Abstract
Bone marrow (BM) is the key hematopoietic organ in mammals and is involved in the homeostatic proliferation of memory CD8+ T cells. Here we expanded on our previous observation that BM is a preferential site for T-cell proliferation in simian immunodeficiency virus (SIV)–infected sooty mangabeys (SMs) that do not progress to AIDS despite high viremia. We found high levels of mature T-cell proliferation, involving both naive and memory cells, in healthy SMs and rhesus macaques (RMs). In addition, we observed in both species that lineage-specific, BM-based T-cell proliferation follows antibody-mediated in vivo CD4+ or CD8+ T-cell depletion, thus indicating a role for the BM in maintaining T-cell homeostasis under depleting circumstances. We also observed that, in SIV-infected SMs, but not RMs, the level of proliferation of BM-based CD4+ T cells is higher than that of circulating CD4+ T cells. Interestingly, limited BM-based CD4+ T-cell proliferation was found in SIV-infected SMs with low CD4+ T-cell counts, suggesting a regenerative failure in these animals. Collectively, these results indicate that BM is involved in maintaining T-cell homeostasis in primates and suggest a role for BM-based CD4+ T-cell proliferation in determining the benign nature of natural SIV infection of SMs.
Introduction
In humans and other mammals, the bone marrow (BM) of healthy adults is the primary anatomic site for hematopoiesis as well as a preferential site for plasma cell homing and antibody production.1,2 In contrast, the role of the BM as an organ involved in regulation of T-cell function is poorly characterized. Although it is widely accepted that BM-based T-cell precursors migrate to the thymus during the early stages of T-cell development, relatively little is known about the function of mature (ie, CD4+ or CD8+ single-positive) T cells that reside within the BM.3,4 This relative lack of knowledge is somewhat surprising because, in humans, the BM harbors a significant proportion of T cells, comprising 5% to 15% of the total nonerythroid cellularity and adding to a total of approximately 25 × 109 T cells (compared with 30 × 109 in spleen and 150 × 109 in lymph nodes [LNs]).5-7 Fully functional mature T cells recirculate from the BM to blood and vice versa.6 Within the BM, T cells show specific phenotypic features compared with blood-derived T cells: (1) decreased ratio of CD4+ and CD8+ T cells7,8 ; (2) higher proportion of cells displaying a memory phenotype, ie, low levels of CD45RA in humans9 and high levels of CD44 in mice8,10,11 ; and (3) higher percentage of CD8+ HLA-DR+ T cells.12 Importantly, BM appears to be an important site of T-cell proliferation in rodents,13-15 with 2 studies in mice suggesting that the total number of proliferating memory CD8+ T cells in the BM exceeds that of spleen, LNs, liver, and lung.14,15 It has been proposed that this T-cell proliferation is stimulated by the unique BM microenvironment where high concentrations of cytokines with T-cell growth factor activity, such as IL-7 and IL-15, are present.16-19
Simian immunodeficiency viruses (SIVs) are primate lentiviruses that naturally infect numerous nonhuman primate (NHP) species in Africa.20,21 Two of these viruses, SIVcpz from chimpanzees (Pan troglodytes) and SIVsmm from sooty mangabeys (SMs; Cercocebus atys), have crossed species barriers on multiple occasions and are the precursors of the HIV types 1 and 2 (HIV-1 and HIV-2), respectively.22,23 Several studies have shown that natural SIV infection of SMs is typically nonpathogenic and usually associated with peripheral CD4 T-cell preservation despite levels of viral replication that are similar to, or even higher than, those observed during pathogenic infections.21,24-27 In contrast, experimental SIV infection of nonnatural host Asian monkey species, such as rhesus macaques (RMs; Macaca mulatta), results in the development of symptoms similar to those described in AIDS patients.28 At present, it is still unclear why SIV infection is nonpathogenic in SMs but induces immunodeficiency in RMs. However, it is widely recognized that a better understanding of the mechanisms underlying the lack of disease in naturally SIV-infected SMs may provide important clues as to the pathogenesis of AIDS in HIV-infected humans.21,29-31
In previous studies, we found that SIV infection of SMs is typically characterized by low levels of immune activation, preservation of the BM and LN structure, and normal numbers of naive T cells.27,32,33 In contrast, pathogenic HIV infection of humans and SIV infection of RMs are commonly associated with progressively severe suppression of BM function, resulting in neutropenia, thrombocytopenia, and lymphopenia.34-36 Based on this preliminary evidence, we hypothesize that (1) the BM-based T-cell compartment plays an important role in maintaining T-cell homeostasis in NHPs; and (2) in naturally SIV-infected SMs, a functioning BM is instrumental in maintaining T-cell homeostasis and avoid AIDS, despite continuous virus replication.
In this study, we tested this hypothesis by performing (1) a detailed cross-sectional analysis of BM-, peripheral blood (PB)-, and LN-derived T cells isolated from healthy, uninfected SMs and RMs; (2) a longitudinal analysis of BM-derived T cells from healthy SMs and RMs that underwent experimental depletion of CD4+ or CD8+ T cells; and (3) a cross-sectional analysis of BM- and PB-derived T cells from SIV-infected SMs and RMs, including a relatively rare subset of SIV-infected SMs with low CD4+ T-cell counts. The results presented here suggest that BM is a preferential anatomic site for homeostatic T-cell proliferation in NHPs and that preservation of the BM-based CD4+ T-cell renewal contributes to lack of disease progression in naturally SIV-infected SMs.
Methods
Animals
SMs and RMs were selected from the colonies housed at the Yerkes National Primate Research Center of Emory University (Atlanta, GA) and maintained in accordance with National Institutes of Health guidelines. The uninfected animals included in the cross-sectional analysis were on average 8.9 and 6.3 years old for SMs and RMs, respectively, whereas the SIV-infected animals were on average 9.7 and 5.6 years old (for SMs and RMs). Anesthesia was used for all blood collections, LN biopsies, and BM aspirations. Either ketamine (10 mg/kg) or Telazol (4 mg/kg) was used. All animal studies were approved by the University of Pennsylvania (Philadelphia) and Emory University Institutional Animal Care and Usage committees.
