Abstract
Acute lymphopenia-induced homeostatic proliferation (HP) of T cells promotes antitumor immunity, but the mechanism is unclear. We hypothesized that this is due to a lack of inhibitory signals that allows activation of T cells with low affinity for self-antigens. Tumors resist immunity in part by expressing inhibitory molecules such as PD-1 ligand 1 (PD-L1), B7-H4, and TGF-β. In irradiated mice undergoing HP, we found that T cells displayed a severe deficit in the activation-induced expression of inhibitory molecules PD-1 and CTLA-4, and TGF-β1–induced expression of Foxp3. HP T cells were also less suppressed by B7-H4/Ig and, unlike control T cells, failed to produce IL-10 in response to this molecule. This deficiency in regulation was reversed as normal T-cell numbers were restored. We conclude that T cells are weakly regulated by inhibitory molecules during the acute phase of HP, which could explain their increased effectiveness in cancer immunotherapy.
Introduction
Under conditions of lymphopenia naive T cells undergo acute homeostatic proliferation (HP)1-4 and have increased reactivity against tumors.1,5-10 In addition, HP has been reported to break anergy,8 ameliorate antitumor vaccination,5-7 and promote impressive response rates (up to 50%) in the clinical immunotherapy of melanoma.9,10 However, the mechanisms for the elicitation of effective antitumor responses are unclear
Malignant tumors are thought to resist immunity at least in part by expressing immune inhibitory molecules such as PD-1 ligand 1 (PD-L1), B7-H4, and TGF-β. In addition, CTLA-4, expressed by T cells, inhibits antitumor immunity.11 Here, we hypothesized that HP T cells resist inhibitory signals and, as a consequence, respond to self-antigens for which they have low affinity, including those expressed by tumor cells. To address this question, we analyzed T cells undergoing HP for the level of expression and/or response to negative regulatory molecules, including CTLA-4, PD-1, TGF-β, and B7-H4.
Methods
The use of mice in this study was approved by the animal review committees of St Michael's Hospital and the University of Toronto. Complete materials and methods are available on the Blood website; see the Supplemental Materials link at the top of the online article
Results and discussion
After cell transfer into irradiated mice, the number of splenic CD4+ cells peaked at day 27, followed by a decline and stabilization (Figure S1). On the other hand, the number of splenic CD8+ cells peaked at day 20, followed by a plateau up to day 27, and a decline and stabilization thereafter (Figure S1). We analyzed cells at 2 or 3 weeks following cell transfer. It should be noted that few of these T cells (< 5%) are newly produced by the thymus,12 and the phenotypic changes we describe occurred in donor cells identified with Thy1 or CD45 allelic markers as described.1
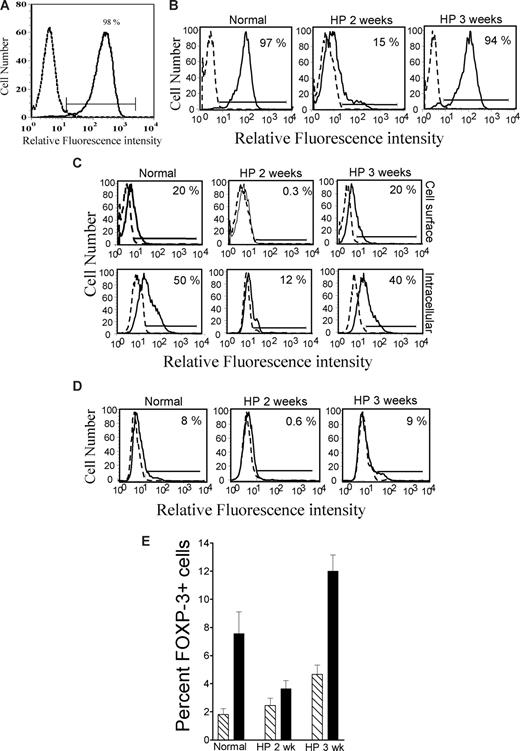
Previous studies showed that HP T cells exhibit a partially activated phenotype.1,6,12 For example, although these cells express several memory markers (eg, CD44, CD122, and Ly6C), they do not up-regulate CD69 and CD25.13 However, HP T cells effectively respond in vitro to CD3/CD28 stimulation by proliferating and producing cytokines (especially IFN-γ)13,14 as early as 9 days after cell transfer.13 Consistent with these findings, we found that, at day 14 after cell transfer, more than 95% of HP CD4+CD25− T cells are activated when stimulated in vitro with anti-CD3/CD28 antibodies, as demonstrated by induction of CD25 expression (Figure 1A). Approximately two thirds of these cells also expressed the early activation marker CD69 (data not shown). Furthermore, upon in vitro stimulation, the HP T cells proliferated as well as the control T cells (Figure S2).
