Abstract
Mitogen-activated protein kinases (MAPK), p38, and extracellular stimuli-responsive kinase (ERK), are acutely but transiently activated in platelets by platelet agonists, and the agonist-induced platelet MAPK activation is inhibited by ligand binding to the integrin αIIbβ3. Here we show that, although the activation of MAPK, as indicated by MAPK phosphorylation, is initially inhibited after ligand binding to integrin αIIbβ3, integrin outside-insignaling results in a late but sustained activation of MAPKs in platelets. Furthermore, we show that the early agonist-induced MAPK activation and the late integrin-mediated MAPK activation play distinct roles in different stages of platelet activation. Agonist-induced MAPK activation primarily plays an important role in stimulating secretion of platelet granules, while integrin-mediated MAPK activation is important in facilitating clot retraction. The stimulatory role of MAPK in clot retraction is mediated by stimulating myosin light chain (MLC) phosphorylation. Importantly, integrin-dependent MAPK activation, MAPK-dependent MLC phosphorylation, and clot retraction are inhibited by a Rac1 inhibitor and in Rac1 knockout platelets, indicating that integrin-induced activation of MAPK and MLC and subsequent clot retraction is Rac1-dependent. Thus, our results reveal 2 different activation mechanisms of MAPKs that are involved in distinct aspects of platelet function and a novel Rac1-MAPK–dependent cell retractile signaling pathway.
Introduction
Mitogen-activated protein kinases (MAPK) are serine/threonine kinases that control cellular responses to proliferative and chemotactic stimuli such as growth factors and hormones. The presence and activity of 3 members of the MAPK family, p38, extracellular stimuli-responsive kinase (ERK), and c-Jun NH2-terminal kinase (JNK) have been demonstrated in blood platelets.1-7 The acute activation of both p38 and ERK in response to the platelet agonists has been reported, and peak activity is detectable within minutes of agonist stimulation.1,2,6-9 The agonist-induced activation of p38 and ERK appears transient, probably because it is negatively regulated by integrin outside-in signaling. Ligand binding to integrin αIIbβ3 has been shown to down-regulate active p387 and ERK4,6,7 in platelets. These findings are intriguing, because it has been reported that outside-in signaling by β1 integrins activates MAPKs in proliferative cells such as fibroblasts,10 endothelial cells,11 and epidermal stem cells,12 although αIIbβ3-dependent MAPK activation induced by integrin ligands has never been demonstrated in platelets
The integrin αIIbβ3 has a low affinity for its ligands in resting platelets. At sites of vascular injury, integrin αIIbβ3 is activated by intracellular signaling (inside-out signaling) initiated by exposure of platelets to the subendothelial adhesive proteins collagen and von Willebrand factor (VWF) or soluble agonists such as thrombin and adenosine diphosphate (ADP) (see reviews13-15 ). Ligand binding to the activated integrin αIIbβ3 not only mediates platelet adhesion and aggregation, but also transmits “outside-in” signals that greatly amplify the platelet response and are critically important in stable platelet adhesion, spreading, and clot retraction.15 The early integrin outside-in signaling leading to cell spreading is thought to be mediated by small G proteins such as Rac and CDC42 in many cells.16 Integrin αIIbβ3-mediated platelet spreading requires Src family of tyrosine kinases17,18 and involves c-Src-dependent inhibition of the RhoA signaling pathway.19,20 After cell spreading, calpain cleavage of integrin β3 by relieving the inhibitory effect of β3-bound c-Src activates the RhoA-dependent retractile signaling, leading to cell retraction.20 However, inhibition of the calpain-mediated switch mechanism only partially inhibited integrin-mediated clot retraction,20 suggesting the presence of a second retractile signaling pathway, the molecular mechanism of which is unclear. With regard to the role of MAPK in platelet integrin signaling, we and other investigators have previously shown that the activation of p38 and ERK by VWF are important for GPIb-IX–mediated integrin activation6,7 and platelet adhesion under flow conditions.21 Other platelet agonists, thrombin, collagen and thromboxane A2 (TXA2), also activate MAPKs, and agonist-induced MAPK activation promotes the second wave platelet aggregation, platelet adhesion, and spreading.2,7,21-24 Despite these recent progresses, the exact roles of these MAPK pathways in integrin signaling in platelets are not totally clear.
In this study, we present data showing that there are 2 distinct MAPK activation mechanisms. In addition to the known agonist-stimulated early activation of p38 and ERK, which are inhibited by ligand binding to integrin as previously shown, integrin outside-in signaling results in a late but sustained activation of both p38 and ERK. More importantly, we found that a primary role of agonist-induced early p38 and ERK activation is to mediate platelet granule release, thus amplifying platelet responses and stimulating the second wave of platelet aggregation. Distinct from the agonist-induced early MAPK activity, the integrin-mediated late MAPK activation phase is important in the late integrin outside-in signaling response leading to clot retraction. Furthermore, we show evidence of a novel Rac-1–dependent integrin outside-in signaling pathway leading to activation of MAPK, which subsequently induces phosphorylation of myosin light chain (MLC), leading to clot retraction. Thus our study reveals dual activation mechanisms of MAPK that play distinct roles in platelet function, and also reveals a Rac1-MAPK–dependent integrin outside-in signaling pathway that stimulates cell retraction.
Methods
Preparation of washed platelets
Platelets were isolated from healthy human donors as previously described.8,25 Institutional Review Board approval was obtained from the University of Illinois at Chicago, and informed consent from volunteers was obtained in accordance with the Declaration of Helsinki. Briefly, fresh blood was anticoagulated with one-seventh volume acid citrate dextrose (ACD; 2.5% trisodium citrate, 2% dextrose, and 1.5% citric acid). Platelets were isolated by differential centrifugation, washed twice with CGS buffer (0.12 M sodium chloride, 0.0129 M trisodium citrate, and 0.03 M glucose, pH 6.5) and resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered Tyrodes solution containing 1 mM CaCl2 and 1 mM MgCl2. They were then allowed to recover to resting state at 25°C for 1 to 2 hours.
Detection of platelet adenosine-5′-triphosphate (ATP) secretion
Platelet secretion of granule ATP was determined as previously described26 in a Lumi-Aggregometer (Chrono-log, Havertown, PA) at 37°C with stirring (1000 rpm) after addition of the luciferin-luciferase reagent and platelet agonists collagen and α-thrombin (Enzyme Research Laboratories, South Bend, IN). To examine the effects of p38 and MEK inhibitors on platelet function, platelets were incubated for 1 minute at 37°C with either 10 μM SB203580 or 3 μM U0126 (Calbiochem, San Diego, CA) or corresponding vehicle control, before addition of platelet agonists. Statistical significance between groups was determined using a Student t test.
