Abstract
Ongoing thymopoiesis requires continual seeding from progenitors that reside within the bone marrow (BM), but the identity of the most proximate prethymocytes has remained controversial. Here we take a comprehensive approach to prospectively identify the major source of thymocyte progenitors that reside within the BM and blood, and find that all thymocyte progenitor activity resides within a rare Flk2+CD27+ population. The BM Flk2+CD27+ subset is predominantly composed of common lymphoid progenitors (CLPs) and multipotent progenitors. Of these 2 populations, only CLPs reconstitute thymopoiesis rapidly after intravenous injection. In contrast, multipotent progenitor-derived cells reconstitute the thymus with delayed kinetics only after they have reseeded the BM, self-renewed, and generated CLPs. These results identify CLPs as the major source of thymocyte progenitors within the BM.
Introduction
T cells develop in the thymus from immature thymocytes throughout life. Thymocytes themselves are not a self-sustaining population but, instead, are supplied from a constant stream of bone marrow (BM)–derived progenitors. However, the identity of the exact progenitors that leave the BM and home to the thymus has remained controversial.1-4
The original identification of the lymphoid-restricted common lymphoid progenitors (CLPs) demonstrated T progenitor activity from this population in vivo, suggesting that it might be the immediate precursor to thymocytes.5 This model has been steadily challenged by an alternative theory, in which subpopulations of multipotent progenitors (MPPs) are the major source of thymocytes.6-13 MPPs were first implicated as the major source of thymic seeding cells on the basis of cell surface phenotype similarity between MPP and early thymic progenitors (DN1), the higher proliferative potential of DN1 and MPPs compared with CLPs, and the phenotype of ikaros mutant mice, which lacked phenotypic CLPs but still had some thymopoiesis.14 Although this study was suggestive, it has since been shown that CLPs rapidly adopt a DN1 phenotype on thymic entry,15 and the time course of intrathymic differentiation of DN1 and CLP is much more similar than that of MPP.14,16,17 Nonetheless, consistent with the idea that MPPs are the major source of thymopoiesis, papers from many laboratories17-23 have shown that subsets of MPPs, when injected intravenously, give rise to more T cells than do CLPs over several weeks. Indeed, a subset of MPPs has been found to home to the thymus 24 hours after intravenous injection, but the ability of those cells to give rise to T-lineage progeny remains unclear.22
Two recent publications supported the MPP progenitor model of thymopoiesis by showing, clonally in vitro, that the majority of DN1 cells have myeloid potential.24,25 Although these results implied that few, if any, DN1 come from the lymphoid restricted CLPs, they contradicted the very low myeloid readout from DN1 observed in other in vitro assays, as well as in vivo.14,26-28 Because a lymphoid-restricted progenitor was not included as a control in the clonal in vitro assay, it remains possible that the myeloid cells derived from DN1 represented an artifact of the culture system.
Most studies of prethymic progenitors to date have compared equivalent numbers of 2 or 3 defined populations to one another. However, this approach does not account for the relative abundance or the possible lineal relationships of these progenitors. Furthermore, the approach necessarily excludes all other putative progenitors that are not included in the selective populations assayed. These studies have identified 4 or 5 distinct BM populations that may seed the thymus but have left undetermined the relative levels of thymic contribution of each of these populations.
Therefore, to identify the major source of thymocyte progenitors in the BM, we performed a plus/minus sorting screen, similar to the approach used for identification of the hematopoietic stem cell,4 in which we divided all BM cells into 2 populations: one that was positive for one or more cell-surface proteins, and the other, which consisted of the remainder of the BM fraction that was negative for those markers. All of the cells in each fraction were then injected into an equal number of recipients. This screen ensured that all relevant BM fractions were considered in each experiment and were compared based on their actual abundance, thus querying directly for the predominant source of thymocyte progenitors in the BM. Importantly, we tested all populations for their ability to give rise to thymocytes over a relatively short period of time (7-14 days), which therefore excluded any progenitor activity that was the result of differentiation of long-term hematopoietic stem cells.17
Using this inclusive approach, we found that all thymic progenitor activity in the BM and blood could be accounted for in a rare population of cells that coexpressed the surface receptors, Flk2 and CD27, and contained predominantly CLPs and MPPs. Thus, these 2 populations contained virtually all the most proximate thymocyte progenitors within the BM. When these 2 populations were compared with one another, CLPs gave rise to a robust, rapid, and brief wave of thymopoiesis, whereas MPPs gave a delayed and prolonged wave, first seeding the BM and giving rise to CLPs. In contrast to the thymic MPP-seeding theory, our results support a model in which MPPs first give rise to CLPs in the BM, and these then drive thymopoiesis. These data define CLP as the major immediate source of thymocyte progenitors within the BM.
Methods
Mice
Strains of mice were all bred to the C57BL6/Ka background and included strains congenic for Thy1, and CD45. IL-7R−/− mice on a BL6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed at the Stanford University Medical Center, fed with standard mouse chow and acidified water ad libitum. Donor and recipient mice were 4 to 10 weeks old. The Stanford Animal Use and Care Committee approved all studies and procedures involving animals.
Antibodies and flow cytometry
Antibodies to CD3 (KT31.1), CD4 (GK1.5), CD8 (53-6.7), CD25 (PC61), CD44 (IM7), Sca-1 (E13-161-7), CD45.2 (AL1-4A2), CD45.1 (A20), IL-7R (A7R34), Ly-76 (Ter-119), Gr-1 (8C5), Mac-1 (M1/70), and B220 (6B2), were affinity purified from hybridoma supernatants and either used directly or were conjugated to biotin (Pierce Chemical, Rockford, IL), Pacific Blue, Pacific Orange, or Alexa Fluor-488, -647, or -680 (Invitrogen, Carlsbad, CA). Cells labeled with biotinylated antibodies were detected using streptavidin-linked Q655 or Q605 (Invitrogen). Antibodies to CD27 (LG.3A10), CD11c (HL3), CD19 (6D5), Flk-2 (A2F10), NK1.1 (136TC), and c-Kit (2B8) were purchased as conjugates to phycoerythrin (PE), PE-Cy5, PE-Cy5.5, PE-Cy7, allophycocyanin (APC), APC-Cy5.5, or APC-Cy7 (eBioscience, San Diego, CA). Anti-Thy1.1-PE (OX-7) was purchased from BD Biosciences PharMingen (San Diego, CA). All stains were performed in staining buffer (phosphate-buffered saline supplemented with 2% heat inactivated calf serum) for 10 to 20 minutes on ice. Cells were washed and resuspended in staining buffer plus propidium iodide before flow cytometry. Flow cytometry was performed on a FACSAria (BD Biosciences, San Jose, CA) using 405-, 488-, and 633-nm lasers to detect 12 fluorescent parameters. Live, single cells were gated by propidium iodide exclusion, forward scatter height, and width. Data analysis was performed using FlowJo (TreeStar, Ashland, OR), and all cells are shown in each plot, but pixel size may vary to facilitate viewing.