BM aspirates and LN biopsies
BM aspirates were performed using an aspiration kit to remove approximately 3 mL BM. To exclude contamination with PB, we routinely assessed the quality of our samples by performing a Wright-Giemsa stain on a glass-slide smear. Samples were accepted and further processed only if the stained smear showed the typical cellular morphology of a BM aspirate, including a sufficient number of bone spicules and significant representation of all hematopoietic lineages with normal distribution of hematopoietic precursors. For LN biopsies, the skin over the inguinal or axillary LN was prepared for aseptic surgery. A small skin incision was made over the node, and blunt dissection was used to isolate and remove the node. The subcutis and skin were then closed with absorbable sutures. LN-derived T cells were isolated as described previously.27
Flow cytometry
The immunophenotype of lymphocytes derived from BM, PB, and LN was analyzed by 4- and 8-color flow cytometric analysis. The staining was performed according to standard procedures using a panel of monoclonal antibodies that we and others have shown to be cross-reactive with SMs and RMs.33,37,38 The antibodies used in this study included: anti-CD4 peridinin chlorophyll protein (PerCP; clone SK3), anti-CD4 allophycocyanin (APC; clone SK3), anti-CD8 PerCp (clone SK1), anti-CD8 APC (clone SK1), anti-CD28 phycoerythrin (PE; clone L293), anti-CD95 APC (clone DX2), anti-CD20 PerCp (clone L27; BD Biosciences, San Jose, CA); and Ki-67 fluorescein isothiocyanate (FITC; clone B56), anti-CD95 CyChrome (clone DX2), anti-CD3 PE (clone SP34-2), anti-CD3 PerCP (clone SP34-2), anti-CD3 APC (clone SP34-2), anti-CD4 PerCp-Cy5.5 (clone L200), anti-CD3 Alexa 700 (clone SP34-2), anti-CD8 Pacific blue (clone RPA-T8), and anti-CD16 PE (clone 3G8; BD Biosciences PharMingen, San Diego, CA). At least 100 000 events were acquired on a FACSCalibur cytometer driven by the CellQuest software (4 colors) and on a LSRII flow cytometer driven by the DiVA software package (7 colors; BD Biosciences). Analyses of the acquired data were performed using FlowJo software (TreeStar, Ashland, OR).
In vitro T-cell proliferation using CFSE staining
In vitro proliferation after stimulation with concanavalin A (ConA; 1 μg/mL) was assessed in mononuclear cells isolated from PB and BM in 3 SIV-uninfected SMs using carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling (3 μM final concentration). The fraction of proliferating cells was measured by flow cytometry at day 5 after activation as a percentage of T lymphocytes that diluted CFSE.
SIV viral load assay
Depletion of CD4+ or CD8+ cells in vivo
Depletion of CD4+ cells was performed in 3 uninfected SMs and 3 uninfected RMs using 10 mg/kg (intravenously with 50 mL saline; B. Braun Medical, Sheffield, United Kingdom) of anti-CD4 mAb (OKT4A) on day −10 and 5 mg/kg on days −7, −3, and 0, a protocol that has been shown to deplete CD4+ cells in vivo in SMs.40 For CD8+ cell depletion, 3 uninfected SMs and 3 uninfected RMs were treated with 4 mg/kg (intravenously with 50 mL saline; B. Braun Medical) of anti-CD8 mAb (OKT8F) on days −2, −1, and 0, a protocol that has been shown to deplete CD8+ cells in vivo in RMs.41-43 Blood and BM aspirates were drawn at baseline (ie, 2 weeks before infusion), and on days 0, 11, 28, and 90 of the study.
Statistical analysis
The performed analyses include the 2-tailed Student t test for comparisons between groups, whereas correlations involving different sets of data within the same group were determined using either the standard Pearson correlation coefficient or the Spearman rank correlation test. Significance was assessed at P less than .05 levels. All analyses were performed using the Prism 4.0 software.
Results
Morphologic and flow cytometric characterization of BM-derived T cells
BM-derived lymphocytes were obtained from BM aspirates of the iliac crest from SMs and RMs. The absence of major PB contamination in BM aspirates was confirmed by the observation of hematopoietic precursors and bone specula as assessed by microscopic examination after Wright-Giemsa staining (in “BM aspirates and LN biopsies”). The presence of resident T cells within the BM tissue architecture was also identified by immunohistochemical staining for CD3 in paraffin-embedded, decalcified sections of BM core biopsy (data not shown). The fact that T lymphocytes present in the BM aspirates represent a specific cell population resident in this organ, and not a contamination from the PB, is indicated also by a phenotypic comparative flow cytometric analysis performed on lymphocytes isolated from the different sites.
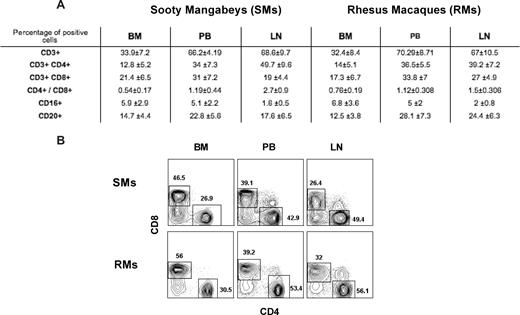
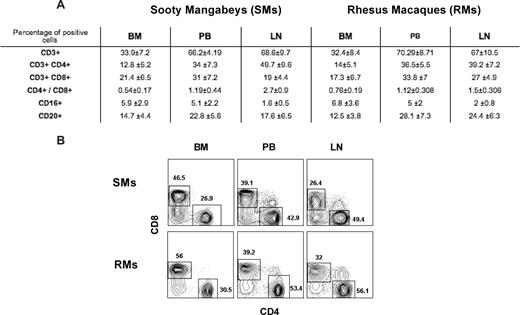
Immunophenotypic analysis indicated that, in healthy uninfected SMs and RMs, BM-derived mononuclear cells include approximately 30% to 35% CD3+ T cells, as opposed to approximately 65% to 70% found in PB and LN (Figure 1A). In addition, BM-derived T cells showed a decreased CD4/CD8 ratio compared with blood (Figure 1A for average in all the animals and Figure 1B for CD4 and CD8 staining in a representative SM and RM), as well as a consistent enrichment in T cells with immunophenotypic features of memory and/or activated lymphocytes (data not shown). These data confirm that BM contains a population of mature T lymphocytes phenotypically distinct from those found in the periphery, and highlight the needs to characterize the role played by these BM-resident T cells in NHPs.
Immunophenotypic characterization of BM-derived T cells. BM-, PB-, and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Flow cytometric analysis was used to assess the percentage of BM-, PB-, and LN-derived mononuclear cells that express CD3, CD4, CD8, CD16, and CD20 in SMs and RMs. The CD4/CD8 ratio is also shown. Numbers indicate mean plus or minus SD. (B) Staining of CD4+ and CD8+ T cells in BM, PB, and LN of a representative SM and RM. BM-derived T cells include an increased proportion of CD8+ T cells with decreased CD4/CD8 ratio compared with the other sites.