Flow cytometric analysis of p-selectin expression in stimulated platelets
Detection of p-selectin expression was performed as previously described.27 Washed platelets from healthy human donors in Tyrode buffer were preincubated with or without 10 μM SB203580 or PD98059 or 1 mM aspirin for 5 minutes, stimulated with various agonists at 37°C for 5 minutes, and fixed by adding paraformaldehyde (final concentration 1%). After incubating with a monoclonal anti-human p-selectin antibody SZ51,27 and washing, the platelets were further incubated with fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse immunoglobulin G (IgG). P-selectin expression was analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Platelet spreading on fibrinogen
Washed platelets (107/mL) in Tyrode buffer were preincubated with 500 μM aspirin and 50 μM 2-methylthioadenosine 5′-monophosphate (2MeSAMP; Calbiochem) for 5 minutes at 25°C. Platelets were further incubated with either 10 μM SB203580, 10 μM PD98059, 20 μM NSC23766, or corresponding vehicle control for 30 minutes at 25°C. Chamber slides (Lab-Tek; Nunc, Thermo-Fisher Scientific, Rochester, NY) were coated with 10 μg/mL fibrinogen and blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Platelets (1.5 × 107/mL) were added to wells (100 μL/well) and allowed to spread for 30, 60, and 90 minutes at 37°C. At each time point, slides were immediately rinsed to detach nonadherent platelets and fixed with 4% paraformaldehyde. Samples were blocked with 5% BSA in PBS and incubated with Alexa Fluor FITC-conjugated phalloidin. Images were collected with a Leica DM IRB microscope using 40× objective (Leica Microsystems, Wetzlar, Germany), and platelet size was quantified using National Institutes of Health (NIH) ImageJ. Statistical significance between groups was determined using a Student t test.
Genetic deletion of Rac1 in murine platelets
The Mx-cre;Rac1loxP/loxP mice were described previously.28 Rac1 gene deletion was induced by intraperitoneal (ip) injection of poly(I:C) as previously described.28 Two weeks after the last injection, washed platelets were obtained from mice as previously described26 and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The expression of Rac1 in platelets was determined by immunoblot using an antibody against Rac1. Animal usage and protocol were approved by the institutional animal care committee of the University of Illinois at Chicago.
Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets
Washed platelets (4 × 107/mL) were preincubated with 500 μM aspirin and 50 μM 2MeSAMP for 5 minutes at 25°C. Furthermore, platelets were preincubated with either 10 μM SB203580, 10 μM PD98059, 20 μM NSC23766, 20 μM Y27632 (Calbiochem), both NSC23766 plus Y27632, or the appropriate vehicle control for 30 minutes at 25°C. Polystyrene dishes (10-cm; Fisher Scientific) were precoated with 100 μg/mL fibrinogen in 0.1 M NaHCO3, and blocked with 5% BSA. Platelets (5 mL) were either kept in suspension or added to the dishes and incubated at 37°C for various lengths of time. Dishes were washed to remove nonadherent cells. Adherent or control platelets were directly solubilized in sample buffer containing 2% SDS, 500 mM NaCl, 50 mM Tris, pH 7.4, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM sodium orthovanadate, 2 mM NaF, and Complete Protease Inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN). After sonication, lysates were analyzed by SDS-PAGE using 4% to 15% gradient gels and immunoblotted using phospho-specific polyclonal antibodies against p38, p42/44, and MLC II (Cell Signaling Technologies, Danvers, MA) or monoclonal antitubulin antibody (Sigma-Aldrich, St Louis, MO). Enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) was used for visualization of antibody reactions. Densitometry was performed using NIH ImageJ, and integrated density of each band was measured. Quantification of each band was normalized with respect to reactivity of unstimulated platelet lysates for each immunoblot, and values were expressed as relative phosphorylation (mean ± SD; 3 separate experiments). Statistical significance was determined using a Student t test.
Platelet-mediated clot retraction
Platelet-rich plasma was anticogulated with 0.38% sodium citrate, preincubated with inhibitors, in the presence or absence of 1 mM aspirin for 30 minutes at 25°C, and induced to coagulate with 0.2 U/mL thrombin. For clot retraction using mouse platelets, citrated human platelet-depleted plasma was mixed with washed murine platelets to a concentration of 4 × 108/mL. Plasma was induced to coagulate with 0.4 U/mL thrombin. The clots were allowed to retract at 37°C and were photographed at various times. Two-dimensional sizes of retracted clots on photographs were quantified using NIH ImageJ software, and retraction was expressed as percentage retraction [1 − (final clot size/initial clot size)]. Statistical significance was determined using a Student t test.