T cells are defective in the expression of PD-1, CTLA-4, and Foxp3 at the early stages of HP. (A) Freshly isolated spleen cells were transfused into irradiated mice. After 2 weeks, splenic CD4+CD25− T cells were isolated and stimulated with anti-CD3/CD28 for 48 hours and analyzed for CD25 expression (IL-2α receptor). Almost all HP T cells were activated, as shown by up-regulation of CD25. Similar results were obtained with conventional (non-HP) T cells (data not shown). (B) Freshly isolated spleen cells were transfused into irradiated mice. After 2 weeks and 3 weeks, splenic CD4+CD25− T cells were isolated and stimulated with anti-CD3/CD28 for 48 hours and analyzed for PD-1 expression. Few HP T cells up-regulated PD-1 expression at 2 weeks, but this was restored to normal levels at 3 weeks. (C) Same as panel B, but analyzed for cytoplasmic and membrane CTLA-4. In panels A-C, dotted line histogram indicates isotype control; solid line histogram, specific antibody. Low expression of CTLA-4 followed the same pattern as PD-1 expression. (D) Same as panel B, but stimulation was in the absence (dotted line histogram) or presence (solid line histogram) of 2 ng/mL TGF-β1, and analyzed for Foxp3 expression. At 2 weeks, Foxp3 was poorly induced in HP T cells compared with control T cells, but this was restored at 3 weeks. (E) Percentage of Foxp3+ cells induced by TGF-β as in panel D (mean ± SEM; n = 4), at 2 weeks (HP 2 wk) or 3 weeks (HP 3 wk) after cell transfer. The expression of PD-1, CTLA-4, and Foxp3 was severely deficient at 2 weeks (n = 4, P < .05), but completely restored at 3 weeks. In all cases, 4 mice per group were examined and a representative histogram is shown.
T cells are defective in the expression of PD-1, CTLA-4, and Foxp3 at the early stages of HP. (A) Freshly isolated spleen cells were transfused into irradiated mice. After 2 weeks, splenic CD4+CD25− T cells were isolated and stimulated with anti-CD3/CD28 for 48 hours and analyzed for CD25 expression (IL-2α receptor). Almost all HP T cells were activated, as shown by up-regulation of CD25. Similar results were obtained with conventional (non-HP) T cells (data not shown). (B) Freshly isolated spleen cells were transfused into irradiated mice. After 2 weeks and 3 weeks, splenic CD4+CD25− T cells were isolated and stimulated with anti-CD3/CD28 for 48 hours and analyzed for PD-1 expression. Few HP T cells up-regulated PD-1 expression at 2 weeks, but this was restored to normal levels at 3 weeks. (C) Same as panel B, but analyzed for cytoplasmic and membrane CTLA-4. In panels A-C, dotted line histogram indicates isotype control; solid line histogram, specific antibody. Low expression of CTLA-4 followed the same pattern as PD-1 expression. (D) Same as panel B, but stimulation was in the absence (dotted line histogram) or presence (solid line histogram) of 2 ng/mL TGF-β1, and analyzed for Foxp3 expression. At 2 weeks, Foxp3 was poorly induced in HP T cells compared with control T cells, but this was restored at 3 weeks. (E) Percentage of Foxp3+ cells induced by TGF-β as in panel D (mean ± SEM; n = 4), at 2 weeks (HP 2 wk) or 3 weeks (HP 3 wk) after cell transfer. The expression of PD-1, CTLA-4, and Foxp3 was severely deficient at 2 weeks (n = 4, P < .05), but completely restored at 3 weeks. In all cases, 4 mice per group were examined and a representative histogram is shown.
As related to antitumor immunity, a relevant question is whether the HP T cells are defective in expressing inhibitory molecules. Therefore, we examined CTLA-4 and PD-1 expression induced by T-cell activation.11,15 CD4+CD25− T cells from normal mice or mice undergoing HP were stimulated in vitro with anti-CD3/CD28 antibodies. Costimulation induced PD-1 expression on almost all control T cells (Figure 1B), as well as cytoplasmic and membrane expression of CTLA-4 on a sizable fraction (50% and 20%, respectively) of these cells (Figure 1C). However, at 2 weeks after cell transfer, up-regulation of these 2 molecules was severely impaired in the HP T cells (Figure 1B,C). This defect was reversible, with almost complete recovery at 3 weeks after cell transfer (Figure 1B,C). Deficiency of CTLA-4 and PD-1 induction was noted in CD4+ T cells, but not CD8+ T cells (data not shown). However, the generation of HP CD8+ effector cells in vivo has been shown to be highly CD4+-cell dependent,16 suggesting that CD4+ T-cell alterations will affect both populations. Interestingly, we found that when T cells were induced to express PD-1 by activation prior to adoptive transfer into lymphopenic mice, they down-regulated PD-1 in vivo (Figure S3). This is in contrast to the increased expression of PD-1 observed in lymphopenic HIV-infected patients.17 In these patients, PD-1+ T cells are characterized by clonal exhaustion or unresponsiveness, which is reversed by blocking PD-1/PD-L1 interactions. Thus, it appears that the T cells undergoing acute HP, as observed in our experiments, are considerably different from those encountered in virally infected lymphopenic patients.