Immunophenotypic characterization of BM-derived T cells. BM-, PB-, and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Flow cytometric analysis was used to assess the percentage of BM-, PB-, and LN-derived mononuclear cells that express CD3, CD4, CD8, CD16, and CD20 in SMs and RMs. The CD4/CD8 ratio is also shown. Numbers indicate mean plus or minus SD. (B) Staining of CD4+ and CD8+ T cells in BM, PB, and LN of a representative SM and RM. BM-derived T cells include an increased proportion of CD8+ T cells with decreased CD4/CD8 ratio compared with the other sites.
Bone marrow is a preferential anatomic site for CD4+ and CD8+ T-cell proliferation in NHPs
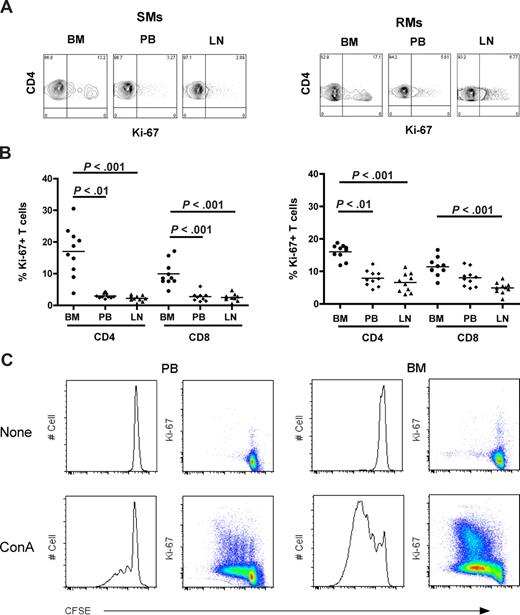
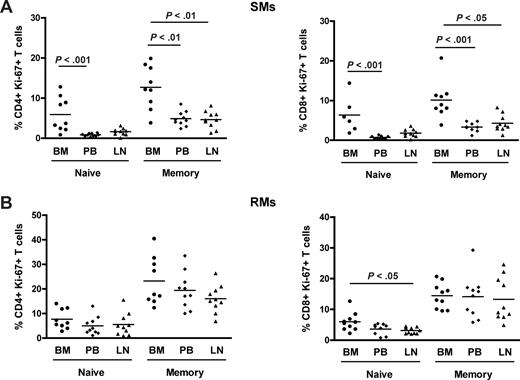
To investigate the level of T-cell proliferation in distinct anatomic compartments of healthy NHPs, we measured the percentage of cells that express the proliferation marker Ki-67 in CD4+ and CD8+ T cells isolated from BM, PB, and LN in 10 SIV-uninfected SMs and RMs. Representative examples of the rates of CD4+Ki-67+ T cells in the different anatomic sites of SMs (left panel) and RMs (right panel) are shown in Figure 2A. In both species, the average fractions of proliferating CD4+ or CD8+ T cells were higher in BM compared with the other sites (Figure 2B). In SMs, T-cell proliferation in the BM was significantly higher (P < .01) compared with PB and LN in both CD4+ and CD8+ cells (Figure 2B left panel), whereas in RMs it was significantly higher (P < .01) compared with PB and LN in CD4+, but only versus LN in CD8+ T cells (Figure 2B right panel). Interestingly, we did not find a significant direct correlation between levels of T-cell proliferation in the BM and those in the PB or LN within the group of either RMs or SMs (data not shown), suggesting that the mechanisms driving T-cell proliferation may be different in these distinct anatomic compartments. Finally, the in vitro proliferative potential of PB- and BM-derived T cells was assessed in 3 uninfected SMs after 5 days of in vitro stimulation with ConA. As shown in Figure 2C and consistent with data relative to ex vivo Ki-67 staining, we found that, in each of the tested animals, BM-resident T cells proliferate, that is, diluted CFSE, to a higher extent compared with T cells isolated from PB. In conclusion, these data confirm that BM is a preferred site for T-cell proliferation in uninfected NHPs.
In NHPs, BM contains higher levels of proliferating CD4+ and CD8+ T cells than PB and LN. The fraction of CD4+ and CD8+ T cells expressing the proliferation marker Ki-67 was assessed in BM, PB, and LN of 10 SIV-uninfected SMs and RMs. (A) Staining of CD4+Ki-67+ T cells in the different anatomic sites of a representative SM (left panel) and RM (right panel). (B) Percentage of CD4+ and CD8+ T cells that express Ki-67 in BM, PB, and LN in 10 SIV-uninfected SMs and RMs. P values are shown when significant differences between groups are present. (C) Assessment of in vitro T-cell proliferation using CFSE staining in PB- and BM-derived CD3+ T lymphocytes in a representative SM after 5 days in vitro stimulation with 1 μg/mL ConA.
In NHPs, BM contains higher levels of proliferating CD4+ and CD8+ T cells than PB and LN. The fraction of CD4+ and CD8+ T cells expressing the proliferation marker Ki-67 was assessed in BM, PB, and LN of 10 SIV-uninfected SMs and RMs. (A) Staining of CD4+Ki-67+ T cells in the different anatomic sites of a representative SM (left panel) and RM (right panel). (B) Percentage of CD4+ and CD8+ T cells that express Ki-67 in BM, PB, and LN in 10 SIV-uninfected SMs and RMs. P values are shown when significant differences between groups are present. (C) Assessment of in vitro T-cell proliferation using CFSE staining in PB- and BM-derived CD3+ T lymphocytes in a representative SM after 5 days in vitro stimulation with 1 μg/mL ConA.
Phenotypic characterization of proliferating T cells in the different anatomic compartments
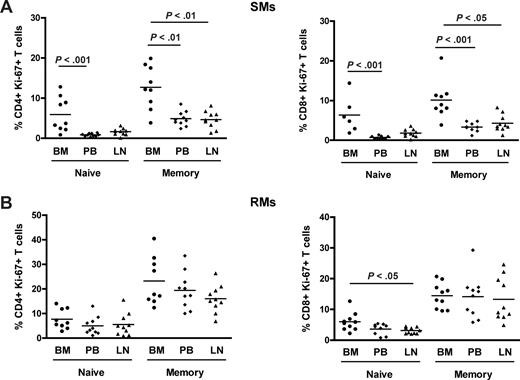
Naive and memory T-cell subpopulations in NHPs may be identified based on the expression of CD28 and CD95.33,44 To better define the nature of proliferating T cells in SIV-uninfected SMs and RMs, naive (CD28+CD95−) and memory (CD28+CD95+) subsets were identified in CD4+ and CD8+ T cells isolated from the different anatomic sites and their levels of proliferation assessed based on Ki-67 expression. We found that, in SMs, memory CD4+ and CD8+ T cells proliferate to a significantly higher extent in BM compared with the other anatomic sites, for example, PB and LN (Figure 3A). Of note, we also observed that, in SMs, naive CD4+ and CD8+ T cells derived from the BM express higher levels of Ki-67 compared with those found in PB (P < .001 for both CD4+ and CD8+ T cells). In contrast, both naive and memory CD4+ and CD8+ T cells isolated from the BM of RMs show a level of proliferation similar to that observed in the same T-cell subsets derived from PB and LN (Figure 3B). Collectively, these data suggest that the increased proliferation of BM-derived T lymphocytes is mainly the result of an enrichment of memory/effector cells in this anatomic site in RMs, whereas in SMs it seems to result from a specific increased proliferative capacity of both subsets of naive and memory CD4+ and CD8+ T cells.