Results
A primary role for agonist-induced p38 and ERK activation is to stimulate platelet granule release
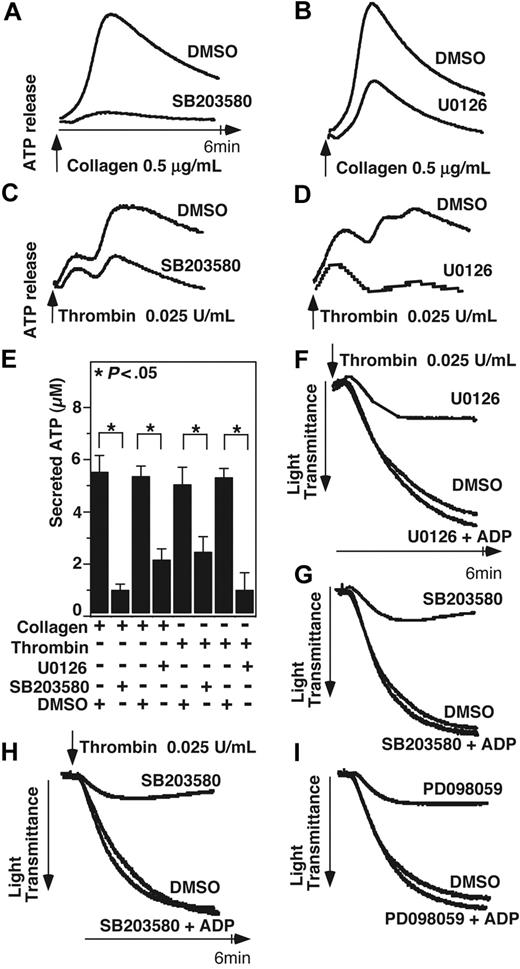
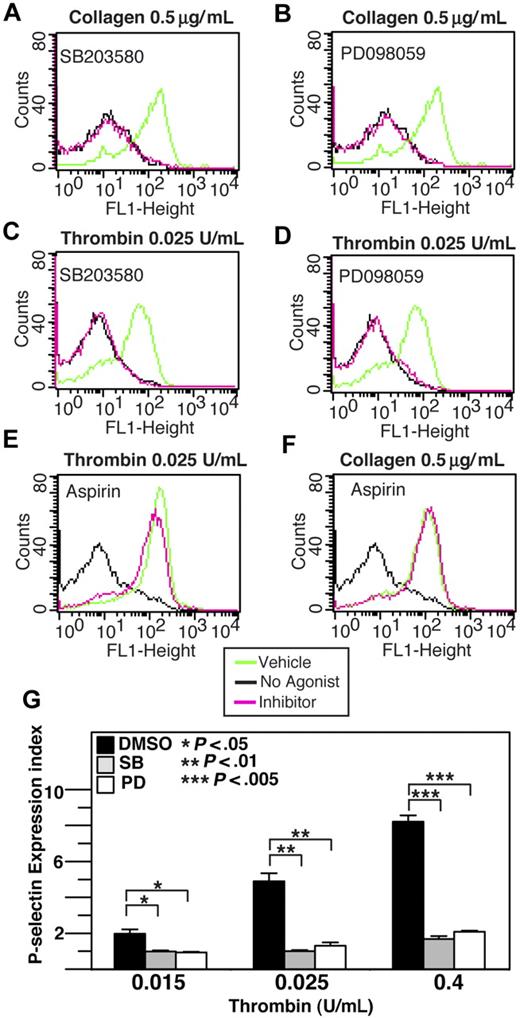
Previous studies have demonstrated that acute and transient activation of platelet p38 and ERK after stimulation by agonists such as thrombin, collagen, VWF, ADP, and TXA2 analog U466192,6,7 plays a role in promoting platelet aggregation. However, with the exception of VWF/GPIb-IX–mediated platelet activation, MAPK inhibitors only partially block integrin-dependent platelet aggregation induced by most agonists,2,6-8 suggesting the possibility that the agonist-induced MAPK activation stimulates the amplification mechanisms of platelet aggregation, such as granule secretion. To investigate whether MAPK is involved in platelet granule secretion, we examined the effects of p38 inhibitor and ERK pathway inhibitors on platelet ATP release and p-selectin expression induced by low-dose collagen and thrombin. ATP release during platelet aggregation in response to low dose collagen or thrombin was significantly (P < .05) inhibited by either SB203580 or U0126, indicating the importance of both p38 and ERK for dense granule release (Figure 1A-D). Similarly, p38 and MEK inhibitors significantly reduced p-selectin expression in platelets in response to low doses of collagen or thrombin (Figure 2A-D), indicating that p38 and ERK are both important in α-granule release induced by these agonists. A previous report suggested that MAPK inhibitors may inhibit cyclooxgenase and thus TXA2 production.29 To examine the role of cyclooxygenase in α-granule secretion, we measured p-selectin expression in the presence or absence of 1 mM aspirin (Figure 2E,F). Our data indicate that p-selectin expression in response to low-dose collagen or thrombin is not affected by high-dose aspirin, suggesting that the inhibitory effect of both p38 inhibitor, SB203580, and MEK inhibitor, PD98059 cannot be due to direct inhibition of cyclooxygenase, and thus is likely to result from inhibition of MAPK pathways. Inhibition of p-selectin expression by MAPK inhibitors was apparently more dramatic than their inhibition of dense granule ATP release, suggesting possible differential involvement of MAPK in the secretion of different granules under these conditions. To determine whether the role of MAPK in stimulating granule secretion of ADP is sufficiently responsible for its effect in promoting platelet aggregation, we determined whether supplement of a low concentration of granule content ADP reverses the inhibitory effect of SB203580 and U0126 on platelet aggregation. It has been previously reported that the ratio of platelet ATP/ADP ranges between 3.7 and 8.5,30 while the ratio of releasable ATP/ADP nucleotides is approximately 7.6.31 Figure 1E shows that 0.025 U/mL thrombin induces the peak release of approximately 5.4 μM ATP and thus approximately 0.7 μM ADP. Therefore, if supplementing up to 0.7 μM exogenous ADP should correct the inhibitory effects of these MAPK inhibitors on platelet aggregation, the effects of these inhibitors would be likely to result from inhibition of ADP secretion. In fact, addition of only 0.5 μM ADP, while alone is insufficient to induce aggregation of washed platelets, restored aggregation of SB203580- or U0126-treated platelets to levels similar to vehicle control–treated platelets (Figure 1F,G). Furthermore, although 10 μM SB203580 and PD98059 already inhibited platelet aggregation, the effects of an extremely high-dose (30 μM) SB203580 or MEK inhibitor, PD98059 on platelet aggregation can still be fully restored by 0.5 μM ADP (Figure 1F-I), suggesting that these inhibitors rather specifically affected platelet secretion. Thus, a primary role for the early agonist-induced MAPK signaling is to stimulate secretion of platelet granule content, ADP, which subsequently amplifies platelet aggregation response to low concentrations of agonists.
The effect of p38 and MEK inhibitors on platelet secretion and aggregation. (A,B) Washed platelets were preincubated at 37°C for 2 minutes in the presence of either 10 μM p38 inhibitor, SB203580 (A), 3 μM MEK inhibitor, U0126 (B), or DMSO vehicle control. Platelet aggregation was induced by 0.5 μg/mL collagen in the presence of luciferase reagent, and ATP release was measured using a platelet lumi-aggregometer. (C,D) Washed platelets were preincubated with either SB203580 (C), U0126 (D), or DMSO. Platelets were stimulated with 0.025 U/mL thrombin, and ATP release was measured. (E) Quantification of peak platelet ATP release (in μM, mean ± SD). Statistical significance was determined using Student t test. Platelets were preincubated with either 10 μM SB203580, 3 μM U0126, or vehicle control (F,G); or 30 μM SB203580, PD98059, or vehicle control (H,I), and stimulated with 0.025 U/mL thrombin in the presence or absence of 0.5 μM ADP in a turbdometric aggregometer. Aggregation traces are shown.