The defects in CTLA-4 and PD-1 expression we are reporting are unlikely to be due to the induction of regulatory T (Tr) cells (possibly suppressing T-cell activation). In vivo, splenic Foxp3+ T-cell numbers were present in normal proportions during the entire period of HP we examined (data not shown). We examined whether HP T cells respond to TGF-β1, which is produced by tumors18 and induces Foxp3+ Tr-cell differentiation.19 Control and HP T cells were equally susceptible to the suppression of proliferation and IL-2 production by TGF-β1 (not shown), but HP CD4+25− T cells obtained at 2 weeks after transfer showed only minor increases in Foxp3+ cells, unlike control T cells (Figure 1D,E). This defect was reversed at 3 weeks, and at that point Foxp3 induction was increased in the HP T cells. Thus, Tr-cell differentiation in vitro is transiently impaired, but in vivo these cells are not deficient and Tr-cell deficiency does not appear to be a major factor in HP. Another important issue is whether HP T cells resist suppression by CD4+CD25+Foxp3+ Tr cells, but the work of other investigators reveals that this is not the case.20 Depletion of CD4+CD25+ Tr cells prior to adoptive transfer into lymphopenic mice resulted in improved antitumor immune responses, in agreement with the conclusion that HP effector T cells are sensitive to Tr-mediated suppression.21
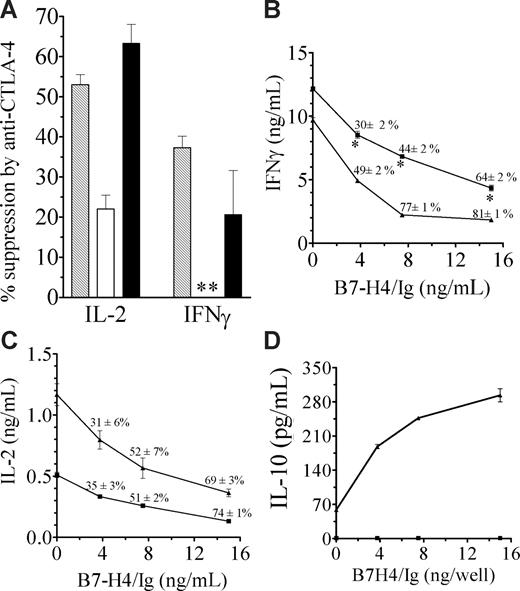
Reduced expression of CTLA-4 and PD-1 suggests HP CD4+ T cells will be less sensitive to negative regulation. To explore this possibility, we first examined CTLA-4–mediated suppression by assessing the effect of cross-linking CD3, CD28, and CTLA-4 with immobilized antibodies. Previous studies have shown that engaging CTLA-4 under this co–cross-linking condition inhibits T-cell activation.22 This contrasts with the use of soluble anti–CTLA-4 antibodies, which block CTLA-4–mediated inhibitory signaling, leading to increased T-cell activation.11 Suppression was examined in restimulated T cells because CTLA-4 is up-regulated by primary CD3/CD28 stimulation, rendering the suppressive effects of CTLA-4 more apparent. As anticipated from the flow cytometric data, we found that early stage HP T cells (2 weeks) were much less sensitive to inhibition by antibody-mediated CTLA-4 cross-linking, as shown by IL-2 and IFN-γ secretion (P < .05; Figure 2A). In fact, IFN-γ production was paradoxically increased by the anti–CTLA-4 antibody during this early phase. However, as expected, HP T cells obtained at later stages (3 weeks), when CTLA-4 expression was restored, were sensitive to suppression by anti–CTLA-4 antibodies (Figure 2A).
