3) were compared with each other and with mouse 3T3 fibroblasts as a positive control. (A-D) Flow cytometry fluorescence in situ hybridization was used to determine telomere length and (E) TRAP to measure telomerase activity. (A,B) Gating strategy of one representative example to select single cells in cell cycle phase G0/1 based on size parameters (FSC-H indicates forward light scatter height; SSC-H, sideward light scatter height; region R1 = 88.6%) and DNA content determined after propidium iodine (PI) staining of hybridized cells (PI fluorescence width vs area, FL2-W, FL2-A; region R2 = 77.7% of region R1). (C) Corresponding histogram showing fluorescein isothiocyanate-tagged peptide nucleic acid (PNA-FITC) binding to telomeres (gray histogram) compared with background fluorescence after mock hybridization (open histogram). (D) Differences between PNA probe specific signal and background fluorescence height (ΔFL1-H; mean ± SD, n = 4 per passage) based on analyses of at least 10 000 single cells in G0/1 phase per sample. (E) Telomerase activity displayed as cycle of threshold (Ct) in a real-time polymerase chain reaction-based TRAP assay. Statistically significant differences: *P < .05, **P < .01.
Telomere length and telomerase activity. Human ECFCs derived from PB (PB ECFCs; n = 4) and umbilical cord blood (UCB ECFC; n = 4) after different culture passages (p1-p3) were compared with each other and with mouse 3T3 fibroblasts as a positive control. (A-D) Flow cytometry fluorescence in situ hybridization was used to determine telomere length and (E) TRAP to measure telomerase activity. (A,B) Gating strategy of one representative example to select single cells in cell cycle phase G0/1 based on size parameters (FSC-H indicates forward light scatter height; SSC-H, sideward light scatter height; region R1 = 88.6%) and DNA content determined after propidium iodine (PI) staining of hybridized cells (PI fluorescence width vs area, FL2-W, FL2-A; region R2 = 77.7% of region R1). (C) Corresponding histogram showing fluorescein isothiocyanate-tagged peptide nucleic acid (PNA-FITC) binding to telomeres (gray histogram) compared with background fluorescence after mock hybridization (open histogram). (D) Differences between PNA probe specific signal and background fluorescence height (ΔFL1-H; mean ± SD, n = 4 per passage) based on analyses of at least 10 000 single cells in G0/1 phase per sample. (E) Telomerase activity displayed as cycle of threshold (Ct) in a real-time polymerase chain reaction-based TRAP assay. Statistically significant differences: *P < .05, **P < .01.
ECFC function after humanized large-scale propagation in vitro and in vivo
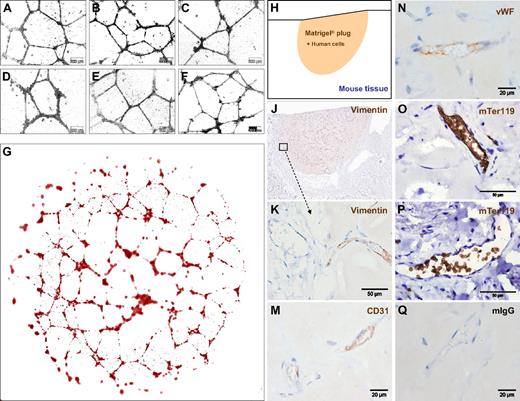
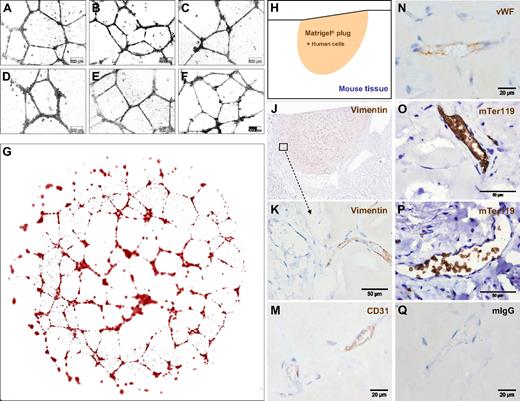
The standardized large-scale expansion of ECFCs offers a great opportunity to test human EPCs in various model systems. Vascular network formation in vitro using Matrigel is one of the most simple, rapid, and reliable models. By seeding 7500 oligoclonal or monoclonal blood-derived ECFCs after large-scale culture, we observed complex vascular network formation comparable with that of UCB-derived EPCs (Figures 5A-F and S5A-F). Computer-assisted image composition demonstrated network complexity over the entire 0.4-cm2 culture area (Figure 5G). Network formation was found to have resulted from ECFC migration and morphogenesis rather than proliferation when monitored using time lapse video microscopy (Videos S2 and S3).
