Abstract
Although Foxp3+ T regulatory cells (Tregs) are well documented for their ability to suppress various immune cells, T-cell subsets capable of counteracting Tregs have not been demonstrated. Here, we assessed phosphoantigen-activated Vγ2Vδ2 T cells for the ability to interplay with Tregs in the context of mycobacterial infection. A short-term IL-2 treatment regimen induced marked expansion of CD4+CD25+Foxp3+ T cells and subsequent suppression of mycobacterium-driven increases in numbers of Vγ2Vδ2 T cells. Surprisingly, activation of Vγ2Vδ2 T cells by adding phosphoantigen Picostim to the IL-2 treatment regimen down-regulated IL-2–induced expansion of CD4+CD25+Foxp3+ T cells. Consistently, in vitro activation of Vγ2Vδ2 T cells by phosphoantigen plus IL-2 down-regulated IL-2–induced expansion of CD4+CD25+Foxp3+ T cells. Interestingly, anti–IFN-γ–neutralizing antibody, not anti–TGF-β or anti–IL-4, reduced the ability of activated Vγ2Vδ2 T cells to down-regulate Tregs, suggesting that autocrine IFN-γ and its network contributed to Vγ2Vδ2 T cells' antagonizing effects. Furthermore, activation of Vγ2Vδ2 T cells by Picostim plus IL-2 treatment appeared to reverse Treg-driven suppression of immune responses of phosphoantigen-specific IFNγ+ or perforin+ Vγ2Vδ2 T cells and PPD-specific IFNγ+αβ T cells. Thus, phos-phoantigen activation of Vγ2Vδ2 T cells antagonizes IL-2–induced expansion of Tregs and subsequent suppression of Ag-specific antimicrobial T-cell responses in mycobacterial infection.
Introduction
Human γδ T cells appear to belong to nonclassical T cells that contribute to both innate and adaptive immune responses. Circulating Vγ2Vδ2 (also termed Vγ9Vδ2) T cells exist only in primates and, in humans, constitute 60% to 95% of total blood γδ T cells. Vγ2Vδ2 T cells in primates can be activated by nonpeptidic phosphorylated metabolites of isoprenoid biosynthesis (eg, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate [HMBPP], isopentenyl pyrophosphate [IPP], and its isomer dimethylallyl pyrophosphate [DMAPP]).1-3 We have recently shown that HMBPP is associated with antigen-presenting cell (APC) membrane and specifically recognized by Vγ2Vδ2 T-cell receptor (TCR) expressed on Vγ2Vδ2 T cells.4 Although HMBPP produced by mycobacteria/other microbes is a potent activator for Vγ2Vδ2 T cells, these γδ T cells possess both innate and adaptive immune features.5-8 The finding that “unprimed” Vγ2Vδ2 T cells can recognize and react to wide ranges of nonpeptide phospholigands with the capability of “naive” production of cytokines has been interpreted as a pattern recognition–like feature of innate immune cells. On the other hand, the capacity of Vγ2Vδ2 T cells to undergo major clonal expansion in primary infection and to mount rapid recall-like expansion upon reinfection has been proposed as adaptive immune response of these γδ T cells.8 Consistent with these memory-type responses is the demonstration of memory phenotypes of Vγ2Vδ2 T cells in the blood of humans.9 Accumulating evidence suggests that Vγ2Vδ2 T cells play a role in mediating immunity against microbial pathogens8 and tumors.10
Foxp3-expressiong CD4+CD25+ regulatory T cells (Tregs) control immune responses to self-antigens and foreign antigens and play a major role in maintaining the balance between immunity and tolerance.11-14 Murine CD4+CD25+ regulatory T cells are induced by transforming growth factor β (TGF-β), although TGF-β plus IL-6 favors the development of Th17 cells.15 Tregs have been shown to broadly suppress activation, proliferation, and/or effector functions of various immune cell populations such as conventional CD4+ and CD8+ T cells,16 natural killer (NK) T cells,17 B cells,18 dendritic cells,19 monocytes/macrophages,20 neutrophils,21 and mast cells.22 Depletion of CD4+CD25+ T cells induces effective antitumor immunity, enhances immune responses to invading microbes, triggers allergic responses to innocuous environmental substances, and breaks fetus-maternal tolerance during pregnancy.11 On the other hand, Tregs have been found to suppress several T cell–mediated immune pathologies, including allergic responses and autoimmune diseases such as type 1 diabetes (T1D), experimental autoimmune encephalomyelitis (EAE), gastritis, colitis, glomerulo-nephritis, and polyarthritis as well as allograft rejection and graft-versus-host disease (GVHD).11
Although Tregs are well defined for their ability to suppress various immune cells, T-cell subsets capable of antagonizing Tregs and their function have not been demonstrated. Although the development of T-cell immune responses may occur as a result of their dominance over existing Tregs, such opposing cellular components have not been elucidated. Development of a useful model system may help to identify potential mutual regulatory effects of Tregs and other immune cells or elements. Interestingly, recent studies have shown that human recombinant IL-2 administration can lead to an increase in the frequency of circulating CD4+CD25+ regulatory T cells in cancer patients.23-26 We and others have also shown that IL-2 plus phospholigand treatment can induce remarkable expansion of Vγ2Vδ2 T cells in nonhuman primates.1,27,28 We therefore took advantage of the IL-2–based in vivo model systems to assess potential interplay or mutual regulation between Vγ2Vδ2 T cells and Tregs during early mycobacterial infection in nonhuman primates. We found that phosphoantigen-activated Vγ2Vδ2 T cells were able to down-regulate IL-2–induced expansion of Tregs, and antagonize Treg-driven suppression of in vivo immune responses.
Methods
Animals
Four- to 8-year-old, 3- to 4-kg cynomolgus macaques (Macaca fascicularis) were used in this study. A total of 18 monkeys were divided into 3 groups, 6 for each. All animals were maintained and used in accordance with the guidelines of the institutional animal care and use committee of all participating institutions. Animals were anesthetized with 10 mg/kg ketamine HCl (Fort Dodge Animal Health, Fort Dodge, IA) intramuscularly for all blood sampling and treatments. EDTA-anticoagulated blood was collected at various time points before and after treatment. Day-0 blood was drawn immediately prior to treatment.