The effect of p38 and MEK inhibitors on platelet secretion and aggregation. (A,B) Washed platelets were preincubated at 37°C for 2 minutes in the presence of either 10 μM p38 inhibitor, SB203580 (A), 3 μM MEK inhibitor, U0126 (B), or DMSO vehicle control. Platelet aggregation was induced by 0.5 μg/mL collagen in the presence of luciferase reagent, and ATP release was measured using a platelet lumi-aggregometer. (C,D) Washed platelets were preincubated with either SB203580 (C), U0126 (D), or DMSO. Platelets were stimulated with 0.025 U/mL thrombin, and ATP release was measured. (E) Quantification of peak platelet ATP release (in μM, mean ± SD). Statistical significance was determined using Student t test. Platelets were preincubated with either 10 μM SB203580, 3 μM U0126, or vehicle control (F,G); or 30 μM SB203580, PD98059, or vehicle control (H,I), and stimulated with 0.025 U/mL thrombin in the presence or absence of 0.5 μM ADP in a turbdometric aggregometer. Aggregation traces are shown.
The effect of p38, MEK inhibitors, and aspirin on thrombin and collagen-induced p-selectin expression. Washed human platelets were resuspended in Tyrode buffer and preincubated with either 20 μM SB203580 (A,C), 10 μM MEK inhibitor, PD98059 (B,D), 1 mM aspirin (E,F), or the appropriate vehicle control for 5 minutes. Platelets were then treated with either 0.5 μg/mL collagen (A,B,F) or 0.025 U/mL thrombin (C-E) at 37°C for 5 minutes and fixed by adding paraformaldehyde. Fixed platelets were incubated with the monoclonal anti–human p-selectin antibody SZ51 at 22°C for 30 minutes and, after washing, were further incubated with a FITC-conjugated goat anti–mouse Ig antibody. Surface expression of the α-granule membrane protein p-selectin was analyzed using flow cytometry. (G) Quantitative results from 3 experiments are expressed as the p-selectin expression index (fluorescence intensity of platelets stimulated with an agonist/fluorescence intensity of unstimulated platelets). Statistical differences between inhibitor-treated samples and controls were examined by Student t test.
The effect of p38, MEK inhibitors, and aspirin on thrombin and collagen-induced p-selectin expression. Washed human platelets were resuspended in Tyrode buffer and preincubated with either 20 μM SB203580 (A,C), 10 μM MEK inhibitor, PD98059 (B,D), 1 mM aspirin (E,F), or the appropriate vehicle control for 5 minutes. Platelets were then treated with either 0.5 μg/mL collagen (A,B,F) or 0.025 U/mL thrombin (C-E) at 37°C for 5 minutes and fixed by adding paraformaldehyde. Fixed platelets were incubated with the monoclonal anti–human p-selectin antibody SZ51 at 22°C for 30 minutes and, after washing, were further incubated with a FITC-conjugated goat anti–mouse Ig antibody. Surface expression of the α-granule membrane protein p-selectin was analyzed using flow cytometry. (G) Quantitative results from 3 experiments are expressed as the p-selectin expression index (fluorescence intensity of platelets stimulated with an agonist/fluorescence intensity of unstimulated platelets). Statistical differences between inhibitor-treated samples and controls were examined by Student t test.
Integrin-mediated activation of MAPK in platelets
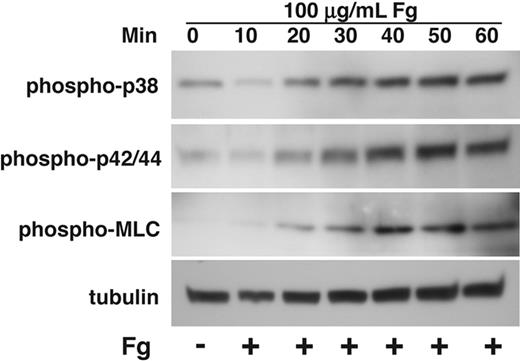
It has been shown that agonist-induced activation of both p38 and ERK in platelets is down-regulated by ligand binding to integrin αIIbβ3.4,7 Ligand binding to integrins in nucleated cells, however, is known to activate MAPK,10 and integrin-mediated MAPK activation plays critical roles in regulating cell proliferation, survival, and migration.32 While the reason for these different effects of integrins has been unclear, integrin-mediated activation of MAPK in nucleated cells was studied in cells that are adherent to integrin ligands for prolonged periods of time. In contrast, platelet MAPK activation induced by platelet agonists is acute and transient. To determine whether this difference is responsible for the different effect of integrins on MAPK activation between platelets and nucleated cells, we investigated whether and how prolonged adhesion of platelets to integrin ligand fibrinogen affects MAPK activation in platelets. To exclude the possible effects of secondary platelet agonist receptors such as P2Y12 ADP receptor and TXA2 receptor that are known to activate MAPKs,22,33 platelets were allowed to adhere to fibrinogen in the presence of high concentrations of cyclooxygenase inhibitor, aspirin, that prevents TXA2 generation, and P2Y12 antagonist, 2MeSAMP. The kinetics of MAPK activation were shown by measuring p38 and ERK phosphorylation of platelets adherent on fibrinogen at 10-minute intervals for 1 hour. In addition, phosphorylation of MLC was also examined in parallel. Consistent with previous observation of an inhibitory role of integrin αIIbβ3 in platelet MAPK phosphorylation, we observed a decrease in p38 and ERK phosphorylation from base line within 10 minutes of platelet adhesion to fibrinogen. Interestingly, longer adhesion time of platelets on fibrinogen results in a marked and persistent increase in phosphorylation of both p38 and ERK (Figure 3). These results indicate that, although the ligand binding to the platelet integrin αIIbβ3 initially inhibits MAPK phosphorylation, integrin-mediated platelet adhesion to fibrinogen, at a later time, induces activation of both p38 and ERK. Thus, MAPK activation in platelets can be induced by agonist-mediated signaling and integrin outside-in signaling during different stages of platelet activation.
Kinetics of activation of p38, ERK, and MLC in platelets spreading on fibrinogen. Washed platelets at a concentration of 4 × 107/mL were incubated with 500 μM aspirin and 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for indicated times or kept in suspension. Dishes were rinsed to remove nonadherent platelets, and platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Activation of p38, ERK, and MLC were analyzed by Western blot analysis using phospho-specific antibodies against phosphorylated Thr180/Tyr182 (p38), Thr-202/Tyr204 (ERK), and Thr18/Ser19 (MLC) (Cell Signaling Technologies). Monoclonal antibody against tubulin was used to verify equal loading.