ECFCs function in vitro and in vivo after large-scale humanized expansion. (A-C) Representative transformed images of vascular network formation from 3 typical independent oligoclonal cultures and (D) 1 monoclonal ECFC culture compared with (E,F) 2 independent oligoclonal UCB-derived EPC networks under the same conditions on Matrigel. Nontransformed original phase-contrast microphotographs are documented in higher magnification (Figure S5). Images were captured with an Olympus Color View III camera on an Olympus IX51 microscope (original magnification 20×/0.4 NA objective) with the Olympus analySIS B acquisition software. (G) Serial image reconstruction of one representative complete vascular network created in a 0.4-cm2 well of a 16-well glass chamber slide is shown (Videos S2,3). For in vivo neovasculogenesis, ECFCs from 2 donors were mixed with MSCs in Matrigel before injecting 0.2 mL of the composite subcutaneously in 4 nude mice per group (n = 24; 4 mice per ECFC source analyzed at 3 time points; a macroscopic view is shown in Figure S6D,E). (H) Topography of the histology is symbolized and (J) shown as a low magnification overview of a vimentin-labeled vascularized plug part. (K) Vimentin reactivity in the border area showing murine tissue (left half) in the direct vicinity of the human cell-containing area of the Matrigel plug as indicated in panel J. (M) Antihuman CD31, (N) antihuman VWF, (O,P) antimouse glycophorin A reactivity detected with antibody mTer119 within (O) human and (P) mouse vessels and (Q) representative isotype control reactivity of mouse red blood cell containing vasculature inside the plug.
ECFCs function in vitro and in vivo after large-scale humanized expansion. (A-C) Representative transformed images of vascular network formation from 3 typical independent oligoclonal cultures and (D) 1 monoclonal ECFC culture compared with (E,F) 2 independent oligoclonal UCB-derived EPC networks under the same conditions on Matrigel. Nontransformed original phase-contrast microphotographs are documented in higher magnification (Figure S5). Images were captured with an Olympus Color View III camera on an Olympus IX51 microscope (original magnification 20×/0.4 NA objective) with the Olympus analySIS B acquisition software. (G) Serial image reconstruction of one representative complete vascular network created in a 0.4-cm2 well of a 16-well glass chamber slide is shown (Videos S2,3). For in vivo neovasculogenesis, ECFCs from 2 donors were mixed with MSCs in Matrigel before injecting 0.2 mL of the composite subcutaneously in 4 nude mice per group (n = 24; 4 mice per ECFC source analyzed at 3 time points; a macroscopic view is shown in Figure S6D,E). (H) Topography of the histology is symbolized and (J) shown as a low magnification overview of a vimentin-labeled vascularized plug part. (K) Vimentin reactivity in the border area showing murine tissue (left half) in the direct vicinity of the human cell-containing area of the Matrigel plug as indicated in panel J. (M) Antihuman CD31, (N) antihuman VWF, (O,P) antimouse glycophorin A reactivity detected with antibody mTer119 within (O) human and (P) mouse vessels and (Q) representative isotype control reactivity of mouse red blood cell containing vasculature inside the plug.