Phosphoantigen compounds: HMBPP and Picostim
The phosphoantigen compound HMBPP was an analytic-pure synthetic compound with chemical structure identical to natural phosphoantigen produced from Escherichia coli or mycobacteria.1 Picostim was another phosphoantigen compound that shares chemical structure and bioactivity with HMBPP except that only 1 atom is different linking the carbon chain and the phosphate moiety (oxygen or carbon). These well-defined phosphoantigens were recognized by human and monkey Vγ2Vδ2 T cells.1 HMBPP and Picostim were produced, characterized, validated, and provided by Dr Hassan Jomaa from Justus-Liebig-Universität Giessen (Giessen, Germany) and Innate Pharma, respectively.
IL-2 alone and IL-2 plus Picostim treatment regimens
For the IL-2 alone treatment regimen, recombinant human IL-2 (rhIL-2; Proleukin; Chiron, Emeryville, CA) was reconstituted with sterile double-distilled (dd) H2O immediately prior to injection. One million IU rhIL-2 was given once daily from days 0 through 5 by subcutaneous injection. This was indeed the low-dose administration compared with the clinical use of rhIL-2 in humans. For the IL-2 plus Picostim treatment regimen, Picostim (22 mg/kg) was administered intramuscularly on day 0. One million IU rhIL-2 was given, exactly as described above, once daily from days 0 through 5 by subcutaneous injection. The Picostim/IL-2 treatment of monkeys reproducibly induced remarkable expansion of Vγ2Vδ2 T cells but not other γδ or αβ T-cell subpopulations.
Mycobacterium bovis bacilli Calmette-Guérin (BCG) infection
Immunofluorescent staining and flow cytometric analysis
EDTA blood (100 μL) was treated with RBC Lysing Buffer (Sigma-Aldrich, St Louis, MO) and washed twice with 5% FBS-PBS before staining. Peripheral blood mononuclear cells (PBMCs) were stained with up to 5 Abs (conjugated to FITC, PE, allophycocyanin, Pacific Blue, and PE-Cy5 or allophycocyanin-Cy7) for at least 15 minutes. After staining, cells were fixed with 2% formaldehyde-PBS (Protocol Formalin, Kalamazoo, MI) prior to analysis on a CyAn ADP flow cytometer (DakoCytomation, Carpinteria, CA). Lymphocytes were gated based on forward and side scatters, and pulse width and at least 40 000 gated events were analyzed using Summit Data Acquisition and Analysis Software (DakoCytomation). Further special gates and quadrants for analyzing the data were determined based on nonstaining, specific Ab staining, and isotype control Ab background staining. Absolute cell numbers were calculated based on flow cytometry data and complete blood counts performed on a hematology system (Advia 120; Siemens, Tarrytown, NY).
The following mouse mAbs were used: Vβ3.1 (8F10), Vβ5a (1C1), and Vβ8a (16G8; Endogen, Woburn, MA); CD25 (BC96; eBioscience, San Diego, CA); and anti–Foxp3-Alexa Fluor 647 (259D, used for intracellular staining; Biolegend, San Diego, CA). The following neutralizing Abs were used: anti–IL-4 (clone 8D4-8; Biolegend), anti–IFN-γ (clone MD-1; eBioscience), and anti–TGF-β1 (clone 9016; R&D Systems, Minneapolis, MN). Other primary and secondary mAbs were listed previously.1
Isolation of CD4+CD25+ T cells and Vγ2+ T cells
PBMCs were isolated from EDTA blood of the monkeys using Ficoll-Paque Plus (Amersham, Piscataway, NJ) density gradient centrifugation. CBC and differentials were performed before CD4+CD25+ T cells or Vγ2 T cells were isolated from PBMCs. CD4+CD25+ T cells were purified using CD4+CD25+ Regulatory T Cell Isolation Kit for nonhuman primates (Miltenyi Biotec, Auburn, CA). Briefly, CD4+ T cells were isolated from PBMCs by depletion of non-CD4+ cells with negative selection. The purified CD4+ T cells were then used to isolate CD4+CD25+ regulatory T cells through positive selection using CD25+ magnetic microbeads. Vγ2+ T cells were purified using purified mouse anti–human Vγ2 Ab (clone: 7A5; Endogen) and goat anti–mouse IgG microbeads (Miltenyi Biotec) with positive selection. The purity of Vγ2+ T cells and CD4+CD25+ T cells isolated using the commercial isolation kit was more than 90%.
In vitro activation of Tregs and Vγ2Vδ2 T cells with IL-2 alone or IL-2 plus HMBPP
PBMCs (106) were divided in 5 wells of Costar (Cambridge, MA) round-bottom 96-well plates and cultured in 10% FBS–RPMI 1640 (Invitrogen-Gibco, Carlsbad, CA) medium and rhIL-2 (Sigma-Aldrich) at one of the concentrations: 10, 20, 40, 80, 120, or 160 U/mL rhIL-2 with or without HMBPP (40 ng/mL HMBPP added at day 0). IL-2 was freshly added at days 0, 3, 5, and 7. At days 8, cells were collected and stained with PB-conjugated anti-CD4, PE-Cy7–conjugated CD8, PE-conjugated anti-CD25, and FITC-conjugated anti-Vδ2 in each tube at 4°C for 20 minutes in dark. After washing, cells were permeabilized in 250 μL Cell Cytofix/Cytoperm solution (BD Pharmingen, San Diego, CA) at 4°C for 45 minutes, washed for 2 times by permeabilization buffer, and then incubated at 4°C in the dark for 45 minutes with 5 μL anti–Foxp3-Alexa Fluor 647 or isotype control Ab. After washes with Perm/Wash buffer, followed by 2 mL 2% formaldehyde-PBS (Protocol Formalin), cells were fixed with 250 μL 2% formaldehyde-PBS buffer.
CFSE and PKH26 labeling
PBMCs (6 × 106), or mixtures of indicated ratios of purified CD4+CD25+ T cells and Vγ2Vδ2 T cells, were labeled with CFSE using the CFSE Cell Proliferation Kit (Invitrogen-Molecular Probes, Eugene, OR) following the manufacturer's protocol. Briefly, cells were suspended gently in 1 mL prewarmed 0.1% BSA-PBS containing CFSE at a 2.0-μM concentration and then incubated for 15 minutes at 37°C in dark. The staining was quenched by adding 5 volumes of ice-cold culture media and incubating for 5 minutes. Purified CD4+CD25+ T cells (106) were also labeled with PKH26 red using the PKH26 red fluorescent cell linker kit (Sigma-Aldrich). Briefly, cells were suspended in a 2-mL volume at 2 × 10−6 M PKH26 dye at room temperature for 5 minutes. The staining reaction was stopped by adding an equal volume of complete medium. The labeled cells were washed 3 to 4 times before use.