Kinetics of activation of p38, ERK, and MLC in platelets spreading on fibrinogen. Washed platelets at a concentration of 4 × 107/mL were incubated with 500 μM aspirin and 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for indicated times or kept in suspension. Dishes were rinsed to remove nonadherent platelets, and platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Activation of p38, ERK, and MLC were analyzed by Western blot analysis using phospho-specific antibodies against phosphorylated Thr180/Tyr182 (p38), Thr-202/Tyr204 (ERK), and Thr18/Ser19 (MLC) (Cell Signaling Technologies). Monoclonal antibody against tubulin was used to verify equal loading.
Integrin-mediated activation of p38 and ERK plays a role in platelet contractile signaling leading to clot retraction
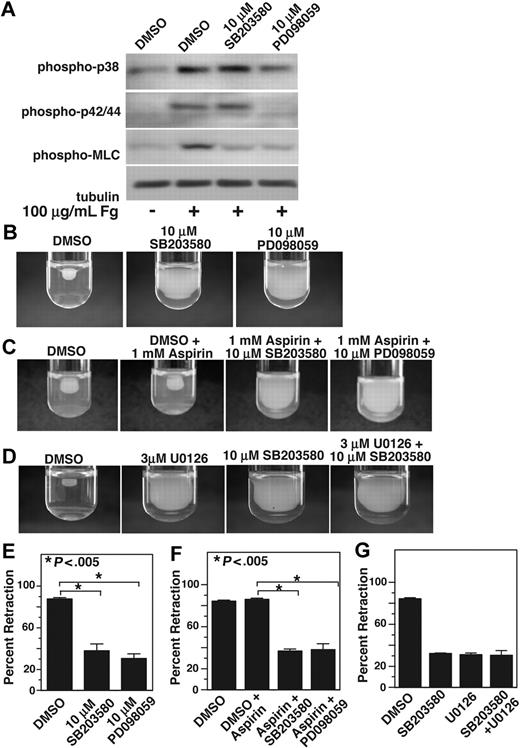
We noted that the kinetics of integrin-mediated late MAPK activation were similar to those of MLC phosphorylation (Figure 3), which indicate a possible relationship between p38, ERK, and MLC activation. MLC phosphorylation is an important retractile signaling event mediated by integrin outside-in signals. To determine whether integrin-dependent MAPK activation plays a role in MLC phosphorylation, we allowed platelets to adhere to fibrinogen-coated surfaces for 1 hour in the presence or absence of either p38 inhibitor, SB203580, or MEK inhibitor, PD98059. To exclude the possible role of secondary platelet agonists such as ADP and TXA2, these platelets were also treated with high concentrations of aspirin and 2MeSAMP. Platelets adherent on integrin ligand fibrinogen showed significant increase in phosphorylation of p38, ERK, and MLC. MLC phosphorylation, however, is markedly reduced in platelets treated with either SB203580 or PD98059, indicating that both p38 and ERK plays an important role in integrin-dependent activation of MLC and thus integrin-dependent contractile signaling (Figure 4A). We have previously shown that activation of ERK during GPIb-IX–mediated signaling requires p38 activation.7 However, integrin-mediated ERK activation does not seem to be affected by p38 inhibitor SB203580, suggesting that p38 and ERK are activated in parallel by integrin outside-in signaling.
p38 and MEK inhibitors inhibit MLC phosphorylation and clot retraction. (A) Washed platelets at a concentration of 4 × 107/mL were preincubated with 500 μM aspirin and with 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were also pretreated with either 10 μM SB203580, 10 μM PD98059, or equal volume DMSO for 30 minutes at 25°C and added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes, or kept in suspension. After washing to remove nonadherent platelets, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B,C) Platelet-rich plasma was anticoagulated with 0.38% sodium citrate and preincubated with (C) or without (B) 1 mM aspirin for 5 minutes at 25°C and with p38 and MEK inhibitor as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (D) Platelet-rich plasma was preincubated with either vehicle control, 10 μM SB203580, 3 μM U0126, or both inhibitors. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E-G) Two-dimensional retraction of clots in panels B through D were measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
p38 and MEK inhibitors inhibit MLC phosphorylation and clot retraction. (A) Washed platelets at a concentration of 4 × 107/mL were preincubated with 500 μM aspirin and with 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were also pretreated with either 10 μM SB203580, 10 μM PD98059, or equal volume DMSO for 30 minutes at 25°C and added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes, or kept in suspension. After washing to remove nonadherent platelets, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B,C) Platelet-rich plasma was anticoagulated with 0.38% sodium citrate and preincubated with (C) or without (B) 1 mM aspirin for 5 minutes at 25°C and with p38 and MEK inhibitor as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (D) Platelet-rich plasma was preincubated with either vehicle control, 10 μM SB203580, 3 μM U0126, or both inhibitors. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E-G) Two-dimensional retraction of clots in panels B through D were measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
One of the functional consequences of integrin outside-in signaling requiring cell contractile signaling is platelet-mediated clot retraction. Therefore, we investigated whether integrin-dependent p38 and ERK activation is important in clot retraction. Indeed both SB203580 and PD98059 significantly (P < .005) inhibited clot retraction compared with dimethyl sulfoxide (DMSO) control (Figure 4B). Similarly, a different MEK inhibitor U0126 (Figure 4D) or a different p38 inhibitor SB202190 (not shown) also inhibited clot retraction, supporting the role of ERK and p38 in integrin-mediated clot retraction. To exclude the possibility that MAPK inhibitors inhibit clot retraction by direct inhibition of cyclooxygenase, we investigated the effects of SB203580 or PD98059 on the clot retraction in the presence of high-dose aspirin. Preincubation of platelets with 1 mM aspirin has no effect on clot retraction (Figure 4C). However, both MAPK inhibitors still significantly (P < .005) inhibited clot retraction in the presence of aspirin. Thus, the inhibitory effects of either SB203580 or PD98059 on clot retraction are unlikely to result from the effects of these inhibitors on TXA2 production. It is interesting to note that we did not observe any additive inhibitory effect of the p38 inhibitor and MEK inhibitor (Figure 4D), suggesting that p38 and ERK pathways promote clot retraction via the same retractile signaling pathway and that both p38 and ERK are required for activating this pathway. Taken together, these data represent a new finding that sustained integrin-mediated p38, and ERK activation plays an important role in mediating platelet contractile signaling and platelet-dependent clot retraction.