Cell purification, sorting, and transplantation
For purification of BM populations, femur, tibia, and humerus from each of 5 to 20 donor mice were crushed with a mortar and pestle in a small volume of staining buffer. Suspensions were cleared of red blood cells (RBCs) using 0.85% ammonium chloride/0.1% potassium bicarbonate and were then centrifuged through a layer of Histopaque 1119 (Sigma-Aldrich, St Louis, MO) to clear residual red cells and bone fragments. Except in Figure 1A in which the BM was stained without lineage depletion, cells were then stained with purified antibodies to lineage markers, and stained cells were depleted using anti–rat IgG–conjugated Dynal beads according to manufacturer's directions (Invitrogen). Remaining cells were stained with PE-Cy5 conjugated anti–rat IgG, subsequently blocked with free rat IgG, and then stained for other markers.
Blood was taken immediately after death of animals by cutting the right atrium, followed by left ventricle puncture with a syringe and perfusion with 10 mM of ethylenediaminetetraacetic acid/phosphate-buffered saline, and collection of blood from the chest cavity. Blood was diluted to 1% Dextran 500 (GE Healthcare, Little Chalfont, United Kingdom), incubated at 37°C for 20 to 30 minutes to pellet the RBCs, and the cells in suspension were centrifuged through a layer of Histopaque 1077 (Sigma-Aldrich) and treated with 0.85% ammonium chloride/0.1% potassium bicarbonate to lyse the remaining RBCs. The remaining cells were then stained and sorted directly. A small fraction of sorted cells were analyzed to ascertain purity, which was generally 90% or greater, with the contaminating cells coming from the bulk population, rather than any population of cells in particular (representative postsort data in Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Sorted cells were briefly centrifuged at 500g and then resuspended in medium with or without lineage-depleted “helper” BM, and then injected retro-orbitally into recipient mice. Recipients were either untreated or were sublethally irradiated (450 cGy) using an X-ray irradiator.
OP9-DL1 culture
Double-sorted CLPs and MPPs (>99% purity) from B6/Ka parent (CD45.2/Thy1.2) were injected retro-orbitally into nonirradiated F1 (CD45.2/CD45.1/Thy1.2/Thy1.1) recipients. Five or 6 days after injection, recipient thymuses were removed and cells were suspended in 1 mL of minimum essential medium-α (MEM-α) 20% fetal bovine serum. A total of 20 to 40 μg of α-Thy1.1 (19XE5) was added to each suspension at 4°C for 30 minutes to kill host cells. Live cells were isolated using Histopaque 1119 (Sigma-Aldrich) and were then either directly plated onto confluent monolayers of OP9-DL1 or were blocked with anti-FcγR (clone93) (eBioscience) and stained with antibodies to CD45.1, CD45.2, Thy1.1, and Thy1.2. Donor cells were then sorted, using a generous donor gate, directly onto confluent wells of OP9-DL1 stroma, with addition of 5 ng/mL of IL-7 and 5 ng/mL of Flt3L (PeproTech, Rocky Hill, NJ). After 8 days of culture, donor-derived thymocytes were detected by flow cytometry using antibodies to Thy1 and CD45.
Results
Cells within the Flk2+CD27+ BM compartment comprise all of the thymic progenitor activity
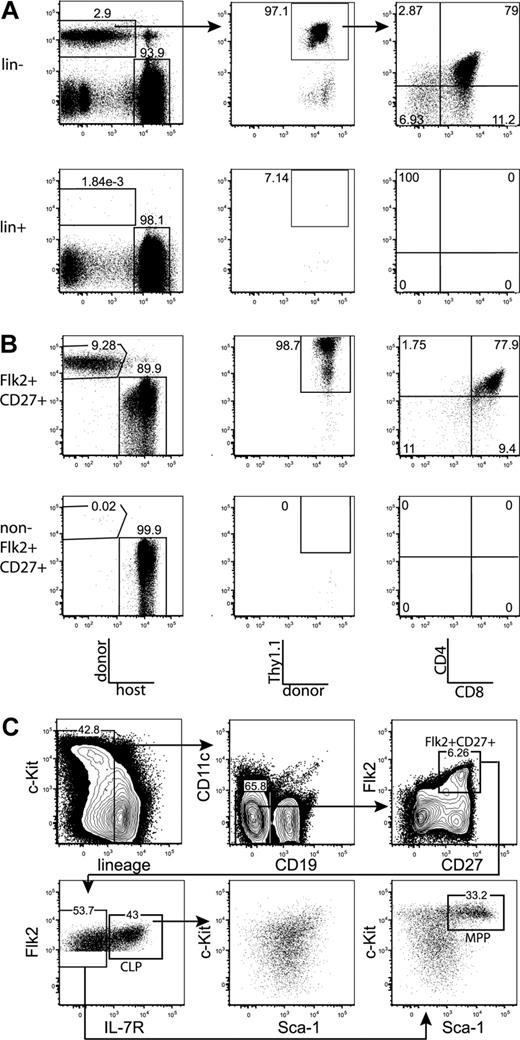
We first tested whether the majority of thymic progenitor activity was contained within the fraction of BM that is negative for markers of mature hematopoietic lineages. BM was sorted as positive or negative for mature lineages, using the markers CD3, CD4, CD8, Mac-1, Gr-1, Ter-119, B220, CD19, CD11c, and NK1.1. Mice were injected intravenously with the sorted populations, plus approximately 1.8 × 105 syngeneic lineage-depleted BM cells, which ensured that neither group experienced differential hematopoietic stress resulting from radioablation, and also ensured that all sorted populations were subjected to equal competition with all other possible prethymic progenitors. Fourteen days after transplantation, Thy1.1+CD4+CD8+ (DP) donor-derived thymocytes were readily detected in mice transplanted with the lineage-negative, but not the lineage-positive, BM fraction (Figure 1A). Therefore, prethymic progenitors within the BM do not express surface proteins that are classically thought to mark mature hematopoietic lineages. All subsequent plus/minus screens were done within the lineage-negative BM fraction.