CFSE-based proliferation assay
This was done following the standard protocols as previously described.31-33 For the suppression assay, CFSE-labeled PBMCs were mixed at 2 × 105 cells/well with PKH26-labeled CD4+CD25+ T cells (0.25-2 × 105/well or at the indicated ratios) in the presence or absence of PPD (15 μg/mL; Colorado Serum, Denver, CO), HMBPP (40 ng/mL), and 5 μg/mL purified antihuman CD28 and anti-CD3 Abs. For the culture using IL-2, rhIL-2 was added at the concentration of 20 U/mL on days 0, 4, and 6. For coculture of CD4+CD25+ Tregs and Vγ2Vδ2 T cells, 1 to 4 × 105 cells of purified Vγ2+ T cells were mixed with 105 CD4+CD25+ T cells/per well, labeled with CFSE, and then cultured in the presence of 20 U/mL IL-2 (added at days 0, 4, and 6). At day 7 or 8, cells were collected and stained with anti-Vδ2 or anti-Vγ2, anti-CD4, anti-CD8, and anti-CD3 Abs as described. Proliferation was analyzed by flow cytometry to determine the dilution of CFSE fluorescence intensity and to exclude PKH26+ cells34 : the percentage of proliferation was calculated based on the number of CFSEdim cells divided by the number of CFSE+ cells.
Proliferation-inhibition assay for attenuating the ability of HMBPP-activated Vγ2Vδ2 T cells to down-regulate Tregs using anticytokine neutralizing antibody
PBMCs (106) were divided in 5 wells of Costar round-bottom 96-well plates and cultured in 10% FBS–RPMI 1640 medium. Cells were cultured, as previously described,35 with rhIL-2 (10 U/mL) only, IL-2 plus HMBPP (40 ng/mL, added on day 0), or IL-2 plus HMBPP with 1 of 3 neutralizing mAbs: anti–TGF-β1 (3 μg/mL), anti–IL-4 (3 μg/mL), and anti–IFN-γ (1 or 3 μg/mL, freshly added at days 0, 3, 5, and 7). At days 8, cells were collected and stained for Tregs and Vγ2Vδ2 T cells.
At days 3 and 5, the supernatants were collected, and the production of IFNγ was measured by enzyme-linked immunosorbent assays (ELISAs), which were done with the kit from BD Biosciences (BD551492 and 555212; San Jose, CA).
Intracellular cytokine staining
For intracellular cytokine staining (ICS), 106 peripheral blood lymphocytes (PBLs) plus costimulatory mAbs CD28 (1 μg/mL) and CD49d (1 μg/mL) were incubated with HMBPP (40 ng/mL), PPD (25 μg/mL), or media alone in 200-μL final volume for 1 hour at 37°C, 5% CO2 followed by an additional 5-hour incubation in the presence of brefeldin A (GolgiPlug; BD Pharmingen). After staining cell-surface CD3, CD4, CD8, and Vγ2 for at least 15 minutes, cells were permeabilized for 45 minutes (Cytofix/Cytoperm; BD Pharmingen) and stained for another 45 minutes for IFNγ and perforin before being resuspended in 2% formaldehyde-PBS.
Statistical analysis
The ANOVA test and the Student t test were exploited to determine the differences between groups, as described.29
Results
A 5-day IL-2 administration regimen induced an apparent expansion of CD4+CD25+Foxp3+ T cells and subsequent suppression of BCG-driven expansion of Vγ2Vδ2 T cells in macaques
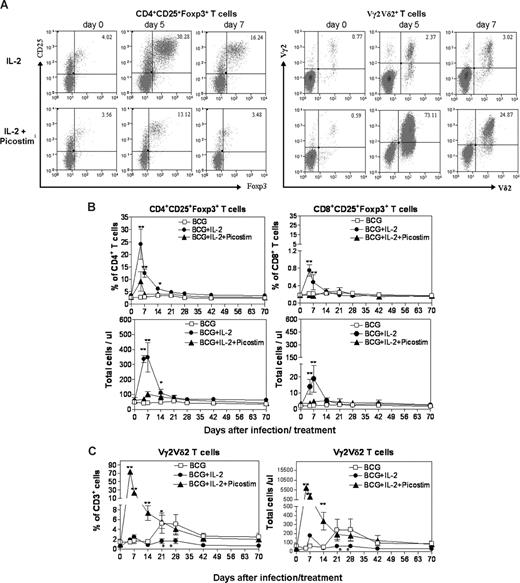
It has been shown that chronic IL-2 treatment in cancer patients can up-regulate Tregs in the blood.23-26 It has also been demonstrated that mycobacterial infections can reproducibly stimulate an expansion of phosphoantigen-specific Vγ2Vδ2 T cells.29,30,36 As an initial effort to study mutual regulatory effects of Tregs and Vγ2Vδ2 T cells, we sought to determine whether an acute IL-2 treatment regimen in the context of BCG infection could induce expansion of Tregs and Treg-driven suppression of BCG-induced expansion of Vγ2Vδ2 T cells in macaques. The acute IL-2 treatment in the initial phase of BCG infection induced up to 8-fold expansion of CD4+CD25+Foxp3+ T cells in PBMCs at days 5, 7, and 14 after the IL-2 treatment (Figure 1A,B). The expanded CD4+CD25+Foxp3+ T cells returned to the baseline level at 21 to 28 days after the IL-2 treatment. The IL-2 treatment also induced transient increases in numbers of selected Vβ3+ and Vβ5+ (not Vβ8+) CD4+Foxp3+ T-cell subpopulations in 5 and 4 of the 6 IL-2–treated monkeys, respectively (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The expansion of selected Vβ+Foxp3+ cells by IL-2 in the outbred macaques appeared to be consistent with the preferential usage of Vβ3 by human Tregs.37,38 Interestingly, whereas BCG infection alone reproducibly induced an expansion of Vγ2Vδ2 T cells at 21 to 42 days after the infection (Figure 1C and Shen et al29 ), the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells led to subsequent suppression of BCG-induced expansion of these γδ T cells in the IL-2–treated BCG-infected monkeys (Figure 1C). These results therefore demonstrated that the 5-day IL-2 treatment regimen induced an apparent expansion of CD4+CD25+Foxp3+ T regulatory cells and subsequent suppression of BCG-induced expansion of Vγ2Vδ2 T cells after BCG infection.