Integrin-dependent activation of p38 and ERK is mediated mainly by Rac1
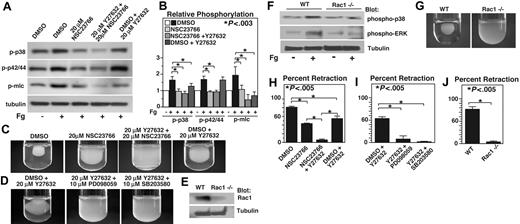
Integrin outside-in signaling that is responsible for cell spreading and retraction requires small GTPases such as Rac1 and RhoA.16 It has been shown that RhoA mediates integrin-dependent retractile signaling through Rho kinase (ROCK), which is inhibited by the compound Y27632.20 Recent studies have also demonstrated that Rac1 inhibitor NSC23766 is similar to Rac1−/− mice in inhibiting platelet aggregation and secretion.28,34 Thus, we investigated the role of Rac1 and RhoA pathways in integrin-mediated p38 and ERK phosphorylation using these inhibitors. Interestingly, phosphorylation of both p38 and ERK during integrin-dependent platelet adhesion on fibrinogen was dramatically inhibited by the Rac1 inhibitor, NSC23766 (Figure 5A). However, ROCK inhibitor Y27632 had no effect on ERK phosphorylation, but slightly inhibited p38 phosphorylation (P < .03). The effect of RhoA inhibitor C3 toxin was similar to that of ROCK inhibitor (not shown). In addition, the presence of both Y27632 and NSC23766 did not cause further inhibition of ERK phosphorylation, but further inhibited phosphorylation of p38 (Figure 5A). These data indicate that integrin-dependent activation of both p38 and ERK is mediated by the Rac1-mediated signaling pathway, but p38 phosphorylation is also promoted by the RhoA-ROCK signaling pathway. In contrast to the results of MAPK phosphorylation, either Rac1 inhibitor NSC23766 or ROCK inhibitor Y27632 significantly inhibited MLC phosphorylation during platelet adhesion to fibrinogen (P < .02), and the presence of both NSC23766 and Y27632 results in additive inhibitory effect on MLC phosphorylation compared with either inhibitor alone. Together with the results that p38 and ERK are both important in MLC phosphorylation, the data here indicate that both Rac1 and RhoA play important but distinct roles in mediating MLC phosphorylation, a key step in retractile signaling. Rac1 appears to mediate MLC phosphorylation via the p38 and ERK signaling pathways, but RhoA-ROCK, as expected, induces MLC phosphorylation mainly via a MAPK-independent pathway.
The effects of Rac1 inhibition and ROCK inhibition on p38, ERK, and MLC phosphorylation and clot retraction. (A)Washed platelets were pretreated with either 20 μM NSC23766, 20 μM ROCK inhibitor, Y27632, both NSC23766 and Y27632, or the appropriate vehicle control for 30 minutes at 25°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B) Densitometry measurements from results in panel A. Values were normalized with respect to resting control for each immunoblot and are expressed as relative phosphorylation (mean ± SD from 3 separate experiments). Statistical significance was determined using Student t test. (C,D) Citrated PRP was preincubated with inhibitors for 30 minutes as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E) Washed platelets from wild-type and Rac1 knockout mice were solubilized, and expression of Rac1 was analyzed by immunoblot with monoclonal antibody against Rac1 (Sigma-Aldrich). (F) Washed platelets from wild-type, and Rac1 knockout mice were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38 and ERK were analyzed by Western blot analysis. A monoclonal antibody against tubulin was used to verify equal loading. (G) Platelets from wild-type or Rac1 knockout mice were mixed with human platelet-poor plasma at a concentration of 4 × 108/mL, induced to coagulate with 0.4 U/mL thrombin, and photographed. (H-J) Extent of clot retraction in panels C, D, and G was measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
The effects of Rac1 inhibition and ROCK inhibition on p38, ERK, and MLC phosphorylation and clot retraction. (A)Washed platelets were pretreated with either 20 μM NSC23766, 20 μM ROCK inhibitor, Y27632, both NSC23766 and Y27632, or the appropriate vehicle control for 30 minutes at 25°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B) Densitometry measurements from results in panel A. Values were normalized with respect to resting control for each immunoblot and are expressed as relative phosphorylation (mean ± SD from 3 separate experiments). Statistical significance was determined using Student t test. (C,D) Citrated PRP was preincubated with inhibitors for 30 minutes as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E) Washed platelets from wild-type and Rac1 knockout mice were solubilized, and expression of Rac1 was analyzed by immunoblot with monoclonal antibody against Rac1 (Sigma-Aldrich). (F) Washed platelets from wild-type, and Rac1 knockout mice were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38 and ERK were analyzed by Western blot analysis. A monoclonal antibody against tubulin was used to verify equal loading. (G) Platelets from wild-type or Rac1 knockout mice were mixed with human platelet-poor plasma at a concentration of 4 × 108/mL, induced to coagulate with 0.4 U/mL thrombin, and photographed. (H-J) Extent of clot retraction in panels C, D, and G was measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
The role of Rac1-MAPK-MLC pathway in clot retraction
Previous studies indicate that Rac is important in cell spreading and RhoA is important in cell retraction.16,20,35 However, our result that Rac1 is important in activation of the MAPK-MLC pathway suggests the possibility that Rac1 may also be important in cell retraction. Indeed, addition of NSC23766 to platelet-rich plasma (PRP) significantly but partially inhibited platelet-dependent clot retraction (P < .005; Figure 5C). Consistent with previous results of an important role for RhoA-ROCK pathway in clot retraction, ROCK inhibitor, Y27632 also significantly but partially inhibited clot retraction. Thus, both Rac1 and RhoA are important in mediating integrin outside-in signaling leading to cell retraction. Furthermore, preincubation of PRP with both NSC23766 and Y27632 together produces almost complete inhibition of clot retraction (P < .005; Figure 5C), suggesting that RhoA- and Rac1-dependent signaling mechanisms play separate roles in stimulating clot retraction. Furthermore, the role of p38 and ERK in Rac1-dependent cell retraction is supported by the observation that preincubation of either MAPK inhibitor SB203580 or PD98059 with Y27632 is similar to preincubation with both Rac1 and ROCK inhibitors, causing almost complete inhibition of clot retraction (P < .005; Figure 5D). Thus the results obtained with the combination of pharmacologic inhibitors in human platelets indicate a novel Rac1-MAPK signaling pathway leading to MLC phosphorylation and platelet-mediated clot retraction.