All thymic progenitor activity resides within the lineage−Flk2+CD27+ subset, which includes MPPs and CLPs. (A) BM was separated into fractions positive and negative for mature lineages (CD3, CD4, CD8, CD11c, Mac-1, Gr-1, B220, CD19, Ter119, and NK1.1). The entirety of each population (9 × 105 lineage+, 3 × 105 lineage−) was injected intravenously into 5 sublethally irradiated CD45-congenic recipients. Thymuses were analyzed for donor chimerism 14 days later, and all thymic progenitor activity was found in the lineage− subset. Each flow cytometry plot is representative of 5 recipients. Arrows in top panels indicate gating hierarchy. (B) Thymic chimerism of sublethally irradiated CD45 congenic mice 14 days after injection of 105 lineage− Flk2+CD27+ cells, or all remaining (3 × 106) lineage− non-Flk2+CD27+ cells. All thymic progenitor activity was found within the Flk2+CD27+ subset. Data are representative of 4 independent experiments. The average donor Thy1.1+ chimerism of the recipients of Flk2+CD27+ and non-Flk2+CD27+ populations were 1.9% and 0.0008%, respectively. (C) Representative BM stain used for sorting. BM was depleted of mature lineages using antibodies to CD3, CD4, CD8, B220, Mac-1, Gr-1, and Ter119 and stained for residual lineage+ cells. CD19+ and CD11c+ cells were excluded in separate channels. A total of 2.5 × 106 events were collected. The lineage− Flk2+CD27+ population was divided into IL-7R− MPP (c-Kithi and Sca-1hi) and IL-7R+ CLP, which had uniform low levels of c-Kit and Sca-1.
All thymic progenitor activity resides within the lineage−Flk2+CD27+ subset, which includes MPPs and CLPs. (A) BM was separated into fractions positive and negative for mature lineages (CD3, CD4, CD8, CD11c, Mac-1, Gr-1, B220, CD19, Ter119, and NK1.1). The entirety of each population (9 × 105 lineage+, 3 × 105 lineage−) was injected intravenously into 5 sublethally irradiated CD45-congenic recipients. Thymuses were analyzed for donor chimerism 14 days later, and all thymic progenitor activity was found in the lineage− subset. Each flow cytometry plot is representative of 5 recipients. Arrows in top panels indicate gating hierarchy. (B) Thymic chimerism of sublethally irradiated CD45 congenic mice 14 days after injection of 105 lineage− Flk2+CD27+ cells, or all remaining (3 × 106) lineage− non-Flk2+CD27+ cells. All thymic progenitor activity was found within the Flk2+CD27+ subset. Data are representative of 4 independent experiments. The average donor Thy1.1+ chimerism of the recipients of Flk2+CD27+ and non-Flk2+CD27+ populations were 1.9% and 0.0008%, respectively. (C) Representative BM stain used for sorting. BM was depleted of mature lineages using antibodies to CD3, CD4, CD8, B220, Mac-1, Gr-1, and Ter119 and stained for residual lineage+ cells. CD19+ and CD11c+ cells were excluded in separate channels. A total of 2.5 × 106 events were collected. The lineage− Flk2+CD27+ population was divided into IL-7R− MPP (c-Kithi and Sca-1hi) and IL-7R+ CLP, which had uniform low levels of c-Kit and Sca-1.
After testing a panel of markers using the same assay, we found that all progenitor activity cleanly fractionated into the Flk2+CD27+ subset of lineage-negative BM (Figures 1B, S1). The Flk2+CD27+ compartment comprises approximately 3% of lineage-negative BM (0.6% of total BM) and is split between IL-7R+ and IL-7R− cells (Figure 1C). The IL-7R− population is predominantly composed of c-Kit+ Sca-1+ cells (MPPs) but also contains a fraction of non-MPP. This IL-7R− non-MPP population gave rise to inconsistent low-level thymic chimerism (T.S. and L.I.R.E., unpublished data, October 17, 2006, and November 8, 2006). The IL-7R+ population is composed of c-Kitint Sca-1int cells. We refer to these cells as CLP because we have recently demonstrated that lineage− Flk2+ IL-7R+ cells are the functional CLP in the BM.15 The Flk2+CD27+ population lacks B220hi or Thy1hi cells (Figure S2), indicating that previously identified thymic seeding populations that express those markers contribute relatively little to thymopoiesis.29-31 In previous studies, both MPPs and CLPs have been shown to possess T-lineage potential5,17,21 ; however, because MPPs are thought to be developmentally upstream of CLP, it has remained uncertain what fraction of thymopoiesis occurs through a CLP intermediate. We were therefore interested in determining whether the majority of the rapid Flk2+CD27+ thymic progenitor activity resided within one or both of these populations.
Kinetics of thymopoiesis is more rapid from CLPs than MPPs
To test the ability of MPPs and CLPs to generate thymocytes over a short time course, BM equivalents of MPPs and CLPs were sorted and transplanted as in Figure 1. We measured the thymic chimerism of recipient mice at weekly time points from 7 to 28 days and determined the developmental stages of donor-derived cells. Seven days after transplantation, donor thymic T-lineage chimerism, as defined by Thy1.1+ cells, was evident from 9 of 9 CLP recipients, but only 1 of 9 MPP recipients (average of 0.005% chimerism for CLPs and 1.6 × 10−5% for MPPs, Figure 2A,B). Most of the T lineage CLP-derived cells were at the CD44+CD25+CD4−CD8− (DN2) and the CD44−CD25+CD4−CD8− (DN3) stages of development and had not progressed to DP cells (Figures 2, S3). These results demonstrate that CLP-, but not MPP-derived thymocytes, can be detected 7 days after intravenous transplantation.