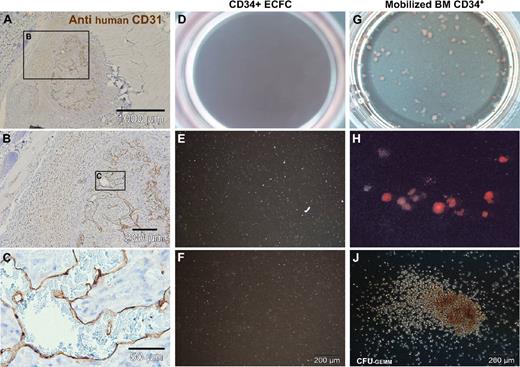
To test in vivo functionality, 1.2 million large-scale expanded ECFCs representing approximately 20 population doublings since culture initiation were mixed with 0.3 million MSCs into Matrigel and injected subcutaneously in nude mice. Matrigel plugs were analyzed after 2, 5, and 7 weeks, respectively. After 2 weeks, there were only sparsely distributed vascular structures detectable in sections of explanted plugs (Figure S6A). After 5 weeks, the implants contained complex networks of human vasculature (Figures 5H,J and S6B). Durable neo-vessel formation was found in human cell-loaded plugs explanted after 7 weeks (Figure S6C). Immune histochemistry verified human origin of the vessels within plugs. At plug borders, human vasculature appeared in close vicinity to murine vessels (Figure 5J,K). The regular red blood cell content in the human vessels, in an amount comparable with that of neighboring mouse vasculature of comparable size, strongly indicates the functional connection of both vascular systems (Figures 5K and S6A-C). Murine origin of the red blood cells was confirmed by antimouse glycophorin A (mTer119) reactivity within the perfused vessels (Figure 5O,P). Cellular distribution of immune reactivity of the human neo-vessels resembled that of human vasculature (Figure 5M,N). Such organized vasculature did not develop when pure ECFCs were injected without matrix and without supporting MSCs. Sole ECFC implantation led to the development of disorganized, but still perfused, vasculature in this particular mouse model, reminiscent of the pathologic picture of hemangiomas (Figures 6A-C and S6F).
Deranged vessel formation of pure ECFCs in vivo and vascular lineage commitment in vitro. (A-C) Representative pure ECFCs (107) derived from large-scale single-step humanized expansion were resuspended in 0.2 mL phosphate-buffered saline and subcutaneously injected into nude mice. After one week, subcutaneous cell deposits (a macroscopic view is shown in Figure S6F) were processed for human CD31 histochemistry. (A) Overview appearance and (B,C) higher magnified regional view as indicated by schematic boxes with scale bars documenting magnification. ECFCs after large-scale expansion were also seeded into methylcellulose for hematopoietic colony-forming cell (CFC) assays. (D,G) Complete (10 cm2) assay plate overview, (E,H), 10× original magnification view, and (F,J) high magnification view that documents (D-F) complete lack of CFCs derived from ECFCs. In comparison, (G,H) regular red blood cell colony formation admixed with a hierarchy of differentially maturating white blood cell colonies and (J) less than 5% colony-forming units of granulocytes, erythrocytes, monocytes, and macrophages (CFU-GEMM) was derived from CD34+ hematopoietic progenitors.
Deranged vessel formation of pure ECFCs in vivo and vascular lineage commitment in vitro. (A-C) Representative pure ECFCs (107) derived from large-scale single-step humanized expansion were resuspended in 0.2 mL phosphate-buffered saline and subcutaneously injected into nude mice. After one week, subcutaneous cell deposits (a macroscopic view is shown in Figure S6F) were processed for human CD31 histochemistry. (A) Overview appearance and (B,C) higher magnified regional view as indicated by schematic boxes with scale bars documenting magnification. ECFCs after large-scale expansion were also seeded into methylcellulose for hematopoietic colony-forming cell (CFC) assays. (D,G) Complete (10 cm2) assay plate overview, (E,H), 10× original magnification view, and (F,J) high magnification view that documents (D-F) complete lack of CFCs derived from ECFCs. In comparison, (G,H) regular red blood cell colony formation admixed with a hierarchy of differentially maturating white blood cell colonies and (J) less than 5% colony-forming units of granulocytes, erythrocytes, monocytes, and macrophages (CFU-GEMM) was derived from CD34+ hematopoietic progenitors.
Most research on EPCs has so far investigated CACs of hematopoietic origin. Analyzing a putative hematopoietic differentiation potential of the CD34+ vascular ECFCs compared with CD34+ hematopoietic cells is therefore a fundamental question of the characterization of EPCs. The vascular lineage-restricted differentiation of ECFCs is supported by a complete lack of hematopoietic colony formation when testing 500 and 1000 ECFCs in standard methylcellulose assays. In clear contrast, equal numbers of purified hematopoietic progenitor cells produced the estimated mixture of red and different white cell lineage colonies (Figure 6D-J). The complete lack of hematopoietic colonies in ECFC-loaded hematopoietic colony assays further argues against even a minute hematopoietic progenitor cell contamination of the large-scale expanded ECFCs.