Phenotypic characterization of proliferating T cells in different anatomic compartments. Levels of proliferation in naive (CD28+CD95−) and memory (CD28+CD95+) CD4+ and CD8+ T cells isolated from the different anatomic sites in 10 SIV-uninfected SMs and RMs. (A) Percentage of naive and memory CD4+ (left graph) and CD8+ (right graph) T cells expressing Ki-67 in BM, PB, and LN in 10 SIV-uninfected SMs. (B) Percentage of naive and memory CD4+ (left graph) and CD8+ (right graph) T cells expressing Ki-67 in BM, PB, and LN in 10 SIV-uninfected RMs. P values are shown when significant differences between groups are present.
Phenotypic characterization of proliferating T cells in different anatomic compartments. Levels of proliferation in naive (CD28+CD95−) and memory (CD28+CD95+) CD4+ and CD8+ T cells isolated from the different anatomic sites in 10 SIV-uninfected SMs and RMs. (A) Percentage of naive and memory CD4+ (left graph) and CD8+ (right graph) T cells expressing Ki-67 in BM, PB, and LN in 10 SIV-uninfected SMs. (B) Percentage of naive and memory CD4+ (left graph) and CD8+ (right graph) T cells expressing Ki-67 in BM, PB, and LN in 10 SIV-uninfected RMs. P values are shown when significant differences between groups are present.
Antibody-mediated CD4+ and CD8+ cell depletion is followed by increased T-cell proliferation in the BM
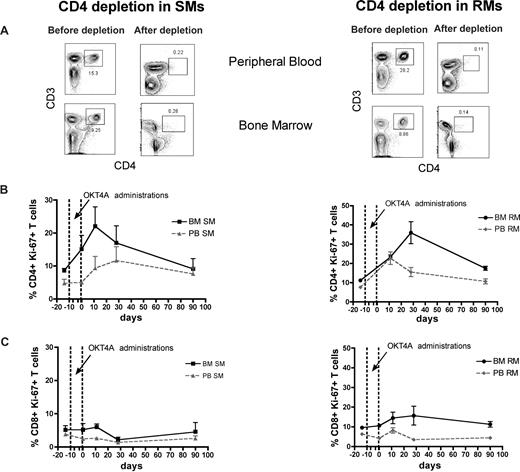
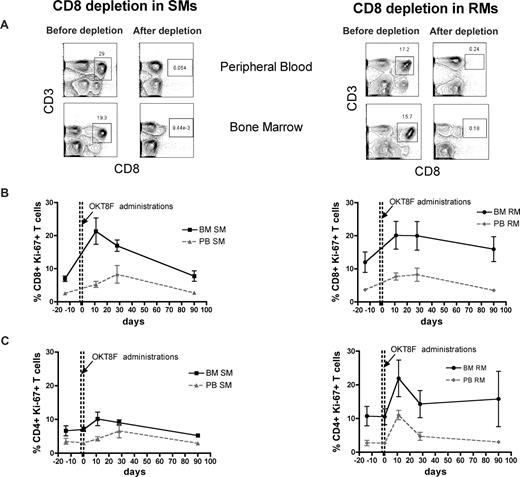
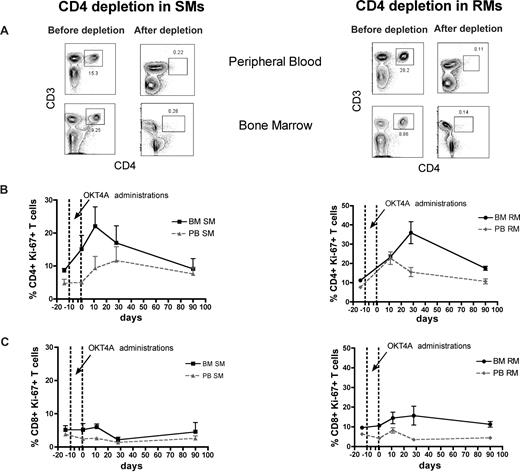
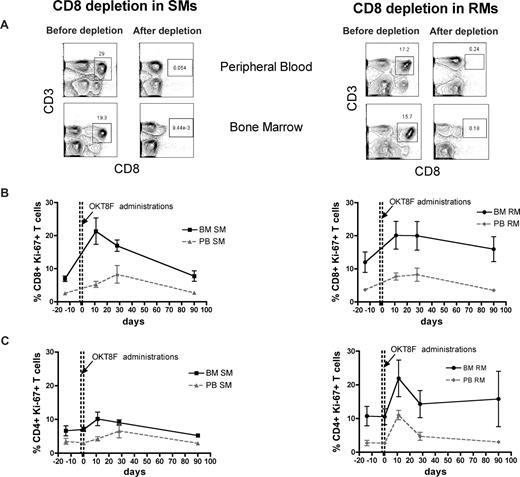
The BM microenvironment is peculiarly rich in cytokines and growth factors, including those involved in the regulation of T-cell homeostasis, such as IL-7 and IL-15.16-19 As such, we hypothesized that the high levels of BM-based T-cell proliferation found in SMs and RMs are, at least in part, driven by homeostatic stimuli. To directly test this hypothesis, we set up an experimental system wherein homeostasis-driven T-cell proliferation would be predicted to be triggered. Specifically, we induced a transient but severe depletion of CD4+ or CD8+ T cells in 3 healthy SMs and 3 healthy RMs by administrating a depleting OKT4A or OKT8F monoclonal antibody, respectively, and then measured the level of T-cell proliferation in BM and PB. As detailed in “Depletion of CD4+ or CD8+ cells in vivo,” for CD4+ T-cell depletion, the OKT4A monoclonal antibody was administered 4 times in a 10-day period (days −10, −7, −3, and 0), whereas for CD8+ T-cell depletion, the OKT8F monoclonal antibody was administered for 3 consecutive days (days −2, −1, and 0). BM- and PB-derived mononuclear cells were collected from the animals before antibody administration (day −14), at the same day of the last antibody administration (day 0), and at days 11, 28, and 90. As expected, both depleting antibodies induced a specific, rapid, and profound depletion of CD4+ or CD8+ T cells in PB and BM (> 95% depletion compared with baseline) of SMs and RMs. Representative examples of the nadir CD4+ and CD8+ T-cell depletion in the PB and BM of SMs and RMs are shown in Figures 4A and 5A, respectively.