CLPs generate thymocytes with faster kinetics than MPPs. (A) MPPs and CLPs were sorted from the BM of CD45.1 mice as indicated in Figure 1C. BM equivalents (1.4 × 104 CLPs and 4 × 103 MPPs per mouse) were transplanted into sublethally irradiated CD45.2 mice along with syngeneic lineage-depleted BM (∼ 1.8 × 105 cells). Representative plots of recipient thymuses at the indicated time points are shown. Arrows in the top panels indicate gating hierarchy. CLP chimerism is evident at day 7, whereas MPP chimerism becomes apparent at day 14. Plots are representative of one of 3 mice in 1 of 3 experiments. For each thymus, 2.5 × 106 events were collected and displayed. In plots with rare events, pixel size has been increased to facilitate visualization. (B) Quantification of donor chimerism pooled from 3 time course experiments as in panel A. Each symbol represents the chimerism of 1 mouse (● indicates MPP recipient: ▲, CLP recipient); bars represent averages of each group. CLPs drive a wave of thymopoiesis that starts by day 7 and peaks at day 21, whereas MPP-derived thymopoiesis starts later and lasts longer. Positive donor gates were drawn using control mice, which were injected in parallel with only syngeneic BM, and the percentage of cells that fell into the donor Ly5+,Thy1.1+ gate of these control animals was zero. As determined by the Student t test, differences in chimerism between MPP and CLP were significant at days 7, 14, and 28 (P = .048, .022, and 10−4, respectively). (C) Faster development of CLP-derived thymocytes. The graph shows the percentage of donor thymocytes in each recipient that have reached the CD4+CD8+ (DP) stage of development. By day 14, an average of 68% of the CLP-derived thymocytes had reached the DP stage, compared with only 19% of the MPP-derived cells. The decrease in the percentage of CLP-derived DP thymocytes at day 28, relative to MPP, reflects their further maturation to the CD4+ and CD8+ SP stages, as seen in panel A. As determined by the Student t test, differences in DP chimerism were significant at days 14 and 28 (P = .006 and .018, respectively).
CLPs generate thymocytes with faster kinetics than MPPs. (A) MPPs and CLPs were sorted from the BM of CD45.1 mice as indicated in Figure 1C. BM equivalents (1.4 × 104 CLPs and 4 × 103 MPPs per mouse) were transplanted into sublethally irradiated CD45.2 mice along with syngeneic lineage-depleted BM (∼ 1.8 × 105 cells). Representative plots of recipient thymuses at the indicated time points are shown. Arrows in the top panels indicate gating hierarchy. CLP chimerism is evident at day 7, whereas MPP chimerism becomes apparent at day 14. Plots are representative of one of 3 mice in 1 of 3 experiments. For each thymus, 2.5 × 106 events were collected and displayed. In plots with rare events, pixel size has been increased to facilitate visualization. (B) Quantification of donor chimerism pooled from 3 time course experiments as in panel A. Each symbol represents the chimerism of 1 mouse (● indicates MPP recipient: ▲, CLP recipient); bars represent averages of each group. CLPs drive a wave of thymopoiesis that starts by day 7 and peaks at day 21, whereas MPP-derived thymopoiesis starts later and lasts longer. Positive donor gates were drawn using control mice, which were injected in parallel with only syngeneic BM, and the percentage of cells that fell into the donor Ly5+,Thy1.1+ gate of these control animals was zero. As determined by the Student t test, differences in chimerism between MPP and CLP were significant at days 7, 14, and 28 (P = .048, .022, and 10−4, respectively). (C) Faster development of CLP-derived thymocytes. The graph shows the percentage of donor thymocytes in each recipient that have reached the CD4+CD8+ (DP) stage of development. By day 14, an average of 68% of the CLP-derived thymocytes had reached the DP stage, compared with only 19% of the MPP-derived cells. The decrease in the percentage of CLP-derived DP thymocytes at day 28, relative to MPP, reflects their further maturation to the CD4+ and CD8+ SP stages, as seen in panel A. As determined by the Student t test, differences in DP chimerism were significant at days 14 and 28 (P = .006 and .018, respectively).
By day 14, T-lineage chimerism in CLP-injected mice expanded to an average of 3.6% chimerism, with the majority of thymocytes at the DP stage (average, 68%; Figure 2). In contrast, MPP-derived thymic chimerism was just beginning to be detected (average, 0.07%), and most cells were still immature DN thymocytes (average, 19% DP, Figures 2C, S3). At day 21, thymocyte chimerism from CLP peaked (average of 20%), with most cells at the DP stage and beyond (average, 79.3% DP; Figure 2). The loss of chimerism in the DN compartment indicates that CLPs generated a single wave of thymopoiesis. In contrast, MPP chimerism continued to rise at day 21 (average, 34.8%), and DN thymocytes continued to be generated (Figures 2A, S3). By day 28, the single wave of CLP-derived T chimerism had diminished (average, 7.8%), with a decreased percentage of DP cells (average, 52.6%) replaced by an increased percentage of mature CD4+CD8− and CD8+CD4− (SP) cells (Figure 2). MPP-derived chimerism continued to rise at day 28 (average 72%, Figure 2A,B) and was still maintained by a large DN population. The persistence of MPP-derived DN thymocytes suggests continual thymic seeding by MPP-derived progenitors, whereas the lack of MPP-derived progenitors at day 7 is inconsistent with substantial direct thymic seeding.
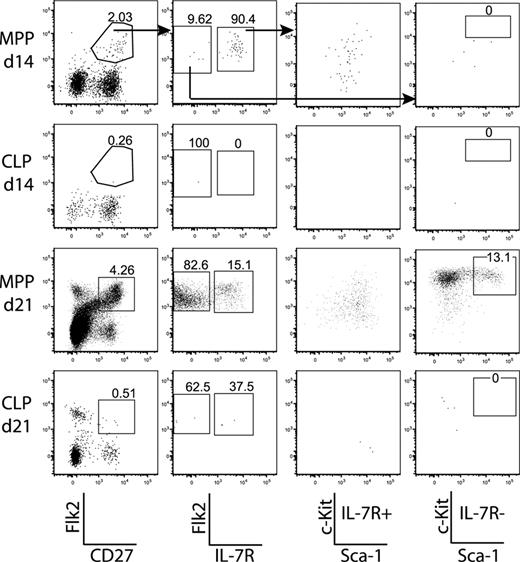
MPPs give rise to CLPs within recipient BM
Because CLPs gave rise to a single wave of thymopoiesis, whereas MPPs continued to generate DN thymocytes, it was possible that MPPs proliferated in the BM and generated a downstream progenitor, probably CLPs, which homed to the thymus. This model predicts that MPPs would give rise to detectable CLPs in the BM after transplantation. Thus, we examined the BM of MPP-recipient mice for donor-derived CLPs during the time course. Because cell-surface levels of c-Kit and Flk2 were reduced after irradiation (Figure S4), we looked at the BM from days 14 and 21. Flk2+CD27+ donor-derived cells were generated efficiently by MPPs, but not CLPs, at both time points. MPPs self-renewed in the BM and gave rise to CLPs throughout the time course, whereas CLPs did not (Figure 3). These results, in combination with the time course of thymopoiesis, support a model in which MPPs home to the BM and give rise to CLP, which then rapidly generate thymocytes.