A 5-day IL-2 administration regimen induced an apparent expansion of CD4+CD25+Foxp3+ T cells (Tregs) in PBMCs; adding Picostim to the IL-2 treatment regimen induced Vγ2Vδ2 T-cell expansion and down-regulated IL-2–induced increases in numbers of Tregs in BCG-infected macaques. (A) The top panel of flow cytometry histograms showed that one representative macaque (7285) exhibited increased numbers of CD25+Foxp3+CD4 T cells after the IL-2 treatment (on the left), with subtle changes in Vγ2Vδ2 T cells (on the right). The bottom panel of flow cytometry histograms showed that the other representative monkey (7287) displayed reduced numbers of CD25+Foxp3+CD4 T cells after the IL-2 + Picostim treatment compared with the IL-2–treated monkey, with marked increases in Vγ2Vδ2 T cells (on the right). CD25+Foxp3+ T cells were gated on CD4. Note that the percentages of CD25+Foxp3+ Tregs in CD3+ T cells were 4% on day 5 after the IL-2 + Picostim treatment. Also see total numbers of these T cells in panel B. (B) Changes in percentage and absolute numbers of CD4+CD25+Foxp3+ T regulatory cells in PBMCs of 3 groups of monkeys (BCG; BCG + IL-2; BCG + IL-2 + Picostim) over time after treatment/infection. Shown are the mean values with SEM from 6 monkeys for each group. **P < .01; *P < .05 for differences between BCG + IL-2 and BCG groups and between BCG + IL-2 and BCG + IL-2. (C) Changes in percentage and absolute numbers of Vγ2Vδ2 T cells in PBMCs of 3 groups of monkeys (BCG; BCG + IL-2; BCG + IL-2 + Picostim) over time after treatment/infection. **P < .01; *P < .05 for differences between BCG + IL-2 and BCG groups, and between BCG + IL-2 + Picostim and BCG groups or BCG + IL-2 + Picostim and BCG + IL-2 groups. Note that IL-2–induced expansion of Tregs (B) led to subsequent suppression of BCG-induced expansion of Vγ2Vδ2 T cells at days 21 to 42 (*P < .05 for differences between BCG + IL-2 and BCG groups as well as BCG + IL-2 and BCG + IL-2 + Picostim groups at days 21 and 28).
A 5-day IL-2 administration regimen induced an apparent expansion of CD4+CD25+Foxp3+ T cells (Tregs) in PBMCs; adding Picostim to the IL-2 treatment regimen induced Vγ2Vδ2 T-cell expansion and down-regulated IL-2–induced increases in numbers of Tregs in BCG-infected macaques. (A) The top panel of flow cytometry histograms showed that one representative macaque (7285) exhibited increased numbers of CD25+Foxp3+CD4 T cells after the IL-2 treatment (on the left), with subtle changes in Vγ2Vδ2 T cells (on the right). The bottom panel of flow cytometry histograms showed that the other representative monkey (7287) displayed reduced numbers of CD25+Foxp3+CD4 T cells after the IL-2 + Picostim treatment compared with the IL-2–treated monkey, with marked increases in Vγ2Vδ2 T cells (on the right). CD25+Foxp3+ T cells were gated on CD4. Note that the percentages of CD25+Foxp3+ Tregs in CD3+ T cells were 4% on day 5 after the IL-2 + Picostim treatment. Also see total numbers of these T cells in panel B. (B) Changes in percentage and absolute numbers of CD4+CD25+Foxp3+ T regulatory cells in PBMCs of 3 groups of monkeys (BCG; BCG + IL-2; BCG + IL-2 + Picostim) over time after treatment/infection. Shown are the mean values with SEM from 6 monkeys for each group. **P < .01; *P < .05 for differences between BCG + IL-2 and BCG groups and between BCG + IL-2 and BCG + IL-2. (C) Changes in percentage and absolute numbers of Vγ2Vδ2 T cells in PBMCs of 3 groups of monkeys (BCG; BCG + IL-2; BCG + IL-2 + Picostim) over time after treatment/infection. **P < .01; *P < .05 for differences between BCG + IL-2 and BCG groups, and between BCG + IL-2 + Picostim and BCG groups or BCG + IL-2 + Picostim and BCG + IL-2 groups. Note that IL-2–induced expansion of Tregs (B) led to subsequent suppression of BCG-induced expansion of Vγ2Vδ2 T cells at days 21 to 42 (*P < .05 for differences between BCG + IL-2 and BCG groups as well as BCG + IL-2 and BCG + IL-2 + Picostim groups at days 21 and 28).
Activation of Vγ2Vδ2 T cells by adding phosphoantigen Picostim to the IL-2 treatment regimen down-regulated the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells
Given that the short-term IL-2 treatment induced Treg expansion and subsequent suppression of BCG-driven expansion of Vγ2Vδ2 T cells, we sought to determine whether an enhanced activation of Vγ2Vδ2 T cells could antagonize IL-2–induced Treg expansion and reverse inhibition of BCG-driven expansion of Vγ2Vδ2 T cells. To this end, a group of BCG-infected monkeys was treated with the single-dose phosphoantigen Picostim plus the 5-day IL-2 regimen (Figure 1), and assessed for the interplay between Tregs and Vγ2Vδ2 T cells. The addition of single-dose Picostim to the IL-2 treatment regimen induced a marked expansion of Vγ2Vδ2 T cells (Figure 1A,C). Surprisingly, the activation/expansion of Vγ2Vδ2 T cells after the Picostim/IL-2 treatment reduced the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells in Picostim/IL-2–treated monkeys, compared with IL-2–treated–only macaques (Figure 1A-C). An addition of HMBPP (50 mg/kg) to the IL-2 regimen also down-regulated IL-2–induced expansion of Tregs (data not shown). Interestingly, although Picostim activation of Vγ2Vδ2 T cells antagonized IL-2–induced expansion of Tregs, the increased numbers of Vγ2Vδ2 T cells were sustained through 42 days after the Picostim/IL-2 treatment and superimposed BCG infection (Figure 1C). These results therefore demonstrated that activation/expansion of Vγ2Vδ2 T cells after the Picostim/IL-2 treatment could remarkably reduce the IL-2–induced expansion of Tregs in the blood.