Antibody-mediated CD4+ T-cell depletion is followed by increased CD4+ T-cell proliferation in the BM. Levels of T-cell proliferation were longitudinally assessed during antibody-mediated depletion of CD4+ cells in 3 healthy SMs and 3 healthy RMs. (A) Representative examples of the levels of CD4+ T cells before and after depletion in the PB and BM of a representative SM (left) and RM (right). (B) Average percentage of CD4+Ki-67+ T cells in the BM and PB before and at different time points after CD4 depletion in SMs (left) and RMs (right). (C) Average percentage of CD8+Ki-67+ T cells in the BM and PB before and at different time points after CD4 depletion in SMs (left) and RMs (right). The dotted lines in panels B and C indicate the timing of anti-CD4 monoclonal antibody administrations. Because of the extremely low level of CD4+ T cells, their levels of proliferation could not be determined at day 0 in RMs.
Antibody-mediated CD4+ T-cell depletion is followed by increased CD4+ T-cell proliferation in the BM. Levels of T-cell proliferation were longitudinally assessed during antibody-mediated depletion of CD4+ cells in 3 healthy SMs and 3 healthy RMs. (A) Representative examples of the levels of CD4+ T cells before and after depletion in the PB and BM of a representative SM (left) and RM (right). (B) Average percentage of CD4+Ki-67+ T cells in the BM and PB before and at different time points after CD4 depletion in SMs (left) and RMs (right). (C) Average percentage of CD8+Ki-67+ T cells in the BM and PB before and at different time points after CD4 depletion in SMs (left) and RMs (right). The dotted lines in panels B and C indicate the timing of anti-CD4 monoclonal antibody administrations. Because of the extremely low level of CD4+ T cells, their levels of proliferation could not be determined at day 0 in RMs.
Antibody-mediated CD8+ cell depletion is followed by increased CD8+ T-cell proliferation in the BM. Levels of T-cell proliferation were longitudinally assessed during antibody-mediated depletion of CD8+ cells in 3 healthy SMs and 3 healthy RMs. (A) Representative examples of the levels of CD8+ T cells before and after depletion in the PB and BM of a representative SM (left) and RM (right). (B) Average percentage of CD8+Ki-67+ T cells in the BM and PB before and at different time points after CD8 depletion in SMs (left) and RMs (right). (C) Average percentage of CD4+Ki-67+ T cells in the BM and PB before and at different time points after CD8 depletion in SMs (left) and RMs (right). The dotted lines in panels B and C indicated the timing of anti-CD8 monoclonal antibody administrations. Because of the extremely low level of CD8+ T cells, it was not possible to analyze their levels of Ki-67 expression at day 0 in both SMs and RMs.
Antibody-mediated CD8+ cell depletion is followed by increased CD8+ T-cell proliferation in the BM. Levels of T-cell proliferation were longitudinally assessed during antibody-mediated depletion of CD8+ cells in 3 healthy SMs and 3 healthy RMs. (A) Representative examples of the levels of CD8+ T cells before and after depletion in the PB and BM of a representative SM (left) and RM (right). (B) Average percentage of CD8+Ki-67+ T cells in the BM and PB before and at different time points after CD8 depletion in SMs (left) and RMs (right). (C) Average percentage of CD4+Ki-67+ T cells in the BM and PB before and at different time points after CD8 depletion in SMs (left) and RMs (right). The dotted lines in panels B and C indicated the timing of anti-CD8 monoclonal antibody administrations. Because of the extremely low level of CD8+ T cells, it was not possible to analyze their levels of Ki-67 expression at day 0 in both SMs and RMs.
The levels of T-cell proliferation were longitudinally assessed in the 2 examined anatomic sites by staining for the Ki-67 marker. We first analyzed the proliferative response in CD4+ T cell–depleted animals and found that, in both species, the depletion of CD4+ cells is followed by a rapid BM-based CD4+ T-cell proliferation that is faster (in SMs) and more sustained (both in SMs and RMs) compared with that found in PB (Figure 4B). We then analyzed the proliferative response in CD8+ T cell–depleted animals and also found that, in both species, monoclonal antibody-induced CD8+ T-cell depletion was followed by a significant and brisk increase in the percentage of proliferating CD8+ T cells in the BM (Figure 5B). It is of note that the BM-based proliferation that follows CD4+ or CD8+ T-cell depletion seems to be predominantly lineage-specific, with an increase in CD4+ T-cell proliferation and limited change in CD8 compartment when CD4+ cells are depleted (in both species, Figure 4C), and a specific increase in CD8+ T-cell proliferation with limited change in the CD4+ T-cell subset when CD8+ T cells are depleted (more evident for SMs, Figure 5C). Taken together, these experiments of in vivo antibody-mediated CD4+ or CD8+ T-cell depletion suggest that BM-based T-cell proliferation plays an important role in regulating the homeostasis of T-lymphocyte compartments in healthy, uninfected NHPs and raise the question of whether and to what extent this role is preserved during SIV infection.