MPPs seed the BM and give rise to CLPs. BM from femurs of the MPP and CLP recipients in the time courses (Figure 2) were pooled and stained to reveal donor chimerism and populations. Plots are from 1 time course experiment and are representative of 2 experiments. A total of 2.5 × 106 events were collected from each sample. Arrows in the top panels indicate gating hierarchy. The far left plots are gated on donor-derived cells, and the various numbers of events in each plot reflect variable donor chimerism. Whereas CLPs do not self-renew appreciably, MPPs do give rise to more Flk2+CD27+ cells, including both MPPs and CLPs.
MPPs seed the BM and give rise to CLPs. BM from femurs of the MPP and CLP recipients in the time courses (Figure 2) were pooled and stained to reveal donor chimerism and populations. Plots are from 1 time course experiment and are representative of 2 experiments. A total of 2.5 × 106 events were collected from each sample. Arrows in the top panels indicate gating hierarchy. The far left plots are gated on donor-derived cells, and the various numbers of events in each plot reflect variable donor chimerism. Whereas CLPs do not self-renew appreciably, MPPs do give rise to more Flk2+CD27+ cells, including both MPPs and CLPs.
Nonirradiated recipient thymuses are more rapidly colonized by CLPs than MPPs
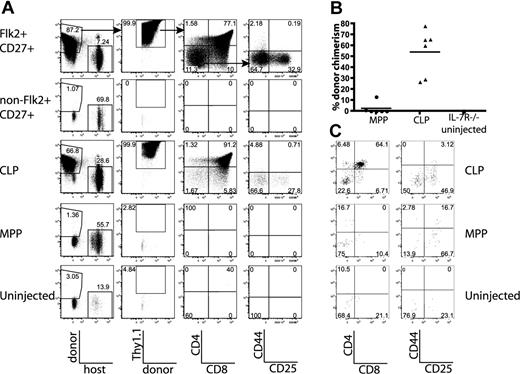
To confirm that the different thymocyte progenitor activities of MPPs and CLPs were not the result of artifacts introduced by irradiation of recipient mice, we examined thymic chimerism in nonirradiated IL-7R−/− and F1 recipients.32 We first used IL-7R−/− as hosts because it has been demonstrated that these thymuses are readily reconstituted by progenitors without irradiation.33 We first confirmed that all thymic progenitor activity was within the lineage− Flk2+CD27+ population of BM 14 days after transplantation (Figure 4A). Chimerism was high because host cells have a competitive disadvantage resulting from the lack of IL-7 signaling. Interestingly, 14 days after transplant, 6 of 6 CLP recipients had high thymic donor chimerism (average, 54%), with most cells at the DP stage. In contrast, only 2 of 6 MPP recipients had any Thy1.1+ donor cells, and chimerism was low (average, 2.4%; Figure 4). MPPs and CLPs sorted in this same experiment were able to generate thymic chimerism 14 days after transplantation in sublethally irradiated recipients, indicating that they were viable and functional (Figure 2B). Furthermore, IL-7R−/− recipients analyzed 18 days after transplantation had thymic chimerism from both MPPs and CLPs, indicating that MPPs engrafted and eventually gave rise to thymocytes, although such delayed development suggests that MPPs proceeded through a prethymic intermediate (Figure S5).
CLPs generate significantly more thymic donor chimerism than MPPs in unirradiated IL-7R−/− and F1 recipients. The BM populations indicated were sorted as in Figure 1C, and BM equivalents (3.8 × 104 Flk2+CD27+, 9.5 × 105 non-Flk2+CD27+, 2.9 × 104 CLP, 1.9 × 104 MPP per IL-7R−/− recipient; 6 × 104 CLP, 4.5 × 104 MPP per F1 recipient) were injected into unirradiated IL-7R−/− or F1 recipients. (A) Flk2+CD27+ cells, and within that population, CLPs represent the major source of prethymic progenitors, 14 days after injection into IL-7R−/− mice. Arrows in the top panel indicate gating hierarchy. (B) Quantification of donor thymocyte chimerism in IL-7R−/− mice from MPPs and CLPs, as shown in panel A. Each symbol represents the chimerism of an individual mouse, and bars represent the average. Data are pooled from 2 separate experiments of 3 recipients each. As determined by the Student t test, differences in chimerism were significant (P = .001). (C) Representative plots of all gated donor thymocytes 14 days after transfer of MPP or CLP from CD45.2, Thy1.2 mice into nonirradiated F1 recipients (CD45.1, Thy1.1x CD45.2, Thy1.2). Donor-gated thymocytes are shown (CD45.2+ CD45.1− Thy1.2+ Thy1.1−). CLP-derived thymocytes are more numerous and have progressed to the DP developmental stage, whereas MPP-derived progeny are relatively rare and are less mature. These plots are representative of 6 mice in 2 separate experiments.
CLPs generate significantly more thymic donor chimerism than MPPs in unirradiated IL-7R−/− and F1 recipients. The BM populations indicated were sorted as in Figure 1C, and BM equivalents (3.8 × 104 Flk2+CD27+, 9.5 × 105 non-Flk2+CD27+, 2.9 × 104 CLP, 1.9 × 104 MPP per IL-7R−/− recipient; 6 × 104 CLP, 4.5 × 104 MPP per F1 recipient) were injected into unirradiated IL-7R−/− or F1 recipients. (A) Flk2+CD27+ cells, and within that population, CLPs represent the major source of prethymic progenitors, 14 days after injection into IL-7R−/− mice. Arrows in the top panel indicate gating hierarchy. (B) Quantification of donor thymocyte chimerism in IL-7R−/− mice from MPPs and CLPs, as shown in panel A. Each symbol represents the chimerism of an individual mouse, and bars represent the average. Data are pooled from 2 separate experiments of 3 recipients each. As determined by the Student t test, differences in chimerism were significant (P = .001). (C) Representative plots of all gated donor thymocytes 14 days after transfer of MPP or CLP from CD45.2, Thy1.2 mice into nonirradiated F1 recipients (CD45.1, Thy1.1x CD45.2, Thy1.2). Donor-gated thymocytes are shown (CD45.2+ CD45.1− Thy1.2+ Thy1.1−). CLP-derived thymocytes are more numerous and have progressed to the DP developmental stage, whereas MPP-derived progeny are relatively rare and are less mature. These plots are representative of 6 mice in 2 separate experiments.