In vitro activation of Vγ2Vδ2 T cells in the PBMC culture, but not in the purified CD25+CD4+ cell culture containing Tregs, down-regulated IL-2–induced expansion of CD4+CD25+Foxp3+ regulatory T cells
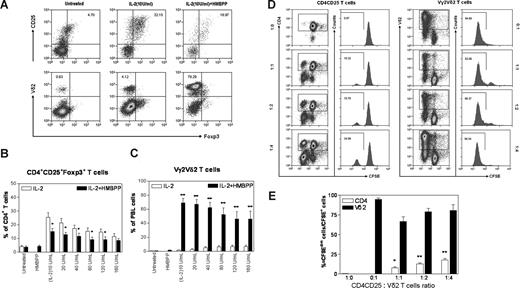
The down-regulation of the IL-2–induced CD4+CD25+Foxp3+ T-cell expansion by Picostim-activated Vγ2Vδ2 T cells raised a question as to whether Vγ2Vδ2 T cells expressed more IL-2 receptors and consumed more IL-2 than Tregs for the in vivo clonal expansion. To address this issue, PBMCs from naive monkeys were cultured in the presence of various concentrations of IL-2 or IL-2 plus phosphoantigen HMBPP, and then assessed for increases in numbers of CD4+CD25+Foxp3+ T cells and phosphoantigen-reactive Vγ2Vδ2 T cells. Consistent with what was seen in vivo, IL-2 also induced increases in numbers of CD4+CD25+Foxp3+ T cells in the PBMC culture. IL-2 at concentrations of 10 to 20 U/mL were more effective than 120 to 160 U/mL to induce Tregs in vitro (Figure 2A,B). However, the addition of phosphoantigen HMBPP to the PBMC culture containing various concentrations of IL-2 not only induced marked activation/expansion of Vγ2Vδ2 T cells, but also significantly down-regulated IL-2–induced expansion of CD4+CD25+Foxp3+ T cells in the cultured PBMCs (Figure 2A-C).
In vitro HMBPP activation of Vγ2Vδ2 T cells in PBMCs, but not in the CD25+CD4+ cell culture containing Tregs, down-regulated IL-2–induced proliferation/expansion of CD4+CD25+Foxp3+ regulatory T cells. (A) Flow cytometry histograms showed changes in percentages of CD4+CD25+Foxp3+ T cells for one representative macaque (7404) after the 8-day culture with IL-2 or IL-2 + HMBPP (top panels were CD4 gated). Panels B and C show percentages of Tregs and Vγ2Vδ2 T cells, respectively, after the 8-day culture with IL-2 only or IL-2 + HMBPP. Data were mean values with SEM derived from 3 independent experiments using PBMCs from 7 naive cynomolgus monkeys. **P < .01; *P < .05 for the comparisons. (D,E) CD4+CD25+ T cells and activated Vδ2 T cells proliferated mutually in the presence of IL-2 in the culture without APCs and other immune cells. Purified Vδ2+ T cells (0 × 105, 1 × 105, 2 × 105, or 4 × 105) were mixed with 105 cells/well of CD4+CD25+ T cells, labeled with CFSE, and then cultured with 20 U/mL IL-2 for 7 days. Proliferation was analyzed by flow cytometry to determine the dilution of CFSE fluorescence intensity: the number of CFSEdim cells divided by the number of CFSE+ cells. (D) Flow histograms of one representative macaque (7716). (E) Percentage rates of proliferation of CD4+CD25+ T cells and Vδ2 T cells after the 7-day culture. Data were mean values with SEM from 3 independent experiments using PBMCs from 6 naive macaques. **P < .01; *P < .05 for differences between CD4+CD25+ T cells only and these cells plus Vδ2 T cells. Purified CD25+CD25+ T cells were shown to inhibit antigen-specific T cells (data not shown).
In vitro HMBPP activation of Vγ2Vδ2 T cells in PBMCs, but not in the CD25+CD4+ cell culture containing Tregs, down-regulated IL-2–induced proliferation/expansion of CD4+CD25+Foxp3+ regulatory T cells. (A) Flow cytometry histograms showed changes in percentages of CD4+CD25+Foxp3+ T cells for one representative macaque (7404) after the 8-day culture with IL-2 or IL-2 + HMBPP (top panels were CD4 gated). Panels B and C show percentages of Tregs and Vγ2Vδ2 T cells, respectively, after the 8-day culture with IL-2 only or IL-2 + HMBPP. Data were mean values with SEM derived from 3 independent experiments using PBMCs from 7 naive cynomolgus monkeys. **P < .01; *P < .05 for the comparisons. (D,E) CD4+CD25+ T cells and activated Vδ2 T cells proliferated mutually in the presence of IL-2 in the culture without APCs and other immune cells. Purified Vδ2+ T cells (0 × 105, 1 × 105, 2 × 105, or 4 × 105) were mixed with 105 cells/well of CD4+CD25+ T cells, labeled with CFSE, and then cultured with 20 U/mL IL-2 for 7 days. Proliferation was analyzed by flow cytometry to determine the dilution of CFSE fluorescence intensity: the number of CFSEdim cells divided by the number of CFSE+ cells. (D) Flow histograms of one representative macaque (7716). (E) Percentage rates of proliferation of CD4+CD25+ T cells and Vδ2 T cells after the 7-day culture. Data were mean values with SEM from 3 independent experiments using PBMCs from 6 naive macaques. **P < .01; *P < .05 for differences between CD4+CD25+ T cells only and these cells plus Vδ2 T cells. Purified CD25+CD25+ T cells were shown to inhibit antigen-specific T cells (data not shown).