BM-based CD4+ T-cell renewal is preserved in naturally SIV-infected SMs but not in SIV-infected RMs
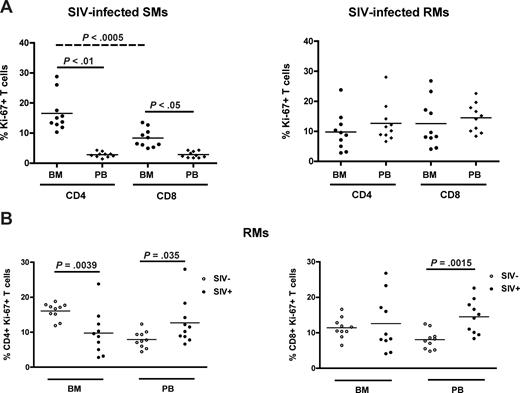
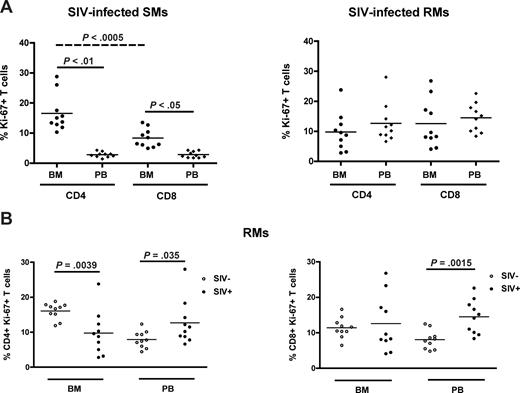
SIV infection in SMs is typically nonpathogenic and associated with preservation of peripheral CD4+ T-cell counts in the majority of animals,25,27,33 thus in sharp contrast to the profound CD4+ T-cell depletion associated with pathogenic HIV or SIV infection in humans and RMs, respectively. As our data suggest that the BM plays a role in the maintenance of T-cell homeostasis in healthy, SIV-uninfected NHPs, we next sought to determine whether BM-based CD4+ T-cell renewal contributes to the different ability to preserve a normal CD4+ T-cell compartment after SIV infection between SMs and RMs. To this end, we measured the percentage of CD4+ and CD8+ T cells that express Ki-67 in the BM and PB of 10 SIV-infected SMs and 10 SIVmac-infected RMs. Similar to uninfected animals, SIV-infected SMs exhibit levels of CD4+ (P < .01) and CD8+ (P < .05) T-cell proliferation significantly higher in BM than in PB (Figure 6A left panel). In contrast, SIV-infected RMs show comparable levels of T-cell proliferation in the BM and PB (Figure 6A right panel). We next compared the level of T-cell proliferation between SIV-infected and uninfected RMs; and, as expected, we found that both CD4+ (left panel) and CD8+ (right panel) T cells isolated from PB expressed significantly higher levels of Ki-67 in SIV-infected animals. Interestingly, we found that the levels of proliferating CD4+, but not CD8+, T cells in the BM of SIV-infected RMs were significantly lower compared with uninfected animals (Figure 6B). Collectively, these data suggest that BM-based CD4+ T-cell proliferation is fully preserved in naturally SIV-infected SMs but compromised in chronically SIV-infected RMs. Of note, we found that, in SIV-infected SMs, the level of CD4+ T-cell proliferation in the BM was significantly higher (P < .001) than the level of CD8+ T-cell proliferation (Figure 6A), and did not correlate with viral load (not shown). These findings support the hypothesis that the CD4+ T-cell proliferation occurring in the BM of SIV-infected SMs is more likely to be homeostasis-driven rather than reflecting immune responses to the virus. Taken together, these results identify a new difference in the way SIV infection affects the immune system in SMs versus RMs, with the former showing higher levels of CD4+ T-cell proliferation in the BM in the context of preserved peripheral CD4+ T-cell counts.
BM-based CD4+ T-cell renewal is preserved in naturally SIV-infected SMs, but not in SIV-infected RMs. The fraction of CD4+ and CD8+ T cells expressing Ki-67 was assessed in the BM and PB of SIV-infected SMs and RMs. (A) Percentage of Ki-67 expression in CD4+ and CD8+ cells isolated from the BM and PB in 10 SIV-infected SMs (left) and RMs (right). (B) Percentage of CD4+ (left) and CD8+ (right) T cells expressing Ki-67 in the BM and PB of 10 uninfected (○) and 10 SIV-infected (●) RMs. P values are shown when significant differences between groups are present.
BM-based CD4+ T-cell renewal is preserved in naturally SIV-infected SMs, but not in SIV-infected RMs. The fraction of CD4+ and CD8+ T cells expressing Ki-67 was assessed in the BM and PB of SIV-infected SMs and RMs. (A) Percentage of Ki-67 expression in CD4+ and CD8+ cells isolated from the BM and PB in 10 SIV-infected SMs (left) and RMs (right). (B) Percentage of CD4+ (left) and CD8+ (right) T cells expressing Ki-67 in the BM and PB of 10 uninfected (○) and 10 SIV-infected (●) RMs. P values are shown when significant differences between groups are present.
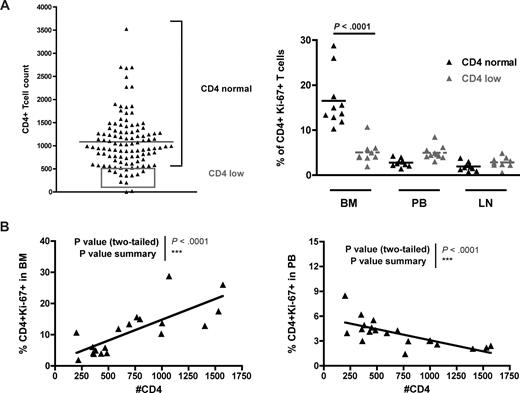
Low levels of BM-based CD4+ T-cell proliferation in SIV-infected SMs with low CD4+ T-cell count
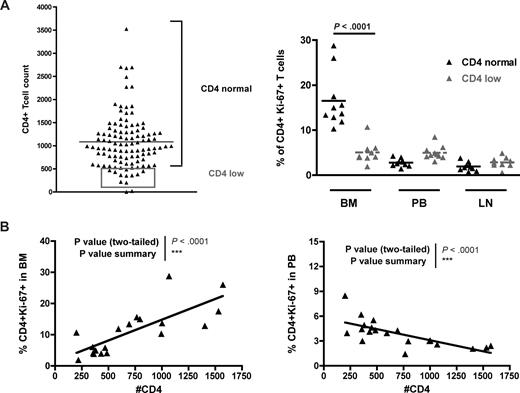
In a previous study,33 we performed a survey of 110 naturally SIV-infected SMs housed at the Yerkes National Primate Research Center and found that 14 of them showed a CD4+ T-cell count less than 500/mm3. It is of note that none of these “CD4 low” naturally SIV-infected SMs progressed to AIDS. Although a severe depletion of CD4+ T cells was observed in a subset of experimentally SV-infected SMs that developed a CXCR4-tropic virus,45 in these naturally SIV-infected “CD4 low” SMs, only CCR5-tropic viruses were isolated (D.L.S. and G.S., unpublished data, 2007). To test the possibility that a defect in BM-based CD4+ T-cell renewal was involved in the loss of CD4+ T-cell homeostasis in the “CD4 low” naturally SIV-infected SMs, we next measured the levels of CD4+ T-cell proliferation in BM, PB, and LN of 10 of these 14 animals (represented in the gray rectangle of Figure 7A) and compared them with those of SIV-infected SMs with normal CD4+ T-cell counts (eg, the same animals analyzed in Figure 6A). We found that “CD4 low” naturally SIV-infected SMs did not show the typical increase in the level of BM-based CD4+ T-cell proliferation (compared with PB or LN) and that, indeed, the percentages of CD4+ Ki-67+ T cells in the BM of “CD4 low” animals were significantly lower than in SIV-infected SMs with preserved CD4+ T-cell counts (Figure 7A). Interestingly, we also observed that, in naturally SIV-infected SMs (both “CD4 low” and “CD4 normal”), CD4+ T-cell counts correlated directly with the level of CD4+ T-cell proliferation in BM (P < .001) but inversely with CD4+ T-cell proliferation in PB (P < .001; Figure 7B). These findings suggest that, in SIV-infected SMs, the CD4+ T-cell proliferation in the BM is involved in maintaining the pool of circulating CD4+ T cells and is consistent with previous findings indicating that the level of CD4+ T-cell proliferation in PB is probably an index of the level of immune activation.33 The facts that “CD4 low” SIV-infected SMs do not exhibit an increased level of BM-based CD4+ T-cell proliferation and that higher levels of CD4+ T-cell proliferation in BM correlate with better preservation of peripheral CD4+ T-cell counts provide further evidence in support of the hypothesis that BM plays a key role in regulating the homeostasis of CD4+ T cells.