We next examined thymic chimerism 14 days after transplantation into unirradiated wild-type F1 recipients after transplantation of CLPs and MPPs. CLPs generated DP thymocytes 2 weeks after transplantation, whereas MPPs yielded less thymic chimerism and a higher fraction of DN thymocytes (Figure 4C). These findings correlate developmentally with those found in irradiated recipients and IL-7R−/− mice, underscoring the role of CLPs as the predominant, proximate BM-derived thymocyte progenitor in multiple models.
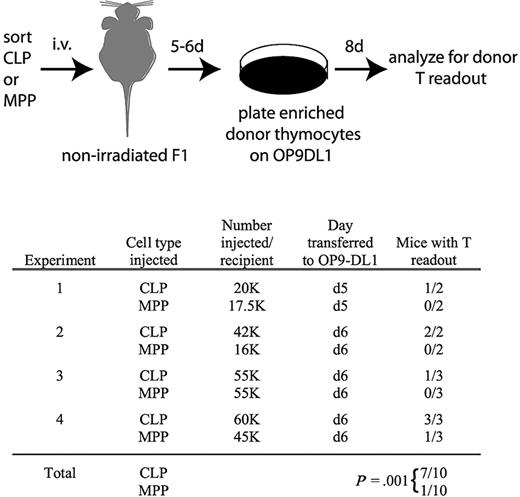
There are 2 possible interpretations for the delayed kinetics with which MPPs yield thymocytes relative to CLPs. Either MPPs seed the thymus directly but undergo thymic development more slowly than CLPs, or MPPs seed the BM first where they produce CLPs that drive thymopoiesis. To distinguish between these models, we analyzed recipient nonirradiated F1 thymuses 5 to 6 days after transplantation of MPPs or CLPs. Donor chimerism was not detected from either population at these early time points. Therefore, we used the OP9-DL1 stromal cell line to amplify rare donor-derived thymocyte progenitors. We removed the thymuses of transplanted mice 5 or 6 days after injection of MPPs or CLPs, enriched donor cells, and plated all resultant cells from each entire thymus on OP9-DL1 stroma (Figure 5). OP9-DL1 cells are highly efficient at promoting proliferation of single thymocyte progenitors, enabling the detection of very rare donor-derived progenitors.24,34 After 8 days on OP9-DL1, the cultures were analyzed by flow cytometry to identify donor-derived cells. Interestingly, after 4 such experiments, donor-derived thymocytes were detected in 7 of 10 CLP recipients but only 1 of 10 MPP recipients (Figure 5), demonstrating that thymus seeding occurs more rapidly from CLPs than MPPs.
Thymocytes are readily detectable in nonirradiated recipients 5 and 6 days after transplantation of CLPs but not MPPs. As schematized, CLPs or MPPs from CD45.2, Thy1.2 mice were injected into unirradiated F1 recipients (CD45.1, Thy1.1xCD45.2, Thy1.2). Five or 6 days after transplantation, as indicated, thymuses were removed and donor cells, which were not detectable over background by flow cytometry, were enriched as described in “OP9-DL1 culture,” and then cultured on OP9-DL1 stroma in the presence of 5 ng/mL of IL-7 and Flt3L for 8 days. Cultures were then analyzed for donor chimerism by flow cytometry of the entire culture (CD45.2+CD45.1−Thy1.2+Thy1.1− cells). As shown in the table, 7 of 10 CLP recipient thymuses contained donor thymocytes that were detected in this assay, whereas only 1 of 10 MPP recipients had detectable donor thymocytes. Readouts were considered positive if the number of cells in the donor gate exceeded the number obtained from the uninjected control mouse in the same experiment. Readouts from the uninjected control ranged from 0 to 36 cells, whereas positive readouts ranged from 300 to 51 000 cells. The statistical significance of the different readouts between CLPs and MPPs was calculated using the Fisher exact test.
Thymocytes are readily detectable in nonirradiated recipients 5 and 6 days after transplantation of CLPs but not MPPs. As schematized, CLPs or MPPs from CD45.2, Thy1.2 mice were injected into unirradiated F1 recipients (CD45.1, Thy1.1xCD45.2, Thy1.2). Five or 6 days after transplantation, as indicated, thymuses were removed and donor cells, which were not detectable over background by flow cytometry, were enriched as described in “OP9-DL1 culture,” and then cultured on OP9-DL1 stroma in the presence of 5 ng/mL of IL-7 and Flt3L for 8 days. Cultures were then analyzed for donor chimerism by flow cytometry of the entire culture (CD45.2+CD45.1−Thy1.2+Thy1.1− cells). As shown in the table, 7 of 10 CLP recipient thymuses contained donor thymocytes that were detected in this assay, whereas only 1 of 10 MPP recipients had detectable donor thymocytes. Readouts were considered positive if the number of cells in the donor gate exceeded the number obtained from the uninjected control mouse in the same experiment. Readouts from the uninjected control ranged from 0 to 36 cells, whereas positive readouts ranged from 300 to 51 000 cells. The statistical significance of the different readouts between CLPs and MPPs was calculated using the Fisher exact test.
Thymic and splenic lineages downstream of CLPs versus MPPs
We observed that CLPs and MPPs gave rise to thymic non-T lineage (Thy1.1−) cells after transplantation (Figure 2A). Further analysis of thymic lineages showed that MPPs gave rise to dendritic cells (CD11c+Thy1.1−), monocyte/macrophage progeny (Thy1.1−CD11c−Mac1+gr-1lo), and rare B cells (CD11c−Mac1−Gr-1−B220+), but no NK1.1+ cells. In contrast, CLPs gave rise to thymic CD11c+ dendritic cells, and B cells, but no Mac-1+ progeny (Figure 6A). Donor MPPs yielded a fairly constant percentage of Mac-1+ progeny between 14 and 28 days, whereas CLPs never yielded thymic monocyte/macrophage lineages (Figure 6B). This is consistent with the differential monocyte/macrophage potentials of MPPs and CLPs observed in spleens of recipient animals (Figure S6). Therefore, thymic monocyte/macrophage lineages must be derived from a distinct progenitor downstream of MPPs.