Next, we sought to explore whether activated Vγ2Vδ2 T cells could directly regulate CD4+CD25+Foxp3+ T cells or vice versa in the absence of APCs and other cells in PBMCs. Vγ2Vδ2 T cells and CD4+CD25+ T cells were purified from PBMCs using antibody-conjugated magnetic beads, and mixed in the culture at various ratios. Initially, we took advantage of anti-Vδ2 Ab/magnetic beads to purify and activate Vγ2Vδ2 T cells simultaneously, since macaque Vδ2 predominantly paired with Vγ2, and anti-Vδ2 Ab cross-linking led to activation of Vγ2Vδ2 T cells. Activated Vγ2Vδ2 T cells purified by anti-Vδ Ab/beads were able to proliferate vigorously in the IL-2–containing medium, and only small reduction in their proliferation was seen when equal or smaller numbers of purified CD4+CD25+ T cells were added to the culture (Figure 2D,E). On the other hand, purified CD4+CD25+ T cells containing Tregs proliferated when equal numbers of Vγ2Vδ2 T cells were added to the culture, and the magnitude of proliferation was augmented over the addition of 2- and 4-fold greater numbers of purified γδ T cells (Figure 2D,E). Similar results were seen when HMBPP-activated Vγ2Vδ2 T cells were mixed with purified CD4+CD25+ T cells in the culture depleted of other cells (data not shown). These findings were different from what was seen in unfractionated PBMCs, demonstrating that in the absence of APCs (monocytes)/other immune cells, activated Vγ2Vδ2 T cells were unable to down-regulate the proliferation of CD4+CD25+ T cells containing Tregs.
Thus, in vitro activation of Vγ2Vδ2 T cells in PBMCs, but not in the purified CD25+CD4+ cell culture containing Tregs only, down-regulated the IL-2–induced expansion of CD4+CD25+Foxp3+ regulatory T cells.
Anti–IFN-γ, but not anti–TGF-β or anti–IL-4, neutralizing antibody reduced the ability of HMBPP-activated Vγ2Vδ2 T cells to down-regulate the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells
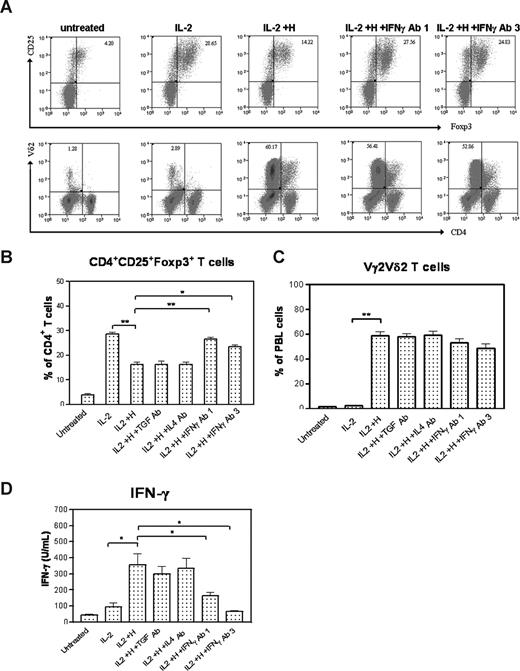
Since activated Vγ2Vδ2 T cells had no inhibiting effect on CD4+CD25+Foxp3+ T cells in the culture without APCs and other cells in PBMCs, we sought to determine whether some cytokines produced by phosphoantigen-activated Vγ2Vδ2 T cells contributed to the down-regulation of the IL-2–induced proliferation of Tregs. We set up cytokine-neutralizing experiments in the proliferation assays using anti–IFN-γ, anti–IL-4, or anti–TGF-β neutralizing antibodies because HMBPP phosphoantigen stimulation could up-regulate many genes including those encoding these cytokines (Wang et al, data not shown). Surprisingly, although anti–TGF-β or anti–IL-4–neutralizing antibodies did not affect the HMBPP-mediated down-regulation of Tregs, anti–IFN-γ–neutralizing antibody significantly reduced the ability of HMBPP-activated Vγ2Vδ2 T cells to antagonize Treg expansion (Figure 3A,B). Anti–IFN-γ– neutralizing antibody allowed the expansion of both CD4+CD25+Foxp3+ and Vγ2Vδ2 T-cell subsets in the HMBPP/IL-2 cultures (Figure 3A-C). Consistently, the anti–IFN-γ mAb neutralized and reduced HMBPP-stimulated increases in IFN-γ (Figure 3D). These results therefore suggested that autocrine IFN-γ or its cytokine networks produced by HMBPP-activated Vγ2Vδ2 T cells might play a role in antagonizing IL-2–induced expansion of CD4+CD25+Foxp3+ T cells.
Anti–IFN-γ, but not anti–TGF-β or anti–IL-4, neutralizing antibody reduced the ability of HMBPP-activated Vγ2Vδ2 T cells to down-regulate the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells. (A) Flow histograms from one representative monkey (7741) show that IL-2–induced expansion of Tregs was inhibited by HMBPP + IL-2, but the inhibition was reversed significantly by adding neutralizing anti–IFN-γ mAb (1 or 3 μg/mL) to HMBPP + IL-2 in PBMC culture. No effect was seen when adding neutralizing anti–TGF-β mAb, or neutralizing anti–IL-4 mAb to the HMBPP + IL-2 culture. (B,C) Numbers of CD4+CD25+Foxp3+ T cells and Vγ2Vδ2 T cells in PBMC cultures treated with medium alone, IL-2, HMBPP + IL-2, HMBPP + IL-2 + anti–TGF-β mAb, HMBPP + IL-2 + anti–IL-4 mAb, and HMBPP + IL-2 + anti–IFN-γ mAb. Data were mean values with error bars of SEM from PBMCs of 3 monkeys in 2 independent experiments. (D) Concentrations of IFN-γ protein in PBMC cultures treated with medium alone, IL-2, HMBPP + IL-2, HMBPP + IL-2 + anti–TGF-β mAb, HMBPP + IL-2 + anti–IL-4 mAb, and HMBPP + IL-2 + anti–IFN-γ mAb. **P < .01; *P < .05.
Anti–IFN-γ, but not anti–TGF-β or anti–IL-4, neutralizing antibody reduced the ability of HMBPP-activated Vγ2Vδ2 T cells to down-regulate the IL-2–induced expansion of CD4+CD25+Foxp3+ T cells. (A) Flow histograms from one representative monkey (7741) show that IL-2–induced expansion of Tregs was inhibited by HMBPP + IL-2, but the inhibition was reversed significantly by adding neutralizing anti–IFN-γ mAb (1 or 3 μg/mL) to HMBPP + IL-2 in PBMC culture. No effect was seen when adding neutralizing anti–TGF-β mAb, or neutralizing anti–IL-4 mAb to the HMBPP + IL-2 culture. (B,C) Numbers of CD4+CD25+Foxp3+ T cells and Vγ2Vδ2 T cells in PBMC cultures treated with medium alone, IL-2, HMBPP + IL-2, HMBPP + IL-2 + anti–TGF-β mAb, HMBPP + IL-2 + anti–IL-4 mAb, and HMBPP + IL-2 + anti–IFN-γ mAb. Data were mean values with error bars of SEM from PBMCs of 3 monkeys in 2 independent experiments. (D) Concentrations of IFN-γ protein in PBMC cultures treated with medium alone, IL-2, HMBPP + IL-2, HMBPP + IL-2 + anti–TGF-β mAb, HMBPP + IL-2 + anti–IL-4 mAb, and HMBPP + IL-2 + anti–IFN-γ mAb. **P < .01; *P < .05.