BM-based CD4+ T-cell proliferation contributes to preserve a normal CD4+ T-cell compartment in SMs after SIV infection. Levels of CD4+ T-cell proliferation in SIV-infected SMs that preserve, or not, the peripheral homeostasis of the CD4+ T-cell compartment. (A, left) Although the majority of SIV-infected SMs maintain a CD4+ T-cell count more than 500/mm3 (CD4 normal), a small percentage of these animals (∼10%) show a CD4+ T-cell count less than 500/mm3 (CD4 low, gray box). Two SMs were not included in the analysis because their levels of CD4+ T cells were too low to accurately determine the expression of Ki-67. (A, right) Percentage of CD4+Ki-67+ T cells in BM, PB, and LN of 10 SIV-infected SMs defined as “CD4 normal” (▲) and 10 SIV-infected SMs defined as “CD4 low” ( ). P values are shown when significant differences between groups are present. (B) In SIV-infected SMs, peripheral CD4+ T-cell count correlates directly with the percentage of proliferating CD4+ T cells in BM (left panel) and inversely with the percentage of proliferating CD4+ T cells in PB (right panel). P values of the correlation coefficient (Spearman rank correlation test) are shown.
). P values are shown when significant differences between groups are present. (B) In SIV-infected SMs, peripheral CD4+ T-cell count correlates directly with the percentage of proliferating CD4+ T cells in BM (left panel) and inversely with the percentage of proliferating CD4+ T cells in PB (right panel). P values of the correlation coefficient (Spearman rank correlation test) are shown.
BM-based CD4+ T-cell proliferation contributes to preserve a normal CD4+ T-cell compartment in SMs after SIV infection. Levels of CD4+ T-cell proliferation in SIV-infected SMs that preserve, or not, the peripheral homeostasis of the CD4+ T-cell compartment. (A, left) Although the majority of SIV-infected SMs maintain a CD4+ T-cell count more than 500/mm3 (CD4 normal), a small percentage of these animals (∼10%) show a CD4+ T-cell count less than 500/mm3 (CD4 low, gray box). Two SMs were not included in the analysis because their levels of CD4+ T cells were too low to accurately determine the expression of Ki-67. (A, right) Percentage of CD4+Ki-67+ T cells in BM, PB, and LN of 10 SIV-infected SMs defined as “CD4 normal” (▲) and 10 SIV-infected SMs defined as “CD4 low” ( ). P values are shown when significant differences between groups are present. (B) In SIV-infected SMs, peripheral CD4+ T-cell count correlates directly with the percentage of proliferating CD4+ T cells in BM (left panel) and inversely with the percentage of proliferating CD4+ T cells in PB (right panel). P values of the correlation coefficient (Spearman rank correlation test) are shown.
). P values are shown when significant differences between groups are present. (B) In SIV-infected SMs, peripheral CD4+ T-cell count correlates directly with the percentage of proliferating CD4+ T cells in BM (left panel) and inversely with the percentage of proliferating CD4+ T cells in PB (right panel). P values of the correlation coefficient (Spearman rank correlation test) are shown.
Discussion
In both humans and mice, a significant proportion of functional mature CD4+ and CD8+ T lymphocytes reside in the BM.6,7 In rodents, these BM-resident T cells contain a high proportion of memory and activated cells8,10,11 and include a percentage of proliferating memory CD8+ T cells that is higher than that found in the blood or secondary lymphoid organs.13-15 In one recent study, 3 different measures of cell division, ie, propidium iodide, bromodeoxyuridine, and CFSE, confirmed that BM contains the most actively dividing pool of memory CD8+ T cells in mice.15 Taken together, these observations indicate that the BM, with its unique microenvironment typically enriched in cytokines with T-cell growth factor activity,16-19 is a preferred site for T-cell proliferation and homeostasis.
The possibility that BM-based T-cell proliferation plays a key role in the maintenance of T-cell homeostatic balance in primates, either human or nonhuman, has not been systematically investigated. Numerous human diseases are associated with failure of T-cell homeostasis, and it could be hypothesized that, in these conditions, specific T-cell defects occurring at the level of BM may be present. Chronic HIV infection is characterized by a profound perturbation of CD4+ T-cell homeostasis that ultimately leads to progressive CD4+ T-cell depletion and AIDS.29,46,47 The mechanisms responsible for the loss of CD4+ T cells in HIV-infected persons are complex and include direct viral infection, death of bystander uninfected T cells (mainly as a result of increased susceptibility to apoptosis), and insufficient T-cell reconstitution. The role of this latter factor in AIDS pathogenesis is emphasized by the observation that disease progression is ultimately associated with depletion of naive and central-memory CD4+ T cells, even though the majority of CD4+ T cells that are either killed by the virus or succumb to apoptosis show an effector or effector-memory phenotype.29,46,48,49 Interestingly, pathogenic HIV infection of humans and SIV infection of RMs are associated with clinical signs of BM suppression, including neutropenia, thrombocytopenia, and lymphopenia.34-36
Important insights into the pathogenesis of HIV infection have been provided by studies of natural, nonpathogenic SIV infection of SMs.21,25-27 Of note, naturally SIV-infected SMs maintain healthy levels of peripheral CD4+ T cells despite levels of virus replication that are as high or even higher than those found in HIV-infected persons who progress to AIDS.25-27 In a previous study, we showed that SIV-infected SMs maintain normal BM architecture and a preserved pool of naive T cells.27 Because BM has been indicated has a preferential site for homeostatic T-cell proliferation in mice13-15 and it shows some level of dysfunction in HIV-infected patients,34-36 we hypothesized that the ability of SIV-infected SMs to preserve BM-based T-cell proliferation is involved in the preservation of CD4+ T-cell homeostasis in these animals after SIV infection.
To test this hypothesis, we performed a comprehensive study of BM as an anatomic site for T-cell proliferation in 2 species of NHPs (RMs and SMs) and investigated its potential role in maintaining T-cell homeostasis under different depleting conditions (ie, antibody-mediated depletion of either CD4+ and CD8+ T cells and SIV infection). In a first set of experiments, we confirmed that, in both uninfected SMs and RMs, BM-derived CD4+ and CD8+ T lymphocytes proliferated to a higher extent compared with those isolated from PB and LNs. In addition, we observed that, in SMs, the increased proliferative capacity of BM-resident T cells was not limited to memory subsets but involved also naive cells.