Only MPPs yield myeloid thymic lineages, whereas CLPs generate lymphoid cells in the thymus. MPPs and CLPs were transferred into sublethally irradiated recipients as in Figure 2. At the indicated times, recipient thymuses were analyzed for donor-derived lineages. (A) Lineages of gated donor thymocytes 14 days after transplantation. Arrows in the top plots indicate gating hierarchy. The left column shows dendritic cells (CD11c+) versus T-committed progeny (Thy1.1+). The middle plots are gated to display myeloid progeny (Mac-1/Gr-1+), and the right plots show B220+ B cells. (B) The percentage of donor myeloid cells was computed as the percentage of total live cells that were CD45.1+Thy1.1−CD11c−Mac-1+. Data are taken from 2 of the time course experiments in Figure 2. Each symbol represents 1 mouse. Thymic myeloid chimerism is apparent from MPPs but not from CLPs over the time course analyzed. As determined by the Student t test, differences in myeloid chimerism were significant at days 14, 21, and 28 (P = .012, .007, and .023, respectively).
Only MPPs yield myeloid thymic lineages, whereas CLPs generate lymphoid cells in the thymus. MPPs and CLPs were transferred into sublethally irradiated recipients as in Figure 2. At the indicated times, recipient thymuses were analyzed for donor-derived lineages. (A) Lineages of gated donor thymocytes 14 days after transplantation. Arrows in the top plots indicate gating hierarchy. The left column shows dendritic cells (CD11c+) versus T-committed progeny (Thy1.1+). The middle plots are gated to display myeloid progeny (Mac-1/Gr-1+), and the right plots show B220+ B cells. (B) The percentage of donor myeloid cells was computed as the percentage of total live cells that were CD45.1+Thy1.1−CD11c−Mac-1+. Data are taken from 2 of the time course experiments in Figure 2. Each symbol represents 1 mouse. Thymic myeloid chimerism is apparent from MPPs but not from CLPs over the time course analyzed. As determined by the Student t test, differences in myeloid chimerism were significant at days 14, 21, and 28 (P = .012, .007, and .023, respectively).
All thymic progenitor activity within blood is found within the Flk2+CD27+ compartment
Because all thymic progenitor activity was restricted to the Flk2+CD27+ BM compartment, we tested whether the thymic seeding cell that passes through the blood also shares the same phenotype. Staining of lineage-depleted blood revealed a rare, discrete population of Flk2+CD27+ cells (∼ 0.008% of white blood cells; Figure 7A). We fractionated lineage− blood into Flk2+CD27+ and non-Flk2+CD27+ subsets, injected sublethally irradiated recipients with the entirety of each fraction, and analyzed thymic chimerism 14 days later. All of the T progenitor activity was in the Flk2+CD27+ population (Figure 7B). Interestingly, this population contains both phenotypic CLPs and MPPs (Figure 7A) and has both lymphoid and myeloid lineage potentials (Figure S7), indicating a parallel between thymic progenitors within BM and blood.
All thymic progenitor activity in blood is found within the rare Flk2+CD27+ subset. (A) Blood was isolated from 20 mice, lineage depleted, and stained to identify lineage−Flk2+CD27+ cells and MPPs and CLPs within this population. The lower left plot shows the enriched lineage−Flk2+CD27+ population. IL-7R+ and IL-7R− cells are present within this population, and the c-Kit versus Sca-1 profiles of both groups are displayed. (B) All lineage−Flk2+CD27+ cells (4500) and all remaining lineage− cells (2.5 × 106) were sorted from the blood of 10 mice and injected into sublethally irradiated congenic recipients as in Figure 2. After 14 days, the contribution of donor thymocytes was determined. All T progenitor activity was within the Flk2+CD27+ blood subset, and thymocytes had reached the CD4+CD8+ DP stage of development. Plots are representative of 3 mice each in each of 2 experiments.
All thymic progenitor activity in blood is found within the rare Flk2+CD27+ subset. (A) Blood was isolated from 20 mice, lineage depleted, and stained to identify lineage−Flk2+CD27+ cells and MPPs and CLPs within this population. The lower left plot shows the enriched lineage−Flk2+CD27+ population. IL-7R+ and IL-7R− cells are present within this population, and the c-Kit versus Sca-1 profiles of both groups are displayed. (B) All lineage−Flk2+CD27+ cells (4500) and all remaining lineage− cells (2.5 × 106) were sorted from the blood of 10 mice and injected into sublethally irradiated congenic recipients as in Figure 2. After 14 days, the contribution of donor thymocytes was determined. All T progenitor activity was within the Flk2+CD27+ blood subset, and thymocytes had reached the CD4+CD8+ DP stage of development. Plots are representative of 3 mice each in each of 2 experiments.
Discussion
Here, we determine that the majority of thymocytes originate from rare Flk2+CD27+ CLPs and MPPs in the BM and blood. These 2 progenitors show drastically different kinetics of thymic seeding, with CLPs giving a rapid single wave of thymocytes, which peaks at approximately 3 weeks, and MPPs giving a prolonged wave of thymocytes that initiates later, after giving rise to CLPs in the BM. The simplest interpretation of this finding is that MPPs give rise to CLPs in the BM, which then seed the thymus, and MPPs do not directly seed the thymus. Alternatively, it is possible that MPPs and CLPs both seed the thymus directly, but MPPs give rise to thymocytes with delayed kinetics. To distinguish between these models, we used OP9-DL1 stromal cultures to detect extremely rare donor cells that were otherwise undetectable at early time points after injection, and we found that only CLPs, but not MPPs, rapidly colonize the thymus with cells that have detectable T-lineage potential. Thus, if MPPs do seed the thymus with T-lineage potent progenitors, they remain undetectable by both flow cytometry and throughout extended culture on OP9-DL1. However, we and others have found that MPPs injected intrathymically readily proliferate and differentiate, and are easily detected by flow cytometry after one week, strongly suggesting that, if MPPs did seed the thymus, we would have detected them in our assays17 (Figure S8). Furthermore, given that the kinetics of thymic development after intravenous versus intrathymic injections of CLP are indistinguishable,17 CLPs either directly seed the thymus or they very rapidly seed another tissue (other than BM) where they rapidly give rise to cells that seed the thymus.