Activation of Vγ2Vδ2 T cells by Picostim/IL-2 treatment not only down-regulated the IL-2–induced expansion of Tregs but also antagonized the subsequent suppression of Ag-specific antimicrobial T-cell responses in mycobacterial infection
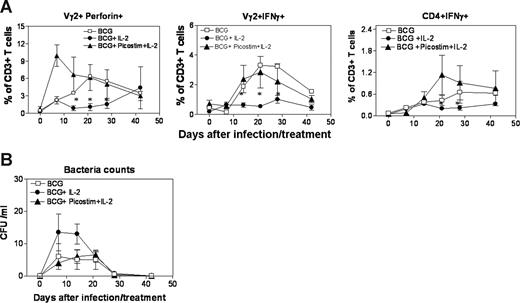
Finally, we sought to determine whether IL-2–induced Tregs suppressed the priming or development of mycobacterium-specific cellular immune responses in early BCG infection, and whether activation of Vγ2Vδ2 T cells by Picostim/IL-2 treatment could antagonize the Treg-mediated immune suppression. The in vivo expansion of CD4+CD25+Foxp3+ Tregs after the IL-2 treatment of BCG-infected monkeys resulted in the suppression of priming and development of mycobacterium-specific Vγ2Vδ2 T effector cells and αβ CD4 T effector cells (Figure 4A). The down-regulation of IL-2–induced Tregs by Picostim activation of Vγ2Vδ2 T cells preserved the development of Vγ2Vδ2 T-cell immune responses (Figure 1C; Figure 4A). The numbers of HMBPP-specific IFN-γ+ and perforin+ Vγ2Vδ2 T cells were significantly greater in the Picostim/IL-2–treated monkeys than those in the IL-2–treated monkeys over time after the BCG infection (Figure 4A). Similarly, the down-regulation of Treg expansion after Picostim activation of Vγ2Vδ2 T cells appeared to reverse the in vivo suppression of mycobacterium-specific αβ T-cell responses, as the numbers of PPD-specific IFNγ+ CD4 T cells detected in PBMCs from the Picostim/IL-2–treated and control monkeys were significantly greater than those from the IL-2–treated monkeys after the BCG infection (Figure 4A). It was worthwhile to mention that functional Tregs were present in PBMCs of all BCG-infected monkeys, as CD4+CD25+ T cells isolated individually from all 3 groups of BCG-infected monkeys similarly suppressed proliferation of HMBPP-specific Vγ2Vδ2 T cells and PPD-specific CD4 and CD8 T cells in the autologous PBMC culture (data not shown). Interestingly, the down-regulation of IL-2–induced Tregs after Picostim activation of Vγ2Vδ2 T cells appeared to lead to lower BCG CFU counts in the blood of the Picostim/IL-2–treated monkeys (Figure 4B). Thus, these results suggested that activated Vγ2Vδ2 T cells after Picostim/IL-2 treatment not only down-regulated IL-2–induced expansion of Tregs but also antagonized subsequent suppression of Ag-specific antimicrobial T-cell responses in mycobacterial infection.
Activation of Vγ2Vδ2 T cells by Picostim/IL-2 treatment not only down-regulated the IL-2–induced expansion of Tregs, but also reversed subsequent Treg-associated suppression of Ag-specific antimicrobial T-cell responses in mycobacterial infection. (A) Intracellular cytokine staining after antigen stimulation showed that numbers of HMBPP-specific Perforin+ (left panel) and IFNγ+ (middle panel) Vγ2Vδ2 T cells were significantly higher in PBMCs of BCG-infected IL-2/Picostim–treated monkeys than those in BCG-infected IL-2–treated macaques. Data were mean values with SEM derived from PBMCs of 6 monkeys in each group. *P < .05 for differences in perforin+ cells between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 2 through 4; P < .05 for differences in IFNSγ+ cells between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 2 and 4. Similarly, numbers of PPD-specific IFN+ CD4 T cells were higher in PBMCs of BCG-infected IL-2/Picostim–treated monkeys than those in BCG-infected IL-2–treated macaques. *P < .05 for differences in IFNγ+ between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 3 and 4. (B) BCG appeared to be more isolable in the blood of BCG + IL-2 group than of BCG + IL-2/Picostim–treated group at week 1. Data were mean values with SEM derived from blood of 6 monkeys per group.
Activation of Vγ2Vδ2 T cells by Picostim/IL-2 treatment not only down-regulated the IL-2–induced expansion of Tregs, but also reversed subsequent Treg-associated suppression of Ag-specific antimicrobial T-cell responses in mycobacterial infection. (A) Intracellular cytokine staining after antigen stimulation showed that numbers of HMBPP-specific Perforin+ (left panel) and IFNγ+ (middle panel) Vγ2Vδ2 T cells were significantly higher in PBMCs of BCG-infected IL-2/Picostim–treated monkeys than those in BCG-infected IL-2–treated macaques. Data were mean values with SEM derived from PBMCs of 6 monkeys in each group. *P < .05 for differences in perforin+ cells between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 2 through 4; P < .05 for differences in IFNSγ+ cells between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 2 and 4. Similarly, numbers of PPD-specific IFN+ CD4 T cells were higher in PBMCs of BCG-infected IL-2/Picostim–treated monkeys than those in BCG-infected IL-2–treated macaques. *P < .05 for differences in IFNγ+ between BCG + IL-2 and BCG + IL-2/Picostim groups at weeks 3 and 4. (B) BCG appeared to be more isolable in the blood of BCG + IL-2 group than of BCG + IL-2/Picostim–treated group at week 1. Data were mean values with SEM derived from blood of 6 monkeys per group.