Discussion
Promising new concepts in vascular regenerative therapy are aiming to support vascular repair by progenitor and stem cell transplantation. However, up to now, a spectrum of experimental and early clinical trials with bone marrow-derived supposed EPCs has shown only limited vessel formation capacity of the transplanted cells.8,18 This reflects, at least in part, our presently restricted understanding of the cell biology underlying vascular homeostasis and regeneration.2,11 The selection of appropriate functional EPCs as a source of cells for experimental test systems or as transplants for vascular regeneration currently represents a major challenge.11,18 Growing evidence now reinforces traditional knowledge that hematopoietic cells facilitate vascular and organ regeneration mainly by humoral and cell-mediated support functions and do not directly form vessels.2,11,36,37
In sharp contrast, ECFCs, which are blood- or vasculature-derived EPCs characterized by robust proliferative potential, can form perfused long-lasting vessels in vivo.8,29,30 We have developed a novel method that for the first time allows us (1) to efficiently recover human ECFCs from unmanipulated blood under humanized entirely animal protein-free conditions; (2) to propagate these ECFCs to a quantity of mean 1.5 plus or minus 0.5 × 108 cells within a single large-scale expansion after oligoclonal primary culture; (3) to cryopreserve expanded ECFCs with intact proliferation potential and function after thawing; and (4) to use human animal serum-free expanded ECFCs in vascular network formation assays in vitro and in 2 different neo-vasculogenesis models in vivo. Despite robust proliferation, these ECFCs proved to be genomically stable as indicated by normal karyotype and array CGH profiles. Monoclonal ECFC progeny could also be generated but were less suited for EPC mass production than oligoclonal ECFCs because of the lower cell number recovered. As a proof of principle, ECFCs were also successfully recovered from unmanipulated UCB and PB of patients with stable CVD using the same optimized animal serum-free procedure. In our experiments, there were no significant differences in time to initial colony outgrowth, colony number, and capacity of large-scale expansion in patients with stable CVD. In a pig model of induced acute myocardial infarction, elevated numbers of ECFCs and increased percentage of HPP-ECFCs were observed immediately after balloon catheter-induced coronary damage.38 Larger prospective studies would be appropriate to address questions regarding ECFC frequency and function in selected patient populations. Our technology could help to determine ECFC frequency and functionality in a standardized fashion. The availability of human ECFCs propagated under completely animal protein-free conditions could also change the focus of cellular therapy concepts for vascular regeneration from hematopoietic CACs to vessel-forming ECFCs.8
Currently, the precise origin of blood-borne ECFCs is not clear. We isolated ECFCs directly from small volumes of heparinized whole blood through an initial adhesion step and subsequent selection of proliferating cells in a specific endothelial growth medium supplemented with human growth factors and pHPL. ECFC phenotype, proliferation potential, maintained progenitor cell hierarchy, and vessel formation in vitro and in vivo clearly meet the criteria established by Ingram and Yoder.20,21,39,40 Previous analyses of blood smears by Hebbel et al with antibody P1H12 already suggested that CD146+ circulating endothelial lineage cells reminiscent of ECFCs by phenotype and profound colony-type proliferation are present in PB as a minute population of 2.6 plus or minus 1.6 cells/mL.22 Consecutive studies by the same authors with blood samples from bone marrow transplantation recipients led to the conclusion that most circulating endothelial lineage cells originate from vessel walls, grow in colonies in vitro, and have limited proliferation potential. Robust late outgrowth resulting from a 1000-fold expansion of blood-derived colony-forming endothelial cells after several weeks of in vitro cell culture was shown to originate from an even tinier subpopulation of transplantable donor cells.41 These circulating transplantable and proliferating endothelial colony-forming cells may be derived from bone marrow vasculature before mobilization into the bloodstream or from an even more immature type of progenitor.9,42,43 Our results, including an endothelial colony recovery of mean 4 colonies per milliliter, may strengthen the assumption that the ECFCs first described by Ingram et al20 and the CD146+ endothelial outgrowth cells originally discovered by the Hebbel group22 are derived from one and the same cell population. We speculate that the higher recovery of ECFCs with our protocol may be the result of a diminished cell loss by avoiding density gradient-based cell separation. Human platelet-derived growth factors (PDGFs) in the pHPL, including PDGF-AA, PDGF-AB/BB, epidermal growth factor, and angiogenin, among others, may further support efficient outgrowth of ECFCs.24,44 ECFCs can be distinguished from hematopoietic progenitors by their lack of the pan-hematopoietic antigen CD45 and the absence of hematopoietic colony formation.7-9 The cells described in this study also meet these criteria. Based on the currently published data, we favor a view of circulating ECFCs as predominantly vessel wall-derived EPCs that can circulate through the bloodstream. Whether ECFCs can really function in an orchestrated interplay with immune cells (including T cells, different activation and differentiation stages of monocytes and other CACs as well as CFU-Hill as a surrogate in vitro marker) to maintain vessel integrity and contribute to adult vasculogenesis during organ regeneration and tumor vascularization needs to be determined before planning future clinical trials.