Flk2 expression was recently used to identify a subset of CLPs that contained all of the T progenitor activity and was more uniform in expression of c-Kit and Sca-1; thus, staining for Flk2 enabled a superior purification of CLPs.15 The use of Flk2 as a marker obviated the need to use c-Kit and Sca-1 expression to identify CLPs because the entire population was shown to be lymphoid committed in vivo. Nevertheless, the controversial nature of the seeding results obtained in the present study led us to repeat the 7-day transplantation and thymocyte chimerism experiment using the CLPs purified as originally defined phenotypically.5 We found that these cells also gave rise to thymocytes at that early time point. Furthermore, we transplanted the IL-7R+ cells that fell into the c-Kit+ and Sca-1+ gates of MPP, or just the IL-7R+ cells that were specifically excluded from this gate. Both populations gave thymocyte chimerism at day 7, and neither yielded splenic or thymic myeloid chimerism (Figure S9). Thus, the CLP population as defined by Flk2 and IL-7R expression used in this study is lymphoid committed and its thymocyte progenitor activity is not restricted to cells that overlap with the c-Kit and Sca-1 levels of MPP.
Many recent studies have suggested that fractions of MPPs, rather than CLPs, are the major sources of thymocyte progenitors in vivo.14,17-19,21,22 In reconciling our conclusions, there are some key differences in the methodologies used that should be considered. One key difference is that most other studies, in comparing CLP to MPP subpopulations, injected equal numbers of each population and then measured thymocyte chimerism.14,17,21 This strategy has the strength of directly comparing the efficiency of thymocyte generation from 2 or more cell types. However, because the populations are almost invariably present at different ratios in the BM, injecting equal cell numbers does not establish the relative importance of the 2 populations as thymocyte progenitors. Furthermore, because MPPs are upstream of CLPs and can self-renew for much longer than CLPs (Figure 3), comparing equal numbers of these 2 populations will invariably yield larger and more sustained readouts from MPPs for all downstream lineages, including T, B, NK, and dendritic cell lineages. Thus, whereas this and other studies have shown that MPPs give rise to more T progenitors than CLPs on a per cell basis over a long time course, our experiments indicate that this is a reflection of the greater proliferative capacity of MPPs and the fact that MPPs efficiently give rise to CLPs in the BM.
Over a long enough time course, the most efficient thymocyte progenitor will be the hematopoietic stem cell, because it self-renews indefinitely. Therefore, we chose to look at early time points to resolve the contributions of downstream progenitors that are closer to thymic seeding. Our studies implicate CLPs as the proximate source of thymocytes. Other recent studies that have implicated MPP subsets as proximate thymocyte progenitors have based their conclusions on peak chimerism obtained 3 weeks or later after transplantation.17,21,22 Whereas we also find that MPPs give rise to more thymocytes than CLPs 3 weeks after transplantation, our data indicate that this is the result of self-renewal of MPPs in the BM, and extended production of CLPs, correlating with extended supply of DN thymocytes.
Because CLPs lack myeloid potential, one implication of this study is that there is probably a separate myeloid progenitor that enters the thymus. Many laboratories have identified macrophage progenitor activity within the DN1 compartment,14,18,24-26 and this has been used as an argument against CLPs as a thymic seeding cell.12,24,25 However, most of these studies show that less than 1% of DN1 cells give rise to myeloid colonies in methylcellulose assays, suggesting that myeloid progenitors are a rare subset within the DN1 population. Two recent studies have shown a high level of myeloid readout downstream of DN1, but it is unclear whether those studies exclude CLPs as a thymic progenitor because CLPs were not included as a control in the in vitro assays.24,25 Because MPP-injected recipients show thymic myeloid readout that is absent in CLP-injected recipients, our data strongly support the idea that a myeloid progenitor enters the thymus separately from CLP-derived cells. Along these lines, intravenously injected common myeloid progenitors yield thymic progeny, including CD11c+ dendritic cells, indicating that at least one myeloid committed lineage enters the thymus and is not derived from DN1.35,36 This implicates 2 separate lineages of thymic seeding cell populations: one that supplies myeloid cells and one that supplies T-lineage cells in the thymus, probably the CLP itself.
If, as these data indicate, CLPs seed the thymus (or rapidly give rise to a seeding cell), CLPs should be present in the thymus and in the blood. We have looked for phenotypic CLPs in the thymus but have not detected these cells. It is interesting to note that DN1 cells in the thymus share common levels of c-Kit and Sca-1 expression with MPPs in the BM, whereas CLPs differ in the expression of these markers.14 However, it has been observed by our laboratory and others that CLPs can rapidly adopt the surface phenotype of DN1 on entering the thymus,15,17 and this rapid change may explain the absence of phenotypic CLPs in the thymus. Therefore, surface phenotypes examined without lineage studies can be misleading in determining the relatedness of cellular populations.
We show that the blood Flk2+CD27+ population contains all of the thymocyte progenitors and is exceedingly rare, consisting of only several hundred cells per mouse. Furthermore, these Flk2+CD27+ cells can be divided into IL-7R+ and IL-7R− subpopulations, consistent with the presence of both CLPs and MPPs in the blood (Figures 6, S7). The existence of blood CLPs has been under some scrutiny, as one early study seemed to show an absence of CLPs in the blood,37 but more recently, blood CLPs have been identified and shown to be functional.22 Therefore, CLPs do exist in the blood and this is probably their route for transiting from the BM to the thymus. Although we have not directly demonstrated that CLPs seed the thymus, the reported B-cell potential of early thymic progenitors is consistent with this model.38-41 In addition, CLPs have been shown to express PSGL-1 and CCR9, 2 receptors known to be important for thymic homing.17,18,42 The plus/minus strategy used here shows that no other BM progenitors, other than CLPs or MPPs, consistently give rise to thymocytes at 14 days after injection. This implies that CLPs, or a subset of cells within the CLP gate, are the thymic seeding cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christina Muscat for generating and providing many antibodies, Libuse Jerabek for facilitating experiments, and Matthew Inlay, Deepta Bhattacharya, and Holger Karsunky for sharing results before publication and for helpful comments on this manuscript.
L.I.R.E. is a senior fellow of the Leukemia & Lymphoma Society.
Authorship
Contribution: T.S. and L.I.R.E. designed, implemented, and analyzed all the experiments and prepared the manuscript; and I.L.W. supervised the research project and contributed to the manuscript.
Conflict-of-interest disclosure: I.L.W. is a director of, owns equity in, and is a consultant for Stem Cells Inc and owns equity in Cellerant Inc. T.S. and L.I.R.E. declare no competing financial interests.
Correspondence: Lauren I. Richie Ehrlich, Stanford University, 279 Campus Drive, Beckman Center B259, Stanford, CA 94305; e-mail: lauren.ehrlich@stanford.edu.
References
Author notes
*T.S and L.I.R.E. contributed equally to this manuscript