Discussion
Our studies provide evidence that the activation of phosphoantigen-specific Vγ2Vδ2 T cells can down-regulate the IL-2–induced expansion of Tregs and reverse subsequent suppression of mycobacterium-specific T-cell immune responses. The finding supports our presumption that T-cell subsets potentially capable of antagonizing Tregs and their immune suppression are indeed present and detectable in the immune system. Although most studies have been focused on developmental and functional aspects of Foxp3+ Tregs, our work emphasizes elucidation of cellular components that regulate and antagonize Tregs. Given the importance for antagonizing Treg-mediated suppressive effects on anticancer and antimicrobial immune responses, our data provide a rationale for a potential application targeting Vγ2Vδ2 T cells for down-regulation of IL-2–induced Tregs.
The capability of activated Vγ2Vδ2 T cells to counteract IL-2–induced Tregs appears to involve APCs (monocytes) or other immune cells in the blood or immune system. Adding Picostim or HMBPP to the systemic IL-2 treatment regimen indeed activated and expanded Vγ2Vδ2 T cells in the circulation or the immune system, and therefore down-regulated the IL-2–induced proliferation of Tregs. Similarly, phosphoantigen HMBPP activation of Vγ2Vδ2 T cells in the unfractionated PBMC culture also counteracted IL-2–induced expansion of Tregs. However, in the absence of APCs and other PBMCs, Vγ2Vδ2 T cells activated by anti-TCR Ab or HMBPP indeed stimulated, not suppressed, proliferation of purified CD4+CD25+ T cells containing Tregs. Since APCs or dendritic cells can optimally present phosphoantigen4 and efficient stimulate ongoing activation of Vγ2Vδ2 T cells,39 cell-cell interaction after phosphoantigen stimulation may generate the environment that limits cell growth or expansion of IL-2–induced Tregs. This scenario might help to explain why phosphoantigen/IL-2 stimulation of Vγ2Vδ2 T cells in the blood circulation or the PBMC culture down-regulated IL-2–mediated Treg expansion.
Although activated Vγ2Vδ2 T cells did not appear to directly inhibit CD4+CD25+ cells containing Tregs when these 2 cell subsets were cultured together without other cells, activated γδ T cells were still able to proliferate in the presence of IL-2. Nevertheless, Treg-mediated suppression of Vγ2Vδ2 T cells was profound when CD4+CD25+ T cells were approximately 200 times more Vγ2Vδ2 T cells in the culture (data not shown). The marked in vivo expansion of Tregs after IL-2 treatment leads to subsequent suppression of BCG-stimulated increases in Vγ2Vδ2 T cells in the BCG infection. However, without IL-2–induced expansion of Tregs, BCG infection elicited an apparent expansion of Vγ2Vδ2 T cells (Figure 1C), and potent PPD-specific αβ T-cell immune responses (Figure 4A). In fact, Vγ2Vδ2 T cells can undergo prolonged expansion in the blood and accumulation in the pulmonary compartment after infections or a single-dose HMBPP plus IL-2 treatment.1,8,29 This unique feature of Vγ2Vδ2 T cells may contribute to the phosphoantigen-stimulated antagonizing effects on Tregs.
The in vivo mechanism by which activated Vγ2Vδ2 T cells antagonize the IL-2–induced Tregs is not precisely known. Our in vitro studies suggest that cytokine networks, but not cytotoxic killing or cell-cell contact inhibition, after activation of Vγ2Vδ2 T cells contribute to the down-regulation of IL-2–induced expansion of Tregs. The neutralizing/blocking experiments using anti–IFN-γ, anti–TGF-β, or anti–IL-4–neutralizing antibodies demonstrate that Vγ2Vδ2 T cell–mediated down-regulation of IL-2–induced Treg expansion involves the Th1 cytokine IFN-γ or its networks, but not TGF-β or IL-4. In fact, Th1 and Th2 polarizing programming including cytokines have been reported to oppose peripheral induction and differentiation of Foxp3+ Tregs from naive cells in mice.40
The finding that phosphoantigen activation of Vγ2Vδ2 T cells can antagonize Tregs and their immune suppression of antigen-specific T-cell responses may provide a tool to down-regulate IL-2–induced Tregs for enhancing immune responses.11 Given the possibility that Tregs inhibit antimicrobial and anticancer immune responses, phosphoantigen activation of Vγ2Vδ2 T cells may counteract Tregs and facilitate development of antimicrobial and anticancer immunity. In fact, the abrogation of proliferation and expansion of Tregs during Mycobacterium tuberculosis can reverse Treg-mediated immune suppression and enhance anti-tuberculosis (TB) immunity in rodents.41 IL-2 immunotoxin denileukin diftitox (a fusion protein of IL-2 and diphtheria toxin) and CD25-directed immunotoxin–LMB-2 have been used to reduce Tregs and augment antitumor immune responses.42,43 Now, the phosphoantigen/IL-2 treatment regimen makes it possible to activate and expand Vγ2Vδ2 T cells and thus down-regulate IL-2–induced Tregs during microbial infections or vaccinations. The down-regulation of Tregs might therefore facilitate the development of microbe-specific immune responses and vaccine-elicited immune responses. Since activation of Vγ2Vδ2 T cells by phosphoantigen/IL-2 treatment regimen itself has been shown to confer anticancer therapy10 and antimicrobial immune responses,1,44 activated Vγ2Vδ2 T cells during phosphoantigen/IL-2 treatment might have a double-edged function: γδ T cell–mediated immunotherapeutics and enhancement of αβ T-cell immune responses or immunity against infection or cancers through down-regulation of Tregs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Hassan Jomaa in Germany for providing phosphoantigen HMBPP for in vitro studies; B. Paige, J. Graves, and Dr K. Hagen for technical assistance with flow cytometry; the UIC Biological Resources Laboratory staff for animal work; and Zahida Ali, Bridgett Ryan, and other members of the Chen Laboratory for technical assistance and discussion.
This work was supported by National Institutes of Health (Bethesda, MD) R01 grants HL64560 (Z.W.C.) and RR13601 (Z.W.C.).
National Institutes of Health
Authorship
Contribution: Z.W.C. designed and supported the project and wrote the paper; G.G., L.S., Y.W., and C.Y.C. helped to design and perform the experiments, analyzed the data, and wrote some parts of the paper; and the remaining authors helped to perform the experiments and to analyze the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zheng W. Chen, 909 S Wolcott Avenue, E704, M/C790, Chicago, IL 60612; e-mail: zchen@uic.edu.
References
Author notes
*G.G., L.S., and Y.W. contributed equally to this work and should be considered first authors.