Clinical trials using hematopoietic CACs appear to be safe but are unfortunately also of limited efficacy and mostly lack durable response.8 Any effective vascular regenerative medicine independent of their mode of action or transplanted cell populations bears the eminent risk of side effects because of the complex and still poorly understood balance between beneficial and deleterious effects. Therefore, at present, a spectrum of various test systems needs to be used to study safety and efficacy before continuing clinical trials with vascular EPCs.8 We observed genomic stability and a normal karyotype of ECFCs expanded for more than 30 population doublings under humanized culture conditions with preserved functionality. The influence of biologic or pharmacologic compounds on endothelial progenitor colony behavior, genomic stability, and vascular network formation can thus be investigated at least in part in vitro. ECFCs accessible through our method in large quantity and with intact functionality also represent a valuable tool to study proangiogenic and antiangiogenic therapeutic strategies in vivo.45 The ECFC large-scale propagation under humanized culture conditions has the potential to facilitate a wide spectrum of future studies to determine their therapeutic applicability and risk profile during vascular homeostasis and repair.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Markus Absenger, Tatjana Kueznik, and Daniela Unterthor for excellent technical assistance, Tina Schreiner for graphics applications, Trevor Devaney for help with large area microscopy applications, Dr Eleonore Froehlich for supporting quantum dot labeling of ECFCs, Dr Kurt Zatloukal for critical discussion and for support during animal in vivo experiments, and Monica Farrell for linguistic editing.
This work has been supported by the Austrian Research Foundation (grant N211-NAN) (D.S.), the European Commission (projects DISMAL LSHC-CCT-2005-018911 and Geninca HEALTH-F2-2008-202230) (M.R.S.), the Austrian National Bank (project 12569) (M.R.S.), GATiB-Genome Austria Tissue Bank GZ200.139/1-VI/1/2006 (K.Z.), and the Adult Stem Cell Research Foundation (A.R.). A.R., A.C.O., and K.F. are fellows of the PhD program Molecular Medicine of the Medical University of Graz.
Authorship
Contribution: A.R. and D.S. designed and performed research, analyzed data, and wrote the paper; N.A.H., E.R., and A.C.O. performed research and analyzed data; K.K., K.S., and K.F. performed research; W.L. and G.L. contributed to research design; and M.R.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dirk Strunk, Stem Cell Research Unit and Department of Hematology and Stem Cell Transplantation, University Clinic of Internal Medicine, Medical University of Graz, Auenbrugger Pl 38, A-8036 Graz, Austria; e-mail: dirk.strunk@klinikum-graz.at; www.meduni-graz.at/stemcellresearch.
References
Author notes
*A.R. and N.A.H. contributed equally to this study.



 ) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated (
) or immediately diluted (□) and seeded in EGM/10% pHPL in 75-cm2 cell culture flasks. Culture surfaces were coated ( ) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in
) with collagen only when indicated (+). PB from patients with stable cardiovascular disease was also immediately diluted and seeded in EGM/10% pHPL (▨). (A) The initial appearance of visible colonies was determined by daily culture observation. (B) Colony number was counted at the end of the primary 7- to 19-day culture period. Results are shown as mean plus or minus SEM of 6 independent experiments. * indicates statistically significant difference, P < .05. (C-H) Representative early colonies (day 8) and parts of large expanded colonies (day 13) from healthy volunteers are depicted with 40× initial magnification corresponding to different recovery strategies as indicated. (A composite picture of 1 representative large ECFC colony is shown in 
 ) or 14 days (
) or 14 days ( ). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies
). Colony plates were then fixed and stained before photo documentation. Precise cell numbers of all imaged colonies were counted in ImageJ software. (B) Examples of typical LPP and HPP colonies