Conceivably, high levels of proliferating T cells in the BM of healthy NHPs could be related to immune response against foreign antigens50 or, alternatively, represent a homeostatic response to compensate for the physiologic loss of senescent cells. To directly assess the role that BM-based T-cell proliferation may play in maintaining the homeostasis of T cells in primates, we longitudinally measured the level of T-cell proliferation in the BM of healthy, SIV uninfected SMs and RMs in which we depleted either CD4+ or CD8+ T cells in vivo using monoclonal antibodies. Consistent with a potential homeostatic nature of the BM-based T-cell proliferation, we observed that, in both species, CD4+ or CD8+ cell depletion was followed by increased level of proliferation within the depleted population that was more rapid and sustained in BM compared with PB. Interestingly, our findings also suggest that the mechanisms regulating T-cell homeostasis seem to be able to differentiate CD4+ from CD8+ T cell losses, with a brisk increase in CD4+ T-cell proliferation and limited changes in the CD8+ compartment after CD4+ T-cell depletion (and, vice versa, increased CD8+ T-cell proliferation and limited changes in CD4+ compartment when CD8+ T cells are depleted). In all, these sets of experiments suggest that, in 2 species of NHP, (1) BM is a preferential site for T-cell proliferation and (2) the BM-based T-cell proliferation plays an important role in regulating T-cell homeostasis in an experimentally induced lymphopenic environment.
Based on these observations, we next sought to determine, in the same NHP species, the impact of SIV infection on the function of BM in regulating T-cell homeostasis. In particular, we investigated whether and to what extent BM-based CD4+ T-cell proliferation was differently affected during pathogenic SIV infection in RMs (where the homeostasis of the peripheral CD4+ T-cell compartment is compromised) and nonpathogenic SIV infection in SMs (where the homeostasis of the peripheral CD4+ T-cell compartment is preserved). Our results indicate that, in SIV-infected SMs, the rates of CD4+ T-cell proliferation were significantly higher in BM compared with the other tested anatomic sites (ie, PB and LN). In contrast, in SIV-infected RMs, CD4+ T cells derived from the BM were found to proliferate to the same level as those in PB and show a fraction of Ki-67+ cells that was significantly lower compared with those found in the BM of uninfected animals. These data suggest that a BM-based homeostatic response to the chronic loss of CD4+ T cells is preserved in naturally SIV-infected SMs but compromised in SIV-infected RMs.
The increased CD4+ T-cell proliferation observed in the BM of SIV-infected SMs may also be interpreted as predominantly reflecting an SIV-specific CD4+ T-cell response. However, 3 lines of indirect evidence argue against this possibility. First, the magnitude of SIV-specific CD4+ T cells measured in SIV-infected SMs indicates that the level of these responses is usually very low and involves less than 1% of total CD4+ T cells.39 Second, no correlation was found between SIV viremia, which probably reflects the overall antigenic load of SIV, and levels of CD4+ Ki-67+ T cells in the BM (data not shown). Third, in SIV-infected SMs, the level of CD4+ T-cell proliferation in the BM was higher than that of CD8+ T cells, even though both CD4+ and CD8+ T cells would be expected to proliferate in response to SIV antigens. Altogether, these data support the hypothesis that the increased CD4+ T-cell proliferation in the BM of SIV-infected SMs represents, at least in part, a lineage-specific homeostatic mechanism that compensates for the chronic, SIV-induced killing of CD4+ T cells. As such, the current data suggest that, in naturally SIV-infected SMs, a functioning BM is instrumental in maintaining T-cell homeostasis and avoids AIDS despite continuous virus replication.
The mechanisms by which HIV infection induces CD4+ T-cell depletion and AIDS are not yet fully understood and probably very complex. The findings reported in this study indicate that a defect in the BM-based homeostatic CD4+ T-cell proliferation may be one of these factors. To further explore this concept, we determined the levels of BM-based CD4+ T-cell proliferation in a rare subset of SIV-infected SMs with peripheral CD4+ T-cell counts less than 500/mm3, and compared them with those found in SIV-infected SMs that preserve normal CD4 count. Consistent with the hypothesis that CD4+ T-cell proliferation in the BM contributes to the maintenance of CD4+ T-cell compartment, we found that naturally SIV-infected SMs with low CD4+ T-cell counts showed lower levels of BM-based CD4+ T-cell proliferation compared with SIV-infected SMs with preserved CD4+ T cells. This latter result suggests that, in a small percentage of SIV-infected SMs, a failure of the BM-based compensatory CD4+ T-cell proliferation may be instrumental in determining a progressive loss of CD4+ T-lymphocyte homeostasis.
Additional studies are required to identify what specific cytokines and growth factors may be primarily responsible for determining the set-point level of CD4+ and CD8+ T-cell proliferation in the BM. In particular, it will be interesting to analyze the effects of cytokines, such as IL-7 and IL-15, which are currently under investigation for the treatment of lymphopenic conditions in humans. In the context of AIDS pathogenesis, additional work will be necessary to determine to what extent a more robust CD4+ T-cell proliferation in the BM contributes to the disease resistance in natural hosts for SIV and, conversely, to investigate whether a specific defect in BM-based homeostatic CD4+ T-cell proliferation is a factor involved in AIDS pathogenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Daniel Andersonn and Mark Feinberg for helpful discussions and critical reading of the manuscript, and Stephanie Ehnert and Dr Elizabeth Strobert for their assistance with animal studies.
This work was supported by National Institutes of Health grants AI-66998 and AI-54234 (G.S.), HL-075766 (S.I.S.), and RR00165 (Yerkes National Primate Research Center).
National Institutes of Health
Authorship
Contribution: M.P., B.C., and G.S. designed the study; M.P. and B.C. performed the immunophenotypic and statistical analyses, analyzed results, and prepared the figures; J.C.E. and N.R.K. performed the CD4 and CD8 cell depletion and contributed to the design of the study; S.N.G. and A.M. provided the data regarding SMs with low CD4+ T cells and contributed to the design of the study; S.I.S. performed the viral load assay and contributed to the design of the study; R.S.M. provided the depleting monoclonal antibodies and contributed to the design of the study; J.E. supervised the housing and care of the animals and contributed to the design of the study; D.L.S. contributed to the design and writing of this paper; and M.P. and G.S. wrote the paper, with contributions from the other authors as appropriate.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guido Silvestri, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 705 Stellar Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19143; e-mail: gsilvest@mail.med.upenn.edu.
References
Author notes
*M.P. and B.C. contributed equally to this study.