To further verify the role of Rac-1-MAPK pathway in retractile signaling in platelets, we examined the ability of Rac1-deficient mouse platelets to activate MAPK and to mediate clot retraction. As previously published, poly(I:C) injection of the Mx-cre;Rac1loxP/loxP mice induces conditional knockout of Rac1 in hemopoietic cells, and thus platelets from these mice are deficient in Rac1 (Figure 5E). Consistent with the effect of Rac1 inhibitors in human platelets, the integrin-dependent sustained phosphorylation of both p38 and ERK during platelet adhesion to fibrinogen was dramatically inhibited in Rac1-knockout platelets compared with control Rac1+/+ platelets from the Mx-cre;Rac1loxP/loxP mice (Figure 5F). Furthermore, Rac1-knockout mouse platelets were also deficient in mediating clot retraction (Figure 5G). These results support the data obtained using pharmacologic inhibitors, and further indicate that Rac1 indeed induces activation of MAPK and is important in retractile signaling. Thus, we have discovered a novel Rac1-dependent signaling pathway that induces sustained integrin-mediated p38 and ERK activation, leading to MLC phosphorylation and clot retraction. This Rac1-MAPK pathway is distinct from the known RhoA-ROCK retractile pathway, and these 2 distinct pathways together are responsible for the integrin-dependent clot retraction signaling.
Inhibitors of Rac1, p38, and ERK do not affect platelet spreading on fibrinogen
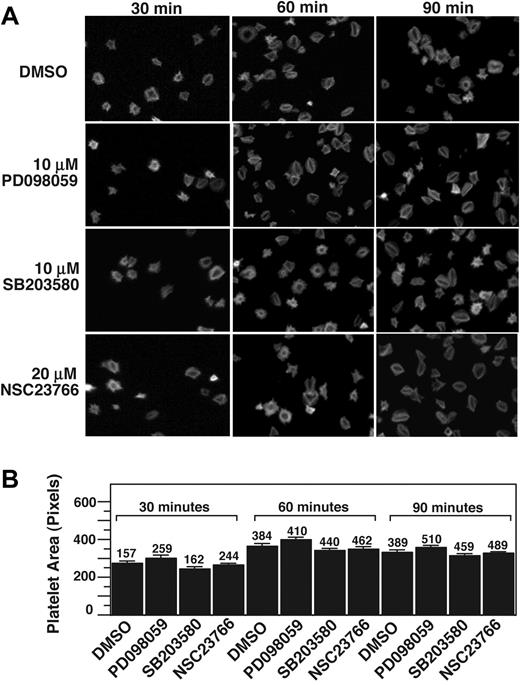
Rac1 has been shown to play an important role in adhesion-dependent lamellipodia formation at the leading edge of nucleated cells and thus cell spreading, which precedes cell retraction and formation of stress fibers.16,35,36 In platelets, it has been reported that Rac1 is important in thrombin- and ADP-stimulated platelet shape change and platelet spreading.37 To understand whether Rac1, p38, and ERK are important in integrin-dependent platelet spreading, we investigated the effects of NSC23766, SB203580, and PD98059 on integrin-dependent platelet spreading on fibrinogen in the absence of platelet agonists. Resting platelets treated with or without inhibitors were allowed to adhere and spread on fibrinogen-coated surfaces for 30, 60, and 90 minutes and were fluorescently labeled with phalloidin to mark cell size and morphology. SB203580 or PD98059 did not significantly affect platelet spreading at any of the observed time intervals (Figure 6). Importantly, NSC23766 also had no inhibitory effect on platelet spreading on fibrinogen in the absence of platelet agonists. Thus, it appears that although Rac1 is important in platelet shape changes stimulated by platelet agonists, it is not directly involved in the platelet spreading signaling downstream of integrin αIIbβ3. Taken together, we conclude that the integrin-mediated Rac1-p38/ERK signaling pathway plays an important role in the platelet retractile signaling pathway leading to clot retraction.
p38, MEK, and Rac1 inhibitors do not affect platelet spreading on fibrinogen. (A) Platelets were preincubated with either 10 μM SB203580, 10 μM PD98059, 20 μM rac 1 inhibitor, NSC23766, or DMSO for 30 minutes at 25°C. Platelets were allowed to spread on fibrinogen-coated slides for 30, 60, and 90 minutes, and were fixed with paraformaldehyde to stop spreading. Platelets were subsequently labeled with FITC-conjugated phalloidin and photographed using a fluorescence microscope. (B) Quantification of area (pixel number) in 4 random fields (mean ± SE; platelet numbers are marked above each column). Statistical analysis performed using Student t test. At all 3 time points, no statistical significance between groups was found.
p38, MEK, and Rac1 inhibitors do not affect platelet spreading on fibrinogen. (A) Platelets were preincubated with either 10 μM SB203580, 10 μM PD98059, 20 μM rac 1 inhibitor, NSC23766, or DMSO for 30 minutes at 25°C. Platelets were allowed to spread on fibrinogen-coated slides for 30, 60, and 90 minutes, and were fixed with paraformaldehyde to stop spreading. Platelets were subsequently labeled with FITC-conjugated phalloidin and photographed using a fluorescence microscope. (B) Quantification of area (pixel number) in 4 random fields (mean ± SE; platelet numbers are marked above each column). Statistical analysis performed using Student t test. At all 3 time points, no statistical significance between groups was found.
Discussion
In this study, we have discovered that there are 2 distinct phases of MAPK activation in platelets, each playing a different role in platelet function. We demonstrate that ligand binding to integrin αIIbβ3 induces a late outside-in signaling phase of p38 and ERK activation, which is important for MLC phosphorylation and clot retraction. We further demonstrate that the acute agonist-induced p38 and ERK activation is important for platelet granule secretion. Importantly, we have discovered a novel integrin-mediated Rac1-dependent MAPK activation pathway that is important in stimulating platelet retraction. Thus, the platelet integrin-dependent clot retraction is mediated by both the Rac1 and RhoA signaling pathways.
Previous studies have highlighted the importance of MAPK activation in agonist-stimulated platelets.2,6,7,21-24 Agonist-induced activation of either p38 or ERK during platelet aggregation is early and transient, which appears to be down-regulated by integrin ligand interactions, as VWF- and thrombin-induced p38 and ERK phosphorylation were significantly enhanced in the presence of integrin inhibitors Arg-Gly-Asp-Ser (RGDS) and anti-αIIbβ3 monoclonal antibody.4,7 There has been recent evidence that αIIbβ3 engagement initiates protein phosphatase 2A-dependent dephosphorylation of ERK238-40 and MLC,39 which may serve as a mechanism of integrin-mediated MAPK deactivation. Consistent with the inhibitory effect of integrins on MAPK, we observed a transient reduction in both p38 and ERK phosphorylation in platelets adherent to substrata coated with fibrinogen at the 10-minute time point, even compared with “unstimulated” washed platelets (Figure 3). Our results suggest that down-regulation of MAPK is an early transient event after ligand binding to the integrin αIIbβ3. More importantly, we demonstrate that platelet adhesion to integrin ligand fibrinogen causes a late but sustained activation of MAPK. This delayed activation of MAPK is unlikely to result from secondary activation of platelets by ADP and TXA2, because the presence of both the inhibitor of the P2Y12 ADP receptor (previously shown to be responsible for ADP-mediated MAPK activation22 ) and aspirin did not affect the outcome. Thus, we present a new finding that the effect of the platelet integrin αIIbβ3 ligation on MAPK (p38 and ERK) is biphasic, consisting an early inhibitory phase and a late stimulatory phase. Consequently, our results also indicate that there are 2 distinct mechanisms of platelet MAPK activation, the previously described agonist-mediated MAPK activation and the novel integrin-mediated MAPK activation in platelets.
We conclude that MAPK activation induced by different mechanisms is important in different aspects of platelet function. A primary role of the early MAPK activation induced by low doses of agonists appears to be in stimulating granule secretion, subsequently amplifying and stabilizing platelet aggregation. This conclusion is supported by the data that (1) both p38 and ERK inhibitors inhibited only the second wave of platelet aggregation, which are secretion-dependent, but did not inhibit the first wave of platelet aggregation, suggesting that the primary activation of integrins are probably not affected; (2) secretion of both dense granules and α-granules are significantly attenuated by either p38 or ERK inhibitors; and (3) the inhibitory effects of p38 and ERK inhibitors on platelet aggregation can be reversed by supplementing low concentrations of granule content ADP, suggesting that the inhibitory effects on platelet aggregation is secondary to inhibition of granule secretion of ADP. However, the conclusion that a primary role of MAPK activation in stimulation of secretion does not exclude that early MAPK activation is also important in other aspects of platelet function. Indeed, VWF stimulates sequential activation of p38 and ERK via the PI3K-Akt-cGMP signaling pathway,6,7,41 which plays important role in GPIb-IX–dependent integrin activation. ERK and p38 are important in platelet adhesion to VWF and/or collagen under shear stress.21
We conclude that the integrin signaling–mediated late p38 and ERK activation are important in stimulating the cellular retractile signaling leading to clot retraction. In support of this conclusion, we show that either p38 or ERK pathway inhibitors inhibited integrin-dependent clot retraction. Furthermore, PD98059 or SB203580 inhibited phosphorylation of MLC, a key step in cell retractile signaling, in the absence of platelet agonists and in the presence of aspirin and P2Y12 antagonists, which prevent secondary platelet activation. Our data not only provide evidence that p38 and ERK play important roles in MLC phosphorylation and clot retraction but, more importantly, also demonstrate that MAPKs serve as downstream mediators of Rac1 in the integrin-mediated retractile pathway. Previously, it has been shown that, after integrin-mediated cell adhesion, integrin outside-in signaling induces cell spreading, which has been thought to involve β3-bound c-Src.17,20 At a later stage, calpain cleavage of the integrin β3 at Y759 dissociates c-Src from β3 and induces a switch in the direction of integrin signaling from spreading to retraction that is mediated by the RhoA-ROCK signaling pathway.20 In many nucleated cells other than platelets, Rac has been shown to mediate integrin-mediated cell spreading rather than retraction.42 However, in platelets, the role of Rac in integrin-mediated platelet spreading has not been demonstrated. Rather, Rac1 knockout and inhibitors had no effect on platelet spreading on fibrinogen in the absence of agonists (Figure 6).37 Here, we show that the integrin-dependent activation of p38 and ERK as well as MAPK-dependent phosphorylation of MLC are inhibited by a Rac1 inhibitor and in Rac1-knockout platelets, which also inhibit platelet-mediated clot retraction. Thus, Rac1 stimulates the MAPK- and MLC-dependent retractile signal. Importantly, several lines of evidence suggest the independence of the Rac1-mediated retractile pathway from RhoA-ROCK pathway. First, while either ROCK inhibitor or Rac1 inhibitor only partially inhibit clot retraction in humans, Rac1 and ROCK inhibitors together produce an additive inhibitory effect on MLC phosphorylation and completely abolished clot retraction. Similarly, while p38 or MEK inhibitor only partially inhibited clot retraction, incubation of either p38 or MEK inhibitors together with ROCK inhibitor caused nearly complete inhibition of clot retraction (Figure 5). In addition, the Rac1 inhibitor or Rac1 knockout dramatically inhibited the integrin-dependent p38 and ERK phosphorylation, while ROCK inhibitors only slightly affected p38 phosphorylation but had no effect on ERK phosphorylation, suggesting that the Rac1 inhibition diminishes MLC phosphorylation and clot retraction via the p38- and ERK-dependent mechanism, while ROCK inhibitor reduces MLC phosphorylation and clot retraction mainly via MAPK-independent mechanisms, which is consistent with previous studies that ROCK inhibits MLC phosphatase, thus enhancing MLC phosphorylation.43,44 Thus, we have identified a novel integrin-mediated platelet retractile signaling pathway that involves sequential activation of Rac1, p38/ERK pathways, and MLC phosphorylation, leading to platelet retraction. Thus, our data suggest both the Rac1-MAPK and RhoA-ROCK pathways are important in platelet-mediated clot retraction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants HL080264, HL062350, and HL068819 from the National Heart, Lung, and Blood Institute/NIH (X.D.). Z.L. is a recipient of the American Heart Association Scientist Development Grant. P.F. is a recipient of the American Heart Association Midwest Affiliate Predoctoral Fellowship Award.
National Institutes of Health
Authorship
Contribution: P.F. performed a major part of experiments, was involved in design of experiments, analyzed data, and wrote the paper; Z.L. performed a major part of experiments on the role of MAPK in secretion, was involved in design of experiments, and data analysis; G.Z. performed important experiments; J.L. contributed to experiments using Rac1 knockout mice; Y.Z. provided Rac-1 conditional knockout mice and discussion; and X.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois at Chicago, 835 S Wolcott Avenue, Room E403, Chicago, IL 60612; e-mail: xdu@uic.edu.
References
Author notes
*P.F. and Z.L. contributed equally to this work.