HP T cells are weakly suppressed by CTLA-4 and B7-H4. (A) Splenic CD4+CD25− T cells recovered 2 or 3 weeks after cell transfer and stimulated with plate-bound anti-CD3/CD28, to induce CTLA-4 expression, and restimulated with anti-CD3 in the presence of anti–CTLA-4 (or control IgG). Supernatants were assayed for IFN-γ and IL-2. Results are presented as percentage suppression = 100 × (cytokine release in the absence of anti–CTLA-4 − cytokine release in the presence of anti-CTLA-4)/cytokine release in the absence of anti–CTLA-4.  indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.
indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.
HP T cells are weakly suppressed by CTLA-4 and B7-H4. (A) Splenic CD4+CD25− T cells recovered 2 or 3 weeks after cell transfer and stimulated with plate-bound anti-CD3/CD28, to induce CTLA-4 expression, and restimulated with anti-CD3 in the presence of anti–CTLA-4 (or control IgG). Supernatants were assayed for IFN-γ and IL-2. Results are presented as percentage suppression = 100 × (cytokine release in the absence of anti–CTLA-4 − cytokine release in the presence of anti-CTLA-4)/cytokine release in the absence of anti–CTLA-4.  indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.
indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.
Because tumors frequently express B7-H4, which has been shown to inhibit T-cell immunity in some experimental models23 by binding to a yet unidentified receptor, we also examined the role of this molecule. As depicted in Figure 2B and C, compared with control T cells, the HP T cells produced more IFN-γ, but moderately less IL-2 in response to CD3/CD28 stimulation. Bound B7-H4/Ig (IgG1-Fc24,25 ) inhibited IL-2 secretion of normal and HP T cells to the same extent (Figure 2C). Proliferation was also inhibited to a similar extent in both cell types (data not shown). However, HP T cells were less sensitive to B7-H4/Ig suppression of IFN-γ production (P < .05; Figure 2B). Importantly, B7-H4 costimulation induced a marked increase in IL-10 production in control T cells, but not HP T cells that completely failed to produce IL-10 (Figure 2D). These defects were still present in HP T cells obtained 25 days after transfer, unlike the CTLA-4 and PD-1 alterations. In view of the many inhibitory effects of IL-10, its production by some regulatory T cells, and its negative role in tumor immunity, deficiency in IL-10 production by HP T cells might contribute to their increased reactivity to tumor antigens.
In conclusion, we found that early HP CD4+ T cells differ markedly from normal T cells in the inducible expression of CTLA-4, PD-1, and Foxp3, and responsiveness to B7-H4 and CTLA-4. This may explain, at least in part, the increased reactivity of HP T cells to tumor antigens in experimental models of cancer, as demonstrated by us1 and others.5-10 Indeed, we have shown that T cells undergoing HP had increased reactivity to tumor cells, as early as 10 days after lymphocytic cell transfer into irradiated mice.1 We have repeatedly observed that HP results in the marked inhibition of melanoma growth in the absence of any procedures to deplete Tr cells (although this might enhance the response21 ), as shown here in a representative experiment (Figure S4). We found that the HP T cells reacted to a tumor cell line transplanted at the same time as the lymphocytes, but not against an unrelated tumor cell line, or against another cell line transplanted later after lymphocyte recovery.1 This suggests that HP T cells were sensitized against tumor antigens during the very early phase of HP, consistent with the lack of inhibitory molecule expression, as presented in this study. However, these abnormalities are reversible and are likely limited to the early phase of HP, as responses were largely restored after only 3 weeks of HP. Interestingly, it has been shown that in humans HP-associated T-cell alterations persist much longer than in mice, because reconstitution by the thymic route is frequently poor,3 with the risk of oligoclonality, autoimmunity, graft-versus-host disease, and weak immunity against infection. In view of this, we hypothesize that defects in negative immune regulation will persist longer in humans undergoing HP than in mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by the Ontario Institute for Cancer Research of the Province of Ontario (Toronto), the Canadian Diabetes Association (Toronto), the Li Ka Shing Knowledge Institute and Keenan Research Center of St Michael's Hospital (Toronto), US Public Health Service grants (AR31203, CA10902; Bethesda, MD), and the Department of Defense (United States) Breast Cancer Research Program (W81XWH-04-1-0454; Fort Detrick, MD). A.S. is a recipient of an Ontario Graduate Scholarship (Toronto).
Authorship
Contribution: A.S. and R.C. contributed equally to this work by designing and performing cell transfer studies and in vitro assays and by writing the paper; R.G.-Q. performed experiments related to T-cell marker expression in vivo; and R.B., A.N.T., and G.J.P. contributed to the design of the experiments and the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rabindranath Chakrabarti, St Michael's Hospital, 30 Bond St, Room 2009CC, Toronto, ON M5B1W8; e-mail: chakrabartir@smh.toronto.on.ca.
References
Author notes
*A.S. and R.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal