Abstract
Acute lymphoblastic leukemia (ALL) is an uncommon but highly fatal disease in adults. We used period analysis to data from the Surveillance, Epidemiology, and End Results (SEER) database to disclose changes in outcomes for patients diagnosed with ALL in the United States in the 2 decades between 1980–1984 and 2000–2004. Major improvement in survival was observed for patients less than 60 years of age. Improvement in survival was greater for women than for men, but was significant for both genders. The greatest improvement was seen in patients aged 15 to 19, in whom 5-year relative survival improved from 41.0% to 61.1%, and 10-year survival improved from 33.0% to 60.4%. Lesser but significant improvements were seen for age groups 20–29, 30–44, and 45–59. Survival for patients aged 60 and over remained essentially unchanged at levels around or below 10%, respectively. Survival has improved for patients with ALL over the time period studied, but treatment of older patients remains a difficult issue.
Introduction
Acute lymphoblastic leukemia (ALL) is a common form of leukemia in childhood but is rare in adults. Nevertheless, in the United States, it is estimated that 5430 people will develop ALL in 2008, and 1460 people died of the disease.1 ALL is frequently curable in children but is much more difficult to treat in adults. Nonetheless, substantial progress has been made in the late 20th century and early 21st century in the treatment of ALL.2 The aim of this study is to monitor progress in long-term survival of adult patients diagnosed with ALL from the early 1980s to the early 21st century. The novel technique of period analysis is used to disclose most recent trends with minimum delay.3,4
Methods
All data presented in this paper are derived from the 1973–2004 limited-use database of the Surveillance, Epidemiology, and End Results (SEER) Program of the United States National Cancer Institute (NCI) issued in April 2007.5 Data included in the 1973–2004 SEER database are from population-based cancer registries in the states of Connecticut, New Mexico, Utah, Iowa, and Hawaii and the metropolitan areas of Atlanta, Detroit, Seattle-Puget Sound, and San Francisco-Oakland, which together cover a population of approximately 30 million people. Geographic areas were selected for inclusion in the SEER Program based on their ability to operate and maintain a high-quality population-based cancer reporting system and for their epidemiologically significant population subgroups.
Overall, 3483 patients aged 15 years or older with a first diagnosis of ALL (and no previous cancer diagnosis) between 1980–2004, who have been followed for vital status until the end of 2004, were included in the dataset. After exclusion of 32 patients who were reported by autopsy or by death certificate only, there remained 3451 patients (99.1%) for the survival analysis.
Five- and 10-year survival were calculated for the calendar periods 1980–1984, 1985–1989, 1990–1994, 1995–1999, and 2000–2004 using the period analysis methodology.3 Furthermore, we tested for statistical significance of trends in 5- and 10-year year survival between 1980–1984 and 2000–2004 by a recently described modeling approach.6 Age is a major prognostic factor in ALL.7 Therefore, all analyses were carried out separately for the following 5 age groups: 15–19, 20–29, 30–44, 45–59, and 60 and over, as well as for men and women.
With period analysis, first proposed by Brenner and Gefeller in 1996,3 only survival experience during the period of interest is included in the analysis. This is achieved by left truncation of observations at the beginning of the period in addition to right censoring at its end. It has been shown by extensive empirical evaluation that period analysis provides more up-to-date long-term survival estimates than traditional cohort-based survival analysis and quite closely predicts long-term survival expectations of cancer patients diagnosed within the period of interest.8,9
According to standard practice in population-based cancer survival analysis, relative survival was calculated in addition to absolute (observed) survival. Relative survival reflects survival of cancer patients compared with survival of the general population. It is calculated as the ratio of absolute survival of cancer patients divided by the expected survival of a group of persons of the corresponding sex, age, and race in the general population.10,11 Estimates of expected survival were derived according to the so-called Ederer II method12 using United States sex-, age-, and race-specific life tables.13
Results
The numbers of patients with a diagnosis of ALL between calendar periods 1980–1984 and 2000–2004 included are shown by age and sex in Table 1. The total number of cases of ALL increased over each time period. Case numbers were most stable in the youngest and oldest age groups. In contrast, case numbers increased by more than 80% in the 30–44 age group and more than doubled in the 45–59 age group. Case numbers increased for both male and female patients with a greater number of male than female patients in all calendar periods.
Changes in survival in ALL are shown in Table 2. Overall, both 5- and 10-year relative survival increased between 1980–1984 and 2000–2004. Improvements were seen for both genders and in all age groups except for the oldest. The greatest improvement was seen in patients aged 15 to 19, in whom 5-year relative survival improved from 41.0% to 61.1% (+ 20.1 percentage points) and 10-year relative survival improved from 33.0% to 60.4% (+ 27.4 percentage points). The increase in 5-year relative survival for the next oldest age group, aged 20 to 29, was nearly as great, going from 25.1% to 44.8% (+ 19.7 percentage points). Overall, the increase was greater among women than among men, particularly for 5-year relative survival (+ 16.2 vs + 8.8 percentage points, respectively).
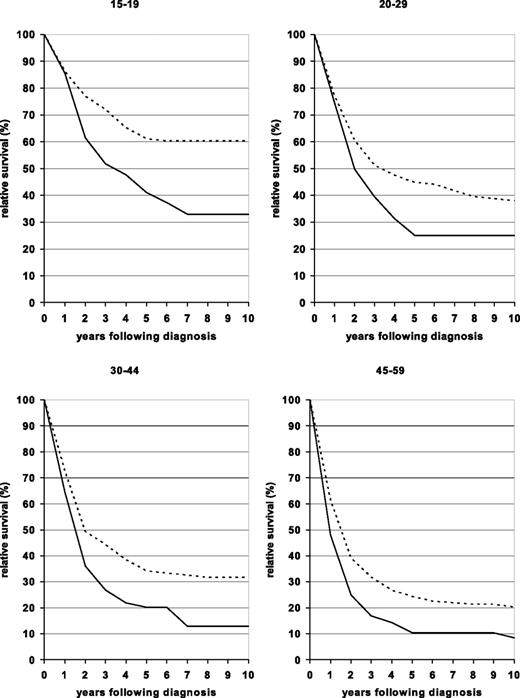
Relative survival curves for patients in the 4 younger age groups diagnosed in one of 2 periods (1980–1984 and 2000–2004) are shown in Figure 1. The curves are essentially flat by 7 to 8 years after diagnosis for both time periods for all age groups, suggesting that a subpopulation of adult ALL patients are cured of their disease. Due to a less steep fall of the survival curves in the early years after diagnosis, this proportion has substantially increased over time.
Ten-year relative survival curves of patients with ALL by major age groups. Period estimates for 1980–1984 (solid curves) and 2000–2004 (dashed curves).
Ten-year relative survival curves of patients with ALL by major age groups. Period estimates for 1980–1984 (solid curves) and 2000–2004 (dashed curves).
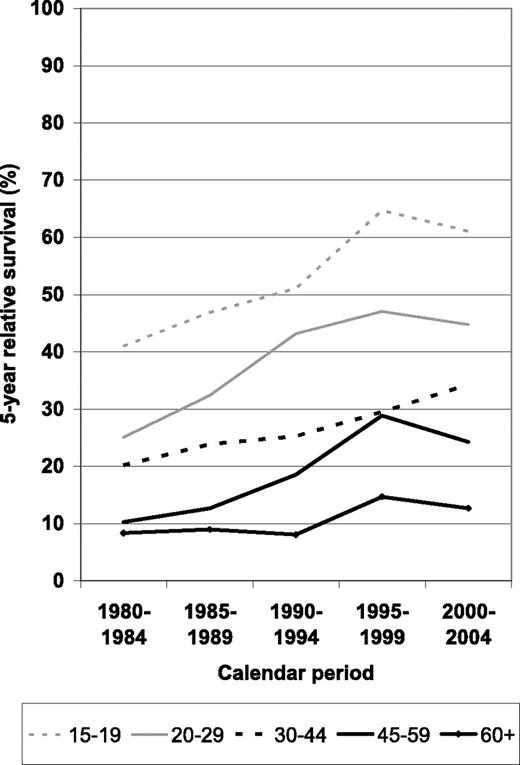
A more comprehensive picture of survival expectations according to time since diagnosis and calendar period for the different age groups is given in Figure 2. Most of the improvement occurred between the 1980 to 1984 and the 1995 to 1999 periods, with little and inconsistent changes (possibly reflecting random variation due to small numbers) between periods 1995–1999 and 2000–2004. Overall, increases seem to have been strongest between periods 1990–1994 and 1995–1999.
Period estimates of 5-year relative survival of patients with ALL by major age groups in defined calendar periods from 1980–1984 to 2000–2004.
Period estimates of 5-year relative survival of patients with ALL by major age groups in defined calendar periods from 1980–1984 to 2000–2004.
Discussion
Our results demonstrate that survival has improved significantly over the past 2 decades for adults diagnosed with ALL. The greatest improvement is observed for the youngest patient population, aged 15 to 19, and for women, but progress is observed for all age groups except the oldest (> 60 years of age) and for both genders.
Outcomes for adults with ALL are generally worse than outcomes for children with ALL. Period analysis of children aged 10 to 14 with ALL shows 5-year relative survival of more than 80% in 2000 to 2004.15 In contrast, in this study, adolescents aged 15 to 19 have a 5-year survival of only slightly more than 60%, and the survival decreases with age, dropping more than 15 points for patients aged 20 to 29. Reasons for this difference in survival include increased toxicity of therapy in adults, making it more difficult to obtain adequate density of treatment and timely administration of consolidation chemotherapy, lower rates of inclusion in clinical trials, and differences in the biology of the leukemia in the adult and pediatric populations, including a higher incidence of Philadelphia chromosome–positive (Ph+) ALL in adults.7,16,17 In addition, adult patients, particularly young adults, may be less likely to have health insurance than children and therefore may be more vulnerable to delays in therapy and incomplete therapy.17,18
Treatment of adolescents and young adults is controversial, but several studies have suggested that patients aged 16 to 21 have better survival rates if treated with pediatric protocols.19 Increased treatment of young adults with ALL using pediatric protocols, which tend to have a more intense use of agents such as vincristine, glucocorticoids, and L-asparaginase,19 as well as more prolonged maintenance therapy,20 may have helped improve the outcomes in this age group.
Philadelphia chromosome positivity occurs in 20% to 30% of adults with ALL (compared with only 3%–5% of children with ALL) and is traditionally associated with a poor prognosis.7,16 Some studies of the use of tyrosine kinase inhibitors suggest that the inclusion of these agents in treatment protocols may lead to improvements in survival of patients with Ph+ ALL in time,19 but the effects of these treatments cannot explain the major improvements in survival that mostly occurred before the period from 2000–2004.
Allogenic stem cell transplantation has been shown to improve survival in standard-risk but not high-risk ALL, and its use may have contributed to the improvement in survival in younger patients (< 35 years of age) over the past several decades.20 Improvements in supportive care and chemotherapeutic protocols as well as improvements in stem cell transplantation protocols, which allow stem cell transplant to be used in older and less healthy patients, may have contributed to the observed improvement in survival as well.
The improvement in survival seen in younger patients does not extend to patients over the age of 60. There may be several overlapping reasons for this. First, the biology of the disease may be different in older patients. Older patients are more likely to have prior malignancies21 and Ph+ ALL than younger patients.22 In addition, older patients are often treated with palliative chemotherapy only, leading to a much lower rate of remission.23,24 Older patients may be less able to tolerate chemotherapy, particularly when multiple comorbidities are present.24 Finally, older patients have, until very recently, generally been excluded from clinical trials, so that treatments tend to be optimized for younger patients.25 Increased registration of older patients in clinical trials, improvements in the treatment of B-cell and Ph+ ALL as specific biologic treatments become available, and improvements in supportive care may lead to improvements in the survival rate for older adults in the next decade.
Clinical trials in ALL conducted in recent years have reported 5-year absolute survival ranging from 28% to 48%, depending on the study population and treatment.26 However, clinical trials necessarily evaluate only a subpopulation of all patients with a given disease. In particular, trial participants are often restricted to younger and generally healthier patients who may have a better prognosis. Only approximately 2.5% of adults diagnosed with malignancy are treated on clinical trials, and several populations including minorities, patients aged 65 or older, and people from lower socioeconomic groups are further underrepresented in NCI-sponsored clinical trials.27 Thus, results from clinical trials may not be representative of results in the general population, and some improvements in survival observed in clinical trials may not translate into better survival in the “average patient,” particularly older patients, with a given condition. Population-based analysis can be used to ensure that changes in survival observed in clinical trials translate to improved survival in the general population of cancer patients. To our knowledge, ours is the only recent study of survival in ALL on a population level. Our results suggest that improvements in survival achieved through clinical trials have translated into improvements on the population level.
In discussing our results, several limitations of the study should be mentioned. Despite being based on the large SEER database, our analysis is limited by rather large standard errors of some of the survival estimates, especially in the age-specific analyses. Because the SEER database does not include information on the use of chemotherapy, participation in clinical trials, or Philadelphia chromosome status, we were unable to include this information in the analysis. Furthermore, although more up-to-date than traditional cohort analysis of survival, even period estimates tend to underestimate survival expectations of newly diagnosed patients to some extent.8,9
In summary, the survival expectations of patients with ALL diagnosed at age less than 60 have substantially improved over time, most likely due to progress in therapy. However, survival in adult ALL remains low compared with survival in children with ALL, and due to the lack of improvement in older patients, the age gradient in survival increased over time. Enhanced therapeutic options for older patients remain a major challenge.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work in this paper was partially supported by a visiting scientist grant from the German Cancer Research Center (DKF2) and a grant from the Cancer Research and Treatment Fund.
Authorship
Contribution: D.P. wrote the paper; A.G. critically reviewed and contributed to the writing of the paper; H.B. designed and performed the research and critically reviewed the paper; and all authors contributed to the analysis of the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Brenner, Division of Clinical Epidemiology & Aging Research, German Cancer Research Center, Bergheimer Strasse 20, D-69115 Heidelberg, Germany; e-mail: h.brenner@dkfz-heidelberg.de.